Abstract
Background
The French RAMSES study is an observational prospective multicentre real-life cohort including severe asthmatic subjects. The objective of the study was to compare the characteristics of patients, in terms of phenotype and asthma care trajectories, between those managed by tertiary referral centres (TRCs) or secondary care centres (SCCs).
Methods
Patients were prospectively recruited and enrolled for a 5-year follow-up. Patients’ characteristics were analysed at inclusion and compared between TRCs and SCCs.
Results
52 centres (24 TRCs and 28 SCCs) included 2046 patients: 1502 (73.4%) were included by a TRC and 544 (26.6%) by a SCC. Patients were mainly women (62%), 53±15 years old, 67% with Asthma Control Test <20; at inclusion, 14% received oral corticosteroids (OCS) and 66% biologics. Compared with the SCC group, the TRC group had more frequent comorbidities and lower blood eosinophil counts (262 versus 340 mm−3; p=0.0036). OCS and biologics use did not differ between groups, but patients in the TRC group benefited more frequently from an educational programme (26% versus 18%; p=0.0008) and received more frequently two or more sequential lines of biologics (33% versus 24%; p=0.0105). In-depth investigations were more frequently performed in the TRC group (allergy tests: 74% versus 62%; p<0.0001; exhaled nitric oxide fraction: 56% versus 21%; p<0.0001; induced sputum: 6% versus 3%; p=0.0390).
Conclusions
Phenotypes and care trajectories differed in the RAMSES cohort between SCCs and TRCs, probably related to different levels of asthma severity and differences in medical resources and practices among centres. This highlights the need for standardisation of severe asthma care.
Shareable abstract
The ongoing French RAMSES cohort includes more than 2000 severe asthma patients, with differences in terms of phenotype and asthma care trajectories between secondary and tertiary referral care centres https://bit.ly/3StCLOY
Introduction
Asthma is a heterogeneous disease characterised by chronic inflammation with airway remodelling and high morbidity. The severe forms of asthma affect 5–10% of an estimated 262 million asthma sufferers worldwide [1]. Severe asthma is defined by the requirement for treatment with high-dose inhaled corticosteroids and a co-controller or systemic corticosteroids for ≥50% of the previous year [2]. The burden of severe asthma appears both clinical and economic.
Severe asthma is a highly heterogeneous disease in terms of phenotypes and endotypes. During the past decade, significant progress has been made in the understanding of its underlying mechanisms. Many of these advances have been achieved through patient phenotyping allowing the development of novel treatments such as biological therapies. Severe asthma registries appear crucial to the global effort to decipher severe asthma mechanisms, identify biomarkers, analyse the real-life effectiveness of therapeutic strategies and participate in the overall improvement of severe asthma management [3, 4]. Recent international severe asthma registries highlighted important differences in treatment strategies across Europe and worldwide [5, 6]. One of the main challenges to face in the next years will be to improve and standardise the delivery of severe asthma care regarding the implementation of treatment strategies and optimisation of comorbidities management in secondary and tertiary care centres.
The RAMSES cohort (Research on Severe Asthma; ClinicalTrials.gov: NCT04077528) is an ongoing study aiming to analyse the use, benefits and risks of Step 5 Global Initiative for Asthma (GINA) treatment strategies in French adults suffering from severe asthma in France. In France, the healthcare system is made up of a fully integrated network of public hospitals, private hospitals and private practice, including secondary and tertiary care centres, among which patients are free to decide where they wish to receive medical care, without financial consideration, since they all belong to the same social security system. The centres participating in the RAMSES study include secondary care centres (SCCs) and tertiary referral centres (TRCs). We hypothesised that although complementary in a shared effort on severe asthma management, SCCs and TRCs may differ in terms of severe asthma population and management, which might be related to geographical location or potential human and material resources. The primary aim of this study was to describe the baseline characteristics of severe asthma patients (including phenotype) and to compare asthma care trajectories between SCCs and TRCs.
Material and methods
Study design
The RAMSES study is a French nationwide observational prospective multicentre cohort that included incident and prevalent severe asthma patients from September 2019 to September 2022. Patient care and choices of treatment were not influenced by participation in RAMSES, reflecting real-life practices. Study participation was open to all pneumologists taking care of severe asthma patients in France. Solicitations were made through the Allergy and Asthma Working Group of the French Society of Pneumology, the national clinical investigation network of severe asthma (CRISALIS) and their contacts. Centres were defined as either SCCs (non-academic general hospitals, or private clinics or practices) or TRCs (university hospitals).
Population and data collection
Adult severe asthmatic subjects defined as per the 2014 European Respiratory Society/American Thoracic Society guideline definition [2] or receiving Step 5 GINA treatment, including long-term oral corticosteroids (OCS), biologics or bronchial thermoplasty, were prospectively recruited and enrolled for 5 years. Baseline and subsequent data collection were scheduled during routine clinical assessments as part of their usual follow-up, usually one visit every 6 months.
Variables
Among all baseline variables collected, we described and compared characteristics and care trajectories of severe asthma patients on key prespecified variables: age at inclusion, gender, body mass index, smoking history, reported comorbidities, asthma characteristics, asthma control score, exacerbations in the past year, lung function (past 12 months), current treatments, treatment adherence as reported by the patient and the investigator, questionnaires assessing asthma-related quality of life and burden (Asthma Quality of Life Questionnaire (AQLQ), Hospital Anxiety and Depression Scale (HADS) and EuroQol EQ-5D-3L), history of asthma-related investigations (blood eosinophils in the past 2 years, antineutrophil cytoplasmic antibody (ANCA), quantitative serum immunoglobulin tests, total IgE, allergy tests, i.e. skin prick test and/or specific IgE, exhaled nitric oxide fraction (FENO), sputum analyses, bronchoscopy and 6-min walk test), and multidisciplinary management (patient education programme and pulmonary rehabilitation) ever performed. Ex-smokers were defined as patients who stopped smoking before the inclusion visit. In case of several measurements available for the same variable in the past 2 years, the highest value was considered for this study. Regarding biological treatment, patients were considered either naive, in a first line of a biologic, or, when the previous line of biologic had to be stopped (e.g. due to side-effects or inefficacy), in a second, third or fourth sequential line of biological treatment.
