Abstract
The pharmacological targeting of the α7 nicotinic acetylcholine receptor (α7) is a promising strategy in the development of new drugs for neurologic diseases. Because α7 receptors regulate cellular calcium, we investigated how the prototypical type II–positive allosteric modulator PNU120596 affects α7-mediated calcium signaling. Live imaging experiments show that PNU120596 augments ryanodine receptor-driven calcium-induced calcium release (CICR), inositol-induced calcium release (IICR), and phospholipase C activation by the α7 receptor. Both influx of calcium through the α7 nicotinic acetylcholine receptor (nAChR) channel as well as the binding of intracellular G proteins were involved in the effect of PNU120596 on intracellular calcium. This is evidenced by the findings that chelation of extracellular calcium, expression of α7D44A or α7345–348A mutant subunits, or blockade of calcium store release compromised the ability of PNU120596 to increase intracellular calcium transients generated by α7 ligand activation. Spatiotemporal stochastic modeling of calcium transient responses corroborates these results and indicates that α7 receptor activation enables calcium microdomains locally and to lesser extent in the distant cytosol. From the model, allosteric modulation of the receptor activates CICR locally via ryanodine receptors and augments IICR through enhanced calcium influx due to prolonged α7 nAChR opening. These findings provide a new mechanistic framework for understanding the effect of α7 receptor allosteric modulation on both local and global calcium dynamics.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are prototype cys-loop receptor channels with the ability to undergo ligand-associated structural changes that translate chemical signals to electrical information (Changeux, 2010). Of the nine different nAChR subunits identified in the mammalian brain, the homomeric α7 nAChR (α7) is one of the most abundant, present in both neuronal and non-neuronal cells and expressed in regions of the brain such as the hippocampus and cortex. The α7 receptors play an important role in signaling that underlies short-term changes in presynaptic neurotransmitter release, as well as postsynaptic dendritic plasticity (Halff et al., 2014). Signaling through α7 can modulate learning, memory, and pain responses and support neuroprotection through an inhibition of inflammation (Shytle et al., 2004; Freitas et al., 2013a, b; King et al., 2017). The α7 nAChR is thus an attractive drug target in the treatment of human diseases such as Alzheimer’s disease, schizophrenia, and human immunodeficiency virus–associated neuroinflammatory disease (Wang et al., 2003; Bencherif et al., 2011; Deutsch et al., 2013; Guerra-Álvarez et al., 2015).
Diverse ligand binding sites on the α7 channel provide an opportunity for selective receptor modulation in different pathologic contexts (Bouzat et al., 2017). Compounds that target allosteric sites on the α7 channel offer a significant advantage over orthosteric-acting compounds because of greater selectivity and lower toxicity. Compounds such as positive allosteric modulators (PAMs) can also directly affect open and close times of the channel. For example, the presence of type I PAMs leads to a greater conduction of ions into the cell than seen with normal agonist alone, whereas type II PAMs prolong the opening of the ligand-bound channel, leading to greater ion influx, while shortening desensitization (Grønlien et al., 2007; McCormack et al., 2010; Williams et al., 2012). The prototypical type II PAM PNU120596 has been shown to promote therapeutic efficacy in animal models of disease, including cognitive, inflammatory conditions, and an animal model of schizophrenia (McLean et al., 2012; Williams et al., 2012; Freitas et al., 2013a,b; Uteshev, 2014; Hashimoto, 2015).
The α7 nAChRs are distinctly known for their short opening duration and rapid transition to a long lasting desensitized state (Séguéla et al., 1993). The α7 channel receptors are also highly calcium permeable and able to activate calcium-induced calcium release (CICR) and inositol-induced calcium release (IICR) in cells (Papke et al., 1996; Liu and Berg, 1999; Campbell et al., 2010; Halff et al., 2014). Studies show that α7 receptors bind and activate the heterotrimeric G protein Gαq, leading to phospholipase C (PLC)–linked IICR and activation of downstream second messenger pathways such as RhoA (Nordman and Kabbani, 2014; King et al., 2015). Studies from our laboratory and others suggest that α7 nAChRs operate through both ionotropic and metabotropic signaling modes, although this distinction remains unclear (Nordman and Kabbani, 2012; Zhong et al., 2013; Kabbani and Nichols, 2018). In this study, we examined the effects of PNU120596 on calcium signaling by the α7 nAChR in pheochromocytoma line 12 (PC12) cells, building on previous studies that indicate that agonists of the α7 nAChR mediate calcium signaling through coupled store release. The current findings confirm that this PAM augments calcium signaling by the α7 receptor through augmented coupling between the channel receptor and the endoplasmic reticulum (ER). A new stochastic computational model depicting spatiotemporal α7 nAChR calcium dynamics in the cell corroborates the findings.
Materials and Methods
Cell Culture and DNA Transfection.
PC12 (American Type Culture Collection, Manassas, VA; CRL1721TM) cells were grown on a poly-D-lysine (100 μg/ml) matrix and differentiated by the addition of 2.5-s mouse nerve growth factor (200 ng/ml; Millipore, Billerica, MA) in PC12 media RPMI media (American Type Culture Collection), 10% horse serum, 5% fetal bovine serum, and 1% penicillin streptomycin (Thermo Fisher, Waltham, MA), as previously described in Nordman and Kabbani (2012). Cells were transfected with plasmids using Lipofectamine 2000, according to the manufacturer’s protocol (Thermo Fisher). Cells were transfected and differentiated for 72 hours before each experiment. Plasmids used in this study have been previously characterized: GCaMP5G (Akerboom et al., 2012), human α7 nAChR (Saragoza et al., 2003), human α7345–348A (King et al., 2015), rat α7D44A (Colón- Sáez and Yakel 2014), and PH-mCherry (Chisari et al., 2009; Nordman and Kabbani, 2014). Plasmid DNA was purified using a maxi prep kit (Xymo Research, Irvine, CA).
Drug Treatment.
Drugs were dissolved into Hanks’ balanced salt solution or dimethylsulfoxide vehicle solution. Drug concentrations were determined based on established pharmacological data and previously published reports: the α7-specific agonist choline (1 and 3 mM; Acros Organics, Geel, Belgium); the α7-specific type II PAM PNU120596 (Zhang et al., 2015) (1 μM) (Tocris, Bristol, United Kingdom); nonmembrane permeable calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (Gu and Yakel, 2011) (10 mM) (Thermo Fisher); the α7-specific antagonist α-bungarotoxin (Bgtx) (Chan and Quik 1993) (50 nM); the ryanodine inhibitor ryanodine (Zhong et al., 2013) (30 μM) (Santa Cruz Biotechnology, Santa Cruz, CA); the inositol triphosphate (IP3)-specific inhibitor Xestospongin C (Xest. C) (Nordman and Kabbani, 2014) (1 μM) (Tocris); the broad α7 agonists nicotine and acetylcholine (Jones and Yakel, 1997) (50 μM); and Rho A inhibitor I (King and Kabbani, 2016) (4 μg/ml) (Cytoskeleton, Denver, CO).
Immunocytochemistry and Fluorescence Detection.
