Abstract
Background and Aim
To date, no randomized trials have compared the efficacy of 7‐day vonoprazan, amoxicillin, and metronidazole triple therapy (VAM) versus 7‐day vonoprazan, amoxicillin, and clarithromycin triple therapy (VAC) as a first‐line treatment for Helicobacter pylori eradication. This study was performed to compare the efficacy of VAM and VAC as first‐line treatments.
Methods
This prospective multicenter randomized trial was performed in Japan and involved 124 H. pylori‐positive patients without a history of eradication. Patients without antibiotic resistance testing of H. pylori were eligible. The patients were randomized to receive either VAC (vonoprazan 20 mg + amoxicillin 750 mg + clarithromycin 200 or 400 mg twice a day) or VAM (vonoprazan 20 mg + amoxicillin 750 mg + metronidazole 250 mg twice a day) for 7 days, with stratification by age and sex. Eradication success was evaluated using the 13C‐urea breath test. We evaluated safety using patient questionnaires (UMIN000025773).
Results
The intention‐to‐treat and per‐protocol eradication rates of VAM were 91.3% (95% confidence interval [CI], 82.0–96.7%) and 92.6% (95% CI, 83.7–97.6%), respectively, and those of VAC were 89.1% (95% CI, 77.8–95.9%) and 96.1% (95% CI, 86.5–99.5%), respectively. No significant difference was observed between VAM and VAC in either analysis (P = 0.76 and P = 0.70, respectively). Abdominal fullness was more frequent in patients who received VAM than VAC.
Conclusions
These findings suggest that VAM as a first‐line treatment in Japan can be categorized as grade B (intention‐to‐treat cure rate of 90–95%) and have potential as a first‐line national insurance ‐approved regimen.
Keywords: 7‐day triple therapy, clarithromycin, Helicobacter pylori, metronidazole, vonoprazan
Randomized trial comparing 7‐day vonoprazan, amoxicillin, and metronidazole triple therapy (VAM) versus 7‐day vonoprazan, amoxicillin, and clarithromycine triple therapy as a first‐line Helicobacter pylori eradication showed that the efficacy of VAM in Japan can be categorized as grade B (ITT cure rate of 90‐95%) and have potential as a first‐line national insurance approved regimen.
Introduction
Signaling pathways triggered by Helicobacter pylori (H. pylori) play a role in the development of gastric carcinogenesis. 1 H. pylori eradication therapy reduces the incidence of gastric cancer, and eradication is recommended for all H. pylori‐infected patients. 2 , 3 , 4 , 5 , 6 Since 2013, the eradication of H. pylori for H. pylori‐associated chronic gastritis has been included in the coverage of national insurance in Japan. Vonoprazan, a type of potassium‐competitive acid blocker, has been commonly employed for eradication purposes since 2015. 7 Japanese insurance coverage is limited to clarithromycin‐based first‐line triple therapy with amoxicillin and vonoprazan or proton pump inhibitors (PPIs), as well as metronidazole‐based second‐line triple therapy with amoxicillin and vonoprazan or PPIs. 8 About the duration, only 7 days is approved in Japan. As for PPI‐based triple therapy with amoxicillin and clarithromycin (PPI‐AC), meta‐analysis showed eradication rate is higher in the order of 14, 10, and 7 days. 9 On the other hand, RCT comparing 7‐day triple therapy with vonoprazan, amoxicillin, and clarithromycin (VAC) and 14‐day PPI‐AC showed no significant difference: 98.3% with 7‐day VAC versus 93.1% with 14‐day PPI‐AC (P = 0.159). 10 Seven‐day duration may be sufficient for VAC. The preference for VAC as a first‐line treatment is due to its avoidance of extensive use of metronidazole, as per the permitted guidelines for the treatment of Trichomonas infection in Japan. 11 Although the mean clarithromycin resistance rate of H. pylori in Japan was 21%, in average, from 1990 to 2020, 12 it has increased to 33.8%, in average, from 2017 to 2019. 13 The mean metronidazole resistance rate of H. pylori in Japan was 11%, in average, from 1990 to 2020, 12 and trends in metronidazole resistance was reported to remain unchanged. 14 Metronidazole‐based triple‐therapy approval in Japan was based on public notice application method without interventional trial. Thus, randomized controlled trial of VAM as first line is important for the first‐line approval of VAM.
