Abstract
Background
General anaesthesia causes atelectasis, which can lead to impaired respiratory function. Positive end‐expiratory pressure (PEEP) is a mechanical manoeuvre that increases functional residual capacity (FRC) and prevents collapse of the airways, thereby reducing atelectasis. It is not known whether intraoperative PEEP alters the risks of postoperative mortality and pulmonary complications. This review was originally published in 2010 and was updated in 2013.
Objectives
To assess the benefits and harms of intraoperative PEEP in terms of postoperative mortality and pulmonary outcomes in all adult surgical patients.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 10, part of The Cochrane Library, as well as MEDLINE (via Ovid) (1966 to October 2013), EMBASE (via Ovid) (1980 to October 2013), CINAHL (via EBSCOhost) (1982 to October 2013), ISI Web of Science (1945 to October 2013) and LILACS (via BIREME interface) (1982 to October 2010). The original search was performed in January 2010.
Selection criteria
We included randomized clinical trials assessing the effects of PEEP versus no PEEP during general anaesthesia on postoperative mortality and postoperative respiratory complications in adults, 16 years of age and older.
Data collection and analysis
Two review authors independently selected papers, assessed trial quality and extracted data. We contacted study authors to ask for additional information, when necessary. We calculated the number of additional participants needed (information size) to make reliable conclusions.
Main results
This updated review includes two new randomized trials. In total, 10 randomized trials with 432 participants and four comparisons are included in this review. One trial had a low risk of bias. No differences were demonstrated in mortality, with risk ratio (RR) of 0.97 (95% confidence interval (CI) 0.20 to 4.59; P value 0.97; 268 participants, six trials, very low quality of evidence (grading of recommendations assessment, development and evaluation (GRADE)), and in pneumonia, with RR of 0.40 (95% CI 0.11 to 1.39; P value 0.15; 120 participants, three trials, very low quality of evidence (GRADE)). Statistically significant results included the following: The PEEP group had higher arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) on day one postoperatively, with a mean difference of 22.98 (95% CI 4.40 to 41.55; P value 0.02; 80 participants, two trials, very low quality of evidence (GRADE)), and postoperative atelectasis (defined as an area of collapsed lung, quantified by computerized tomography scan) was less in the PEEP group (standard mean difference ‐1.2, 95% CI ‐1.78 to ‐0.79; P value 0.00001; 88 participants, two trials, very low quality of evidence (GRADE)). No adverse events were reported in the three trials that adequately measured these outcomes (barotrauma and cardiac complications). Using information size calculations, we estimated that a further 21,200 participants would have to be randomly assigned to allow a reliable conclusion about PEEP and mortality.
Authors' conclusions
Evidence is currently insufficient to permit conclusions about whether intraoperative PEEP alters risks of postoperative mortality and respiratory complications among undifferentiated surgical patients.
Keywords: Adult; Humans; Anesthesia, General; Anesthesia, General/adverse effects; Pneumonia; Pneumonia/etiology; Pneumonia/mortality; Pneumonia/prevention & control; Positive‐Pressure Respiration; Positive‐Pressure Respiration/adverse effects; Positive‐Pressure Respiration/methods; Postoperative Complications; Postoperative Complications/mortality; Postoperative Complications/prevention & control; Pulmonary Atelectasis; Pulmonary Atelectasis/etiology; Pulmonary Atelectasis/mortality; Pulmonary Atelectasis/prevention & control; Randomized Controlled Trials as Topic; Respiratory Insufficiency; Respiratory Insufficiency/etiology; Respiratory Insufficiency/mortality; Respiratory Insufficiency/prevention & control
Plain language summary
Applying positive pressure at the end of each breath during anaesthesia for prevention of mortality and postoperative pulmonary complications
Review question
We reviewed the evidence on the effects of positive end‐expiratory pressure (PEEP) during general anaesthesia in adult patients 16 years of age and older.
Background
PEEP is a mechanical technique that is often used for ventilating an unconscious patient. The technique involves adding a quantity of pressure into the lungs at the end of each breath. This process causes a degree of deflation in the lungs and can collapse some areas because between breaths, the lungs contain less air than usual. By adding positive pressure at that time, we aim to reinflate the collapsed areas of the lung (atelectasis). Although PEEP can be used during general anaesthesia, some lung areas collapse at the end of the anaesthetic procedure. We do not know whether patients who receive PEEP have lower risks of postoperative mortality (approximately 3% to 5% of adult patients) or respiratory complications. In this review, we aimed to assess the postoperative benefits and harms of using PEEP during general anaesthesia.
Study characteristics
The evidence is current to October 2013. We found 10 randomized clinical trials involving 432 participants. The main limitation of our review was our inability to identify studies analysing intraoperative data.
Key results
Six trials reported mortality. We pooled these data and found no differences between the group of patients who received PEEP and those who did not, but because of the small number of patients, and the fact that this outcome may be rare, these results did not allow us to make a conclusion about the effect of PEEP on mortality. Two results suggested some benefit of PEEP. First, oxygenation was better on the day after surgery in the PEEP group. Second, radiological imaging showed less atelectasis after surgery in the PEEP group. The studies that we found did not suggest that intraoperative PEEP causes harm.
Because of the small number of studies, this finding is inconclusive. We performed calculations to predict how many more participants would be needed before reliable conclusions can be made about the effect on mortality of the application of PEEP. This number was 21,200.
Evidence is currently insufficient to allow conclusions about how intraoperative PEEP affects postoperative mortality and respiratory complications.
Quality of the evidence
The quality of the evidence is very low because of poorly conducted studies, small numbers of participants and low event rates.
Summary of findings
Summary of findings for the main comparison. PEEP compared with zero end‐expiratory pressure (ZEEP) during mechanical ventilation for adult participants given general anaesthesia.
| PEEP compared with zero end‐expiratory pressure (ZEEP) during mechanical ventilation for adult participants given general anaesthesia | ||||||
| Patient or population: adult participants given general anaesthesia Settings: surgery Intervention: PEEP Comparison: zero end‐expiratory pressure (ZEEP) during mechanical ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Zero end‐expiratory pressure (ZEEP) during mechanical ventilation | PEEP | |||||
| Postoperative mortality Follow‐up: mean 30 days | Study population | RR 0.97 (0.2‐4.59) | 268 (6 studies1) | ⊕⊝⊝⊝ very low2,3 | Effect is uncertain | |
| 18 per 1000 | 17 per 1000 (4‐81) | |||||
| Oxygen efficiency (PaO2/FiO2) day 1 postoperative Follow‐up: mean 1 day | Mean oxygen efficiency (PaO2/FiO2) day 1 postoperative in the intervention groups was 22.98 higher (4.4‐41.55 higher) | 80 (2 studies) | ⊕⊝⊝⊝ very low2,4 | ncrease in oxygenation suggests improvement with the intervention (statistically significant). However, this is a surrogate outcome; effect size is very small and a very small number of participants were included in the analysis | ||
| Oxygen efficiency (PaO2/FiO2) day 3 postoperative Follow‐up: mean 3 days | Mean oxygen efficiency (PaO2/FiO2) day 3 postoperative in the intervention groups was 12.59 higher (6.78 lower‐31.96 higher) | 80 (2 studies) | ⊕⊝⊝⊝ very low2,4 | Effect is uncertain | ||
| Pneumonia Follow‐up: mean 7 days | Study population | RR 0.4 (0.11‐1.39) | 120 (3 studies5) | ⊕⊝⊝⊝ very low2,6 | Effect is uncertain | |
| 121 per 1000 | 48 per 1000 (13‐168) | |||||
| Moderate | ||||||
| 50 per 1000 | 20 per 1000 (5‐69) | |||||
| Postoperative atelectasis Using a scale from 0‐67 Follow‐up: mean 7 days | Mean postoperative atelectasis in the intervention groups was 1.29 standard deviations lower (1.78‐0.79 lower)8 | 88 (2 studies) | ⊕⊝⊝⊝ very low2,4 | SMD ‐1.29 (‐1.78 to ‐0.79) Decreased score in the intervention group represents a decrease in atelectasis (statistically significant). However, this is a surrogate outcome and a small number of participants were included in the analysis |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
13 of these trials had zero events and therefore could not be included in the analysis. 2Only 1 of these trials was assessed as having low risk of bias. 3Precision was very low because of the small numbers and the low rate of events (only 268 participants and 4 events). 4Precision was very low because of the small numbers (only 80 participants). 5One of these trials had zero events and therefore could not be included in the analysis. 6Precision was very low because of the small numbers (only 120 participants). 7The Wilcox severity score used in Azab 2005 (the representative study) was used. 8Calculated by multiplying the standardised mean difference by the standard deviation of the control group in the representative study (Azab 2005).
Background
Positive end‐expiratory pressure (PEEP) is an easily implemented intervention that can be used in ventilating a patient with positive pressure ventilation. It is a mechanical manoeuvre by which positive pressure is exerted in the lungs at the end of each exhalation. This end‐expiratory pressure increases the functional residual capacity (FRC) and prevents collapse of the small airways, thereby reducing atelectasis. In patients with healthy lungs, PEEP leads to improved lung compliance, decreased shunting and higher arterial oxygen pressure (PaO2) (Maisch 2008; Maracaja‐Neto 2009; Meininger 2005; Tusman 2004).
General anaesthesia causes a reduction in FRC (Hedenstierna 1985; Hewlett 1974). This reduction is thought to be due to a decrease in inspiratory muscle tone, an increase in abdominal pressure and a change in thoracic blood volume. Movement from the erect position to the supine position, when a patient is lying on the operating table, causes a loss of about 20% of FRC. Induction of anaesthesia usually causes a further loss of 10% (Lumb 2000). Furthermore, general anaesthesia causes atelectasis (Brismar 1985; Eichenberger 2002; Lindquist 1995; Rothen 1998). Ventilatory strategies used in general anaesthesia with high tidal volume, high plateau pressure and no PEEP can cause an inflammatory injury due to atelectasis, even in healthy patients (Tusman 2012). Many other factors may contribute to the development of atelectasis during general anaesthesia. Supine positioning and surgical elements can increase abdominal pressure, leading to direct pressure on the airways and compression atelectasis. Increased abdominal pressure during laparoscopic procedures is one example. Rapid absorption of oxygen, or of nitrous oxide, from occluded airways can cause absorption atelectasis; therefore, both the inspired concentration of oxygen and the use of nitrous oxide may affect the amount of atelectasis (Hedenstierna 2010). Obese patients have greater loss of FRC during anaesthesia, with a linear relationship between increasing body mass index (BMI) and decreasing FRC (Pelosi 1998). Obese patients therefore are likely to have more atelectasis (Eichenberger 2002). Lung disease, patient age and duration of surgery may also be important variables.
Use of PEEP has been shown to be effective in preventing atelectasis during anaesthesia (Brismar 1985; Neumann 1999; Tokics 1987). Atelectasis impairs gas exchange during the intraoperative period (Hedenstierna 1986; Lindberg 1992; Rothen 1998; Tokics 1987), and atelectasis has been hypothesized as a main cause of postoperative hypoxaemia (Hedenstierna 2005). Moreover, atelectasis impairs the clearing of secretions (Hedenstierna 2003) and may impede lymphatic flow (Pearse 2005). Both of these effects may predispose to infection. Atelectasis can continue for several days after general anaesthesia (Hedenstierna 2010). In light of all these factors, it seems likely that an increase in atelectasis may lead to an increase in clinically relevant postoperative adverse outcomes, such as respiratory failure, pneumonia and mortality. PEEP, as an intervention that reduces atelectasis, may reduce the risk of these postoperative outcomes.
PEEP, as with most medical interventions, has the potential to do both harm and good. In damaged lungs, PEEP may cause overdistension of normal lung areas without improvement in abnormal areas (Carvalho 2006; Terragni 2007). So it is possible that PEEP may actually impair respiratory function. The increased intrapleural pressure caused by PEEP may increase the risk of barotrauma and cause changes to cardiovascular dynamics. Cardiovascular effects can change the intraoperative management of a patient's condition, sometimes causing an increase in the need for cardiovascular support. In the context of postoperative implications, which are the focus of this review, these changes may translate into changes in postoperative cardiac risk.
It is unclear how intraoperative PEEP affects postoperative outcomes for adult patients receiving general anaesthesia. This intervention may reduce the risk of postoperative respiratory complications. PEEP is easily implemented, and its use does not result in significant economical costs. Respiratory complications, on the other hand, can lead to large costs in terms of morbidity, mortality and economy (Ferreyra 2009). This review is needed to determine both whether PEEP does indeed reduce postoperative respiratory complications and whether it causes a postoperative increase in harm.
Objectives
To assess the benefits and harms of intraoperative PEEP in terms of postoperative mortality and pulmonary outcomes in all adult surgical patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that evaluated the effects of PEEP versus zero PEEP during general anaesthesia on postoperative mortality and postoperative respiratory complications. We included studies irrespective of language and publication status. We excluded prospective cohort studies and quasi‐randomized studies.
Types of participants
We included adult patients (16 years of age and older) undergoing any kind of surgical procedure with general anaesthesia. We included patients managed with both laryngeal mask airways and endotracheal tubes and patients ventilated both with and without continuous muscle relaxation.