Cells were fixed in 1× of the following: 80 mM 1,4-piperazinediethanesulfonic acid, 5 mM EGTA, and 1 mM MgCl2, pH 6.8, containing 0.3% glutaraldehyde, and then permeabilized (following cell surface labeling) with 0.05% Triton X-100 (He et al., 2005). Two milligrams per milliliter sodium borohydride was used for glutaraldehyde quenching prior to immunoblocking with 10% goat serum (Life Technologies, Carlsbad, CA). An anti-protein disulfide isomerase (PDI) antibody was used for ER labeling (Enzo Scientific, Ann Arbor, MI), and carbocyanine 2 (Jackson ImmunoResearch, West Grove, PA) was used as a secondary antibody. Cell surface detection of α7 nAChRs was performed, as previously described (Nordman and Kabbani, 2012), in nonpermeabilized cells using 50 nM Alexa Fluor 488 conjugated to Bgtx. Cells were visualized using an inverted Zeiss LSM800 confocal microscope, and images were captured with Zen software (Carl Zeiss AG, Oberkochen, Germany) and processed in ImageJ (National Institutes of Health, Bethesda, MD). Signals were obtained using 405- and 488-nm excitation lasers, respectively. Heat maps showing colocalization were generated using Photoshop CS5 (Adobe Systems, San Jose, CA). Imaging experiments were repeated in three separate trials (n = 20).
Calcium Imaging.
PC12 cells were transfected with the calcium sensor protein GCaMP5G and differentiated with nerve growth factor, as previously described (Nordman and Kabbani, 2012). Imaging was done in clear Hanks’ balanced salt solution media (Thermo Fisher) equilibrated to room temperature, and drugs were added using a gravity-fed profusion system. Calcium transients were measured above the threshold of 3 S.D. from the baseline average that was obtained from the first 20 frames of each recording. Transient duration was measured from the first to last response value above the threshold. Changes in intracellular calcium were measured using an inverted Zeiss LSM800 confocal microscope at an acquisition rate of 1 frame per 256 ms for 45 s at 2 × 2 binning. All measures were done at room temperature. Phototoxicity and photobleaching were minimized using low-wavelength and neutral density light filters. Drugs were applied after a 50-frame baseline recording. Changes in the intracellular calcium signal were calculated from changes in the fluorescence of GCaMP5G (ΔF/Fθ) using ImageJ (National Institutes of Health), and all measures are the result of examining regions of interest in the growth cone. When drug preincubations are indicated, the preincubation step occurred for 30 minutes prior to the imaging experiment. Experiments were repeated in three independent trials (n = 20).
PLC Assay.
PLC activation was detected by imaging the translocation of the Pleckstrin homology (PH) domain of PLC-δ tagged with mCherry (PH-mCherry), as previously reported (Chisari et al., 2009; Nordman and Kabbani, 2014). PH-mCherry fluorescence was measured at the excitation wavelength λ = 555 nm, and the dynamics of translocation were visualized at an acquisition rate of 1 frame per 10 s for 1 minute with 2 × 2 binning. Drugs were applied 20 s after a one-frame (10-s) baseline recording within the same region of interest. PLC activation can be inferred from the translocation of PH-mCherry, as shown (Chisari et al., 2009), using the equation (Fm − Fc)/(Fm + Fc), where Fm and Fc refer to fluorescence at the plasma membrane and in the cytosol, respectively. Fluorescence values were normalized for surface area (square micrometer) measured with ImageJ (National Institutes of Health). The PLC assay was performed in three separate experiments (n = 8).
Computational Model Generation and Analysis.
A new model that integrates the stochastic Monte Carlo models for ion channels is the IP3 receptor (IP3R) model of Ullah and Ullah, the RyR model developed by Williams et al. (2011), with an improved model for the α7 channel based upon the published model of Anderson et al., 2016; and Corradi and Bouzat, 2016 (McCormack et al., 2010; Williams et al., 2011; Andersen et al., 2016; Corradi and Bouzat, 2016; Ullah and Ullah, 2016). These models were chosen because they are well constrained by experimental data. For computational simplicity, a one-dimensional simulation was performed. Detailed equations and parameters for the model are presented in Supplemental Material. Supplemental Tables 1 and 2 detail symbols used in the Supplemental Material equations.
Statistical Analysis.
Data are averaged as mean ± S.D. and are representative of independent experiments in each assay. Statistical analysis was obtained via a one-way analysis of variance (ANOVA), or, where applicable, a paired two-tailed Student’s t test, to determine significance between mean values. All statistical values were obtained using SPSS 24 (IBM, Armonk, NY). A Fischer least significant difference post hoc test was used for individual comparisons. A minimum value P < 0.05 was considered significant.
Results
Allosteric Modulation of the α7 nAChR by PNU120596 Increases Intracellular Calcium.
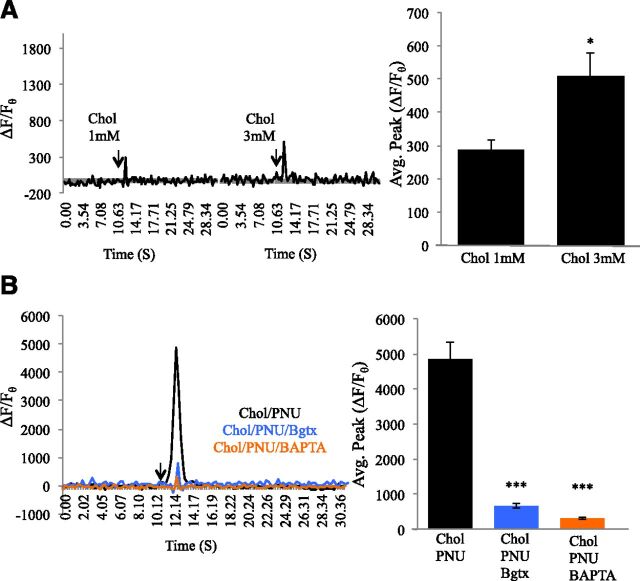
The α7 nAChR maintains the greatest calcium permeability among the nAChR types, contributing to not only cellular calcium level activity but also various forms of calcium signaling in cells (Le Novère et al., 2002). We have shown an important role for α7 activation in neurite growth through calcium signaling in the growth cone of differentiating PC12 cells (Nordman and Kabbani, 2014). To examine the effects of the PNU120596 on α7-mediated calcium activity, we measured calcium changes in the growth cone using the genetically encoded calcium sensor protein GCaMP5G under conditions of α7 activation with the specific agonist choline or α7 activation by choline in the presence of PNU120596. In all of our experiments, PNU120596 was applied at the established effective concentration of 1 μM (Zhong et al., 2013). Because the EC50 for choline at the α7 receptor site is 1.6 mM (Alkondon et al., 1997), we tested the effects of 3 mM choline on calcium transients throughout the study. Indeed, choline application was associated with a dose-dependent increase in the average peak of the calcium transient signal within PC12 cells (t test, F = 5.440, P = 0.012) (ΔF/Fθ for 1 mM choline = 288.11% ± 27.01%; ΔF/Fθ for 3 mM = 507.57% ± 59.51%) (Fig. 1A). This effect of choline on calcium transients was reduced by 91.23% (average change) by the α7-specific antagonist Bgtx (50 nM), confirming that pharmacological activation of the α7 receptor produces a robust cellular calcium transient (Nordman and Kabbani, 2014).
Fig. 1.