Statistical analyses
Continuous variables were described by mean with standard deviation or median (interquartile range (IQR)) and categorical variables by frequency (percentage). To compare baseline characteristics or patient care trajectories between types of centres, Pearson's Chi-squared test or Fisher's exact test (for categorical variables) and the t-test or Wilcoxon test (for continuous variables) were performed as appropriate. Correction for multiple comparisons was carried out using the Benjamini–Hochberg method. p-values<0.05 were considered statistically significant (two-tailed test). A mapping comparison was conducted to compare the distance between the patient's home and centre according to the type of centre. Statistical analyses were performed using R version 4.1.0 (www.rstudio.com).
Ethics and regulatory
The study protocol was approved by an independent ethics committee (Comité De Protection Des Personnes Sud-Est IV, ID-RCB: 2018-A03282-53). Before enrolment, all patients were informed and orally consented to participate in the study.
Results
RAMSES cohort
From September 2019 to September 2022, a total of 2046 patients were included in the RAMSES cohort in 52 centres. Patients’ characteristics are detailed in table 1. Briefly, patients were mainly women (62%), with a mean±sd age of 53±15 years. Reported comorbidities were frequent, including chronic rhinosinusitis (58.7%), nasal polyps (42.2%), gastro-oesophageal reflux disease (GORD) (32.8%), obesity (28.1%), allergic rhinitis (17.4%) and a history of anxiety and/or depression (15.6%). Cardiovascular diseases affected 21.3% of the patients and 10.4% suffered from osteoporosis.
TABLE 1.
Baseline patient characteristics
| Total | TRC | SCC | p-value | |
| Patients | 2046 | 1502 | 544 | |
| Age, years | 53.3±15.2 | 53.5±15.3 | 52.7±15.0 | 0.3134 |
| Female | 1271 (62.1) | 931 (62) | 340 (62.5) | 0.8761 |
| BMI, kg·m−2 (n=1983) | 27.5±5.8 | 27.4±5.9 | 27.8±5.6 | 0.1342 |
| <18.5 kg·m−2 | 47 (2.4) | 39 (2.7) | 8 (1.6) | 0.1903 |
| 18.5–24.9 kg·m−2 | 704 (35.5) | 538 (36.6) | 166 (32.3) | |
| 25–29.9 kg·m−2 | 674 (34.0) | 482 (32.8) | 192 (37.4) | |
| ≥30 kg·m−2 | 558 (28.1) | 410 (27.9) | 148 (28.8) | |
| Smoking history (n=1966) | ||||
| Current smoker | 113 (5.7) | 72 (5.0) | 41 (7.9) | 0.0621 |
| Never-smoker | 1121 (57.0) | 843 (58.2) | 278 (53.7) | |
| Ex-smoker | 732 (37.2) | 533 (36.8) | 199 (38.4) | |
| Comorbidities | ||||
| Chronic rhinosinusitis | 1202 (58.7) | 911 (60.7) | 291 (53.5) | 0.0133 |
| Nasal polyps | 863 (42.2) | 662 (44.1) | 201 (36.9) | 0.3914 |
| GORD | 651 (31.8) | 529 (35.2) | 122 (22.4) | <0.0001 |
| Allergic rhinitis | 356 (17.4) | 257 (17.1) | 99 (18.2) | 0.6459 |
| Obstructive sleep apnoea | 298 (14.6) | 219 (14.6) | 79 (14.5) | 0.9776 |
| Anxiety and/or depression history | 319 (15.6) | 240 (16.0) | 79 (14.5) | 0.5447 |
| Osteoporosis | 213 (10.4) | 184 (12.3) | 29 (5.3) | <0.0001 |
| Cardiovascular disease | 435 (21.3) | 323 (21.5) | 112 (20.6) | 0.7225 |
| Diabetes mellitus | 182 (8.9) | 130 (8.7) | 52 (9.6) | 0.6256 |
| Treatment adherence | ||||
| Drug intake as prescribed (n=1105) | 707 (64.0) | 468 (67.7) | 239 (57.7) | 0.0035 |
| Correct technical use of inhaler device | 1614 (78.9) | 1184 (78.8) | 430 (79) | 0.9362 |
| ACT score (n=1966) | 15.9±5.9 | 15.9±5.9 | 15.7±5.8 | 0.5977 |