A meta‐analysis of randomized controlled trials (RCTs) indicated that VAC may be superior to PPI‐AC as a first‐line regimen in populations exhibiting approximately 30% clarithromycin resistance, 13 whereas no RCT comparing VAC and 7‐day triple therapy with vonoprazan, amoxicillin, and metronidazole (VAM) as first line was reported. The superiority of VAC compared with PPI‐AC in approximately 30% clarithromycin resistance setting can be evaluated by the combination of VAC versus PPI‐AC for clarithromycin‐resistant H. pylori and VAC versus PPI‐AC for clarithromycin‐susceptible H. pylori. Based on the review, VAC is superior to PPI‐AC for clarithromycin‐resistant H. pylori, whereas VAC is not superior to PPI‐AC for clarithromycin‐susceptible H. pylori. Thus, the total superiority is based on superiority for clarithromycin‐resistant H. pylori. Importantly, the eradication rate of clarithromycin‐resistant H. pylori using VAC triple therapy remains unacceptably low. The evidence for VAM triple therapy versus triple therapy with PPI, amoxicillin, and metronidazole (PPI‐AM) as a second‐line regimen is almost entirely based on retrospective cohort trials; no RCTs have been performed to date. 13 A recent meta‐analysis of non‐RCTs showed slight (approximately 2.6%) superiority of VAM over PPI‐AM as second‐line eradication, but the result was unreliable because of several biases. 13 We conducted a prospective study of first‐line VAM for clarithromycin‐resistant H. pylori to investigate the use of VAM as a first‐line regimen, and we confirmed VAM (100%, 95% CI, 90–100%, n = 35, both intention‐to‐treat [ITT] and per‐protocol [PP]) was superior to VAC (76.5%, 95% CI, 66.9–84.5%, n = 98 in ITT) (77.3%, 95% CI, 67.7–85.2%, n = 97 in PP) for clarithromycin‐resistant H. pylori (P = 0.00052 in ITT, P = 0.00095 in PP). 15 In addition, a recent study showed that dual therapy with vonoprazan and amoxicillin as a first‐line regimen produced an eradication rate of 84.5% in ITT analysis and 87.1% in the PP analysis. 16 Therefore, we consider that VAM has the potential to be the standard first‐line regimen when information on antibiotic resistance is unavailable.
Our hypothesis in the present study was that VAM is superior to VAC in the clinical setting in Japan. Because we concluded in our prior studies that VAC should not be used as a first‐line regimen for clarithromycin‐resistant H. pylori 17 and that VAM should be used for clarithromycin‐resistant H. pylori, 15 we needed to exclude patients in whom the antibiotic resistance status of H. pylori was known.
In the present RCT, therefore, the efficacy and safety of VAM triple therapy as a first‐line regimen were compared with those of VAC triple therapy as a first‐line regimen in patients with no available antibiotic resistance or susceptibility information.
Methods
Study design and ethical issues
This RCT was carried out across multiple centers and conducted in compliance with both the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects outlined by the Japanese Ministry of Health, Labour and Welfare. The study was conducted by Yokohama City University Hospital, Yokohama Minami Kyosai Hospital, Yokohama Ekisaikai Hospital, Yokosuka City Hospital, and Kanagawa Prefectural Ashigarakami Hospital. The research received approval from the institutional review board at each participating hospital and was registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (www.umin.ac.jp/ctr/) (UMIN000025773, January 2017). The institutional review board of Yokohama City University Hospital granted approval for the protocol on 16 December 2016 (no. B161201004).
Participants
The inclusion criteria for this study were age of >20 years, current H. pylori infection, no history of H. pylori eradication, no H. pylori susceptibility test results, and the ability to perform a urea breath test (UBT) 8 weeks after treatment completion. The exclusion criteria for this study were a history of allergy to the drugs used in the study, pregnancy, lactation, severe renal dysfunction, severe liver dysfunction, severe heart dysfunction, infectious mononucleosis, brain and spinal cord disease, and disqualification by the physicians. All patients provided written informed consent.