PNU120596 augments choline-associated calcium transients. (A) Traces: calcium transient responses to application of the α7-specific agonist choline at 1 and 3 mM concentrations. The arrow points to the time of drug application. Histogram: average calcium transient peak. (B) Traces: 3 mM choline with 1 μM PNU120596, pretreated with 50 nM Bgtx, in the presence of the nonmembrane permeant calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (10 mM). *P < 0.05; ***P < 0.001. Error bars represent S.D.; n = 22 cells.
We determined the effect of allosteric regulation at the α7 receptor on the cellular calcium transient by coapplying PNU120596 and choline. As shown in Fig. 1B, PNU120596 coapplication increased the calcium transient by 958.18% compared with choline alone (ΔF/Fθ = 4858.25% ± 468.66%) (t test, F = 88.878, P < 0.001). In addition, PNU120596 coapplication was associated with prolonging the choline-driven calcium signal by 1.45 s, which represents a twofold increase in duration (Fig. 1). The effect of PNU120596 on the choline-mediated calcium transient was reduced by −86.35% in the presence of Bgtx (50 nM) (ΔF/Fθ = 662.99% ± 62.04%) or the nonmembrane permeable calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (10 mM) (ΔF/Fθ = 311.82% ± 37.17%) (Fig. 1B) (ANOVA: F(2,92) = 41.881, P < 0.001; post hoc P < 0.001 for both groups compared with control). These results indicate that allosteric modulation of the α7 receptor with PNU120596 strongly augments the intracellular calcium transient response through extracellular calcium influx.
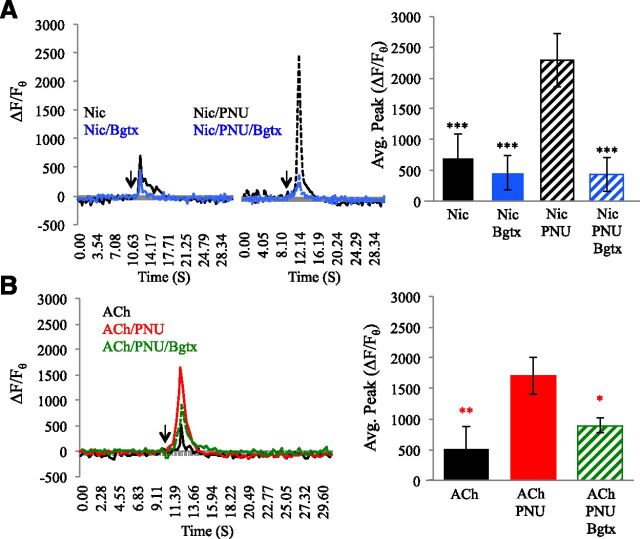
These findings were corroborated by experiments aimed at examining the effects of PNU120596 on α7 receptor activation by other agonists. Average calcium transient peak values (ΔF/Fθ) for these studies are presented in Table 1. As shown in Fig. 2A, application of the broad nAChR agonist nicotine (50 μM) enhanced intracellular calcium levels (ΔF/Fθ = 705.53% ± 89.91%) above baseline, an effect that was diminished by Bgtx. Coapplication of PNU120596 increased the nicotine-associated calcium transient by 324.14%, and this too was diminished by Bgtx treatment (ANOVA: F(3,57) = 14.190, P < 0.00, all post hoc comparison of nicotine + PNU120596 P < 0.001). Treatment with acetylcholine (ACh; 50 μM) was also associated with a rise in intracellular calcium (ΔF/Fθ = 520.61% ± 86.85%) above baseline (Fig. 2B). ACh-mediated calcium transients were also dramatically augmented by PNU120596 coapplication, which raised them by 327.16% when compared with ACh treatment alone (ANOVA: F(2,44) = 6.119, P = 0.005, post hoc comparison of ACh + PNU120596 to ACh alone, P = 0.001). Application of Bgtx reduced the ability of PNU120596 to increase calcium transient generation by ACh (Fig. 2B), confirming the essential role of the α7 nAChR in calcium signaling.
TABLE 1.
Average peak calcium transient measures following the application of nicotine (Nic, 50 μM), ACh (50 μM), or α-Bgtx (Bgtx, 50 nM)
PNU120596 (1 μM) was coapplied.
| Drug | ΔF/Fθ |
|---|---|
| Nic | 705.52% ± 89.91% |
| Nic + Bgtx | 462.35% ± 83.15% |
| Nic + PNU120596 | 2286.88% ± 429.91% |
| Nic + PNU120596 + Bgtx | 437.01% ± 76.68% |
| ACh | 520.61% ± 85.85% |
| ACh + PNU120596 | 1702.21% ± 302.48% |
| ACh + PNU120596 + Bgtx | 888.96% ± 392.74% |
Fig. 2.
PNU120596 enhances calcium transients by the α7 nAChR when activated by nicotine or ACh. (A) Traces: left, calcium transient responses to application of the α7 agonist nicotine (50 μM; black) or nicotine and Bgtx (50 nM; light blue). Right, coapplication of nicotine and PNU120596 (1 μM) or nicotine, Bgtx, and PNU120596. Histogram: average calcium transient peak. (B) Traces: left, calcium transient responses to application of ACh (50 μM; black) or ACh and PNU120596 (1 μM; red), or ACh, PNU120596, and Bgtx (50 nM; green). *P < 0.05; **P < 0.01; ***P < 0.001 (asterisk color represents significant comparison with individual group); error bars represent S.D.; n = 22 cells.
PNU120596 Augments α7 nAChR Calcium Transients through Intracellular Store Release.
Upon ligand activation, the α7 channel conducts sodium and calcium into the cell, with the latter contributing to calcium-induced calcium release from nearby ER (Zhong et al., 2013; Nordman and Kabbani, 2014; King et al., 2015). In various types of cells, α7 nAChRs are targeted close to the ER and thus positioned to regulate calcium store activity (Shen and Yakel, 2009). We confirmed colocalization of the α7 nAChR and the ER in PC12 cells using a dual labeling method in which the fluorescent-tagged Bgtx (fBgtx) is used in conjunction with the ER marker protein PDI, respectively. In this assay, we detected α7 nAChRs at the cell surface by labeling prior to Triton permeabilization of the membrane. Noticeable coexpression of the two signals was seen throughout the cell; however, distinct subcellular colocalization was also observed. Specifically, strong overlap in the α7/ER signal was seen within the soma and the growth cone (Fig. 3A). The strongest colocalization between the α7 nAChR and the ER was in the growth cone, consistent with earlier studies on functional coupling between the receptor and calcium stores during neurite growth (Nordman and Kabbani, 2014).
Fig. 3.