Types of interventions
Participants who had extrinsically applied PEEP of any quantity greater than zero constituted the intervention group. To be included, participants needed to have had PEEP commenced either from induction or immediately post induction and had to have PEEP continued throughout the duration of surgery. The intervention group was compared with a control group. Participants who had zero PEEP (ZEEP) throughout the duration of general anaesthesia constituted the control group. Because we were interested in the effect of PEEP when applied throughout the entire duration of general anaesthesia, we excluded cross‐over studies using both PEEP and ZEEP in individual participants.
Types of outcome measures
Primary outcomes
Mortality, all causes.
We used the longest follow‐up data available in each RCT.
Secondary outcomes
Respiratory failure: defined as an arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) ratio of less than 200.
Oxygen efficiency: defined as the value of the ratio of PaO2/FiO2.
Mechanical respiratory support: defined as intubation and ventilatory support after discharge from the postoperative care unit.
Pneumonia: defined as a new or progressive radiographic infiltrate on chest radiograph that was associated with clinical features of pneumonia, or as defined by the primary investigators.
Atelectasis: defined as an area (percentage) of collapsed lung, quantified by a computerized tomography (CT) scan.
Barotrauma: defined as the clinically diagnosed presence or absence of pneumothorax, pneumomediastinum or subcutaneous emphysema.
Postoperative cardiac complications: defined as clinically diagnosed unstable angina, acute myocardial infarction or acute left ventricular failure requiring pharmacological or invasive support.
Length of stay in post‐anaesthesia care unit (PACU).
Length of stay in hospital.
Postoperative admission to intensive care.
Search methods for identification of studies
Electronic searches
The authors of the original review (Imberger 2010) searched the databases from inception to January 2010.
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 10, part of The Cochrane Library, (see Appendix 1 for the detailed search strategy), as well as MEDLINE (via Ovid, January 2010 to October 2013, see Appendix 2), EMBASE (via Ovid, January 2010 to October 2013, see Appendix 3), CINAHL (via EBSCOhost,bJanuary 2010 to October 2013, see Appendix 4), ISI Web of Science (January 2010 to October 2013, see Appendix 5) and LILACS (via BIREME interface, January 2010 to October 2013, see Appendix 6).
We applied no language restrictions. We combined our subject search terms with the highly sensitive strategies of The Cochrane Collaboration to identify RCTs described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for our search of MEDLINE. We modified this RCT filter for our search of EMBASE. We assessed retrieved studies to look for any free‐text terms or medical subject heading (MeSH) terms for PEEP intensive that had not been used, and we incorporated into the search strategy any new terms that were identified. We used the free‐text terms in all databases and in combination with subject headings when thesauri were a component of a database.
Searching other resources
We screened the reference lists of included trials for additional trials and contacted trials authors when necessary.
Data collection and analysis
Selection of studies
Using the results of the above searches, two review authors independently screened all titles and abstracts for eligibility, documenting the reason for exclusion for each excluded trial. In the original review, Georgina Imberger and David McIlroy undertook the selection of studies (Imberger 2010). Fabiano T Barbosa and Aldemar A Castro independently screened all titles and abstracts for eligibility in the 2013 update. We constructed a list of possible inclusions according to eligibility criteria. Full copies of all selected studies were retrieved, and the studies reviewed for inclusion. When published information was insufficient to allow a decision about inclusion, we attempted to contact the study authors for clarification. We resolved disagreements by discussion between the two review authors.
Data extraction and management
Two review authors (FTB and AAC) independently extracted and collected data on a paper form. A copy of this paper form is provided in Appendix 7. When additional information was required, we attempted to contact the study authors. We resolved discrepancies in extracted data by discussion between the two data‐retrieving review authors.
Assessment of risk of bias in included studies
The same two review authors (FTB and AAC) independently assessed the methodological quality of eligible trials using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and by Jüni (Jüni 2001). A copy of the form used in this assessment is provided in Appendix 8. Discrepancies in this assessment were resolved by a consensus meeting. As the result of changes to the ’Risk of bias’ tool in RevMan 5.2, we reconsidered the risk of bias for all included trials. These changes included separation of blinding of participants and personnel from blinding of outcome assessors. We assessed the risk of bias on the basis of information presented in these papers. We did not contact study authors for clarification. We judged the risk of bias as 'low' or 'high.' We considered 'unclear' risk of bias when available information was insufficient for 'low' or 'high' risk of bias to be considered.
1. Random sequence generation
Low: if sequence generation was reported using computer‐generated random numbers, codes or sealed envelopes. We judged other processes, such as tossing of a coin, as adequate if the whole sequence was generated before the start of the trial, and if it was performed by a person not otherwise involved in participant recruitment.
High: if a system was used that was generated by a non‐random approach (e.g. dates, names, identification numbers).
2. Allocation concealment
Low: if the process that was used prevented participant recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study.
High: if the allocation method allowed participant recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
3. Blinding of participants and personnel
Low: if participants and personnel were reported as blinded.
High: if participants and personnel were not blinded.
4. Blinding of outcome assessors
Low: if outcome assessors were reported as blinded.
High: if outcome assessors were not blinded.
5. Incomplete outcome data
Low: if numbers of withdrawals or exclusions per group, with reasons, were provided, or if the study authors reported no withdrawals or exclusions.
High: if numbers of withdrawals or exclusions per group, with reasons, were not provided, or if study authors did not report reasons for withdrawal or exclusion per group when evident.
6. Selective reporting bias
Low: if all outcomes were reported.
High: if outcomes were measured but not reported.
7. Intention‐to‐treat
We considered intention‐to‐treat (ITT) adequate if all dropouts or withdrawals were accounted for. We considered ITT inadequate if the number of dropouts or withdrawals was not stated, or if the reason for any dropout or withdrawal was not stated.
We considered a trial as having a low risk of bias if random sequence generation, allocation concealment and blinding were assessed as 'low' risk of bias and ITT was assessed as adequate. We considered a trial as having a high risk of bias if one or more of these three criteria were assessed as 'high' risk of bias and ITT was assessed as inadequate.
Measures of treatment effect
We presented categorical data as a risk ratio (RR). We presented numerical comparisons as differences between means. When the unit of continuous measurement was different, we used a standardized mean difference (SMD) as the effect measure. For outcomes with data from more than one trial, we assessed the data qualitatively and then generated a quantitative summary measure. We performed these pooled analyses using Review Manager software (RevMan 5.2) and attempted to perform all analyses according to the ITT principle. No trials were found in which participants crossed over into the alternative intervention group. When data from only a single trial were provided for a given outcome, we calculated estimates of effect using data from the single trial.
Unit of analysis issues
We had no unit of analysis issues.
Dealing with missing data
We contacted the trial authors to clarify missing or unclear data. We attempted to include data on participants who were enrolled and were excluded for a variety reasons. In this case, we followed the ITT principle.
Assessment of heterogeneity
We assessed statistical heterogeneity in the results of trials using tests of heterogeneity. The tests used were the Chi2 test and the I2 statistic; I2 > 50% and Chi2 with a P value > 0.1 implied significant heterogeneity (Higgins 2002).
A random‐effects model seemed appropriate from a clinical perspective because planned comparisons had the potential for at least moderate heterogeneity; the population for inclusion varied, including all types of operations, and the intervention effect was therefore likely to vary across different studies. However, when little information is available, a random‐effects analysis will provide poor estimates of the width of the distribution of intervention effects (Higgins 2002).
Assessment of reporting biases
We planned to assess publication bias and small study effects in a qualitative manner, using a funnel plot (Egger 1997). This plot has an inverted funnel shape if publication bias is absent. If more than nine studies were included in any meta‐analysis, funnel plots were examined visually and by using Egger’s test to look for asymmetry.
We were also concerned about outcome reporting bias, as we knew that many trials looked at this intervention and reported only intraoperative outcomes. For this reason, we carefully reviewed all randomized trials that reported the correct participants and interventions, irrespective of which outcomes were described. In all cases in which relevant outcomes were not reported, we attempted to contact the study authors to confirm that no relevant outcomes had been measured.
Data synthesis
We included small numbers of participants in all of our pooled comparisons, and we used a fixed‐effect model for the meta‐analyses. We had planned to assess statistical heterogeneity using the I2 statistic, thereby estimating the percentage of total variance across studies that was due to heterogeneity rather than to chance (Higgins 2002). We performed the analysis using RevMan 5.2 (RevMan 5.2).
Trial sequential analysis
We planned to perform trial sequential analysis (TSA) (Brok 2008; Thorlund 2009; Wetterslev 2008) for the primary outcome and for two of the secondary outcomes (respiratory failure and pneumonia). In a similar way to using sequential monitoring boundaries in a single trial, this technique aims to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of any conclusions drawn.
As part of this analysis, we planned to calculate the information size required for these three outcomes, thereby obtaining an estimate of how many more participants would be required before a reliable conclusion could be drawn. We used a conventional calculation for sample size estimation, with conventional values for α and β error (0.05 and 0.20) and assuming a two‐sided test. We performed calculations for relative risk reductions of 25%. We planned to use control event rates from our own results in performing these calculations. To calculate an information size as part of TSA, the sample size is multiplied by a 'heterogeneity‐adjustment factor' (Brok 2008; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). We planned to use the heterogeneity (or diversity) in our results to calculate heterogeneity adjustment factors.
Subgroup analysis and investigation of heterogeneity
We planned to conduct analyses on the following subgroups of participants.
Increased age (> 60 years).
Obesity (BMI > 30).
Lung disease (preoperative diagnosis of a chronic lung disease requiring ongoing management).
Increased cardiac risk (one or more clinical risk factors as defined by the American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines (Fleisher 2007)).
Operations of prolonged duration (> four hours).
High inspired oxygen (> 50%).
Use of nitrous oxide.
Laparoscopic procedures.
We planned to perform analyses on the following subgroups of the intervention.
Different values of PEEP (5 cm H2O and 10 cm H2O)
Statistical heterogeneity was estimated by using the Chi2 test and the I2 statistic. Clinical heterogeneity was considered through evaluation of the populations, interventions and outcomes within each study.
Sensitivity analysis
We planned to perform sensitivity analyses of trials with low risk of bias versus those with high risk of bias.
Results
Description of studies
Results of the search
The original search revealed eight trials that met the inclusion criteria (Imberger 2010). During an updated search of electronic databases (January 2010 to October 2013), we identified 560 titles and abstracts. We excluded 552 studies by considering the inclusion criteria. Full papers were retrieved for eight abstracts, and two studies were added. For a summary of the search, see Figure 1. A search of the references of included studies yielded no new studies.
1.
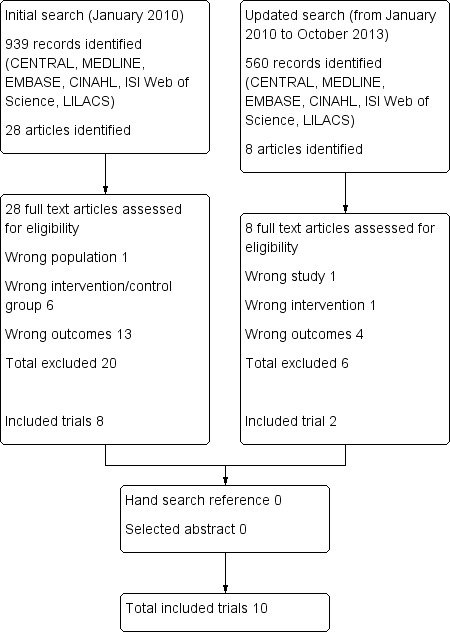
Study flow diagram.
Included studies
We included studies with 432 participants that compared PEEP versus ZEEP in participants 16 years of age or older (Almarakbi 2009; Azab 2005; Berthelsen 1979; Choi 2006; Pang 2003; Severgnini 2013; Talab 2009; Tusman 1999; Weingarten 2010; Wetterslev 2001).
Of the included studies, two included participants with obesity who were undergoing laparoscopic gastric banding (Almarakbi 2009; Talab 2009). Three studies included participants undergoing elective cholecystectomies: one with American Society of Anesthesiologists (ASA) I participants having laparoscopic procedures (Azab 2005); one with ASA II/III participants having laparoscopic procedures (Pang 2003); and one with participants having open procedures (Berthelsen 1979). One study included participants scheduled to undergo surgery for longer than two hours (Severgnini 2013), and the other study included those undergoing surgery for longer than five hours (Choi 2006). Two studies included participants older than 60 years of age who were having surgery that was non‐laparoscopic and was not expected to affect the thorax or the diaphragm (Tusman 1999; Weingarten 2010). One study included participants scheduled for elective upper abdominal surgery (Wetterslev 2001).
We had planned to include all trials in which PEEP was commenced at induction or post induction and was continued throughout the surgery. The control group needed to receive ZEEP. We noted variations in our included trials with regard to the timing of commencement of PEEP, ranging from immediately post induction to 30 minutes after induction. The amount of PEEP varied from 5 cm H2O to 12 cm H2O. Recruitment manoeuvres (involving isolated sustained application of positive pressure to the lungs) were used in six trials (Almarakbi 2009; Pang 2003; Severgnini 2013; Talab 2009; Tusman 1999; Weingarten 2010).
We contacted four authors of the included studies. Weingarten 2010 answered our request. Details of participants and interventions for this study can be found in Characteristics of included studies.