Assessment of H. pylori infection
H. pylori infection was confirmed by a UBT, 18 detection of anti‐H. pylori antibody, 19 a rapid urease test, 20 pathology (histology), 21 or H. pylori culture 22 (same procedure as in our previous studies 15 , 17 ) (Table 1). Latex agglutination immunoturbidimetry kit was used with 10 U/mL cutoff for anti‐H. pylori antibody test, which was reported with sufficient sensitivity and specificity for diagnosis. 23 The anti‐H. pylori test was performed by external agency. Endoscopy was also performed in all patients within 1 year, and H. pylori infection was suspected based on endoscopic gastritis in all patients (Table 1). These diagnostic methods are stated in the Japanese Helicobacter guideline and generally used as national insurance covered medical examination in Japan.
Table 1.
Baseline characteristics
| VAM triple therapy (n = 69) | VAC triple therapy (n = 55) | P | |
|---|---|---|---|
| Age, years | 65.3 ± 12.3 | 63.1 ± 10.7 | |
| Male | 38 (55.1) | 33 (60.0) | 0.59 |
| CAM 400 mg/day | NA | 41 (74.5) | NA |
| Endoscopic findings | |||
| Gastroduodenal ulcer | 8 (11.6) | 8 (14.5) | |
| Gastritis only | 61 (88.4) | 47 (85.5) | 0.79 |
| Diagnosis of infection | |||
| Anti‐H. pylori antibody | 35 (50.7) | 24 (43.6) | |
| H. pylori culture (without susceptibility test) | 16 (23.2) | 14 (25.5) | |
| Urea breath test | 11 (15.9) | 11 (20.0) | 0.46 |
| Rapid urease test | 4 (5.8) | 6 (10.9) | |
| Pathology (histology) | 3 (4.3) | 0 (0.0) | |
Data are presented as the mean ± SD or n (%).
CAM, clarithromycin; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; NA, not applicable; VAC triple therapy, vonoprazan + amoxicillin + clarithromycin 1‐week eradication therapy; VAM triple therapy, vonoprazan + amoxicillin + metronidazole 1‐week eradication therapy.
Randomization and treatment
Eligible patients were randomly assigned to receive one of the following two regimens: VAM (triple therapy, administered twice daily for a duration of 7 days, the combination consisted of 20 mg of vonoprazan, 750 mg of amoxicillin, and 250 mg of metronidazole.) or VAC (triple therapy, administered twice daily for a duration of 7 days, the combination consist of 20 mg of vonoprazan, 750 mg of amoxicillin, and 200 mg or 400 mg clarithromycin). Clarithromycin 200 mg twice daily and 400 mg twice daily are both national insurance covered and double‐blind RCT showed no difference between 200 mg and 400 mg. 7 Amoxicillin 750 mg twice daily and metronidazole 250 mg twice daily is the Japanese national insurance covered doses for H. pylori eradication, and commonly used in Japan. These dose settings are based on previous studies in Japan, especially vonoprazan phase III double‐blind RCT. 7 Randomization was performed using the minimization method with reference to age (20–39, 40–64, 65–75, or >75 years) and sex (male or female). The detail of minimization method is indeterministic type, in which random assignment was performed while adjusting to increase the probability of being assigned to a setting. Compared with deterministic type minimization method, indeterministic type retains unpredictability, but does not converge to a 1:1 ratio. We used the easily accessible web‐based allocation system “QMinim”: online version of MinimPy, as in our previous studies. 15 , 21 MinimPy is a free, open source, minimization program, with which nearly all aspects of a minimization model can be configured. This was an open‐label trial because the primary endpoint (the eradication rate) was an objective parameter.
Outcome
We tested the following hypothesis: VAM is superior to VAC as a first‐line regimen for eradication of H. pylori.
The primary endpoint of this study was the eradication success rate of H. pylori with first‐line therapy, assessed using the 13C‐UBT at least 8 weeks after the end of eradication therapy. All patients were instructed not to take PPIs or vonoprazan from eradication until completion of the UBT. This is settled to prevent false negative of UBT. All UBTs were performed by an external agency or inspection department that was independent from the researchers' institution.
The primary analysis was established based on both the ITT and PP analyses. The ITT analysis included all patients who began the eradication therapy. In the ITT analysis, patients who were lost to follow‐up or did not undergo the UBT after eradication were considered to have experienced treatment failure. Eradication success was defined as a UBT result of <2.5‰.