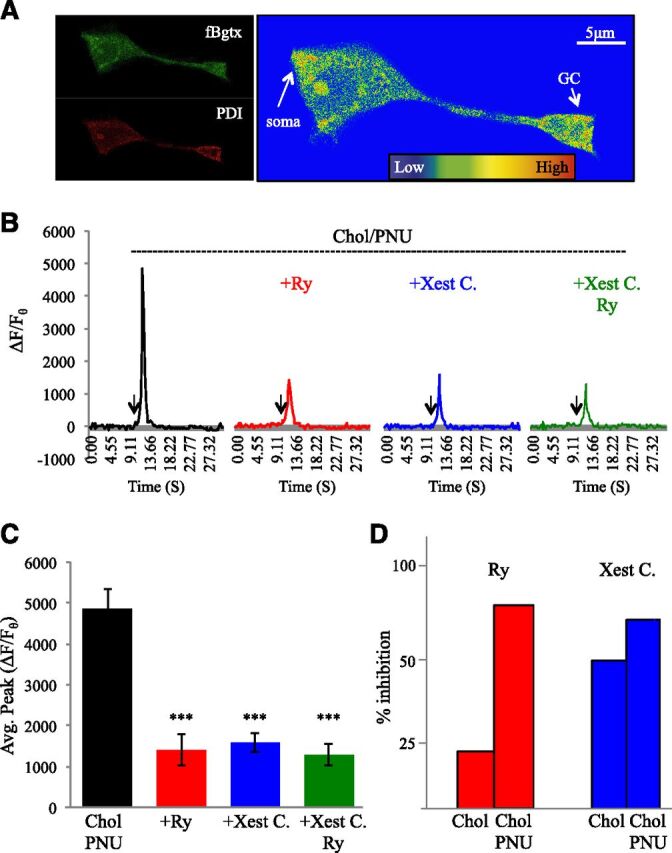
Calcium signaling through the ER is enhanced by PNU120596. (A) Fluorescent detection of cell surface α7 nAChRs labeled with fBgtx and intracellular labeling of the ER using PDI. Heat map colocalization of the two signals shows strong overlap in the soma and growth cone (arrow). (B) Comparison of transient measures following coapplication of PNU120596 (1 μM) and choline (3 mM) in cells preincubated with Ry (30 μM; red), Xest. C (1 μM; blue), or both (green). (C) Average peak calcium transient for each group. (D) Percent inhibition of the average peak calcium transient response in cells treated with choline (3 mM), or 3 mM choline and PNU20596, when inhibitied by Ry or Xest. C (1 μM). ***P < 0.001; error bars represent S.D.; n = 22 cells.
We assessed the ability of coapplication of PNU120596 and choline 3 mM to modulate intracellular calcium levels in the presence of pharmacological agents that block calcium release from the ER. As shown in Fig. 3B and Table 2, pretreatment of cells with 30 μM ryanodine (Ry) to block the Ry receptor (RyR) was associated with a 71.21% decrease in the calcium transient generated by the application of PNU120596 and choline. Inhibition of the IP3 receptor by pretreatment with 1 μM Xest. C was associated with a 67.40% loss in the calcium transient generated by PNU120596 and choline coapplication. Coapplication of Ry and Xest C. also significantly decreased the calcium transient (−73.67%). This is confirmed by ANOVA, showing that blocking the RyR, the IP3R, or both the RyR and IP3R leads to a significant loss in calcium signal generation by PNU120596 and choline coapplication (Fig. 3C) [ANOVA: F(3,135) = 23.980, P < 0.001; post hoc: P < 0.001 for all pretreatment groups].
TABLE 2.
Average peak calcium transient measures following the coapplication of 3 mM choline and 1 μM PNU120596 in cells transfected with variants of the α7 receptor
Cells were pretreated with Ry (30 μM) or Xest. C (1 μM).
| Receptor | Vehicle (ΔF/Fθ) | Ry (ΔF/Fθ) | Xest. C (ΔF/Fθ) |
|---|---|---|---|
| α7endogenous | 4858.25% ± 468.65% | 1398.76% ± 382.33% | 1583.34% ± 240.76% |
| α7D44A | 2654.13% ± 498.24% | 1115.99% ± 234.19% | 1696.25% ± 259.46% |
| α7345–348A | 3322.11% ± 474.83% | 1965.54% ± 282.33% | 2647.60% ± 432.65% |
A relative comparison of the effect of IP3R blockade by Xest. C and RyR blockade by Ry between choline and choline with PNU120596 indicates that modulation alters α7 coupling to the ER. In particular, in the presence of PNU120596, choline treatment drives a calcium transient with greater dependence on the RyR, as evidenced by the ability of Ry to inhibit calcium signals to a greater extent in cotreated cells (Fig. 3D). Interestingly, PNU120596 coapplication appears to also increase IP3R-mediated IICR in comparison with choline alone. The experiments suggest a nonlinear relationship between RyR- and IP3R-mediated calcium release in the presence of PNU120596 consistent with known interactions between RyR and IP3R pathways in cells (Verkhratsky, 2002). These findings support evidence on the ability of PNU120596 to prolong the open α7 channel state leading to enhanced calcium influx (Andersen et al., 2016).
Calcium Influx through the α7 nAChR Is Necessary for PNU120596-Mediated Calcium Signaling.
Ligand activation of the α7 nAChR enables channel transition to an open state that conducts calcium ions across the plasma membrane; however, this state is short-lived because the receptor quickly transitions into a desensitized state (Andersen et al., 2016; Corradi and Bouzat, 2016). PNU120596 is a prototypical type II PAM, which increases the open time of the α7 nAChR channel, promoting greater calcium conductance across the plasma membrane (Williams et al., 2012).
We tested the contribution of calcium entry through the α7 channel in PNU120596-associated calcium transient responses by utilizing the α7D44A mutant, which has been shown to be impaired in permeability to calcium (Colón-Sáez and Yakel, 2014). The α7D44A subunit expresses in PC12 cells and can operate as a dominant negative for calcium entry through the α7 channel (Nordman and Kabbani, 2014; King and Kabbani, 2016). Cell surface detection with fBgtx confirms α7D44A expression at the cell surface and colocalization with the ER (Fig. 4A). Analysis of the α7 nAChR overexpression in (α7+) cells revealed no statistical significance in the peak calcium transient when compared with the endogenous α7 nAChR baseline condition (α7) (Fig. 4B). Calcium-imaging experiments, however, showed a significant difference in the duration of the calcium transient between α7 and α7+ cells when cells were cotreated with PNU120596 and 3 mM choline consistent with previous findings (King et al., 2015).
Fig. 4.