Excluded studies
We excluded 26 studies during the review process. In one case, the population was incorrect. In another case, the study was not a randomized trial. In nine cases, the intervention was incorrect. In most cases—15 studies—review of the entire paper and confirmation with study authors revealed that the outcomes rendered the trials ineligible for our review. In most cases, outcomes were measured only during the intraoperative period.
Details of all exclusions can be found in Characteristics of excluded studies.
Risk of bias in included studies
One study had low risk of bias (Wetterslev 2001). The remaining included studies were assessed as having high risk of bias. See Figure 2 and Figure 3.
2.
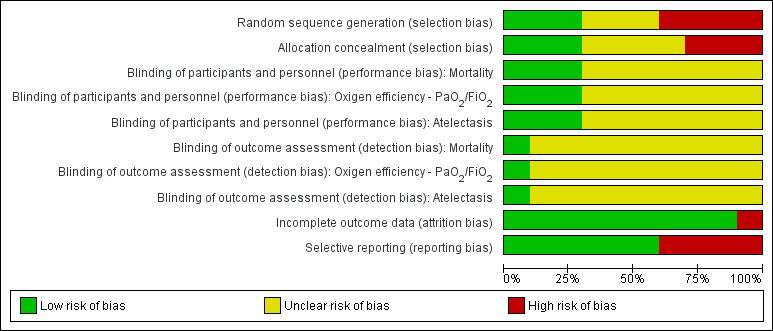
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
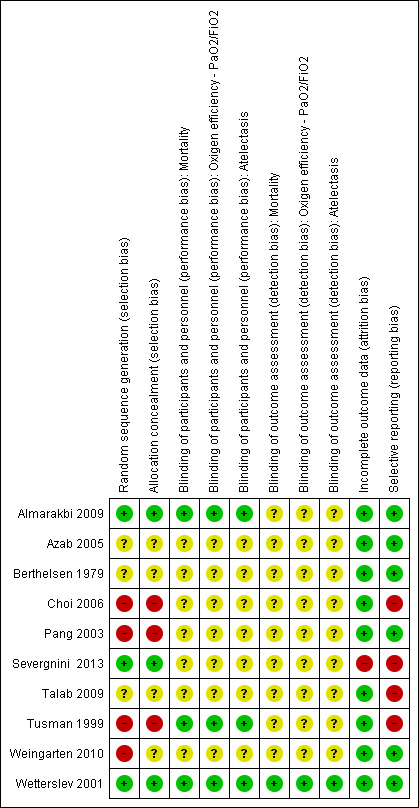
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Generation of allocation sequence was adequately reported in three studies (Almarakbi 2009; Severgnini 2013; Wetterslev 2001). Two studies reported an inadequate technique (Pang 2003; Weingarten 2010), and the remainder did not describe a technique. Allocation concealment was adequately reported in five studies (Almarakbi 2009; Choi 2006; Severgnini 2013; Tusman 1999; Wetterslev 2001) and was not described in the others.
Blinding
We considered blinding of participants and personnel adequate if the authors of the RCTs reported an adequate method of blinding participants and personnel. None of the studies actually stated that participants were blinded to the intervention. PEEP is an intervention that is implemented when a patient is anaesthetized, and it is possible that many patients would not understand what PEEP is, or what its implications could be. Therefore, if a study described that participants were randomly assigned after they were anaesthetized, we thought it reasonable to assume that participants were blinded to the intervention. On the basis of this assumption, two trials were assessed by the review authors as describing adequate blinding (Almarakbi 2009; Wetterslev 2001).
We considered blinding of outcome assessors adequate if authors of RCTs reported this information for all outcome assessors. On this basis, it was determined that one trial described adequate blinding (Wetterslev 2001).
Incomplete outcome data
In six of the 10 trials, all randomly assigned participants were analysed for all relevant outcomes (Almarakbi 2009; Azab 2005; Berthelsen 1979; Pang 2003; Tusman 1999; Weingarten 2010). In Wetterslev 2001, for all of the relevant outcomes reported in this paper, all randomly assigned participants were analysed. But for this study, we also retrieved from the trial authors raw data on two outcomes, which revealed that for four participants, outcome data were incomplete (10% of sample size). These four participants were all accounted for in the text. In Choi 2006, six dropouts were reported (13% of sample size), and all were explained. Analysis was done per protocol. Severgnini 2013 reported one dropout from the analysis, and this was explained (1.8% of sample size). Analysis was done per protocol. In Talab 2009, eight dropouts were reported (12% of sample size); again, all dropouts were explained, but analysis was done per protocol.
Selective reporting
In six of the 10 trials, risk of reporting bias was rated as low (Almarakbi 2009; Azab 2005; Berthelsen 1979; Pang 2003; Weingarten 2010; Wetterslev 2001). Two studies did not report mortality in the methods section (Choi 2006; Severgnini 2013). One study did not report postoperative complications in the methods section (Talab 2009). One study did not report parameters of lung mechanics in the methods section (Tusman 1999).
Other potential sources of bias
We had planned to assess publication bias and small study effects in a qualitative manner, using a funnel plot (Egger 1997). We did not make this assessment because of the small number of trials that were included.
With regard to potential outcome reporting bias, of the 26 trials reviewed in full, 15 did not report outcomes that were relevant for our review. In all 15 cases, we attempted to contact the trial authors to confirm that no relevant outcomes had been measured. In eight cases, we were able to get this confirmation. Details of this process are provided in Characteristics of excluded studies. It is possible that relevant outcomes had been measured in the remaining five trials but were not reported, representing a source of reporting bias.
Summary of risk of bias
One study was judged as having low risk of bias in all domains (Wetterslev 2001). Because details were omitted in eligible papers, we assessed risk as unclear for almost all items in the Risk of bias table for included studies.
Effects of interventions
See: Table 1
See the Summary of findings table for PEEP compared with ZEEP during mechanical ventilation for adult participants given general anaesthesia (Table 1).
1. Mortality
Six trials provided data on postoperative mortality (Almarakbi 2009; Choi 2006; Severgnini 2013; Tusman 1999; Weingarten 2010; Wetterslev 2001). Three of these studies reported zero event rates in both groups and therefore did not contribute to the pooled analysis. The pooled analysis showed no statistically significant difference in risk ratio (RR 0.97, 95% CI 0.20 to 4.59;, P value 0.97; 268 participants, very low quality of evidence) (Analysis 1.1). See also Figure 4.
1.1. Analysis.
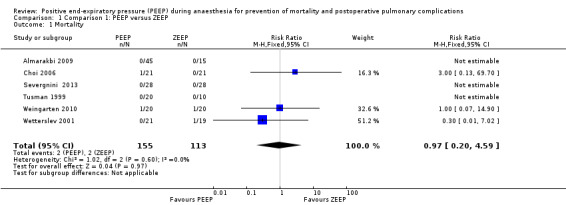
Comparison 1 Comparison 1: PEEP versus ZEEP, Outcome 1 Mortality.
4.
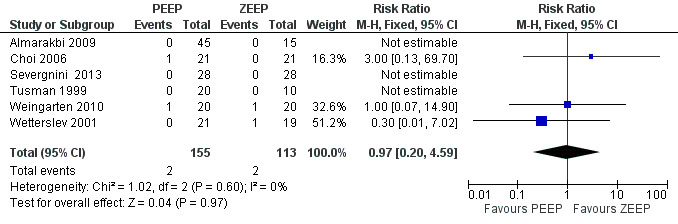
Forest plot of comparison 1.1: mortality.
2. Respiratory failure
We defined respiratory failure as PaO2/FiO2 < 200. This definition was not used in any of the included trials. To measure this outcome for our review, we needed to be able to retrieve individual values of PaO2 with known values of FiO2. Severgnini 2013 defined this outcome while considering acute respiratory distress syndrome. We were able to obtain data for respiratory failure during the first four postoperative days from one trial involving 40 participants (Wetterslev 2001), yielding an RR of 0.18 (95% CI 0.01 to 3.56). See Table 2.
1. Estimates of effect for outcomes reported in only one trial.
| Outcome | Trial | Participants | Effect measure |
Estimate of effect (95% CI) |
| Respiratory failure | Wetterslev 2001 | 40 | RR | 0.18 (0.01‐3.56) |
| Oxygen efficiency in PACU (PaO2/FiO2) |
Weingarten 2010 | 40 | Mean difference | 4.8 (‐3.10 to 12.70) |
| Oxygen efficiency day 2 (PaO2/FiO2) |
Wetterslev 2001 | 40 | Mean difference | 22 (‐9.87 to 53.87) |
| Oxygen efficiency day 4 (PaO2/FiO2) |
Wetterslev 2001 | 40 | Mean difference | ‐1 (‐38.79 to 36.79) |
| Mechanical respiratory support | Wetterslev 2001 | 40 | RR | 0.18 (0.01‐3.56) |
| Cardiac complications | Wetterslev 2001 | 40 | RR | 0.30 (0.01‐7.02) |
| LOS in PACU (minutes) | Talab 2009 | 44 | Mean difference |
‐21.05 (favouring PEEP) (‐37.73 to ‐4.37) |
| LOS in hospital (hours) (laparoscopic gastric banding surgery) |
Almarakbi 2009 | 60 | Mean difference |
‐19.9 (favouring PEEP) (‐27 to ‐12.8) |
| LOS in hospital (days) (major abdominal surgery) |
Weingarten 2010 | 40 | Mean difference | 5.31 (‐1.37 to 11.57) |
| LOS in hospital (hours) (open upper abdominal surgery) |
Wetterslev 2001 | 40 | Mean difference |
24 (favouring ZEEP) (18.2‐29.8) |
| ICU admission | Wetterslev 2001 | 40 | RR | 0.45 (0.04‐4.60) |
FiO2 = fraction of inspired oxygen.
ICU = intensive care unit.
LOS = length of stay.
PEEP = positive end‐expiratory pressure.
RR = risk ratio.
PACU = post‐anaesthesia care unit.
PaO2 = partial pressure of oxygen in arterial blood.
Bold indicates statistically significant findings.
3. Oxygen efficiency
We defined oxygen efficiency as PaO2/FiO2. Five trials measured data that could be used for this outcome (Berthelsen 1979; Pang 2003; Severgnini 2013; Weingarten 2010; Wetterslev 2001). Pang 2003 reported this outcome incorrectly. Severgnini 2013 defined PaO2/FiO2 according to acute respiratory distress syndrome, and these data could not be used. We analysed data from 80 participants. See Figure 5.
5.
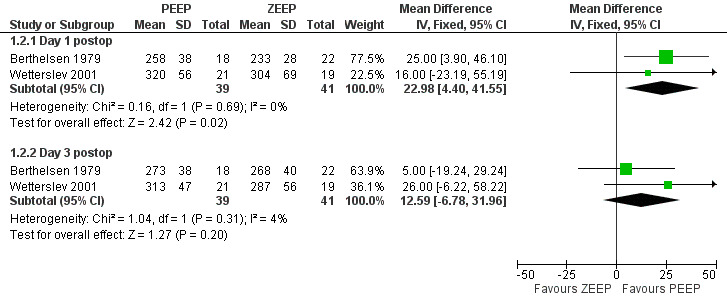
Forest plot of comparison 1.2: oxygen efficiency—PaO2/FiO2.
In the post‐anaesthesia care unit (PACU)
Two studies measured FiO2 and PaO2 in the PACU (Pang 2003; Weingarten 2010). We calculated an effect estimate using the data from Weingarten 2010 involving 40 participants and found a small decrease in the PaO2/FiO2 ratio in the PEEP group; this difference was not statistically significant (mean difference (MD) 4.80, 95% CI ‐3.10 to 12.70) (Table 2) .
Postoperative day one
Three studies measured FiO2 and PaO2 on postoperative day one (Berthelsen 1979; Severgnini 2013; Wetterslev 2001). The pooled analysis showed a statistically significant increase in the PaO2/FiO2 ratio in the PEEP group (MD 22.98, 95% CI 4.40 to 41.55; P value 0.02; 80 participants, two trials, very low quality of evidence (GRADE)) (Analysis 1.2).
1.2. Analysis.
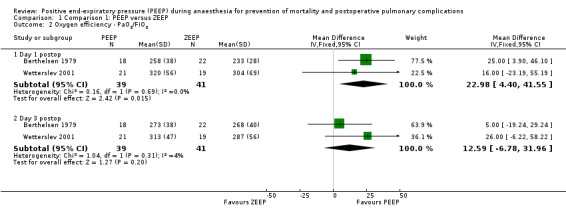
Comparison 1 Comparison 1: PEEP versus ZEEP, Outcome 2 Oxygen efficiency ‐ PaO2/FiO2.
Postoperative day two
One study involving 40 participants measured FiO2 and PaO2 on postoperative day two (Wetterslev 2001). We calculated an effect estimate using the data from this single trial (Table 2) and found an increase in the PaO2/FiO2 ratio in the PEEP group; this difference was not statistically significant (MD 22, 95% CI ‐9.87 to 53.87).
Postoperative day three
Three studies measured FiO2 and PaO2 on postoperative day three (Berthelsen 1979; Severgnini 2013; Wetterslev 2001). The pooled analysis showed an increase in the PaO2/FiO2 ratio in the PEEP group; this difference was not statistically significant (MD 12.59, 95% CI ‐6.78 to 31.96; P value 0.02; 80 participants, two trials, very low quality of evidence) (Analysis 1.2).