Safety
The safety assessment for the secondary endpoint involved the completion of an adverse events questionnaire (AEQ) by each patient during the course of therapy. We used the same AEQ as in our previous studies. 8 , 15 , 17 , 24 , 25 , 26 The AEQ contained questions regarding fatigue, vomiting, eructation, abdominal fullness, headache, urticaria, heartburn, abdominal pain, anorexia, nausea, dysgeusia, diarrhea, and other symptoms. These were categorized as none, weak, moderate, or strong, corresponding to AEQ scores of 0, 1, 2, and 3, respectively. All adverse events questionnaires (AEQs) were gathered at the commencement of the clinical examination, ensuring the absence of reporting bias.
Sample size calculation
The sample size was planned with reference to vonoprazan phase III data revealing a second‐line VAM eradication rate of 98% (95% confidence interval [CI], 89.4–99.9%; n = 50). When designing this study, we expected the eradication rate of first‐line VAM to be 98% because the first‐line eradication rate is higher than the second‐line eradication rate. We also estimated the eradication rate of VAC to be 85% based on our previous prospective observational study, which showed an eradication rate of 84.9% (95% CI, 81.9–87.6%; n = 623). 8 The number of patients needed to achieve a power of 80% with a type I error of 0.05 was 56 in each group (112 in total). We estimated a dropout rate of about 10%, and the sample size was thus calculated as 62 patients in each group (124 in total). We preplanned an interim analysis at 124 cases, at which time we would determine whether statistical significance existed. If the VAM eradication rate was around 97% and no significant difference was observed, we planned to continue the study to 190 cases. Based on the 97% expected eradication rate of VAM and 85% estimated eradication rate of VAC, we achieved a power of 80% with a type I error of 0.05, allowing for an approximately 10% dropout rate.
Statistical analyses
Fisher's exact test was applied for the analysis of categorical data, while Student's t‐test was utilized to analyze continuous data, presented as the mean ± SD.
The primary endpoints' 95% CIs were computed. In both the ITT and PP analyses, the disparity in eradication rates between VAM and VAC was assessed using Fisher's exact test, accompanied by two‐sided 95% CIs. Statistical significance was defined as a P value of less than 0.05. We performed all statistical analyses using SPSS ver. 24 (IBM Corp., Armonk, NY, USA).
Results
Recruitment and follow‐up periods
We registered this study in the UMIN registry in January 2017. Patient enrollment began in January 2017, and the last follow‐up occurred in February 2019.
As shown in Figure 1, 124 patients with H. pylori infection without an eradication history were enrolled in this study, and they were randomly assigned using the minimization method with respect to age and sex. Sixty‐nine patients were assigned to VAM, and 55 were assigned to VAC. In the VAM arm, one patient was not included in the PP analysis due to loss of follow‐up. In the VAC arm, four patients were excluded from the PP analysis due to loss to follow‐up.
Figure 1.
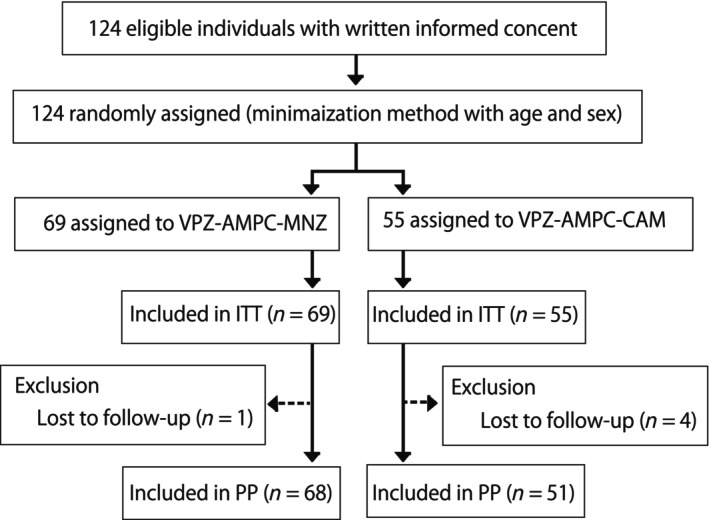
Flowchart of patient enrollment. In total, 124 patients were randomized and treated with either VAM triple therapy or VAC triple therapy. Five patients dropped out or interrupted their therapy. VAC triple therapy: vonoprazan + amoxicillin + clarithromycin 1‐week eradication therapy; VAM triple therapy: vonoprazan + amoxicillin + metronidazole 1‐week eradication therapy.
Baseline characteristics
The patients' baseline characteristics are summarized in Table 1. The patients in both groups were similar in age, sex, endoscopic findings, and diagnostic technique. There were no statistically significant differences in any characteristics between the two groups.