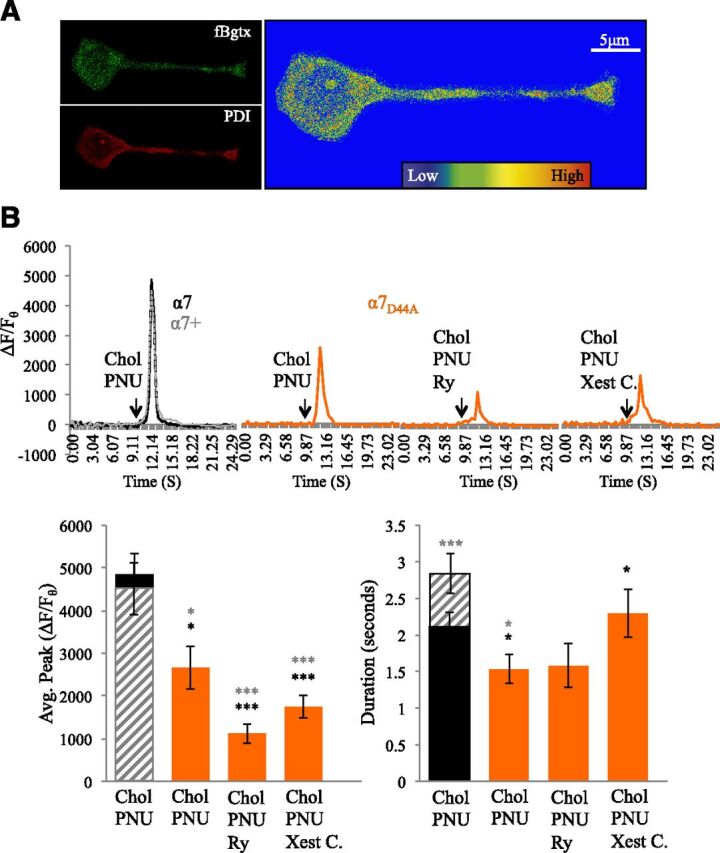
Decrease in calcium conductance following α7D44A expression attenuates calcium signaling by PNU120596. (A) Fluorescent detection of cell surface α7 nAChRs in α7D44A-transfected cells labeled with fBgtx and intracellular labeling of the ER using PDI. Heat map colocalization of the two signals shows strong overlap in the soma and growth cone. (B) Traces: a comparison of transient measures following coapplication of PNU120596 (1 μM) and choline (3 mM) in α7D44A-transfected cells (orange) preincubated with Ry (30 μM) or Xest. C (1 μM). Control conditions: endogenous α7 expression (black solid); transfected with human α7 (α7+) (gray broken line). Histogram: left, average calcium transient peak; right, average duration of the calcium transient. *P < 0.05; ***P < 0.001 (asterisk color represents significant comparison with individual group); error bars represent S.D.; n = 24 cells.
In previous studies, we have shown that α7D44A expression is associated with a reduction in the ability of choline to enhanced intracellular calcium in PC12 cells (Nordman and Kabbani, 2014). Calcium-imaging experiments in this study indicate that transfection with α7D44A is associated with a 45.37% decrease from α7 control and a 41.14% decrease from α7+ control cells when PNU120596 and 3 mM choline are coapplied [ANOVA: F(4,148) = 13.439, P < 0.001; post hoc to endogenous P = 0.012; post hoc to α7+ P = 0.024) (Fig. 4B; Table 2). Pretreatment of α7D44A-transfected cells with either Ry or Xest C. led to a greater reduction in calcium signaling following choline/PNU120596 coapplication (post hoc P < 0.001 when compared with both controls). A comparison of the effects of α7D44A expression on the intracellular calcium transients following pretreatment reveals a greater loss in calcium transient responses in the presence of Ry when compared with the presence of Xest C. (Fig. 4B). Transfection with α7D44A also significantly decreased the duration of the calcium transient relative to controls [ANOVA: F(4,148) = 2.453, P = 0.048; post hoc to endogenous P = 0.012; post hoc to α7+ P = 0.020].
Enhanced Calcium Signaling Is Lost When the α7 nAChR Does Not Bind G Proteins.
α7 nAChRs operate through ionotropic and metabotropic modes and can regulate immediate as well as long-term signal transduction in cells (King et al., 2015; King and Kabbani, 2016). Recent studies suggest a role for allosteric modulators in altering metabotropic signaling by nAChRs (Andersen et al., 2016; Corradi and Bouzat, 2016). We have shown that the α7 receptor can directly activate Gαq, leading to downstream signaling via PLC- and IP3-associated IICR in PC12 cells (King et al., 2015). We tested the ability of PNU120596 to modulate calcium through G proteins by transfecting cells with a dominant-negative α7 subunit harboring a mutation at the G protein–binding cluster located within the M3–M4 intracellular loop region (α7345–348A) (King et al., 2015). As shown in Fig. 5A, expression of the α7345–348A subunit does not interfere with cell surface localization nor the distribution of fBgtx labeling. In addition, colocalization of the fBgtx and the PDI signal appears similar in cells transfected with the α7345–348A mutant as control cells [cells transfected with an empty vector or native (nontransfected) cells] (Fig. 5A and data not shown).
Fig. 5.
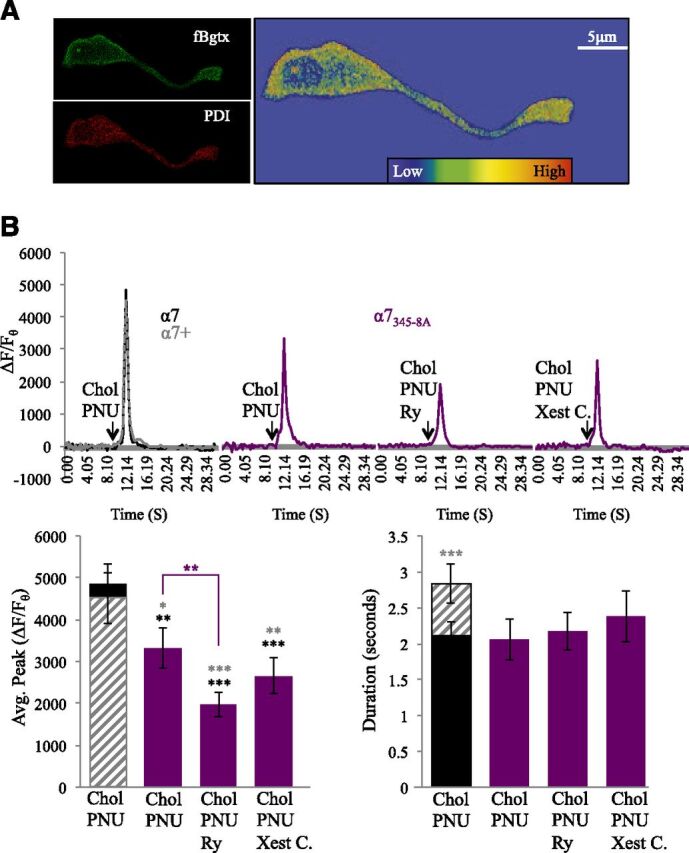
Loss of G protein binding in α7345–348A-expressing cells reduces calcium signaling by PNU120596. (A) Fluorescent detection of cell surface α7 nAChRs in α7345–348A labeled with fBgtx and intracellular labeling of the ER using PDI. Heat map colocalization of the two signals shows strong overlap in the soma and growth cone. (B) Traces: a comparison of transient measures following coapplication of PNU120596 (1 μM) and choline (3 mM) in α7345–348A-transfected cells (purple) preincubated with Ry (30 μM) or Xest. C (1 μM). Control conditions: endogenous α7 expression (black solid); transfected with human α7 (α7+) (gray broken line). Histogram: left, average calcium transient peak; right, average duration of the calcium transient. **P < 0.01; ***P < 0.001 (asterisk color represents significant comparison with individual group); error bars represent S.D.; n = 24 cells.