| <15 | 839 (42.7) | 613 (42.3) | 226 (43.8) | 0.5431 |
| 15–19 | 468 (23.8) | 339 (23.4) | 129 (25.0) | |
| ≥20 | 659 (33.5) | 498 (34.3) | 161 (31.2) | |
| Exacerbations | ||||
| In the past year | 1275 (62.3) | 959 (63.8) | 316 (58.1) | 0.0457 |
| Per patient, n (if at least one per patient) | 4.0±4.1 | 4.0±4.2 | 4.1±3.7 | 0.2238 |
| Emergency room visit in the past year | 400 (19.6) | 266 (17.7) | 134 (24.6) | 0.0022 |
| Hospitalisation in the past year | 367 (17.9) | 241 (16.0) | 126 (23.2) | 0.0011 |
| Intensive care unit, ever | 442 (21.6) | 312 (20.8) | 130 (23.9) | 0.2476 |
| Intubation, ever | 155 (7.6) | 121 (8.1) | 34 (6.2) | 0.3134 |
| Asthma phenotype | ||||
| Blood eosinophils, mm−3 (n=1568) | 290 (100–600) | 261.5 (100.0–570.0) | 340.0 (132.0–692.5) | 0.0036 |
| <150 mm−3 | 501 (32.0) | 393 (33.2) | 108 (28.0) | 0.0068 |
| 150–299 mm−3 | 291 (18.6) | 234 (19.8) | 57 (14.8) | |
| ≥300 mm−3 | 776 (49.5) | 555 (47.0) | 221 (57.3) | |
| FENO, ppb (n=752) | 32.0 (17.0–64.1) | 31.0 (17.0–64.0) | 37.0 (18.1–83.0) | 0.4164 |
| ≥20 ppb | 523 (69.5) | 474 (69.3) | 49 (72.1) | 0.7090 |
| Aeroallergen sensitisation (n=1454) | 793 (54.5) | 582 (52.2) | 211 (62.4) | 0.0036 |
| Lung function | ||||
| FEV1, % pred (n=1712) | 77.1±21.7 | 77.8±21.8 | 75.1±21.6 | 0.0634 |
| FEV1/FVC, % (n=1678) | 67.2±17.7 | 66.9±19.3 | 68.3±11.9 | 0.0296 |
| Fixed airflow limitation (FEV1/FVC <70%) (n=1678) | 936 (55.8) | 719 (57.6) | 217 (50.6) | 0.0345 |
| RV/TLC (n=644) | 77.0±41.3 | 63.2±36.6 | 96.5±39.7 | <0.0001 |
| Asthma burden | ||||
| AQLQ score (n=1950) | 4.7±1.3 | 4.8±1.3 | 4.4±1.3 | <0.0001 |
| EQ-5D-3L score (n=1904) | 0.73±0.27 | 0.74±0.27 | 0.70±0.29 | 0.0120 |
| HADS Anxiety score >7 at inclusion (n=1885) | 742 (39.4) | 545 (39.2) | 197 (39.9) | 0.8398 |
| HADS Depression score >7 at inclusion (n=1911) | 414 (21.7) | 296 (21.0) | 118 (23.5) | 0.3998 |
Data are presented as n, mean±sd, n (%) or median (interquartile range), unless otherwise stated. Unless specified, values are available for all 2046 patients. TRC: tertiary referral centre; SCC: secondary care centre; BMI: body mass index; GORD: gastro-oesophageal reflux disease; ACT: Asthma Control Test; FENO: exhaled nitric oxide fraction; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; RV: residual volume; TLC: total lung capacity; AQLQ: Asthma Quality of Life Questionnaire; EQ-5D-3L: EuroQol EQ-5D-3L; HADS: Hospital Anxiety and Depression Scale. p-values are from Pearson's Chi-squared test or Fisher's exact test (for categorical variables) and the t-test or Wilcoxon test (for continuous variables), as appropriate. Correction for multiple comparisons was carried out using the Benjamini–Hochberg method.
At inclusion, 63.9% of the subjects had uncontrolled asthma (Asthma Control Test (ACT) <20); 62.3% had at least one exacerbation in the past year, with a mean annual exacerbation rate of 4.0±4.1. Type 2 (T2) biomarkers were high in more than half of patients: 49.5% had blood eosinophil counts ≥300 mm−3, 69.5% had FENO levels ≥20 ppb and sensitisation to at least one aeroallergen was identified in 53.4%.
Treatment adherence was considered optimal by investigators in 64% of the patients, the technical use of an inhaler device was correct for 79% of the subjects. Step 5 GINA treatments included long-term OCS (≥6 months in the past year; 14.2%) and biologics (66%), with 12.7% receiving both OCS and biologics at inclusion (table 2).