Postoperative day four
One study involving 40 participants measured FiO2 and PaO2 on postoperative day four (Wetterslev 2001). We calculated an effect estimate using the data from this single trial (Table 2) and found a small decrease in the PaO2/FiO2 ratio in the PEEP group; this difference was not statistically significant (MD ‐1, 95% CI ‐38.79 to 36.79).
4. Pneumonia
Four studies reported pneumonia as an outcome (Choi 2006; Severgnini 2013; Weingarten 2010; Wetterslev 2001). The event rate was zero in both groups in Choi 2006. This trial did not contribute to the pooled analysis. Severgnini 2013 used a score to report pulmonary complications without stating the number of participants with pneumonia in each group. This trial did not contribute to the pooled analysis. The pooled analysis showed no statistically significant differences (RR 0.40, 95% CI 0.11 to 1.39; P value 0.15; 120 participants, three trials, very low quality of evidence) (Analysis 1.3).
1.3. Analysis.
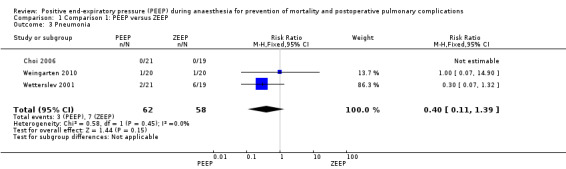
Comparison 1 Comparison 1: PEEP versus ZEEP, Outcome 3 Pneumonia.
5. Mechanical respiratory support
Two studies reported this outcome (Almarakbi 2009; Wetterslev 2001). The event rate was zero in both groups in Almarakbi 2009, so no pooled analysis was done. The data from Wetterslev 2001 involving 40 participants were obtained through correspondence with the study authors. We calculated an estimate of effect (Table 2): The RR for the PEEP group was 0.18 (95% CI 0.01 to 3.56).
6. Atelectasis
Three trials reported measurements of atelectasis as postoperative outcomes (Azab 2005; Severgnini 2013; Talab 2009). Severgnini 2013 measured this outcome using chest radiography. This trial did not contribute to the pooled analysis. Azab 2005 and Talab 2009 measured this outcome using a CT scan done immediately after discharge from the PACU. Both used ordinal scales: Azab 2005 used a six‐point scale, with classifications for the severity of atelectasis taken from the Joyce et al modification of Wilcox severity scoring (Joyce 1995); Talab 2009 used a five‐point scale that included similar radiological classifications (Westcott 1985). These data were treated as quantitative, and the standardized mean difference (SMD) was used as the descriptive parameter. Pooled analysis showed a statistically significant result, with a reduction in the PEEP group of SMD ‐1.2 (95% CI ‐1.78 to ‐0.79; P value 0.00001; 88 participants, two trials, very low quality of evidence) (Analysis 1.4). See also Figure 6.
1.4. Analysis.
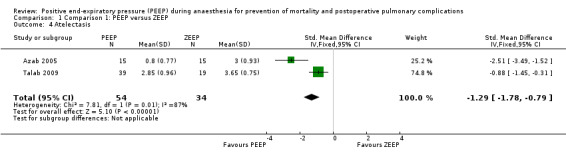
Comparison 1 Comparison 1: PEEP versus ZEEP, Outcome 4 Atelectasis.
6.
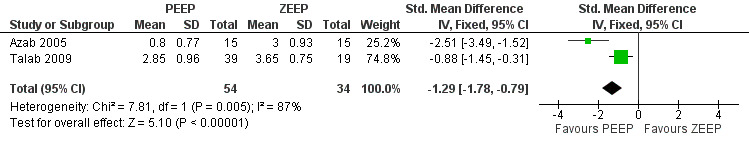
Forest plot of comparison 1.3: atelectasis.
7. Barotrauma
Two trials reported postoperative barotrauma in the PEEP and ZEEP groups (Almarakbi 2009; Talab 2009). In both trials, the event rate was zero in both groups.
8. Cardiac complications
Two studies reported this outcome (Pang 2003; Wetterslev 2001). The event rate was zero in both groups in Pang 2003, so no pooled analysis was done. The data from Wetterslev 2001 involving 40 participants were reported in this paper, and no statistical comparison was performed. We calculated an effect estimate (Table 2), and our comparison revealed an RR of 0.3 for the PEEP group; this result was not statistically significant (95% CI 0.01 to 7.02).
9. Length of stay (LOS) in PACU
Only one study involving 66 participants reported this outcome (Talab 2009). We calculated an effect measure (Table 2) and found a statistically significant difference between the two groups, with the PEEP group in the PACU for 21 minutes less than the ZEEP group (95% CI ‐37.73 to ‐4.37).
10. Length of stay (LOS) in hospital
Four studies reported this outcome (Almarakbi 2009; Severgnini 2013; Weingarten 2010; Wetterslev 2001). Because of clinical and statistical heterogeneity, a pooled analysis was not performed. We calculated effect estimates for three trials (Table 2). In Almarakbi 2009, 60 participants had undergone laparoscopic gastric banding surgery and a significant difference in LOS was reported, with PEEP participants staying for ‐19.9 hours less (95% CI ‐27 to ‐12.8). In Weingarten 2010, 40 elderly participants had undergone major abdominal surgery and no significant difference in LOS was described, with an MD of 5.1 days (95% CI ‐1.37 to 11.57). In Wetterslev 2001, 40 participants had undergone open upper abdominal surgery with a significant difference in LOS, but with the PEEP group staying for 24 hours longer (95% CI 18.2 to 29.8). Severgnini 2013 used a Kaplan‐Meier curve to analyse these data and did not report the LOS in hospital for each group.
11. Intensive care unit (ICU) admissions
Two studies reported this outcome (Talab 2009; Wetterslev 2001). The event rate was zero in both groups in Talab 2009, so no pooled analysis was done. We calculated an effect measure using the data from Wetterslev 2001 (Table 2) and found the RR for the PEEP group to be 0.45 (95% CI 0.04 to 4.60). We analysed data from 40 participants.
Trial sequential analysis (TSA)
Because available data on these outcomes were limited, we were unable to perform TSA. We did, however, calculate the information size required for these three outcomes, thus obtaining an estimate of how many more participants would be required for a reliable conclusion to be drawn.
Mortality
The control event rate in our review was 3.3%. A 25% RR reduction would lower this event rate to 2.5%. Sample size calculation (with α = 0.05 and β = 0.20) yielded a sample size of 7642 participants.
To calculate an information size as part of TSA, the sample size is multiplied by a 'heterogeneity‐adjustment factor' (Brok 2008; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). We had planned to use heterogeneity (or diversity) in our own results to estimate this factor. However, as a result of the small number of trials included in our review, we had to estimate the extent of heterogeneity (or diversity) that might exist as further trials accumulate. On the basis of clinical context, we anticipated that heterogeneity (or diversity) would be at least moderate (D2= 50%) and assigned a value of two as our heterogeneity adjustment factor. With heterogeneity adjustment, the information size for these parameters was therefore estimated at 15,284 participants.
We had planned to use just the control event rates from our own results to perform these calculations. Again, because of the small numbers included in our review, we decided that we needed a more robust estimate of control event rates for mortality. To get this estimate, we surveyed the 76 reviews currently published by the Cochrane Anaesthesia Review Group (CARG 2013) and found that six reviews reported postoperative mortality in participants who had received general anaesthesia, with a total number of 11,497 participants included in these mortality outcomes. The median event rate was 4% (range 0.7% to 20.97%); we used this event rate as our estimate for the control rate for mortality.
When 4% is used as the control event rate for mortality, a 25% RR reduction would lower it to 3%. Sample size calculation (with α = 0.05 and β = 0.20) revealed a sample size of 10,602 participants. With heterogeneity adjustment, the information size was estimated at 21,204 participants.
Respiratory failure
Data for respiratory failure were available from one trial (Wetterslev 2001); three events and 19 participants revealed a control event rate of 16%. A 25% RR reduction would lower this event rate to 12%. Sample size calculation (with α = 0.05 and β = 0.20) yielded a sample size of 2362 participants. With heterogeneity adjustment, the information size was therefore estimated at 4724 participants.
Pneumonia
With a control event rate for pneumonia of 18%, our review determined that a 25% RR reduction would lower it to 13.5%. Sample size calculation (with α = 0.05 and β = 0.20) revealed a sample size of 1640 participants. With heterogeneity adjustment, the information size was therefore estimated at 3280 participants.
Subgroup analyses
We pooled data from six trials for mortality analyses (Almarakbi 2009; Choi 2006; Severgnini 2013; Tusman 1999; Weingarten 2010; Wetterslev 2001). Almarakbi 2009, Severgnini 2013 and Tusman 1999 reported no events in each group. We did not use data from these three trials in this analysis. Data from two trials could be used in participant subgroup analyses (Weingarten 2010; Wetterslev 2001). Weingarten 2010 analysed participants who were older than 60 years of age. Wetterslev 2001 analysed data from participants who received nitrous oxide during general anaesthesia. One trial could be used in intervention subgroup analyses (Choi 2006). Choi 2006 analysed participants who received 10 cm H2O PEEP. Therefore it was not possible to perform any of the planned subgroup analyses.
Data from only two trials were provided on oxygen efficiency, pneumonia and atelectasis. Therefore it was not possible to perform the planned subgroup analyses.
Sensitivity analyses
Sensitivity analysis was planned on the basis of adequate random sequence generation, allocation concealment and blinding by separating studies with an unclear risk from those with a high risk of bias. Mortality data were obtained from six trials (Almarakbi 2009; Choi 2006; Severgnini 2013; Tusman 1999; Weingarten 2010; Wetterslev 2001). Three of the contributing studies reported zero event rates in both groups, and only four deaths were reported among 122 participants. It was not possible to perform the planned sensitivity analyses.
Oxygen efficiency, pneumonia and atelectasis data were available from only two trials. Therefore it was not possible to perform the planned sensitivity analyses.
Discussion
Summary of main results
The focus of our review was to assess the benefits and harms of intraoperative PEEP, for all adult surgical patients, in terms of postoperative mortality and pulmonary outcomes. Evidence was found to be insufficient to support or refute the use of PEEP with respect to postoperative mortality and pulmonary outcomes. The small number of participants and the small event rate were reflected in a risk ratio reduction that was close to one and had wide confidence intervals. The same conclusion can be drawn for the two secondary outcomes that we considered clinically important: respiratory failure and pneumonia. Given the small frequency of events, the small number of included studies and the low quality of most of these studies, available evidence until this moment is graded as very low.
For two secondary outcomes, a pooled analysis revealed a statistically significant benefit in the PEEP group. The first was postoperative atelectasis, for which the results from two trials (Azab 2005; Talab 2009) showed an SMD of ‐1.29 (95% CI ‐1.78 to ‐0.79). Both of these trials measured atelectasis using a CT scan, and both performed these measurements immediately after discharge from postoperative care. Some guides are available on how to interpret the size of SMD effect measurements. One guide suggests that a value greater than 0.8 represents a large effect, while a value less than 0.2 represents a small effect (Cohen 1988), suggesting that we have observed a large difference in atelectasis in the PEEP group. However, our result was based on only two trials, with a total of 88 participants. Empirical evidence suggests that effect size can easily be overestimated in the early stages of a meta‐analysis (Gehr 2006; Ioannidis 2001; Thorlund 2009; Trikalinos 2004), so this result cannot be considered as definitive. Moreover, atelectasis is a surrogate outcome. If PEEP can reduce postoperative atelectasis, this fact does suggest possible clinical benefit. Whether this effect is large or small, the important question remains: Does this reduction in atelectasis lead to meaningful clinical improvements?
The second outcome with a statistically significant result was oxygen efficiency (PaO2/FiO2) on postoperative day one. For this comparison, the pooled analysis from two trials (Berthelsen 1979; Wetterslev 2001) showed a higher PaO2/FiO2 ratio in the PEEP group (MD 22.98, 95% CI 4.40 to 41.55). Three things are important to consider regarding the importance of this finding. First, pooled analyses were done for two time points postoperatively: postoperative day one and postoperative day two. Effect estimates were calculated from single trials for a further three time points: in PACU, on postoperative day two and on postoperative day four. From these five times, only one comparison yielded a significant result. It is possible that we did not find a significant difference at any other time point because of lack of power. However, it may also be true that the single statistically significant result was a chance finding. Second, like atelectasis, PaO2/FiO2 is a surrogate outcome. Improved oxygen uptake into the bloodstream is an encouraging observation. However, it is not necessarily the case that improved oxygenation leads to clinical benefit; in fact, it is possible that this finding is associated with harm. Once again, clinical outcomes need to be correlated with these surrogate findings. Finally, the quantity of the effect needs comment. The lower end of the 95% confidence interval includes a PaO2/FiO2 of 4.40. If a patient is breathing room air, this result equates to an increase of about 1 mm Hg of oxygen in the blood. On the other end of the 95% confidence interval, a value of 41.55 corresponds to an increase of about 9 mm Hg of oxygen in the blood. In a clinical context, this amount of increase in PaO2 is of questionable value.