Coapplication of choline and PNU120596 was associated with a significant decrease in the calcium transient within α7345–348A-expressing cells relative to α7 control cells (31.62%) as well as α7+ control cells (26.33%) (Fig. 5B; Table 2) [ANOVA: F(4,191) = 9.824, P < 0.001; post hoc P = 0.014 to control; post hoc P = 0.025 to α7+-expressing cells]. Experiments indicate that expression of α7345–348A is associated with a loss in the ability of PNU120596 to modulate choline-associated calcium transients through disrupting coupling between the α7 nAChR and the ER. Pretreatment with Ry led to a significant decrease in calcium transients in α7 (−59.55%) and α7+ (−56.42%) cells (post hoc P < 0.001). In cells transfected with α7345–348A, pretreatment with Ry was also associated with a reduction (−40.83%) in the ability of PNU120596 to modulate choline-evoked calcium release (post hoc P = 0.024 compared with α7345–348A PNU120596/choline none–pretreated cells). Pretreatment with Xest C. weakened the ability of PNU120596 to modulate the choline calcium transient signal in α7345–348A-transfected cells (post hoc P < 0.001 to α7; post hoc P = 0.001 to α7+) (Fig. 5B; Table 2). Because a loss in G protein coupling within α7345–348A attenuates the ability of the α7 receptor to activate IP3R (King et al., 2015), these findings support earlier experiments showing that PNU120596 modulation of the α7 nAChR augments RyR-mediated CICR.
Positive Allosteric Modulation of the α7 nAChR Activates PLC Signaling.
α7 nAChR activation leads to second messenger signaling PLC in hippocampal axons, growing neurites, and dorsal root ganglia neurons, leading to calcium signaling and release (Santos et al., 2013; Nordman and Kabbani, 2014; King et al., 2015). We tested the ability of PNU120596 to impact PLC signaling through α7 receptor activation in the growth cone. To do this, we used live cell imaging to track the translocation of the fluorescent protein sensor PLC (δ) tagged with mCherry (PH-mCherry) from the cell surface to the cytoplasm within the growth cone (Chisari et al., 2009; Nordman and Kabbani, 2014; King et al., 2015). At 3 mM, choline was associated with a translocation of the PH-mCherry probe from the membrane to the cytoplasm, where it largely localized to the cytoplasm at 50 s after drug application (Fig. 6). Coapplication of PNU120596 and choline enhanced the rate of PH-mCherry translocation with maximal signal detection in the cytoplasm at 20 s after the start of treatment (Fig. 6). These results demonstrate that PNU120596 augments α7 nAChR signaling through PLC, likely contributing to faster and greater IP3 production and IICR.
Fig. 6.
PNU120596 augments PLC signaling. PLC activation was measured by translocation of the PH-mCherry sensor from the cell surface to the cytosol. (A) A heat map of PH-mCherry imaging at the growth cone at 0, 20, and 50 s of treatment with choline (3 mM) or choline and PNU120596 (1 μM). Black line surrounding region of interest areas of strong signal measure. (B) Time course of PH-mCherry translocation in response to choline (3 mM) or choline and PNU120596 (1 μM). Arrow: time of drug application; n = 10 cells.
A Computational Model of Allosteric Mechanisms Underlying Calcium Signaling by the α7 Receptor.
Experiments in this study, as well as those conducted previously, suggest dynamic interplay between α7, IP3, and Ry receptor proteins in the growth cone. We hypothesize that under normal full agonist activation of the α7 nAChR, metabotropically generated IP3 is produced and diffuses to activate Ca2+ release from nearby IP3Rs through a process dominated by IICR and G protein signaling (Nordman and Kabbani, 2014). The application of α7 open channel enhancers such as PNU120596, however, results in an increase in the open time of the channel, thus fostering a larger calcium influx in the cell. This process favors a shift to CICR through RyR opening in nearby ER. Given the varied kinetics of IP3R and RyR and the altered time course of α7 channel opening, the actions of a PAM may significantly impact local calcium dynamics. To test this, a spatiotemporal computational model of Ca2+ dynamics in the growth cone was developed. The model accurately simulates growth cone Ca2+ signal responses based on α7 channel kinetics and rate constants published in literature (Corradi and Bouzat, 2016). Simulation experiments indicate that PNU120596 modulation is associated with enhanced intracellular calcium relative to agonist application alone (Fig. 7, C–E). PNU120596 coapplication was in fact associated with a fourfold increase in the concentration of intracellular calcium similar to calcium measures in PC12 cells (Fig. 7D). The model also predicts that calcium concentration near the nAChR channel (local calcium concentrations) is much higher, however, and peaks at ∼25 μM in free calcium concentration within the subcellular microdomain space between the nAChR and the ER membrane (Fig. 7E). Thus, α7 receptor placement is critical for ER-associated Ca2+ release, and ligand-activated calcium signals in the model are best matched to experimental findings when the nAChR and the ER are 10–15 nm apart. Computational simulations reveal that increases in local calcium concentrations following PNU120596 modulation are sufficient to activate the RyR, leading to CICR. Measures of calcium flux through the RyR show that a considerable level of calcium is released from the ER for longer time periods when PNU120596 is bound to the α7 channel (Fig. 7G). The α7-mediated IICR is also enhanced by PNU120596 in the model, and in cellular experiments, but in this work too IICR appears less impacted by the presence of the PAM than CICR (Fig. 7F).
Fig. 7.
Modeling mechanisms of ligand-induced calcium signaling. (A) Model schematic showing the spatial dynamics of the α7 channel, the RyR, and IP3R. (B) Kinetic scheme for the α7 channel indicating closed (C), open (O), and desensitized (D) states. Transition rates between various states are indicated in Supplemental Material. (C) Simulated Ca2+ fluorescence is represented by the amount of Ca2+ bound to indicator dye [([Ca2+F] − [Ca2+F]baseline)/[Ca2+F]baseline]. The addition of PNU120596 increases the magnitude of the measured calcium transient over the ACh baseline (green) and ACh with PNU120596 (black). (D) The model allows visualization of the increase in the intracellular calcium concentration ([Ca2+]i) with the addition of PNU120596. (E) The model predicts local calcium concentrations ([Ca2+]local) near the α7 receptor, which are elevated by PNU120596. (F) Simulation of [Ca2+]i generated by PNU120596 and ACh (black) is reduced by blocking the RyR (blue) or blocking the IP3R (red). (G) Simulations of calcium flux through open α7 (black) and RyR (red) clusters in the presence of ACh and PNU120596. Inset: integrated α7 calcium flux in the presence of the agonist (black) is augmented by the addition of PNU120596 (red). (H) Simulations of IP3R calcium flux generated by ACh activation of the α7 nACh with (blue) or without (yellow) PNU120596. Inset: the integrated fluxes for the period of agonist stimulation (approximately 2.5–3.0 s).
Discussion
Although it has been proposed that many different states of the α7 nAChR may exist following ligand binding, it remains unclear how these states affect global signaling within cells (Le Novère et al., 2002; Bocquet et al., 2009; Bouzat et al., 2017). In this study, we demonstrate that activation of the α7 nAChR is tightly coupled to ER calcium store release and that this process is subject to regulation by allosteric-acting compounds such as PNU120596. Specifically, choline activation of the α7 nAChR either by itself, or in combination with PNU120596, leads to a significant rise in intracellular calcium through calcium store release. This process can be directly measured as robust calcium transient with a discreet peak in its amplitude and duration across various experimental conditions. Similar to choline treatment, the application of nicotine or the endogenous neurotransmitter ACh can mediate α7 nAChR calcium signaling that is altered by PNU120596 coapplication, suggesting that allosteric site occupancy impacts calcium signaling. Interestingly, calcium signaling was seen even in the presence of Bgtx when ACh or nicotine was applied, consistent with the finding of other types of cholinergic receptors in PC12 cells, which express several other nAChR subunits, including α3, α5, β2, β3, and β4, and muscarinic receptors (Rogers et al., 1992).