TABLE 2.
Patient care trajectories
| Total | TRC | SCC | p-value | |
| Patients | 2046 | 1502 | 544 | |
| Time between inclusion and event, years | ||||
| First visit in the centre (n=1977) | 3.3 (0.7–7.6) | 3.1 (0.5–7.1) | 4.0 (1.1–8.9) | <0.0001 |
| Onset of symptoms (n=1342) | 23.0±17.0 | 23.5±17.1 | 21.3±16.4 | 0.0954 |
| Asthma diagnosis (n=1867) | 22.5±17.1 | 22.6±17.2 | 22.0±16.9 | 0.5977 |
| Severe asthma diagnosis (n=1878) | 9.3±10.8 | 10.2±11.4 | 6.9±8.6 | <0.0001 |
| Investigations performed | ||||
| Spirometry | 1995 (97.5) | 1468 (97.7) | 527 (96.9) | 0.4164 |
| Blood eosinophils | 1806 (88.3) | 1328 (88.4) | 478 (87.9) | 0.7910 |
| ANCA | 1145 (56.0) | 896 (59.7) | 249 (45.8) | <0.0001 |
| Quantitative serum immunoglobulin tests | 1561 (76.3) | 1208 (80.4) | 353 (64.9) | <0.0001 |
| Allergy tests (skin prick tests, specific IgE) | 1454 (71.1) | 1116 (74.3) | 338 (62.1) | <0.0001 |
| FENO | 952 (46.5) | 840 (55.9) | 112 (20.6) | <0.0001 |
| 6-min walk test | 145 (7.1) | 110 (7.3) | 35 (6.4) | 0.5977 |
| Bronchoscopy | 395 (19.3) | 299 (19.9) | 96 (17.6) | 0.4101 |
| Induced sputum | 109 (5.3) | 91 (6.1) | 18 (3.3) | 0.0390 |
| Treatment at inclusion | ||||
| Inhaled corticosteroid | 1859 (90.9) | 1398 (93.1) | 461 (84.7) | <0.0001 |
| Long-acting β2-agonist | 1865 (91.2) | 1400 (93.2) | 465 (85.5) | <0.0001 |
| Long-acting muscarinic antagonist | 1109 (54.2) | 834 (55.5) | 275 (50.6) | 0.1041 |
| Montelukast | 699 (34.2) | 475 (31.6) | 224 (41.2) | 0.0003 |
| Long-term azithromycin | 194 (9.5) | 163 (10.9) | 31 (5.7) | 0.0020 |
| Long-term oral corticosteroid | 290 (14.2) | 219 (14.6) | 71 (13.1) | 0.5079 |
| Biologic | 1356 (66.3) | 1010 (67.2) | 346 (63.6) | 0.2407 |
| First line | 944 (69.6) | 681 (67.4) | 263 (76.0) | 0.0105 |
| Second line or higher# | 412 (30.4) | 329 (32.6) | 83 (24.0) | |
| Interventional clinical trial | 178 (8.7) | 146 (9.7) | 32 (5.9) | 0.0214 |
| Bronchial thermoplasty | 32 (1.6) | 30 (2.0) | 2 (0.4) | 0.0272 |
| Multidisciplinary management | ||||
| Patient education (n=1971) | 475 (24.1) | 379 (26.3) | 96 (18.1) | 0.0008 |
| Pulmonary rehabilitation (n=1980) | 412 (20.8) | 348 (24.0) | 64 (12.1) | <0.0001 |
Data are presented as n, mean (interquartile range), mean±sd or n (%), unless otherwise stated. Unless specified, values are available for all 2046 patients. TRC: tertiary referral centre; SCC: secondary care centre; ANCA: antineutrophil cytoplasmic antibody; FENO: exhaled nitric oxide fraction. #: second, third or fourth sequential line of biological treatment. p-values are from Pearson's Chi-squared test or Fisher's exact test (for categorical variables) and the t-test or Wilcoxon test (for continuous variables), as appropriate. Correction for multiple comparisons was carried out using the Benjamini–Hochberg method.
Asthma burden appeared to be important: the patients exhibited a marked alteration in quality of life (AQLQ 4.7±1.3 and EQ-5D-3L 0.73±0.28). The HADS questionnaire revealed symptom scores suggesting anxiety and depression in 39.4% and 21.7% of the patients, respectively (table 1).
Severe asthma in SCCs and TRCs
Among the 2046 patients included, 1502 (73.4%) were included by 24 TRCs and 544 (26.6%) by 28 SCCs (tables 1 and 2, and figure 1).
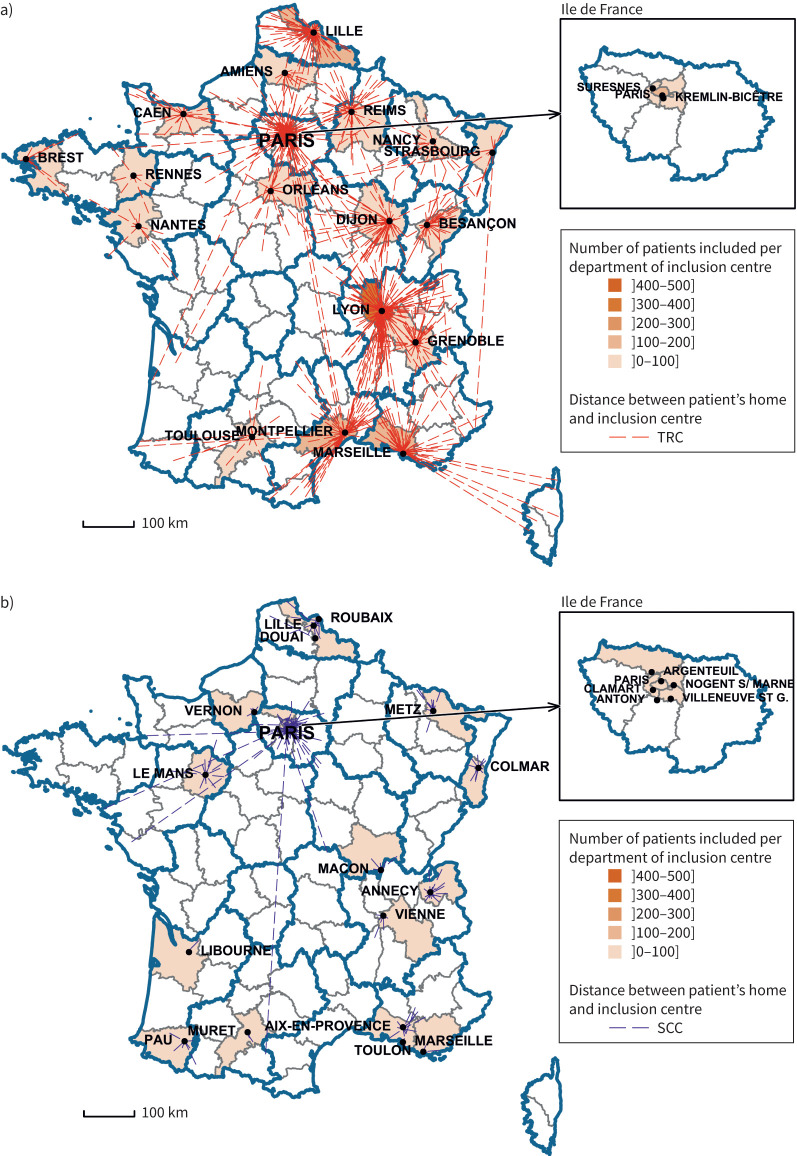
FIGURE 1.