With regard to potential adverse events, two trials measured barotrauma (Almarakbi 2009; Talab 2009). Both groups in both of these trials recorded zero events. Cardiac complications were reported in only two trials (Pang 2003; Wetterslev 2001). In Pang 2003, zero events were reported in both groups. An effect estimate from Wetterslev 2001 yielded a non‐significant result. Lack of evidence of adverse effects of PEEP is reassuring. However, evidence from randomized trials is insufficient to conclude that PEEP does not cause postoperative harm. Because any potential benefit of PEEP therapy is probably small in an undifferentiated surgical population, even a very low level of increase in adverse events would be clinically meaningful. Given the practical limitations of studying these outcomes with randomized trials, other forms of evidence may be appropriate to guide practical and clinical decision making.
The limitations of this review are that identified studies reported more intraoperative data with low precision. RCT authors have to consider the effects of interventions during follow‐up time if they are to consolidate the validity of their results. Frequency of event rates in this systematic review were small and the range of confidence intervals large, showing low precision in our results. This low precision reduces the confidence that we can have in statistically significant results.
The strengths of this review are that details omitted in papers assessed as having high risk of bias were identified, thus alerting authors of new studies in this area of knowledge to avoid this problem. Random sequence generation and allocation concealment were adequately reported in three studies (Almarakbi 2009; Severgnini 2013; Wetterslev 2001). Random sequence generation is one method that implies that each individual or unit entered into a trial has the same chance of receiving each of the possible interventions (Green 2005). Allocation concealment is the process that does not allow one to know the comparison groups into which participants will be allocated (Green 2005). Included studies used terms such as randomized study or randomization to describe their processes, but these terms are not adequate to describe how sequence generation and allocation concealment were ensured. Blinding protects the sequence of randomization after allocation (Schulz 2002). Only one study correctly reported blinding (Wetterslev 2001). It would be useful if authors of future RCTs will clearly describe the blinding of participants, personnel and outcome assessors.
Overall completeness and applicability of evidence
Our review aimed to assess the postoperative benefit of intraoperative PEEP. Evidence from randomized trials is insufficient to allow any definitive conclusions on this topic. We had planned to perform trial sequential analysis—a method that aims to adjust for the multiplicity caused by repeated updates in systematic reviews (Brok 2008; Thorlund 2009; Wetterslev 2008). Because of the small number of eligible trials, we did not perform this analysis. However, we did use this method to calculate an 'information size,' which predicts the number of extra participants that would have to be randomly assigned to allow a reliable conclusion. We made these calculations for the primary outcome and for the two important clinical outcomes. We calculated that 21,200 more participants would be needed to estimate mortality, 4700 for respiratory failure and 3200 for pneumonia. These numbers are high because our population of interest included all adult patients presenting for general anaesthesia. The baseline risk of postoperative complications is low in this broad population, and a large amount of heterogeneity exists. Both of these factors increase the sample sizes needed to provide enough power to detect true benefit. We used a risk ratio reduction of 25% in our calculations. Given the huge number of patients who could benefit from this intervention, even a small improvement in these postoperative outcomes would be important. It would be reasonable to look for differences smaller than 25%, and this effect size would require even larger numbers. Alternatively, information sizes could be considered for less heterogeneous groups. In a surgical population with increased risk of pulmonary complications, the information size required for the same effect size would be smaller.
Quality of the evidence
We considered the quality of evidence using the measurement of 'Risk of bias' tools. Wetterslev 2001 was assessed to have adequate random sequence generation, allocation concealment and blinding. Wetterslev 2001 used intention‐to‐treat analysis. We considered this study as having low risk of bias. This RCT did not report all prespecified outcomes of this systematic review. Sample size of the included studies was small.
Potential biases in the review process
Different levels of PEEP were tested in included studies, such as four, five, eight, 10 and 12 cm H2O. Some authors of the included studies combined PEEP with an alveolar recruitment manoeuvre. These differing strategies for ventilating a patient could improve lung compliance, decrease shunting and decrease the collapse of the small airways in a different way. We did not consider these differences in our subgroup analysis. Differences in mechanical ventilation strategies during general anaesthesia could affect outcomes.
Agreements and disagreements with other studies or reviews
It seems unlikely that in the near future, enough participants will be randomly assigned to fulfil the information size requirements for reliable answers to questions about mortality and postoperative respiratory complications. Physicians providing anaesthesia care need to make a decision about whether they will use PEEP when they ventilate their patients. In the absence of level one evidence for postoperative clinical outcomes, physicians need to use other information in making this decision. In the setting of intensive care, for patients with existing lung pathology, PEEP is an essential part of ventilatory care (Mercat 2008). In intensive care research, the question now focuses more on how much PEEP should be used (Brower 2004; Phoenix 2009; Villar 2006). The population of patients in intensive care is different from that of all patients receiving general anaesthesia. Differences include the co‐morbidity of patients, the lack of surgical intervention and the duration of mechanical ventilation. It is not reasonable to conclude that demonstrated benefits of PEEP in intensive care ventilation translate into benefits for all patients receiving general anaesthesia. However, it is reasonable to consider the adverse effects of PEEP in the intensive care population. In a recent meta‐analysis comparing high levels of PEEP versus low levels of PEEP in intensive care, no increase in barotrauma was detected (Oba 2009). This finding makes it unlikely that PEEP would cause a meaningful increase in barotrauma in an undifferentiated surgical population.
In the setting of general anaesthesia for surgery, available information does support the use of PEEP. PEEP is able to correct the decrease in FRC that is caused by anaesthesia, thereby preventing atelectasis (Brismar 1985; Neumann 1999; Tokics 1987). Atelectasis causes decreased lung compliance, impaired oxygenation, increased pulmonary vascular resistance and lung injury (Duggan 2005). Evidence shows that PEEP improves intraoperative respiratory function (Clarke 1998; Maracaja‐Neto 2009; Meininger 2005), especially when used in combination with recruitment maneuvers (Dyhr 2004; Maisch 2008; Tusman 2004). Observational evidence also indicates that atelectasis persists well into the postoperative period (Lindberg 1992), especially in obese patients (Eichenberger 2002). Finally, the finding of our systematic review, albeit inconclusive, does suggest that PEEP improves postoperative atelectasis and oxygenation.
Authors' conclusions
Implications for practice.
PEEP is an intraoperative intervention that is easy and cheap to implement. A physiological rationale for the efficacy of PEEP has been demonstrated intraoperatively. Our review aimed to assess whether the benefits of PEEP extend into the postoperative period. We found insufficient evidence to allow review authors to comment on whether PEEP affects the risk of postoperative mortality, respiratory failure or pneumonia. Some evidence suggests that intraoperative PEEP reduces postoperative atelectasis and may improve postoperative gas exchange. Evidence is insufficient to allow a conclusion about whether intraoperative PEEP increases the risk of barotrauma or postoperative cardiac complications.
Current evidence is insufficient to permit conclusions about the postoperative benefits of intraoperative PEEP. Physicians providing anaesthetic care need to make their decision about whether to use PEEP on the basis of known physiological effects, known intraoperative benefits and known benefits for the intensive care population.
Implications for research.
More randomized trials are needed to confirm the postoperative benefit of using intraoperative PEEP. It does seem likely that this intervention improves patient care. However, in the absence of level one evidence, this benefit continues to be unproven, and the potential remains that this intervention may cause adverse effects. Because of the enormous number of patients who receive general anaesthesia and the low rate of serious postoperative complications in an undifferentiated population, very large sample sizes would be needed to demonstrate any benefit and to exclude any adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2014 | New citation required but conclusions have not changed | We found eight new studies. After we assessed the full articles for eligibility criteria, we included two studies in our updated review (Severgnini 2013; Weingarten 2010). These two studies are randomized clinical trials. Severgnini 2013 assessed the effectiveness of protective mechanical ventilation during open abdominal surgery. Weingarten 2010 compared two ventilatory strategies in elderly study participants undergoing major abdominal surgery. These studies focused on assessment of intraoperative outcomes, and they did not assess most of the outcomes listed in this review. The conclusions were not changed. |
| 20 May 2014 | New search has been performed | The previous review authors (Imberger 2010) decided not to update the review.
Three new review authors updated this version: Fabiano T Barbosa, Aldemar A Castro and Célio F Sousa‐Rodrigues. We reran the searches until October 2013. We updated the methods section. We included selective reporting in the risk of bias table. Bias related to blinding of participants and personnel was assessed separately from bias related to blinding of outcome assessment. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 12 October 2010 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Drs Georgina Imberger, David McIlroy, Nathan Leon Pace, Jørn Wetterslev and Jesper Brok, the first review team, who started this systematic review (Imberger 2010).
We thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)) and Karen Hovhannisyan (Trials Search Co‐ordinator (CARG)).
We thank CC Lopes de Jesus, AN Atallah, AT Paes and H Sacconato, who were initially responsible for the concept of this review and developed a preliminary protocol.
We thank Mathew Zacharias (Content Editor); Helen Worthington (Statistical Editor); Stephan Kettner, Felix Ram and Yaseen Arabi (Peer Reviewers for the protocol); Janet Wale (Cochrane Consumer Network Representative); and Rodrigo Cavallazzi and Maureen Meade (Peer Reviewers for the review) for help and editorial advice provided during preparation of the protocol for this systematic review.
We thank Ewa Zasada for assistance with translation.
We thank the following authors for providing extra information about their trials: Jamal Alhashemi, Goda Choi, Göran Hedenstierna, Cher‐Ming Liou, Lennart Magnusson, Toshiki Mizobe, Jamie Sleigh, Esther Wolthuis and Hermann Wrigge.