Efficacy
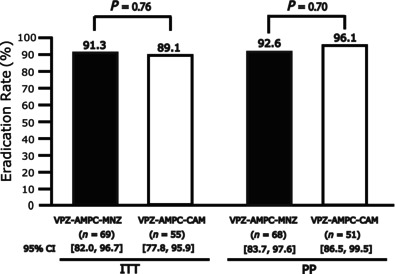
In the ITT analysis, the eradication rates were 91.3% (95% CI, 82.0–96.7%, n = 69) with VAM and 89.1% (95% CI, 77.8–95.9%, n = 55) with VAC. In the PP analysis, the eradication rates were 92.6% (95% CI, 83.7–97.6%, n = 68) with VAM and 96.1% (95% CI, 86.5–99.5%, n = 51) with VAC. No significant differences were observed between VAM and VAC in either the ITT analysis (P = 0.76) or PP analysis (P = 0.70) (Fig. 2).
Figure 2.
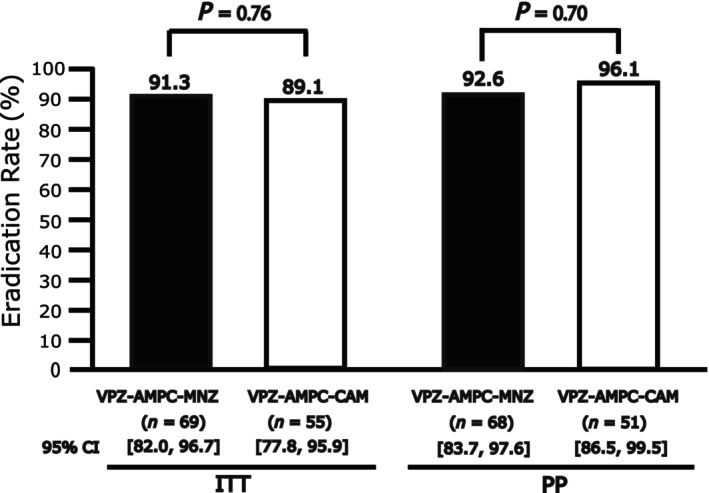
Eradication rates of VAM triple therapy and VAC triple therapy in ITT and PP analyses. No significant differences were observed between VAM and VAC in either the ITT or PP analyses. ITT, intention‐to‐treat; PP, per‐protocol; VAC triple therapy: vonoprazan + amoxicillin + clarithromycin 1‐week eradication therapy; VAM triple therapy: vonoprazan + amoxicillin + metronidazole 1‐week eradication therapy.
Adverse events
The frequencies of adverse events during therapy are shown in Table 2. The frequency of any grade (score of 1, 2, or 3) of abdominal fullness was significantly higher in the VAM than VAC arm (56% vs 14%, respectively). The frequency of any grade of anorexia was also significantly higher in the VAM than VAC arm (23% vs 5%, respectively). The frequencies of any grade of other symptoms (diarrhea, dysgeusia, nausea, abdominal pain, heartburn, hives, headache, belching, vomiting, general malaise, and others) were not significantly different between the groups. Moreover, there were no significant differences in strong symptoms (score of 3) between the groups. All adverse events resolved spontaneously without any intervention, and no patients required hospitalization due to these events.
Table 2.