Distance between the patient's home and inclusion centre for a) tertiary referral centres (TRCs) and b) secondary care centres (SCCs) in metropolitan France.
Severe asthma phenotypes in the SCC and TRC groups
We first compared patients’ phenotypes between the SCC and TRC groups. Patients did not differ in terms of age, gender or smoking history, but did differ in terms in comorbidities: compared with the SCC group, the TRC group was characterised by more frequent GORD (35.2% versus 22.4%; p<0.0001), chronic rhinosinusitis (60.7% versus 53.5%; p=0.0133) and osteoporosis (12.3% versus 5.3%; p<0.0001). Asthma control was similar in both groups in terms of ACT scores. However, the proportion of patients requiring an emergency room visit or hospitalisation for severe exacerbation in the past year was higher in the SCC group (24.6% versus 17.7%; p=0.0022 and 23.2% versus 16.0%; p=0.0011 respectively).
The stratification of severe asthma patients depending on blood eosinophil counts per class (<150, 150–299 and ≥300 mm−3) identified different distributions between the TRC and SCC groups (p=0.0068) with more TRC patients exhibiting low blood eosinophil counts. The median blood eosinophil count was lower in the TRC group compared with the SCC group (261.5 versus 340 mm−3; p=0.0036).
A more pronounced alteration of quality of life was observed in the SCC group compared with the TRC group (AQLQ 4.8±1.3 versus 4.4±1.3; p<0.0001 and EQ-5D-3L 0.74±0.27 versus 0.70±0.29; p=0.0120).
Asthma care trajectories in the SCC and TRC groups
We next analysed asthma care trajectories in the SCC and TRC groups. The time between inclusion and first symptoms, and between inclusion and asthma diagnosis, did not differ between groups. The patients in the TRC group had an older diagnosis of severe asthma (median (IQR) 6 (3–13) versus 4 (1–9) years; p<0.0001) and a shorter follow-up in the recruiting centre (3.1 (0.5–7.1) versus 4.0 (1.1–8.9) years; p<0.0001). The median distance between the patient's home and inclusion care centre was 25 (7–67) km for the TRC group and 7 (3–17) km for the SCC group (p<0.0001) (figure 1).
The patients in the TRC group more frequently underwent investigations for severe asthma differential diagnosis or phenotyping, including allergy tests (74.3% versus 62.1%; p<0.0001), FENO measurements (55.9% versus 20.6%; p<0.0001), induced sputum analysis (6.1% versus 3.3%; p=0.0390) or ANCA (59.7% versus 45.8% p<0.0001) (table 2). No differences were observed for blood analyses for eosinophils (88.4% versus 87.9% p=0.7910) or bronchoscopy (19.9% versus 17.6%; p=0.4101).
The analysis of therapeutic management identified different treatment strategies between both groups: at inclusion, patients in the TRC group were more likely to receive long-term azithromycin treatment (10.9% versus 5.7%; p=0.0020) and more frequently benefited from a multidisciplinary care approach, including patient education (26.3% versus 18.1%; p=0.0008) and pulmonary rehabilitation (24.0% versus 12.1%; p<0.0001). Patients in the SCC group were more frequently treated with montelukast (31.6% versus 41.2%; p=0.0003). Although the number of patients with past or current treatment with biologics did not differ between groups, patients in the TRC group received more frequently several sequential lines of biologics compared with the SCC group (32.6% versus 24.0%; p=0.0105). Patients in the TRC group were also more frequently included in a therapeutic clinical trial (9.7% versus 5.9%; p=0.0214).
Discussion
In this study, we present the first description of the RAMSES cohort and point out important differences in terms of severe asthma phenotype and asthma care trajectories between SCCs and TRCs.
RAMSES is currently one of the largest nationwide registries of severe asthmatic subjects in Europe [7–11]. The main demographic and phenotypic features of the RAMSES cohort appear similar to the other national and international registries: female predominance, 50–60 years old, asthma onset in the second decade, T2-high phenotype in half of patients, and most frequent comorbidities including GORD, ear/nose/throat (ENT) diseases and obesity [5, 7–13]. The rate of ex- and current smokers in RAMSES was slightly higher than in the COBRA (38%) and U-BIOPRED (26%) cohorts [9, 13]. Asthma was frequently uncontrolled (66.5% with ACT <20; 42.7% with ACT <15), with a mean of 4 exacerbations in the past year. The objectives of the RAMSES study, focusing on efficacy and security of biologics, and thus favouring the inclusion of patients initiating such treatments, may represent a selection bias for uncontrolled subjects with a recent history of exacerbation, as in the recently published UK Severe Asthma Registry [11]. However, similar rates of uncontrolled asthma were reported in the French academic COBRA (60%) and non-academic FASE-CPHG (70%) cohorts [9, 10], and across European registries included in SHARP (54–100%) [5]. Maintenance OCS use (14.2%) was much lower than in previous severe asthma registries: 34–50% in studies including patients before 2017 [6, 9, 13]. This could be due either to differences between countries in prescription habits over time, in the availability of biologics or maybe in self-limitation in OCS use in the context of the coronavirus disease 2019 (COVID-19) pandemic [14, 15]. Despite a decrease in maintenance OCS use, this 14.2% rate remains important, with several potential side-effects as observed in our population which exhibited frequent long-term corticosteroid-induced or -worsened diseases, including osteoporosis and diabetes, that are known to be associated with the level of the daily dose used [16]. These results highlight the still unmet needs for severe asthma management improvement.