Appendices
Appendix 1. Search strategy for CENTRAL, part of The Cochrane Library
#1 MeSH descriptor Positive‐Pressure Respiration explode all trees #2 MeSH descriptor Positive‐Pressure Respiration, Intrinsic explode all trees #3 MeSH descriptor Continuous Positive Airway Pressure explode all trees #4 MeSH descriptor Intermittent Positive‐Pressure Ventilation explode all trees #5 MeSH descriptor Intermittent Positive‐Pressure Ventilation explode all trees #6 positive pressure ventil* #7 positive‐pressure ventil* #8 positive airway pressure #9 PEEP* #10 autoPEEP #11 (occult or auto or nontherapeutic) near PEEP #12 positive pressure respirat* #13 positive‐pressure respirat* #14 endexpiratory pressure #15 APRV or CPAP or nCPAP #16 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Anesthesia, General explode all trees #18 MeSH descriptor Anesthesia, Conduction explode all trees #19 MeSH descriptor Anesthesia, Inhalation explode all trees #20 MeSH descriptor Anesthesia, Closed‐Circuit explode all trees #21 general an?est* #22 general near an?esth* #23 an?esthesia:ti,ab #24 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25 (#16 AND #24)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Positive‐Pressure‐Respiration/ 2. exp Positive‐Pressure‐Respiration‐Intrinsic/ 3. exp Continuous‐Positive‐Airway‐Pressure/ 4. exp Intermittent‐Positive‐Pressure‐Breathing/ 5. exp Intermittent‐Positive‐Pressure‐Ventilation/ 6. ((occult or auto or non?therapeutic) adj6 PEEP).mp. 7. (positive pressure adj3 (respirat* or ventil*)).mp. 8. ((positive airway or end?expiratory) adj3 pressure).mp. 9. (APRV or CPAP or nCPAP or PEEP* or auto?PEEP or positive?pressure).mp. 10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 11. exp Anesthesia‐General/ 12. exp Anesthesia‐Conduction/ 13. exp Anesthesia‐Inhalation/ 14. exp Anesthesia‐Closed‐Circuit/ 15. (general adj3 an?esth*).mp. 16. an?esthesia.ti,ab. 17. 11 or 16 or 13 or 12 or 15 or 14 18. 10 and 17 19. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (animals and humans)).sh. 20. 18 and 19
Appendix 3. Search strategy for EMBASE (Ovid SP)
1 exp positive‐end‐expiratory‐pressure/ 2 airway‐pressure/ 3 ((occult or auto or non?therapeutic) adj6 PEEP).mp. 4 (positive pressure adj3 (respirat* or ventil*)).mp. 5 ((positive airway or end?expiratory) adj3 pressure).mp. 6 (APRV or CPAP or nCPAP or PEEP* or auto?PEEP or positive?pressure).mp. 7 1 or 2 or 3 or 4 or 5 or 6 8 exp General Anesthesia/ 9 exp Inhalation Anesthesia/ 10 Anesthesia Induction/ 11 (general adj3 an?esth*).mp. 12 an?esthesia.ti,ab. 13 8 or 11 or 10 or 9 or 12 14 7 and 13 15 (RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/ or (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab.) not (animal not (animal and human)).sh. 16 15 and 14
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 (MM "Continuous Positive Airway Pressure") S2 (MH "Intermittent Positive Pressure Breathing") or (MH "Intermittent Positive Pressure Ventilation") S3 (MH "Positive‐Pressure Respiration, Intrinsic") S4 (MM "Positive End‐Expiratory Pressure") S5 (MH "Positive Pressure Ventilation+") S6 TX positive pressure ventil* S7 TX positive airway pressure S8 TX PEEP* S9 TX positive pressure respirat* S10 TX end‐expiratory pressure S11 TX APRV or CPAP or nCPAP S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 ("anaesthesia") or (MH "Anesthesia, Conduction+") or (MH "Anesthesia, General+") S14 (MM "Anesthesia, Inhalation") S15 TX anesthesia S16 S13 or S14 or S15 S17 S12 and S16 S18 (MH "Random Assignment") S19 TX random* S20 (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies") S21 (MM "Placebos") S22 (MM "Clinical Trials+") S23 S18 or S19 or S20 or S21 or S22 S24 S17 and S23
Appendix 5. Search strategy for ISI Web of Science
# 1TS=(positive SAME pressure) AND TS=ventil* # 2TS=positive airway pressure # 3TS=positive pressure ventil* # 4TS=(airway SAME pressure) # 5TS=PEEP* # 6TS=positive pressure respirat* # 7TS=(positive pressure) SAME TS=respirat* # 8TS=end?expiratory pressure # 9TS=end$expiratory pressure # 10#9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 11TS=an$esth* SAME TS=general # 12TS=an$esth* SAME TS=(general OR conduct* OR closed$circuit OR inhalat*) # 13TS=general an$esth* OR TS=an$esth* general # 14TS=an$esth* conduct* # 15TS=an$esth* inhalat* # 16#15 OR #14 OR #13 OR #12 OR #11 # 17#16 AND #10 # 18TS=random* or TS=clinical trial* or TS=(CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) or TS=MULTICENTER or TS=((SINGL* or DOUBL* or TREBL* or TRIPL*) SAME (BLIND* or MASK*)) # 19TS=(animal* not (animal* and human*)) # 20#18 not #19 # 21#17 and #20
Appendix 6. Search strategy for LILACS (via BIREME interface)
("RESPIRATION, ARTIFICIAL/" or "AIRWAY PRESSURE RELEASE VENTILATION/" or "AIRWAY PRESSURE RELEASE VENTILATION/" or "AIRWAYS" or "PEEP" or "PEEP INTRINSECA/" or "PEEP OCULTA/" or "PEEP, INTRINSIC/" or "PEEP, OCCULT" or "PEEP, OCCULT/" or "PEEP/" or "POSITIVE END‐EXPIRATORY PRESSURE/" or "POSITIVE‐PRESSURE RESPIRATION" or "POSITIVE‐PRESSURE RESPIRATION, INTRINSIC/" or "POSITIVE‐PRESSURE RESPIRATION, OCCULT" or "POSITIVE‐PRESSURE RESPIRATION/" or "VENTILATION, INTERMITTENT POSITIVE‐PRESSURE/" or "VENTILATION, MECHANICAL/") and ("ANESTHESIA/" or "ANESTHETICS, INHALATION/" or "ANESTICIA" or "ANETESIA" or "CLOSED‐CIRCUIT ANESTHESIA/" or "GENERAL ANESTHETICS")
Appendix 7. Data extraction form
PEEP during anaesthesia for prevention of postoperative pulmonary complications and mortality
Data extraction form
| Outcomes | Reported in paper (circle) | Subgroups | Information available in paper (circle) |
| Primary outcome—mortality | Yes/No | PEEP 5 cm H2O | Yes/No |
| Secondary outcomes: | PEEP 10 cm H2O | Yes/No | |
| Outcome 1—respiratory failure | Yes/No | Age > 60 | Yes/No |
| Outcome 2—oxygen efficiency | Yes/No | Obesity | Yes/No |
| Outcome 3—mechanical respiratory support | Yes/No | Participants with lung disease | Yes/No |
| Outcome 4—pneumonia | Yes/No | Participants with increased cardiac risk | Yes/No |
| Outcome 5—atelectasis | Yes/No | Operations > 6 hours | Yes/No |
| Outcome 6—barotrauma | Yes/No | FiO2 > 50% | Yes/No |
| Outcome 7—postoperative cardiac complications | Yes/No | Use of nitrous oxide | Yes/No |
| Outcome 8—LOS in PACU | Yes/No | Laparoscopic procedure | Yes/No |
| Outcome 9—LOS in hospital | Yes/No | ||
| Outcome 10—postoperative admission to intensive care | Yes/No |
| For continuous data (with a separate copy for each relevant subgroup) | |||||||
| Code of paper |
Outcomes |
Unit of measurement |
Intervention group | Control group | Details if outcome described only in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Outcome 2 Oxygen efficiency |
|||||||
| Outcome 5 Atelectasis |
|||||||
| Outcome 8 LOS in PACU |
|||||||
| Outcome 9 LOS in hospital |
|||||||
| For dichotomous data (with a separate copy for each relevant subgroup) | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Primary outcome Mortality |
|||
| Outcome 1 Respiratory failure |
|||
| Outcome 3 Mechanical respiratory support |
|||
| Outcome 4 Pneumonia |
|||
| Outcome 5 Atelectasis |
|||
| Outcome 6 Barotrauma |
|||
| Outcome 7 Postoperative cardiac complications |
|||
| Outcome 10 Postoperative admission to intensive care |
|||
| Trial characteristics | |
| Further details | |
| Single centre/multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? |
|
| How many participants were randomly assigned? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/frequency of administration | |
| Duration of treatment (state weeks/months, etc; if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan 5.2 | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Appendix 8. Quality assessment of eligible trials form
PEEP during anaesthesia for prevention of postoperative pulmonary complications and mortality
Methodological quality
Trial:
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Comment on allocation by review authors or included study quote concerning allocation: |
Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Comment on allocation concealment by review authors or included study quote concerning allocation: | Adequate |
| Inadequate | |
| Unclear | |
| Blinding | |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
| Comment on blinding by review authors or included study quote concerning allocation: | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it. | |
| All participants entering trial | |
| 15% or less excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes No Not clear
Discuss if appropriate
Data and analyses
Comparison 1. Comparison 1: PEEP versus ZEEP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.20, 4.59] |
| 2 Oxygen efficiency ‐ PaO2/FiO2 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 1 postop | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 22.98 [4.40, 41.55] |
| 2.2 Day 3 postop | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 12.59 [‐6.78, 31.96] |
| 3 Pneumonia | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.39] |
| 4 Atelectasis | 2 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.78, ‐0.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almarakbi 2009.
| Methods | Parallel randomized controlled trial Low risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | SIngle centre Follow‐up was continued until discharge, and the study authors reported no major adverse events. We thought it reasonable to assume from these statements that no mortality occurred in either group during admission Could be included in the following subgroup analyses: PEEP 10 cm H2O, obesity, laparoscopic procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomized, using a computer‐generated randomization schedule and sealed opaque envelopes..." Comment: clear |
| Allocation concealment (selection bias) | Low risk | Quote: as above Comment: clear |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Quotes: "... Ten minutes after the pneumoperitoneum... patients were randomized...", "... patients were discharged from hospital at the discretion of the surgeon who was blinded to patient's group assignment..." Comment: Given that participants were anaesthetized when the intervention was randomly assigned and implemented, we are assuming that participants were blinded |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Low risk | Quotes: "... Ten minutes after the pneumoperitoneum... patients were randomized...", "... patients were discharged from hospital at the discretion of the surgeon who was blinded to patient's group assignment..." Comment: Given that participants were anaesthetized when the intervention was randomly assigned and implemented, we are assuming that participants were blinded |
| Blinding of participants and personnel (performance bias) Atelectasis | Low risk | Quotes: "... Ten minutes after the pneumoperitoneum... patients were randomized...", "... patients were discharged from hospital at the discretion of the surgeon who was blinded to patient's group assignment..." Comment: Given that participants were anaesthetized when the intervention was randomly assigned and implemented, we are assuming that participants were blinded |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Azab 2005.
| Methods | Parallel randomized controlled trials High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre Study authors did not report arterial blood gas data in text Could be included in the following subgroup analyses: PEEP 5 cm H2O, laparoscopic procedures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... patients were randomly allocated into two groups..." Comment: not described adequately |
| Allocation concealment (selection bias) | Unclear risk | Quote: as above Comment: not described |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: unclear whether participants were aware of intervention groups |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: unclear whether participants were aware of intervention groups |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: unclear whether participants were aware of intervention groups |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: unclear whether all outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: unclear whether all outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Quote: "...CT scan of the chest was performed by a radiologist who was unaware of the experimental protocol and patient assignment..." Comment: Unclear whether all outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Berthelsen 1979.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre Oxygen efficiency was also measured on postoperative day 5. 11 participants were missing from this day 5 measurement: 8 from the PEEP group and 3 from the ZEEP group. It was not clear why these data were missing. Given that participants had an elective open cholecystectomy, all may be explained by the fact that participants were discharged home. Given the disparity between the number of dropouts in each group and the consequential potential for confounding, this comparison was not included in our review Could be included in the following subgroup analyses: PEEP 10 cm H2O and use of nitrous oxide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... the patients were then allocated randomly to one of two groups..." Comment: not described adequately |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... the patients were then allocated randomly to one of two groups..." Comment: not described |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | No description of blinding |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | No description of blinding |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | No description of blinding |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | No description of blinding |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | No description of blinding |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed up until day 3 postop. After this time, a substantial reduction in numbers was seen, more so in the PEEP group. No reason for this reduction was provided. Presumably, it can be explained by discharge of participants. Data after day 3 were not used in our analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Choi 2006.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre Mortality was not specifically described as an outcome. 1 death was described; it was stated clearly that all other participants in the trial were discharged home Could be included in the following subgroup analyses: PEEP 10 cm H2O |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...Randomization was performed by drawing a presealed envelope..." Comment: inadequate sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: as above Comment: sealed envelopes, but allocation concealment would have been compromised towards the end, given the randomization technique |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants analysed |
| Selective reporting (reporting bias) | High risk | Mortality not described in methods section |
Pang 2003.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre Could be included in the following subgroup analyses: PEEP 5 cm H2O, use of nitrous oxide, laparoscopic procedure We did not localize the study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...Patients were randomized..., using shuffled envelopes" Comment: inadequate sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: as above Comment: Allocation concealment would have been compromised towards the end, given the randomization technique |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Severgnini 2013.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | SIngle centre
The ratio PaO2/FiO2 was reported in light of acute respiratory distress syndrome
Atelectasis was analysed by chest x‐ray
Authors used modified Clinical Pulmonary Infection Score and did not report the number of participants with pneumonia Pulmonary complications included cough, increased secretions, dyspnoea, chest pain, temperature greater than 38°C and pulse rate greater than 100 beats/min Mortality was not specifically described as an outcome Could be included in the following subgroup analysis: PEEP 10 cm H2O We emailed the study authors to remove our doubts. The study authors did not respond to our request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... to select patients for treatment we generated a randomization list by Random Allocation Software (Windows software, version 1.0, May 2004, Saghaei, licensee BioMed Central Ltd.) (allocation ratio 1:1) and inserted the group‐identification paper in envelopes, which were then sealed and clouded to not reveal allocations" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: as above Comment: adequate |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Quote: "... blinded way by an independent specialist in radiology, who was not involved in the study" Comment: only radiologists reported |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Quote: "... blinded way by an independent specialist in radiology, who was not involved in the study" Comment: only radiologists reported |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Quote: "... blinded way by an independent specialist in radiology, who was not involved in the study" Comment: only radiologists reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant in the intervention group was excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Mortality was not reported in the methods section |
Talab 2009.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre The paper presented means and standard deviations of LOS in PACU for the ZEEP group, the PEEP group (5 cm H2O) and the PEEP group (10 cm H2O). We contacted the study authors to attempt to obtain the raw data and calculate an accurate standard deviation for both PEEP groups pooled together. We were unable to get this information and therefore used the ZEEP group and the PEEP group (10 cm H2O) for our estimate of effect This trial also measured PaO2 in the PACU. Investigators used these data to calculate alveolar‐to‐arterial oxygen gradients, so they must also have recorded FiO2. These data could have contributed to 2 further outcomes in our review: respiratory failure and oxygen efficiency. Unfortunately, we were unable to get this information from the authors of the paper. Could be included in the following subgroup analyses: PEEP 5 cm H2O, PEEP 10 cm H2O, obesity, FiO2 > 50%, laparoscopic procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...We randomly allocated 66 adult obese patients..." Comment: not described adequately |
| Allocation concealment (selection bias) | Unclear risk | Quote: as above Comment: not described |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: no information about participant blinding |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: no information about participant blinding |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: no information about participant blinding |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: inclear whether all assessors were blinded |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: unclear whether all assessors were blinded |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Quote: "... CT scans were interpreted by radiologists who were aware of the experimental protocol but unaware of patient group assignment..." Comment: unclear whether all assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants were excluded after randomization (12%): 3 from the ZEEP group and 5 from the PEEP group. Reasons for exclusion were well described, including 2 participant refusals (both from the PEEP group) and surgical complications, which prevented performance of CT (3 in each group) |
| Selective reporting (reporting bias) | High risk | Postoperative complications were not described in the methods section |
Tusman 1999.
| Methods | Parallel randomized controlled trial High risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre The study authors stated that all participants were followed up until discharge and that no complications occurred in any participant. We thought it reasonable to assume from these statements that no mortality occurred in either group during the admission Could be included in the following subgroup analyses: PEEP 5 cm H2O, age > 60 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...patients... were randomized prospectively to one of three groups of 10 by opening sealed envelopes..." Comment: inadequate sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: as above Comment: Allocation concealment would have been compromised towards the end, given the randomization technique |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Low risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Blinding of participants and personnel (performance bias) Atelectasis | Low risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Random assignment occurred after participants were anaesthetized. No comment was made about blinding of the outcome assessor. BUT only outcome used in our review was mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants analysed |
| Selective reporting (reporting bias) | High risk | Parameters of lung mechanics not described in the methods section |
Weingarten 2010.