Adverse events based on a questionnaire
| Any (score of 1, 2, or 3) | VAM triple therapy | VAC triple therapy | P |
|---|---|---|---|
| Diarrhea | 33% | 37% | 0.82 |
| Dysgeusia | 21% | 17% | 1 |
| Nausea | 14% | 2% | 0.11 |
| Anorexia | 23% | 5% | 0.03 |
| Abdominal pain | 23% | 23% | 1 |
| Heartburn | 16% | 12% | 0.76 |
| Hives | 0% | 5% | 0.49 |
| Headache | 16% | 16% | 1 |
| Abdominal fullness | 56% | 14% | <0.001 |
| Belching | 21% | 12% | 0.38 |
| Vomiting | 0% | 0% | 1 |
| General malaise | 16% | 9% | 0.52 |
| Other | 2% | 5% | 1 |
| Any (score of 2 or 3) | |||
| Diarrhea | 16% | 7% | 0.31 |
| Dysgeusia | 21% | 12% | 0.38 |
| Nausea | 7% | 0% | 0.24 |
| Anorexia | 9% | 0% | 0.12 |
| Abdominal pain | 7% | 2% | 0.62 |
| Heartburn | 5% | 2% | 1 |
| Hives | 0% | 2% | 1 |
| Headache | 5% | 2% | 1 |
| Abdominal fullness | 28% | 2% | 0.007 |
| Belching | 9% | 2% | 0.36 |
| Vomiting | 0% | 0% | 1 |
| General malaise | 2% | 0% | 1 |
| Other | 0% | 0% | 1 |
| Score of 3 | |||
| Diarrhea | 5% | 5% | 1 |
| Dysgeusia | 2% | 9% | 0.36 |
| Nausea | 0% | 0% | 1 |
| Anorexia | 2% | 0% | 1 |
| Abdominal pain | 2% | 0% | 1 |
| Heartburn | 0% | 0% | 1 |
| Hives | 0% | 2% | 1 |
| Headache | 2% | 2% | 1 |
| Abdominal fullness | 7% | 2% | 0.62 |
| Belching | 0% | 0% | 1 |
| Vomiting | 0% | 0% | 1 |
| General malaise | 0% | 0% | 1 |
| Other | 0% | 0% | 1 |
VAC triple therapy, vonoprazan + amoxicillin + clarithromycin 1‐week eradication therapy; VAM triple therapy: vonoprazan + amoxicillin + metronidazole 1‐week eradication therapy. The bold showes less than 0.05.
Discussion
To our knowledge, this is the first RCT to compare the efficacy of VAM versus VAC triple therapy as a first‐line regimen for H. pylori eradication. This study showed no significant difference in the eradication rates of VAM and VAC as first‐line therapy in both the ITT and PP analyses. In addition, based on the point estimate of the ITT results, VAM as first‐line therapy was categorized as good (cure rate of 90–95%), and VAC as first‐line therapy was categorized as fair (cure rate of 85–89%). 27
As in our previous studies, we used an AEQ to avoid evaluator bias in the comparison of adverse events. The rates of abdominal fullness and anorexia of any severity were higher in VAM than VAC therapy, but the symptoms were cured and compliance was good. There were no significant differences in score 3 symptoms between the groups. We believe that the use of VAM as a first‐line regimen is as safe as the use of VAM as a second‐line regimen.
This result is consistent with previous studies of VAM as a second‐line therapy, which showed a slightly higher eradication rate than that of PPI‐AM, 28 , 29 as well as a recent study of dual therapy with vonoprazan and amoxicillin as a first‐line regimen. 16 First, the eradication rate of VAM as a first‐line therapy in the present study was higher than that of dual VPZ and AMPC therapy: 84.5% in the ITT analysis and 87.1% in the PP analysis. Second, a meta‐analysis showed that PPI‐AC and PPI‐AM as first‐line therapies were equivalent worldwide, and PPI‐AM therapy has the potential to reach an eradication success rate of >90% in geographical areas of low metronidazole resistance. Possible explanation for lower‐than‐expected eradication rate of VAM is metronidazole resistance rate in this study.
By contrast, the VAC eradication rate of 89.1% (95% CI, 77.8–95.9%) in the ITT analysis and that of 96.1% (95% CI, 86.5–99.5%) in the PP analysis were higher than expected. We previously reported a VAC eradication rate of 87.3% (95% CI, 75.5–94.7%) in the ITT analysis and 88.9% (95% CI, 77.4–95.8%) in the PP analysis for CAM‐susceptible H. pylori. 17 We also reported a VAC eradication rate of 82.9% (95% CI, 67.9–92.8%) for clarithromycin‐resistant H. pylori. 17 A possible explanation for this difference is the MIC of amoxicillin. In the previous study, we set the amoxicillin‐resistance cutoff at ≥0.5 mg/L, and no cases were resistant. 17 , 25 However, a recent study of VAC as a first‐line therapy showed a difference in the eradication rate between the MIC levels of amoxicillin in the non‐resistance category: 92.7% (n = 151) for an MIC of <0.03, 77.8% (n = 9) for an MIC of 0.03, and 25.0% (n = 4) for an MIC of 0.06. 16 Thus, not only clarithromycin susceptibility or resistance but also the MIC of amoxicillin is important in the treatment of H. pylori with very low amoxicillin resistance.