Important differences in severe asthma management exist across Europe and worldwide [5, 6], including differences in national regulations and health system structures [17]. These differences might also be in part due to different human and material resources and biologic availability. As an example, a recent survey focusing on the use of biologics and performed in 28 European countries participating in SHARP identified disparities in the number of biologics available (two to five drugs), the requirement for patient financial contribution in nine of the 28 countries and limitations regarding prescribers, restricted to pulmonologists in four of the 28 countries [18]. Previous studies identified that severe asthma care organisation within dedicated severe asthma centres led to a significant improvement in asthma symptoms [19]. We identified, at a national scale, differences in severe asthma phenotypes between SCCs and TRCs. In France, patients are free to choose their care centre and a referral from a general practitioner, although usual, is not mandatory to access a SCC or TRC. In accordance with French regulations, the initial biologic prescription must be issued by a physician affiliated with a hospital (either secondary or tertiary), while subsequent renewals are permissible through pulmonologists, allergologists, paediatricians, dermatologists, ENT or internal medicine specialists. Non-compliance with this regulation may subject patients to the risk of non-reimbursement of drug costs by the national health insurance. In our study, TRCs appeared to manage more complex severe asthma patients, characterised by a long history of severe asthma, more frequent comorbidities, low blood eosinophil counts and multiple previous lines of biologics. As a result, those TRC-managed patients benefit from more frequent investigations for differential diagnoses or associated comorbidities. It must be pointed out that despite national [20] and international [2, 21] guidelines for severe asthma management, 29% of the subjects did not benefit from allergy testing and 12% were not assessed for blood eosinophils counts. Other phenotyping tools, depending on the local availability of material resources, were more scarcely used, including FENO measurement, which is not reimbursed by health insurance in France (46.5%), or induced sputum (5.3%), with important differences between the TRC and SCC groups, confirming the FASE-CPHG cohort description where 4.4% of the patients were assessed for FENO [10] and reflecting the national disparity of resources.
In addition to different phenotypes, we observed different care trajectories: TRC patients were more likely to receive less conventional treatment strategies, including azithromycin and bronchial thermoplasty, and to participate in clinical trials. A recent meta-analysis including 25 studies versus placebo (about 2000 patients) suggested an effect of macrolides on reducing severe exacerbations and asthma symptoms [22]. However, those studies were performed on highly selected patients. Moreover, long-term macrolide treatment raises concerns about antimicrobial resistance, which is a major public health issue [21]. Such treatment strategy should be discussed on a case-to-case basis in multidisciplinary severe asthma-dedicated meetings, as recommended in France.
Less than 25% of the patients benefited from patient education. Patient education aims to encourage adherence, and provide skills in inhaler device use and self-management, to control symptoms and reduce the risk of exacerbations. It is recommended for all patients with asthma, with a special focus on difficult-to-treat asthma [23]. Among the 52 RAMSES investigation centres, only 21 (40%) were able to provide a patient education programme certified by national health authorities, representing half of TRCs (12 out of 24 (50%)) and one-third of SCCs (nine out of 28 (32%)) involved in the current study [24]. This appears largely undersized, especially in SCCs, given the estimated 4 million people affected by asthma in France, of which 4.5% have severe asthma [25].
Several limitations must be pointed out. First, as already mentioned, the objectives of the RAMSES cohort focusing on efficacy and security of biologics favouring the inclusion of patients initiating such treatments, by centres experienced in severe asthma management, may represent a selection bias for uncontrolled subjects. Second, the reasons why some investigations were not performed were not collected, whether they were deemed unnecessary, too complex to organise or not available locally. In addition, data regarding the history of care centres attended by patients before their inclusion in the cohort were not available. Whether the differences observed in our study can be extended to other European countries, although suggested, remains to be investigated [17]. Despite those limitations, our study reveals that TRCs and SCCs provide complimentary management of severe asthmatic subjects and suggests that a shared effort is necessary to improve severe asthma care.
Conclusion
In conclusion, this first analysis of the RAMSES cohort, one of the largest national registries of severe asthmatic subjects in Europe, identified different phenotypes and care trajectories depending on the type (secondary or tertiary) of the care centre. These results suggest different levels of asthma severity and differences in medical resources and practices among centres. They also highlight the need for standardisation of severe asthma care at a national level.
Acknowledgements
We thank Karima Bourayou, Nessima Yelles, Sarra Pochon, Amal Gouider, Hadj Kaci Medina, Sellali Yasmine, Dahmani Djouher, Diakhou Ndao and Yannick Vacher (AP-HP, Centre de Pharmacoépidémiologie (Cephepi), Paris, France) for project management, regulatory, financial, data monitoring and data management tasks; and all clinical research associates from RAMSES centres and patients participating in the RAMSES cohort.
Provenance: Submitted article, peer reviewed.
RAMSES Study Group: G. Devouassoux, C. Taillé, P. Chanez, P. Bonniaud, A. Bourdin, C. Saint Raymond, C. Maurer, A. Beurnier, P. Roux, V. Margelidon, A. Boudjemaa, G. Mangiapan, N. Freymond, T. Didi, M. Russier, G. Garcia, E. Popin Meyer, C. Dupin, F. Fouquet, S. Jouveshomme, W. Gaspard, S. Dury, S. Habib Maillard, A. Izadifar, E. Cuvillon, G. Deslée, C. Barnig, J-M. Perotin, A.S. Gamez, J.P. Oster, N. Khayat, C. Chenivesse, X. Li, C. Appere de Vecchi, A. Gicquello, H. Rami, G. Vignal, N. Just, X. Blanc, C. Leroyer, L. Wemeau, A. Achkar, C. Sattler, E. Catherinot, L. Guilleminault, M. Gaillot-Drevon, C. Rochefort-Morel, F. Couturaud, P. Martin, A. Chabrol, H. Pegliasco, L. Sese, S. Romanet, B. Caverstri, C. Tcherakian, A. Magnan, E. Ahmed, F. Allibe, G. Beltramo, K. Michaux, N. Paleiron, S. Martinez, C. Begne, C. Tummino, C. Givel, G. Mourin, H. Salvator, M. Volpato, M. Drucbert, N. Rossignoli, S. Keddache, A. Justet, C. Andrejak, J. Valcke, J. Perrin, M. Mercy, M. Jouvenot, T. Soumagne, X. Elharrar, B. Douvry, B. Godbert, B. Maitre, C. Goyard, A. Didier, E. Cadet, F. Chabot, J. Gonzalez, L. Mattei, M. Gouitaa, S. Chauveau, C. Saint Raymond, S. Dirou, S. Fry, A. Briault, A. Moui, A. Paris, E. Noel-Savina, C. Olivier, E. Caradec, N. Roche, G. Picart, L. Belmont, L. Portel, M. Rocca Serra, N. Guibert, R. Jean, S. Hadjadj, S. Guillo, L. Gauquelin, C. Estellat, A. Prigent, M. Larrousse and D. Jaffuel.