| Methods | Parallel randomized controlled trial High risk of bias | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | SIngle centre Atelectasis was analysed by chest x‐ray Study authors defined postoperative respiratory failure as unanticipated mechanical ventilation for > 48 hours after operation or the need for reinstitution of mechanical or non‐invasive ventilation after extubation Could be included in the following subgroup analyses: increased age (> 60 years) We emailed the study authors to remove our doubts. Study authors provided data about length of hospital stay | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomized to one of the two ventilatory management strategies using a randomization schedule provided by the Division of Biostatistics" Comment: not clear |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Mortality | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Atelectasis | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Atelectasis | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Wetterslev 2001.
| Methods | Parallel randomized controlled trial Low risk of bias |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Single centre Some missing data in the raw results used. As per our protocol, we analysed these data according to the ITT principle. Data were missing for 4 participants (10% sample size); 2 from the PEEP group and 2 from the ZEEP group. All 4 dropouts were explained in the paper In the ZEEP group, 1 participant refused any further blood sampling from day 2 onwards. In the PEEP group, 1 participant was discharged before the measurement could be made on day 4. We used the last observation carried forward method for both of these participants 2 participants were unable to have their blood sampled while breathing room air because 1 was being mechanically ventilated and 1 had a very low PaO2 despite supplemental oxygen. 1 of these participants came from the PEEP group and 1 from the ZEEP group. Given the description of clinical conditions, we believed it was reasonable to assume that both of these participants were in respiratory failure and would have had a PaO2/FiO2 ratio of less than 200. Therefore, both were assigned a ratio of 199 for our analysis. This number was chosen as it fulfilled our criteria for respiratory failure Could be included in the following subgroup analysis: use of nitrous oxide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patients were randomly allocated, by means of sealed envelopes numbered according to computer‐generated random digits..." Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: as above Comment: adequate |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that participants were blinded |
| Blinding of participants and personnel (performance bias) Oxigen efficiency ‐ PaO2/FiO2 | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that participants were blinded |
| Blinding of participants and personnel (performance bias) Atelectasis | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that participants were blinded |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Oxigen efficiency ‐ PaO2/FiO2 | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Atelectasis | Low risk | Quotes: "... The investigators were unaware of the results, an the surgeons (unaware of ... perioperative PEEP/ZEEP status) diagnosed and recorded any complications...which needed treatment, or death, and the length of stay in hospital..." Comment: Description of outcome assessor blinding was adequate. Methods section clearly defines that participants were anaesthetized when the intervention was randomly assigned and implemented. We therefore assumed that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For most of our outcomes, outcome data were available for all randomly assigned participants. For our outcome of oxygen efficiency, we used raw data provided by the study authors. These data revealed 5 dropouts. The reasons for 4 of these dropouts were described in the paper. (See Outcomes section above for details) |
| Selective reporting (reporting bias) | Low risk | All outcomes expected reported |
ASA = American Society of Anesthesiologists classification system.
BMI = body mass index.
COAD = chronic obstructive airways disease.
CT = computerized tomography scan.
FiO2 = fraction of inspired oxygen.
ICP = intracranial pressure.
ICU = intensive care unit.
ITT = intention‐to‐treat.
LOS = length of stay.
M/F = male/female.
PaO2 = partial pressure of oxygen in arterial blood.
PACU = post‐anaesthesia care unit.
PEEP = positive end‐expiratory pressure.
VTE = venous thromboembolism.
ZEEP = zero end‐expiratory pressure.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Clarke 1998 | Wrong outcomes Alveolar‐arterial oxygen gradients measured postoperatively were reported. However, no inspired oxygen fractions were reported, so these measurements did not fulfil our outcome criteria for oxygen efficiency or respiratory failure. We emailed the study authors and confirmed that they did not have the extra data to allow us to include this trial |
| Cohendy 1994 | Wrong intervention PEEP was not continued throughout surgery |
| Coussa 2004 | Wrong intervention PEEP was continued for only five minutes. Confirmed by email correspondence with study author |
| Dyhr 2002 | Wrong population Looking at patients in ICU, after cardiac surgery |
| Futier 2010 | Wrong study Participants were not randomly assigned. |
| Gander 2005 | Wrong intervention PEEP was not continued throughout surgery |
| Goldmann 2011 | Wrong outcomes This paper mainly reports intraoperative outcomes |
| Grzybowska 2005 | Wrong outcomes This paper was read by a Polish speaker (Ewa Zasada), who confirmed that no postoperative outcomes were reported in the text. An attempt was made to contact study authors via email to confirm that no relevant outcomes were measured, unfortunately with no success |
| Khanam 1973 | Wrong outcomes No postoperative outcomes reported. Written mail sent to the correspondence address. No reply, which was not surprising, given the date of the paper |
| Kim 2010 | Wrong outcomes This paper mainly reports intraoperative outcomes |
| Kim 2013 | Wrong intervention Effects of inspiratory time on pulmonary gas exchange and respiratory mechanics were analysed |
| Liou 1993 | Wrong outcomes No postoperative outcomes reported in the paper. Email correspondence with study author confirmed that no postoperative outcomes were measured |
| Meininger 2005 | Wrong outcomes No postoperative outcomes reported in the paper. Paper clearly describes that last measurements were taken while participants were still on the operating table. An email was sent to study authors to confirm that no unpublished postoperative measurements were made. We did not receive a reply |
| Mizobe 2005 | Wrong outcomes This paper reported the effects of PEEP (and clonidine) on intraoperative thermodynamics. We contacted study authors to make sure that they had not made any postoperative measurements that were relevant to our review. Email correspondence with the lead study author confirmed that no relevant outcomes were measured |
| Nabil 2005 | Wrong outcomes This paper reports mainly intraoperative outcomes. Study authors state that no pulmonary or haemodynamic complications occurred. However, they do not define how this measurement was made, or over what time period. They also state that no barotrauma occurred, but CXRs were taken only in the intervention group. We were unable to find an email contact for any of the study authors. A letter was posted to the corresponding address to try to clarify the above. However, we received no reply. It was therefore decided that these data should be excluded |
| Neumann 1999 | Wrong outcomes No postoperative outcomes reported. Email correspondence with one of the study authors (Hedenstierna) confirmed that no postoperative measurements were made |
| Park 2009 | Wrong intervention PEEP not continued throughout surgery |
| Reinius 2009 | Wrong outcomes No postoperative outcomes reported. It was clearly stated in the paper that final measurements were taken 40 minutes after the intervention, when participants were still anaesthetized. Email correspondence with corresponding author confirmed that no postoperative measurements were taken |
| Rusca 2003 | Wrong intervention PEEP applied only during induction |
| Russo 2013 | Wrong intervention No postoperative outcomes reported |
| Turgut 2007 | Wrong outcomes This paper reported pneumocephalus as the study outcome. We wanted to be sure that no postoperative outcomes relevant to our review had also been measured, most likely mortality Such outcomes were not reported in the text. We emailed the corresponding study author but received no reply |
| Wolthuis 2008 | Wrong outcomes This paper looked at pulmonary inflammation intraoperatively as the study outcome. No outcomes relevant to our review were reported in the paper. We emailed the corresponding study author, who confirmed that no relevant outcomes were measured |
| Wrigge 2000 | Wrong outcomes This paper looked at inflammatory responses as study outcomes. No outcomes relevant to our review were reported in the paper. We emailed the corresponding study author, who confirmed that no relevant outcomes were measured |
| Wrigge 2004 | Wrong outcomes This paper looked at inflammatory responses as study outcomes. No outcomes relevant to our review were reported in the paper. We emailed the corresponding study author, who confirmed that no relevant outcomes were measured |
| Ye 2011 | Wrong intervention No postoperative outcomes reported |
| Yu 2006 | Wrong intervention Cross‐over study. PEEP was not continued throughout surgery |
ICU = intensive care unit.
PEEP = positive end‐expiratory pressure.
ZEEP = zero end‐expiratory pressure.
Differences between protocol and review
In the 2013 update, the review team changed from Imberger G, McIlroy D, Pace NL, Wetterslev J, Brok J and Møller AM to Barbosa FT, Castro AA and Sousa‐Rodrigues CF.
We included one item in the Risk of bias table: selective reporting.
The bias related to blinding of participants and personnel was assessed separately from the bias related to blinding of outcome assessment.
Contributions of authors
Updated review: Fabiano T Barbosa (FTB), Aldemar A Castro (AAC), Célio Fernando de Sousa‐Rodrigues (CFSR)
Conceiving of the original review: Georgina Imberger (GI)
Co‐ordinating the review: FTB
Undertaking manual searches: FTB
Screening search results: FTB, AAC
Organizing retrieval of papers: FTB
Screening retrieved papers against inclusion criteria: FTB, AAC
Appraising quality of papers: FTB, AAC
Abstracting data from papers: FTB, CFSR
Writing to authors of papers for additional information: FTB
Providing additional data about papers: FTB
Managing data for the review: FTB, AAC
Entering data into Review Manager (RevMan 5.2): FTB
Checking data entry: FTB, CFSR
Handling RevMan statistical data: FTB, AAC
Performing other statistical analysis not using RevMan: GI, Jesper Brok (JB), Jørn Wetterslev (JW)
Interpreting data: FTB, AAC, CFSR
Making statistical inferences: FTB
Writing the review: FTB
Serving as guarantor for the review (one author): FTB
Taking responsibility for reading and checking the review before submission: FTB
Original review (Imberger 2010)
GI conceived of the idea for the review and wrote the protocol. David McIlroy (DM) and Nathan Leon Pace (NLP) helped to design the protocol. GI, DM, NLP, Jørn Wetterslev and Jesper Brok conducted the review.
Declarations of interest
The original review (Imberger 2010) was a recipient of the National Institute for Health Research (NIHR) Cochrane Review Incentive Scheme 2009 award.
Fabiano T Barbosa: none known.
Aldemar A Castro: none known.