Our experiments clearly show that the α7-driven calcium transient consists of a RyR-mediated CICR and an IP3R-mediated IICR component operating in a synergistic manner to augment calcium release in response to α7 nAChR activation. Compounds such as PNU120596 thus appear to operate, at least in part, through an ability to enhance coupling between the α7 nAChR and the ER when the receptor resides in close proximity to this organelle. We tested our hypothesis through pharmacological, genetic, and computational methods, building upon a well-established body of literature that demonstrates α7 specificity for calcium signaling (Zhong et al., 2013; Nordman and Kabbani, 2014; Cheng and Yakel, 2015; King et al., 2015). In one set of experiments, we explored the ability of PNU120596 to regulate calcium signaling in cells transfected with the α7D44A subunit, which harbors a mutation at amino acid position 44 in the amino terminus extracellular receptor region, resulting in a 64% lowered channel conductance of calcium (Colón-Sáez and Yakel, 2014). From earlier work, we knew that expression of the α7D44A mutant in the PC12 cell is possible and associated with aberrances in α7 signaling during neurite growth (King and Kabbani, 2016). Experiments in this study show that calcium entry through the α7 channel is critical for PNU120596 modulation of calcium release from the ER involving both CICR and IICR. In a second series of studies, we examined the capacity of PNU120596 to alter metabotropic G protein signaling by α7 receptors by transfecting the α7345–348A subunit, which is impaired in G protein binding (King et al., 2015). Experiments using the α7345–348A subunit confirm the involvement of G protein–coupled IICR in the mechanism of action of PAM modulation because the effects of PNU120596 on calcium signaling were also diminished in cells expressing this mutant. Based on the data, however, G protein–associated metabotropic signaling by the α7 receptor appears relatively less impacted by the modulatory actions of PNU120596 than CICR. Based on these findings, we propose that calcium influx through the open α7 channel leading to CICR is an essential component of PNU120596 actions on cellular calcium signaling and may be a mechanism for its efficacy in drug development (Fig. 8).
Fig. 8.
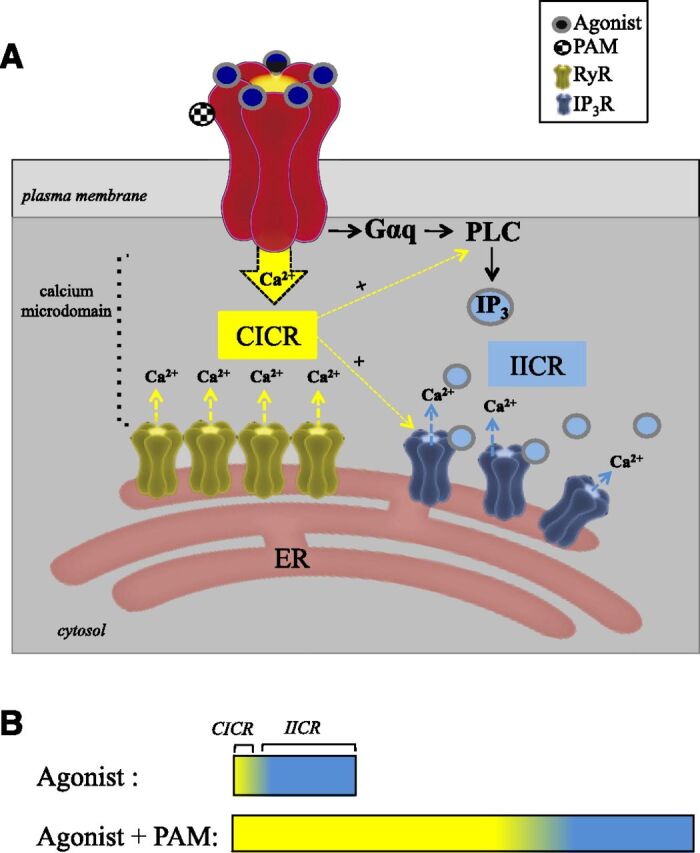
α7 nAChR calcium signaling and its modulation. α7 nAChRs localize to calcium microdomains, which enable coupling between the receptor and the ER. (A) Allosteric modulation by PNU120596 promotes enhanced calcium entry through the open α7 channel and the activation of RyR-associated CICR. Direct interaction between the α7 nAChR and Gαq activates PLC, resulting in IP3R-mediated IICR, which can be further augmented by elevated local calcium acting on PLC as well as IP3R. (B) Intracellular calcium signaling by the α7 nAChR is differentially regulated by the binding of orthosteric and allosteric compounds.
These studies also show an effect of PNU120596 on intracellular calcium signaling at the growth cone of PC12 cells that is corroborated by a new computational model of α7 nAChR calcium signaling through the ER. This computational model is established on published single channel recording for the α7 receptor in mammalian cells (McCormack et al., 2010; Corradi and Bouzat, 2016). The model accurately predicts intracellular calcium transients with similar time and amplitude properties as our experimental measures. The model extends our experimental data by showing that an ability of the α7 receptor to regulate CICR (as well as IICR) depends on colocalization between α7 nAChRs and Ry as well as IP3 receptors in regions where the plasma membrane and the ER are in close proximity. At such sites, PNU120596 modulation is associated with enhanced time in the open channel state and decreased time in the desensitized state; this is matched by greater peak current and open probability (21-fold) within the model similar to experimental results. PNU120596 modulation of the α7 channel drives strong calcium influx that is critical for activating nearby RyRs through CICR. Earlier studies in neurons and brain slices support this idea by showing that α7 receptors localize to ER-rich cellular domains and can directly regulate calcium release from the ER, which is essential for their function at neuronal synapsis (Zhong et al., 2013). Experiments and computational studies provided in this study elucidate a mechanism of α7/ER coupling that can guide future studies on the effects and therapeutic potential of α7-targeting compounds.
Taken together, our findings support the notion that PAMs act at the α7 nAChR site through enhanced ionotropic flux through the open channel, which in turn activates CICR, leading to calcium signal transduction. We show that this effect strongly depends on spatial and functional coupling between the α7 receptor and the ER within the cell. In some cells (such as immune cells), however, it is possible that PAM modulation of the α7 nAChR is less paired to CICR especially if the α7 receptor is not in the vicinity of the ER. In such a case, it is plausible that a PAM such as PNU120596 operates via enhanced G protein–mediated metabotropic signaling and through a nonconducting α7 state, which has been suggested (Bouzat et al., 2017). In fact, our study supports the role of PNU120596 in augmented metabotropic G protein signaling by the α7 receptor through enhanced PLC activity and IP3R-driven IICR (Fig. 8). PNU120596-mediated increases in ionotropic-driven CICR within cells do not occlude metabotropic coupling, which appears to also increase in the presence of this compound. In fact, PAM-associated CICR leading to elevated calcium within the α7/ER microdomain may support metabotropic G protein signaling through increased PLC activation and IP3R conductance, because both molecules are positively driven by calcium ions. Based on this study, it is thus important to consider ionotropic and metabotropic α7 nAChR modes when assessing the effectiveness and function of an allosteric modulator (Kabbani and Nichols, 2018). In neurons, where α7 nAChRs are regulators of synaptic activity that underlies learning and memory through various forms of intracellular calcium signaling (Ge and Dani, 2005; Shen and Yakel, 2009; Gomez-Varela and Berg, 2013), the mechanisms of pharmacological neuromodulation maybe translate to specific measured outcomes in enhanced cognition and learning (Deutsch et al., 2013; Bouzat et al., 2017).