Author contributors: Study concept: C. Taillé. Study design: J-M. Perotin, N. Just and C. Taillé. Acquisition of data: J-M. Perotin, N. Just, G. Devouassoux, C. Chenivesse, A. Bourdin, G. Garcia, C. Saint Raymond, A. Boudjemaa, P. Bonniaud, P. Chanez, C. Barnig, A. Beurnier, C. Maurer, N. Freymond, T. Didi, C. Tcherakian, M. Russier, M. Drucbert and C. Taillé. Analysis and data interpretation: J-M. Perotin, L. Gauquelin, S. Guillo, C. Estellat and C. Taillé. Revision of the manuscript: J-M. Perotin, L. Gauquelin, N. Just, G. Devouassoux, C. Chenivesse, A. Bourdin, G. Garcia, P. Bonniaud, S. Guillo, C. Estellat and C. Taillé. Manuscript writing: J-M. Perotin, L. Gauquelin, N. Just, S. Guillo, C. Estellat and C. Taillé.
Conflict of interest: J-M. Perotin reports lecture honoraria from AstraZeneca, support for attending meetings from AstraZeneca and Chiesi, and membership of working groups and associations receiving financial support from AstraZeneca, Chiesi, Novartis and Sanofi; outside the submitted work. N. Just participated or participates as an investigator in clinical trials with Chiesi, and reports honoraria from Chiesi and AstraZeneca. G. Devouassoux reports lecture honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundi Pharma, Novartis Pharma, Vivisol, Sanofi, ALK and Menarini; consultancy fees from AGIR Adom, ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, MSD, Novartis Pharma, Orkyn, Takeda, TEVA, Sanofi and Cipla; has served as a principal investigator and/or study coordinator for AB Science, ALK, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Novartis Pharma, Roche, Regeneron Pharmaceuticals Inc., Sanofi, TEVA, VitalAire, Gossamer and Zambon; and reports research grants from AGIR Adom, ALLP, Chiesi, GlaxoSmithKline, MSD, Novartis Pharma, Orkyn, Takeda and Vivisol. C. Chenivesse reports grants from AstraZeneca, GlaxoSmithKline, Novartis and Santelys, personal fees from ALK-Abello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, and congress support from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis and Sanofi. C. Saint Raymond reports lecture honoraria, support for attending meetings, and membership of working groups and associations receiving financial support from AstraZeneca, Novartis, GlaxoSmithKline and Sanofi. A. Bourdin reports grants from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, AB Science, Celltrion, Cipla, Areteia, Novartis, Sanofi Regeneron and Chiesi, consulting fees, lecture fees and support for meetings from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, AB Science, Celltrion, Cipla, Novartis, Sanofi Regeneron and Chiesi, and participation on an advisory board for AB Science. G. Garcia reports personal fees from AstraZeneca, Sanofi, Novartis, Chiesi and GlaxoSmithKline, and congress support from Sanofi and Oxyvie. A. Boudjemaa reports personal fees from AstraZeneca, Sanofi, Chiesi and GlaxoSmithKline, and congress support from GlaxoSmithKline and Oxyvie. P. Bonniaud reports research grants from AstraZeneca, personal fees from AstraZeneca, Boehringer Ingelheim, Sanofi, Novartis and GlaxoSmithKline, and congress support from AstraZeneca, Sanofi, Boehringer Ingelheim, Stallergenes and Novartis. P. Chanez reports grants, consulting fees, lectures fees and support for meetings from ALK, AstraZeneca, Biopharm, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis and Sanofi-Aventis. C. Barnig reports support for attending meetings from AstraZeneca, GlaxoSmithKline, Sanofi, ALK and Chiesi. A. Beurnier reports lecture fees from AstraZeneca and Sanofi. N. Freymond reports lecture honoraria from AstraZeneca, Sanofi and GlaxoSmithKline, and support for attending meetings from AstraZeneca and Sanofi; outside the submitted work. C. Tcherakian reports a grant from Air Liquide Foundation, lecture fees from AstraZeneca, GlaxoSmithKline, Chiesi, Novartis and Sanofi, and meeting support from Sanofi and GlaxoSmithKline. M. Drucbert reports lecture honoraria from GlaxoSmithKline and AstraZeneca, and membership of working group receiving financial support from AstraZeneca; outside the submitted work. S. Guillo reports no personal fees, congress support or research grants with conflicting interests to disclose other than the funding of the RAMSES cohort. C. Estellat reports no personal fees, congress support or research grants with conflicting interests to disclose other than the funding of the RAMSES cohort. C. Taillé reports lecture or advisory board fees and grants from AstraZeneca, Sanofi, GlaxoSmithKline, Chiesi, Stallergenes and Novartis. All other authors have nothing to declare. There are no further conflicting interests to disclose.