Célio F Sousa‐Rodrigues: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Almarakbi 2009 {published data only}
- Almarakbi WA, Fawzi HM, Alhashemi JA. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. British Journal of Anaesthesia 2009;102(6):862‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Azab 2005 {published data only}
- Azab TO, El‐Masry A, Salah M, Azab AO. Effects of intraoperative use of positive end expiratory pressure on lung atelectasis during laparoscopic cholecystectomy. Egyptian Journal of Anaesthesia 2005;21(3):219‐25. [EMBASE: 2005550041] [Google Scholar]
Berthelsen 1979 {published data only}
- Berthelsen P, Husum B, Kortsen H. Postoperative arterial oxygen tension after perioperative PEEP‐ventilation. Acta Anaesthesiologica Scandinavica 1979;23(3):253‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2006 {published data only}
- Choi G, Wolthuis EK, Bresser P, Levi M, Poll T, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end‐expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 2006;105(4):689‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pang 2003 {published data only}
- Pang CK, Yap J, Chen PP. The effect of an alveolar recruitment strategy on oxygenation during laparoscopic cholecystectomy. Anaesthesia and Intensive Care 2003;31(2):176‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Severgnini 2013 {published data only}
- Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118(6):1307‐21. [PUBMED: 23542800] [DOI] [PubMed] [Google Scholar]
Talab 2009 {published data only}
- Talab HF, Zabani IA, Abdelrahman HS, Bukhari WL, Mamoun I, Ashour MA, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesthesia and Analgesia 2009;109(5):1511‐6. [PUBMED: 19843790] [DOI] [PubMed] [Google Scholar]
Tusman 1999 {published data only}
- Tusman G, Bohm SH, Vazquez De Anda GF, Do Campo JL, Lachmann B. 'Alveolar recruitment strategy' improves arterial oxygenation during general anaesthesia. British Journal of Anaesthesia 1999;82(1):8‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weingarten 2010 {published data only}
- Weingarten TN, Whalen FX, Warner DO, Gajic O, Schears GJ, Snyder MR, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. British Journal of Anaesthesia 2010;104(1):16‐22. [PUBMED: 19933173] [DOI] [PubMed] [Google Scholar]
Wetterslev 2001 {published data only}
- Wetterslev J, Hansen EG, Roikjaer O, Kanstrup IL, Heslet L. Optimizing perioperative compliance with PEEP during upper abdominal surgery: effects on perioperative oxygenation and complications in patients without preoperative cardiopulmonary dysfunction. European Journal of Anaesthesiology 2001;18(6):358‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Clarke 1998 {published data only}
- Clarke JP, Schuitemaker MN, Sleigh JW. The effect of intraoperative ventilation strategies on perioperative atelectasis. Anaesthesia and Intensive Care 1998;26(3):262‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohendy 1994 {published data only}
- Cohendy R, Ripart J, Eledjam JJ. The effect of positive end‐expiratory pressure on respiratory resistive properties in anaesthetized paralysed humans. European Respiratory Journal 1994;7(2):286‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coussa 2004 {published data only}
- Coussa M, Proietti S, Schnyder P, Frascarolo P, Suter M, Spahn DR, et al. Prevention of atelectasis formation during the induction of general anesthesia in morbidly obese patients. Anesthesia and Analgesia 2004;98(5):1491‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dyhr 2002 {published data only}
- Dyhr T, Laursen N, Larsson A. Effects of lung recruitment maneuver and positive end‐expiratory pressure on lung volume, respiratory mechanics and alveolar gas mixing in patients ventilated after cardiac surgery. Acta Anaesthesiologica Scandinavica 2002;46:717‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Futier 2010 {published data only}
- Futier E, Constantin JM, Pelosi P, Chanques G, Kwiatkoskwi F, Jaber S, et al. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum‐induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 2010;113(6):1310‐9. [PUBMED: 21068660] [DOI] [PubMed] [Google Scholar]
Gander 2005 {published data only}
- Gander S, Frascarolo P, Suter M, Spahn DR, Magnusson L. Positive end‐expiratory pressure during induction of general anesthesia increases duration of nonhypoxic apnea in morbidly obese patients. Anesthesia and Analgesia 2005;100(2):580‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldmann 2011 {published data only}
- Goldmann K, Gerlach M, Bornträger C. ProSeal™ laryngeal mask in normal weight and obese patients: oxygenation under pressure‐controlled ventilation and different end‐expiratory pressures [ProSeal®‐Kehlkopfmaske in normalgewichtigen und adipösen Patienten. Oxygenierung unter druckkontrollierter Beatmung mit verschiedenen endexspiratorischen Drücken]. Anaesthesist 2011;60(10):908‐5. [PUBMED: 21796447] [DOI] [PubMed] [Google Scholar]
Grzybowska 2005 {published data only}
- Grzybowska K, Duda I, Kwiek S, Luszawski J, Dyaczyńska‐Herman A, Karpel E. Air embolism during posterior fossa craniectomy performed in the sitting position. Anestezjologia Intensywna Terapia 2005;37(2):100‐4. [Google Scholar]
Khanam 1973 {published data only}
- Khanam T, Branthwaite MA. Arterial oxygenation during one‐lung anaesthesia. Anaesthesia 1973;28(3):280‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim JY, Shin CS, Kim HS, Jung WS, Kwak HJ. Positive end‐expiratory pressure in pressure‐controlled ventilation improves ventilatory and oxygenation parameters during laparoscopic cholecystectomy. Surgical Endoscopy 2010;24(5):1099‐103. [PUBMED: 19915912] [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim WH, Hahm TS, Kim JA, Sim WS, Choi DH, Lee EK, et al. Prolonged inspiratory time produces better gas exchange in patients undergoing laparoscopic surgery: a randomised trial. Acta Anaesthesiologica Scandinavica 2013;57(5):613‐22. [PUBMED: 23496092] [DOI] [PubMed] [Google Scholar]
Liou 1993 {published data only}
- Liou CM, Lin CH, Kang HM, Liu YC, Tso HS. The influence of position and PEEP on arterial blood gas during operation. Acta Anaesthesiologica Sinica 1993;31(2):103‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Meininger 2005 {published data only}
- Meininger D, Byhahn C, Mierdl S, Westphal K, Zwissler B. Positive end‐expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiologica Scandinavica 2005;49:778‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mizobe 2005 {published data only}
- Mizobe T, Nakajima Y, Sunaguchi M, Ueno H, Sessler DI. Clonidine produces a dose‐dependent impairment of baroreflex‐mediated thermoregulatory responses to positive end‐expiratory pressure in anaesthetized humans. British Journal of Anaesthesia 2005;94(4):536‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabil 2005 {published data only}
- Nabil S, Gamil M, El‐Gohary M, El‐Agaty A. The effect of alveolar recruitment on arterial oxygenation in anaesthetized morbidly obese patients. Egyptian Journal of Anaesthesia 2005;21(1):47‐52. [Google Scholar]
Neumann 1999 {published data only}
- Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Positive end‐expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiologica Scandinavica 1999;43(3):295‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park HP, Hwang JW, Kim YB, Jeon YT, Park SH, Yun MJ, et al. Effect of pre‐emptive alveolar recruitment strategy before pneumoperitoneum on arterial oxygenation during laparoscopic hysterectomy. Anaesthesia and Intensive Care 2009;37(4):593‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reinius 2009 {published data only}
- Reinius H, Jonsson L, Gustafsson S, Sundbom M, Duvernoy O, Pelosi P, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology 2009;111(5):979‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rusca 2003 {published data only}
- Rusca M, Proietti S, Schnyder P, Frascarolo P, Hedenstierna G, Spahn DR, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesthesia and Analgesia 2003;97(6):1835‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Russo 2013 {published data only}
- Russo A, Stasio E, Scagliusi A, Bevilacqua F, Isgrò MA, Marana R, et al. Positive end‐expiratory pressure during laparoscopy: cardiac and respiratory effects. Journal of Clinical Anesthesia 2013;25(4):314‐20. [PUBMED: 23712068] [DOI] [PubMed] [Google Scholar]
Turgut 2007 {published data only}
- Turgut N, Turkmen A, Gokkaya S, Hatiboglu MA, Iplikcioglu AC, Altan A. Positive end‐expiratory pressure reduces pneumocephalus in spinal intradural tumor surgery. Journal of Neurosurgical Anesthesiology 2007;19(3):161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolthuis 2008 {published data only}
- Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end‐expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 2008;108(1):46‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wrigge 2000 {published data only}
- Wrigge H, Zinserling J, Stber F, Spiegel T, Hering R, Wetegrove S, et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 2000;93(6):1413‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wrigge 2004 {published data only}
- Wrigge H, Uhlig U, Zinserling J, Behrends‐Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesthesia and Analgesia 2004;98:775‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ye 2011 {published data only}
- Ye FF, Li LW. Effects of different ventilation modes for one‐lung ventilation anesthesia on respiratory function and F(A)/F(I) changes during sevoflurane inhalation. Journal of Southern Medical University 2011;31(4):714‐7. [PUBMED: 21515479] [PubMed] [Google Scholar]
Yu 2006 {published data only}
- Yu G, Yang K, Baker AB, Young I. The effect of bi‐level positive airway pressure mechanical ventilation on gas exchange during general anaesthesia. British Journal of Anaesthesia 2006;96(4):522‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Brismar 1985
- Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L. Pulmonary densities during anesthesia with muscular relaxation—a proposal of atelectasis. Anesthesiology 1985;62(4):422‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brower 2004
- Brower RG, Lanken PN, Macintyre N, Matthay MA, Morris A, Ancukiewicz M. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. The New England Journal of Medicine 2004;351(4):327‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARG 2013
- Cochrane Anaesthesia Group Reviews. www.thecochranelibrary.com ('By Review Group').
Carvalho 2006
- Carvalho AR, Jandre FC, Pino AV, Bozza FA, Salluh JI, Rodrigues R, et al. Effects of descending positive end‐expiratory pressure on lung mechanics and aeration in healthy anaesthetized piglets. Critical Care 2006;10:R122. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Duggan 2005
- Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005;102:838‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dyhr 2004
- Dyhr T, Nyga E, Laursen N, Larsson A. Both lung recruitment maneuver and PEEP are needed to increase oxygenation and lung volume after cardiac surgery. Acta Anaesthesiologica Scandinavica 2004;48:187‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;350:326‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichenberger 2002
- Eichenberger AS, Proietti S, Wicky S, Frascarolo P, Suter M, Spahn R, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesthesia and Analgesia 2002;95:1782‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferreyra 2009
- Ferreyra G, Long Y, Ranieri VM. Respiratory complications after major surgery. Current Opinion in Critical Care 2009;15:342‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fleisher 2007
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA guidelines on perioperative cardiovascular evaluation and care for non‐cardiac surgery: executive summary. Circulation 2007;116:1971‐96. [DOI] [PubMed] [Google Scholar]
Gehr 2006
- Gehr B, Weiss C, Porzsolt F. The fading of reported effectiveness. A meta‐analysis of randomised controlled trials. BMC Medical Research Methodology 2006;6:25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2005
- Green S, Higgins J, editors. Glossary. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. http://www.cochrane.org/resources/handbook/ (accessed 1 August 2009).
Hedenstierna 1985
- Hedenstierna G, Strandberg A, Brismar B. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 1985;62(3):247‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedenstierna 1986
- Hedenstierna G, Tokics L, Strandberg A, Lundquist H, Brismar B. Correlation of gas exchange impairment to development of atelectasis during anaesthesia and muscle paralysis. Acta Anaesthesiologica Scandinavica 1986;30(2):183‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedenstierna 2003
- Hedenstierna G. Atelectasis and gas exchange during anaesthesia. Electromedica 71 2003;1:70‐3. [Google Scholar]
Hedenstierna 2005
- Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Medicine 2005;31:1327‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedenstierna 2010
- Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Practice & Research. Clinical Anaesthesiology 2010;24(2):157‐69. [PUBMED: 20608554] [DOI] [PubMed] [Google Scholar]
Hewlett 1974
- Hewlett A, Hulands G, Nunn J, Milledge J. Functional residual capacity during anaesthesia III: artificial ventilation. British Journal of Anaesthesia 1974;46(7):495‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thomson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration 2011. www.cochrane‐handbook.org.
Ioannidis 2001
- Ioannidis J, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proceedings of the National Academy of Science USA 2001;98:831‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joyce 1995
- Joyce CJ, Baker AB. What is the role of absorption atelectasis in the genesis of perioperative pulmonary collapse?. Anaesthesia and Intensive Care 1995;23:691‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindberg 1992
- Lindberg P, Gunnarsson L, Tokics L, Secher E, Lundquist H, Brismar B, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiologica Scandinavica 1992;36(6):546‐53. [PUBMED: 1514340] [DOI] [PubMed] [Google Scholar]
Lindquist 1995
- Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT‐assessment of dependent lung densities in man during general anaesthesia. Acta Radiologica 1995;36(6):626‐32. [PUBMED: 8519574] [PubMed] [Google Scholar]
Lumb 2000
- Lumb A. Anaesthesia. Nunn's Applied Respiratory Physiology. Burlington, MA: Butterworth‐Heinemann, 2000. [Google Scholar]
Maisch 2008
- Maisch S, Reissmann H, Fuellekrug B, Weismann D, Rutkowski T, Tusman G, et al. Compliance and dead space fraction indicate an optimal level of positive end‐expiratory pressure after recruitment in anesthetized patients. Anesthesia and Analgesia 2008;106(1):175‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maracaja‐Neto 2009
- Maracaja‐Neto LF, Vercosa N, Roncally AC, Giannela A, Bozza FA, Lessa MA. Beneficial effects of high positive end‐expiratory pressure in lung respiratory mechanics during laparoscopic surgery. Acta Anaesthesiologica Scandinavica 2009;53:210‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mercat 2008
- Mercat A, Richard JCM, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress: syndrome: a randomized controlled trial. JAMA 2008;299(6):646‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oba 2009
- Oba Y, Thameem DM, Zaza T. High levels of PEEP may improve survival in acute respiratory distress syndrome: a meta‐analysis. Respiratory Medicine 2009;103:1174‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pearse 2005
- Pearse DB, Searcy RM, Mitzner W, Permutt S, Sylvester JT. Effects of tidal volume and respiratory frequency on lung lymph flow. Journal of Applied Physiology 2005;99:556‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pelosi 1998
- Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anaesthesia. Anesthesia and Analgesia 1998;87:654‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phoenix 2009
- Phoenix SI, Paravastu S, Columb M, VIncent JL, Nirmalan M. Does a higher positive end expiratory pressure decrease mortality in acute respiratory distress syndrome?. Anesthesiology 2009;110:1098‐105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rothen 1998
- Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G. Airway closure, atelectasis and gas exchange during general anaesthesia. British Journal of Anaesthesia 1998;81(5):681‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Annals of Internal Medicine 2002;136(5):254‐9. [PUBMED: 11827510] [DOI] [PubMed] [Google Scholar]
Terragni 2007
- Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. American Journal of Respiratory Critical Care Medicine 2007;175:160‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tokics 1987
- Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H. Lung collapse and gas exchange during general anesthesia: effects of spontaneous breathing, muscle paralysis, and positive end‐expiratory pressure. Anesthesiology 1987;66:2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trikalinos 2004
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57:1124‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tusman 2004
- Tusman G, Böhm SH, Suarez‐Sipmann F, Turchetto E. Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Canadian Journal of Anesthesia 2004;51(7):723‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tusman 2012
- Tusman G, Böhm SH, Warner DO, Sprung J. Atelectasis and perioperative pulmonary complications in high‐risk patients. Current Opinion in Anaesthesiology 2012;25(1):1‐10. [PUBMED: 22113182] [DOI] [PubMed] [Google Scholar]
Villar 2006
- Villar J, Kacmarek RM, Pérez‐Méndez L, Aguirre‐Jaime A. A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomised, controlled trial. Critical Care Medicine 2006;34(5):1311‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Westcott 1985
- Westcott JL. Plate atelectasis. Radiology 1985;155:1‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463 ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analysis. BMC Medical Research Methodology 2009;9:86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Imberger 2010
- Imberger G, McIlroy D, Pace NL, Wetterslev J, Brok J, Møller A. Positive end‐expiratory pressure (PEEP) during anaesthesia for the prevention of mortality and postoperative pulmonary complications. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007922.pub2] [DOI] [PubMed] [Google Scholar]