The present study has three main limitations. First, this study was conducted as superiority study and non‐inferiority of VAM to VAC was not proved. Further RCT with non‐inferiority design is needed. Second, the patients who did not have amoxicillin, clarithromycin, or metronidazole susceptibility testing result were recruited. Susceptibility testing was not conducted after recruitment due to ethical concerns arising from randomizing patients to an unacceptably low regimen. For clarithromycin‐resistant H. pylori, we showed VAM is superior to VAC, and VAC showed unacceptably low eradication rate for clarithromycin‐resistant H. pylori. 15 Third, this multicenter RCT was conducted only in Japan, limiting the generalization of its results beyond Japanese patients with H. pylori.
Conclusions
The present registered prospective RCT showed efficacy of VAM triple therapy as a first‐line H. pylori eradication therapy in Japan. No significant difference in the eradication rate was observed between VAM triple therapy and standard VAC triple therapy as a first‐line treatment. We suggest that, similar to VAC triple therapy, VAM triple therapy has potential to become a standard first‐line regimen without information regarding antibiotic resistance in Japanese patients, and further non‐inferiority RCT of first‐line VAM is desirable.
Informed consent statement
All participants provided written informed consent before study enrollment.
Declaration of conflict of interest: The authors declare no conflict of interest.
Author contribution: Soichiro Sue and Shin Maeda contributed to the study concept and design. Soichiro Sue, Hiroyuki Oka, Yosuke Kunishi, Yuichi Suzuki, Shingo Suzuki, Takashi Kaneko, Kazuo Komatsu, Makoto Naito, Yoshio Kato, Tomohiko Sasaki, Hiroaki Kaneko, Kuniyasu Irie, Masaaki Kondo, and Shin Maeda were involved in data acquisition. Soichiro Sue and Shin Maeda conducted data analysis and interpretation. Soichiro Sue drafted the manuscript. Hiroyuki Oka, Yosuke Kunishi, Yuichi Suzuki, Shingo Suzuki, Takashi Kaneko, Kazuo Komatsu, Makoto Naito, Yoshio Kato, Tomohiko Sasaki, Hiroaki Kaneko, Kuniyasu Irie, Masaaki Kondo, and Shin Maeda contributed to critical revision of the manuscript; and funding was provided by Shin Maeda. All authors have read and agreed to the published version of the manuscript.
Financial support: This research was funded by Yokohama City University (basic research expenditures).
Data availability statement
The data are not available for public access because of ethical restrictions, but are available from the corresponding author on reasonable request.
References
- 1. Sue S, Shibata W, Maeda S. Helicobacter pylori‐induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis. Biomed. Res. Int. 2015; 2015: 737621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta‐analysis. Gut. 2020; 69: 2113–2121. [DOI] [PubMed] [Google Scholar]
- 3. Lee YC, Chiang TH, Chou CK et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta‐analysis. Gastroenterology. 2016; 150: 1113–1124.e5. [DOI] [PubMed] [Google Scholar]
- 4. Malfertheiner P, Megraud F, Rokkas T et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; 71: 1724–1762. [Google Scholar]
- 5. Kato M, Ota H, Okuda M et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. 2019; 24: e12597. [DOI] [PubMed] [Google Scholar]
- 6. Chey WD, Leontiadis GI, Howden CW, Moss SF. Correction: ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2018; 113: 1102. [DOI] [PubMed] [Google Scholar]
- 7. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium‐competitive acid blocker, as a component of first‐line and second‐line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double‐blind study. Gut. 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sue S, Kuwashima H, Iwata Y et al. the superiority of vonoprazan‐based first‐line triple therapy with clarithromycin: a prospective multi‐center cohort study on Helicobacter pylori eradication. Intern. Med. 2017; 56: 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan Y, Ford AC, Khan KJ et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2013; 12: CD008337. [DOI] [PubMed] [Google Scholar]
- 10. Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7‐day vonoprazan‐based versus 14‐day omeprazole‐based triple therapy for Helicobacter pylori . J. Gastroenterol. Hepatol. 2021; 36: 3308–3313. [DOI] [PubMed] [Google Scholar]