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- Bgtx
α-bungarotoxin
- CICR
calcium-induced calcium release
- ER
endoplasmic reticulum
- fBgtx
fluorescent-tagged Bgtx
- IICR
inositol-induced calcium release
- IP3
inositol triphosphate
- IP3R
IP3 receptor
- nAChR
nicotinic acetylcholine receptor
- PAM
positive allosteric modulator
- PC12
pheochromocytoma cell line 12
- PDI
protein disulfide isomerase
- PH
Pleckstrin homology domain
- PLC
phospholipase C
- Ry
ryanodine
- RyR
Ry receptor
- Xest. C
Xestospongin C
Authorship Contributions
Participated in research design: King, Ullah, Jafri, Kabbani.
Conducted experiments: King, Bak, Ullah.
Contributed new reagents or analytic tools: Ullah, Jafri.
Performed data analysis: King, Ullah, Jafri, Kabbani.
Wrote or contributed to the writing of the manuscript: King, Ullah, Jafri, Kabbani.
Footnotes
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, et al. (2012) Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32:13819–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997) Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9:2734–2742. [DOI] [PubMed] [Google Scholar]
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR, Bouzat C (2016) Exploring the positive allosteric modulation of human α7 nicotinic receptors from a single-channel perspective. Neuropharmacology 107:189–200. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lippiello PM, Lucas R, Marrero MB (2011) Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci 68:931–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux J-P, Delarue M, Corringer P-J (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Lasala M, Nielsen BE, Corradi J, Esandi MDC (2017) Molecular function of α7 nicotinic receptors as drug targets. J Physiol DOI: 10.1113/JP275101 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK (2010) Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci 30:8734–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Quik M (1993) A role for the nicotinic α-bungarotoxin receptor in neurite outgrowth in PC12 cells. Neuroscience 56:441–451. [DOI] [PubMed] [Google Scholar]
- Changeux J-P (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11:389–401. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL (2015) Activation of α7 nicotinic acetylcholine receptors increases intracellular cAMP levels via activation of AC1 in hippocampal neurons. Neuropharmacology 95:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Saini DK, Cho J-H, Kalyanaraman V, Gautam N (2009) G protein subunit dissociation and translocation regulate cellular response to receptor stimulation. PLoS One 4:e7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Sáez JO, Yakel JL (2014) A mutation in the extracellular domain of the α7 nAChR reduces calcium permeability. Pflugers Arch 466:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi J, Bouzat C (2016) Understanding the bases of function and modulation of α7 nicotinic receptors: implications for drug discovery. Mol Pharmacol 90:288–299. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Brown CH, Rosse RB, Rosse SM (2013) Targeting alpha-7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res 148:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI (2013a) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther 344:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M (2013b) Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Dani JA (2005) Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci 25:6084–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Varela D, Berg DK (2013) Lateral mobility of presynaptic α7-containing nicotinic receptors and its relevance for glutamate release. J Neurosci 33:17062–17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J (2007) Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL (2011) Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 71:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Álvarez M, Moreno-Ortega AJ, Navarro E, Fernández-Morales JC, Egea J, López MG, Cano-Abad MF (2015) Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca(2+) release from the endoplasmic reticulum. J Neurochem 133:309–319. [DOI] [PubMed] [Google Scholar]
- Halff AW, Gómez-Varela D, John D, Berg DK (2014) A novel mechanism for nicotinic potentiation of glutamatergic synapses. J Neurosci 34:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K (2015) Targeting of α7 nicotinic acetylcholine receptors in the treatment of schizophrenia and the use of auditory sensory gating as a translational biomarker. Curr Pharm Des 21:3797–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW (2005) Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol 168:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Yakel JL (1997) Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol 504:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA (2018) Beyond the channel: metabotropic signaling by nicotinic receptors. Trends Pharmacol Sci 39:354–366. [DOI] [PubMed] [Google Scholar]
- King JR, Gillevet TC, Kabbani N (2017) A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio 7:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Kabbani N (2016) Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J Neurochem 138:532–545. [DOI] [PubMed] [Google Scholar]
- King JR, Nordman JC, Bridges SP, Lin M-K, Kabbani N (2015) Identification and characterization of a G protein-binding cluster in α7 nicotinic acetylcholine receptors. J Biol Chem 290:20060–20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Corringer P-J, Changeux J-P (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53:447–456. [DOI] [PubMed] [Google Scholar]
- Liu Qs, Berg DK (1999) Actin filaments and the opposing actions of CaM kinase II and calcineurin in regulating alpha7-containing nicotinic receptors on chick ciliary ganglion neurons. J Neurosci 19:10280–10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack TJ, Melis C, Colón J, Gay EA, Mike A, Karoly R, Lamb PW, Molteni C, Yakel JL (2010) Rapid desensitization of the rat α7 nAChR is facilitated by the presence of a proline residue in the outer β-sheet. J Physiol 588:4415–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, Pemberton DJ, Neill JC (2012) PNU-120596, a positive allosteric modulator of α7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol 26:1265–1270. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Kabbani N (2012) An interaction between α7 nicotinic receptors and a G-protein pathway complex regulates neurite growth in neural cells. J Cell Sci 125:5502–5513. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Kabbani N (2014) Microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation of a Gαq and IP3 receptor pathway. FASEB J 28:2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P (1996) An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett 213:201–204. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Mandelzys A, Deneris ES, Cooper E, Heinemann S (1992) The expression of nicotinic acetylcholine receptors by PC12 cells treated with NGF. J Neurosci 12:4611–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MdeS, Naal RM, Baird B, Holowka D (2013) Inhibitors of PI(4,5)P2 synthesis reveal dynamic regulation of IgE receptor signaling by phosphoinositides in RBL mast cells. Mol Pharmacol 83:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragoza PA, Modir JG, Goel N, French KL, Li L, Nowak MW, Stitzel JA (2003) Identification of an alternatively processed nicotinic receptor alpha7 subunit RNA in mouse brain. Brain Res Mol Brain Res 117:15–26. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JX, Yakel JL (2009) Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin 30:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89:337–343. [DOI] [PubMed] [Google Scholar]
- Ullah G, Ullah A (2016) Mode switching of inositol 1,4,5-trisphosphate receptor channel shapes the spatiotemporal scales of Ca2+signals. J Biol Phys 42:507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV (2014) The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol 727:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A (2002) The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium 32:393–404. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421:384–388. [DOI] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol 82:746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GSB, Chikando AC, Tuan H-TM, Sobie EA, Lederer WJ, Jafri MS (2011) Dynamics of calcium sparks and calcium leak in the heart. Biophys J 101:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Du Y, Zhang J, Xu X, Xue F, Guo C, Huang Y, Lukas RJ, Chang Y (2015) Functional impact of 14 single nucleotide polymorphisms causing missense mutations of human α7 nicotinic receptor. PLoS One 10:e0137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Talmage DA, Role LW (2013) Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS One 8:e82719. [DOI] [PMC free article] [PubMed] [Google Scholar]