Support statement: The RAMSES cohort is funded by AstraZeneca, GlaxoSmithKline, Sanofi, Boston Scientific, Novartis and the French Pneumology Society (SPLF). The initiative, the statistical analysis plan and the results of the present study have not been submitted for approval to the industrial sponsors funding the RAMSES cohort. Funding information for this article has been deposited with the Crossref Funder Registry.
Ethics statement: The study protocol was approved by an independent ethics committee (Comité De Protection Des Personnes Sud-Est IV, ID-RCB: 2018-A03282-53). Before enrolment, all patients were informed and given the opportunity to decline participation in the study.
References
- 1.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 3.Kupczyk M, Wenzel S. U.S. and European severe asthma cohorts: what can they teach us about severe asthma? J Intern Med 2012; 272: 121–132. doi: 10.1111/j.1365-2796.2012.02558.x [DOI] [PubMed] [Google Scholar]
- 4.Paoletti G, Pepys J, Casini M, et al. Biologics in severe asthma: the role of real-world evidence from registries. Eur Respir Rev 2022; 31: 210278. doi: 10.1183/16000617.0278-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bragt JJMH, Adcock IM, Bel EHD, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J 2020; 55: 1901163. doi: 10.1183/13993003.01163-2019 [DOI] [PubMed] [Google Scholar]
- 6.Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest 2020; 157: 790–804. doi: 10.1016/j.chest.2019.10.053 [DOI] [PubMed] [Google Scholar]
- 7.Senna G, Latorre M, Bugiani M, et al. Sex differences in severe asthma: results from Severe Asthma Network in Italy-SANI. Allergy Asthma Immunol Res 2021; 13: 219–228. doi: 10.4168/aair.2021.13.2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff S, Vanwynsberghe S, Brusselle G, et al. Chronic oral corticosteroids use and persistent eosinophilia in severe asthmatics from the Belgian severe asthma registry. Respir Res 2020; 21: 214. doi: 10.1186/s12931-020-01460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pretolani M, Soussan D, Poirier I, et al. Clinical and biological characteristics of the French COBRA cohort of adult subjects with asthma. Eur Respir J 2017; 50: 1700019. doi: 10.1183/13993003.00019-2017 [DOI] [PubMed] [Google Scholar]
- 10.Portel L, Parrat E, Nocent-Ejnaini C, et al. FASE-CPHG study: a panoramic snapshot of difficult-to-treat, severe asthma in French nonacademic hospitals. ERJ Open Res 2019; 5: 00069-2019. doi: 10.1183/23120541.00069-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansur AH, Gonem S, Brown T, et al. Biologic therapy practices in severe asthma; outcomes from the UK Severe Asthma Registry and survey of specialist opinion. Clin Exp Allergy 2023; 53: 173–185. doi: 10.1111/cea.14222 [DOI] [PubMed] [Google Scholar]
- 12.Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract 2018; 6: 545–554. doi: 10.1016/j.jaip.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46: 1308–1321. doi: 10.1183/13993003.00779-2015 [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Sadatsafavi M, Tran TN, et al. Characterization of patients in the International Severe Asthma Registry with high steroid exposure who did or did not initiate biologic therapy. J Asthma Allergy 2022; 15: 1491–1510. doi: 10.2147/JAA.S377174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porsbjerg CM, Menzies-Gow AN, Tran TN, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract 2022; 10: 1202–1216. doi: 10.1016/j.jaip.2021.12.027 [DOI] [PubMed] [Google Scholar]
- 16.Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J 2018; 52: 1800703. doi: 10.1183/13993003.00703-2018 [DOI] [PubMed] [Google Scholar]
- 17.Santos-Valente E, Buntrock-Döpke H, Abou Taam R, et al. Biologicals in childhood severe asthma: the European PERMEABLE survey on the status quo. ERJ Open Res 2021; 7: 00143-2021. doi: 10.1183/23120541.00143-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frix AN, Heaney LG, Dahlén B, et al. Heterogeneity in the use of biologics for severe asthma in Europe: a SHARP ERS study. ERJ Open Res 2022; 8: 00273-2022. doi: 10.1183/23120541.00273-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redmond C, Heaney LG, Chaudhuri R, et al. Benefits of specialist severe asthma management: demographic and geographic disparities. Eur Respir J 2022; 60: 2200660. doi: 10.1183/13993003.00660-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raherison-Semjen C, Guilleminault L, Billiart I, et al. Mise à jour des recommandations (2021) pour la prise en charge et le suivi des patients asthmatiques adultes sous l’égide de la Société de pneumologie de langue française (SPLF) et de la Société pédiatrique de pneumologie et allergologie (SP2A). Version longue. [Update of the 2021 recommendations for the management and follow-up of adult asthmatic patients under the guidance of the French Society of Pulmonology and the Paediatric Society of Pulmonology and Allergology. Long version]. Rev Mal Respir 2021; 38: 1048–1083. doi: 10.1016/j.rmr.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 21.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55: 1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 22.Undela K, Goldsmith L, Kew KM, et al. Macrolides versus placebo for chronic asthma. Cochrane Database Syst Rev 2021; 11: CD002997. doi: 10.1002/14651858.CD002997.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. Available from: http://ginasthma.org/
- 24.Asthme & Allergies Association . Official list of asthma centres in France. [Liste officielle des écoles de l'Asthme en France.] 2023. https://asthme-allergies.org/liste-officielle-ecoles-de-lasthme-france Date last accessed: 18 February 2024.
- 25.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract 2019; 7: 1477–1487. doi: 10.1016/j.jaip.2018.12.029 [DOI] [PubMed] [Google Scholar]