- 11. Satoh K. Indications for Helicobacter pylori eradication therapy and first‐line therapy regimen in Japan: recommendation by the Japanese Society for Helicobacter Research. J. Gastroenterol. 2002; 37: 34–38. [DOI] [PubMed] [Google Scholar]
- 12. Hong TC, El‐Omar EM, Kuo YT et al. Primary antibiotic resistance of Helicobacter pylori in the Asia‐Pacific region between 1990 and 2022: an updated systematic review and meta‐analysis. Lancet Gastroenterol. Hepatol. 2024; 9: 56–67. [DOI] [PubMed] [Google Scholar]
- 13. Sue S, Maeda S. Is a potassium‐competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021; 15: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horie R, Handa O, Ando T et al. Helicobacter pylori eradication therapy outcome according to clarithromycin susceptibility testing in Japan. Helicobacter. 2020; 25: e12698. [DOI] [PubMed] [Google Scholar]
- 15. Sue S, Suzuki Y, Sasaki T et al. Prospective study of vonoprazan‐based first‐line triple therapy with amoxicillin and metronidazole for clarithromycin‐resistant Helicobacter pylori . J. Clin. Med. 2023; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki S, Gotoda T, Kusano C et al. Seven‐day vonoprazan and low‐dose amoxicillin dual therapy as first‐line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020; 69: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sue S, Ogushi M, Arima I et al. Vonoprazan‐ vs proton‐pump inhibitor‐based first‐line 7‐day triple therapy for clarithromycin‐susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter. 2017; 23: e12456. [DOI] [PubMed] [Google Scholar]
- 18. Ohara S, Kato M, Saito M et al. Comparison between a new 13C‐urea breath test, using a film‐coated tablet, and the conventional 13C‐urea breath test for the detection of Helicobacter pylori infection. J. Gastroenterol. 2004; 39: 621–628. [DOI] [PubMed] [Google Scholar]
- 19. Kosunen TU, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori . Lancet. 1992; 339: 893–895. [DOI] [PubMed] [Google Scholar]
- 20. Nishikawa K, Sugiyama T, Kato M et al. A prospective evaluation of new rapid urease tests before and after eradication treatment of Helicobacter pylori, in comparison with histology, culture and 13C‐urea breath test. Gastrointest. Endosc. 2000; 51: 164–168. [DOI] [PubMed] [Google Scholar]
- 21. Laine L, Lewin DN, Naritoku W, Cohen H. Prospective comparison of H&E, Giemsa, and Genta stains for the diagnosis of Helicobacter pylori . Gastrointest. Endosc. 1997; 45: 463–467. [DOI] [PubMed] [Google Scholar]
- 22. Cutler AF. Diagnostic tests for Helicobacter pylori infection. Gastroenterologist. 1997; 5: 202–212. [PubMed] [Google Scholar]
- 23. Inui M, Ohwada S, Inui Y, Kondo Y. Analysis of the gray‐zone cutoff values of new serum Helicobacter pylori antibody kits using latex immunoassay. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro‐enterology. 2017; 114: 1968–1977. [DOI] [PubMed] [Google Scholar]
- 24. Sue S, Shibata W, Sasaki T et al. Randomized trial of vonoprazan‐based versus proton‐pump inhibitor‐based third‐line triple therapy with sitafloxacin for Helicobacter pylori . J. Gastroenterol. Hepatol. 2019; 34: 686–692. [DOI] [PubMed] [Google Scholar]
- 25. Sue S, Kondo M, Sato T et al. Vonoprazan and high‐dose amoxicillin dual therapy for Helicobacter pylori first‐line eradication: a single‐arm, interventional study. JGH Open. 2023; 7: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sue S, Sasaki T, Kaneko H, Irie K, Kondo M, Maeda S. Helicobacter pylori rescue treatment with vonoprazan, metronidazole, and sitafloxacin in the presence of penicillin allergy. JGH Open. 2021; 5: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007; 12: 275–278. [DOI] [PubMed] [Google Scholar]
- 28. Dong SQ, Singh TP, Wei X, Yao H, Wang HL. Review: a Japanese population‐based meta‐analysis of vonoprazan versus PPI for Helicobacter pylori eradication therapy: is superiority an illusion? Helicobacter. 2017; 22: e12438. [DOI] [PubMed] [Google Scholar]
- 29. Shinozaki S, Kobayashi Y, Osawa H et al. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second‐line Helicobacter pylori eradication therapy: systematic review and meta‐analysis. Digestion. 2020; 1‐7: 319–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not available for public access because of ethical restrictions, but are available from the corresponding author on reasonable request.


