Abstract
Despite limited clinical efficacy in large trials, dendritic cells (DC)-based immunization has yielded impressive responses in some patients. Key questions remain to be solved in order to optimize this therapeutic vaccine. Among them, the nature of the DC type used and its state of maturation are pivotal. Besides myeloid DC which are mostly used in clinical trials, a new DC type has been recently described resulting from the differentiation of monocytes in the presence of type I IFNs. In the present study, we analyze the features of type I IFNs DC generated in the presence of either IL-3 (IL-3-DC) or GM-CSF (GM-CSF-DC) and compare their capacity to respond to poly(I:C) and to subsequently trigger T-cell activation. The two DC types disclose a similar immunophenotype characterized by high levels of chemokines receptors, co-stimulatory and HLA molecules expression. After poly(I:C) maturation, both DC types display a marked upregulation of CD80, CD83 and CD86 and the same pattern of gene expression. In addition, poly(I:C) stimulated them to secrete IFN-α and IL-12p70. Both DC types elicit potent allogeneic reactions. Priming of autologous T cells by IL-3-DC or GM-CSF-DC pulsed with an HLA-A2 restricted melan-A derived peptide, lead to the expansion of peptide specific CTL secreting high amounts of IFN-γ.We conclude that poly(I:C) matured IL-3-DC and GM-CSF-DC share similar phenotype and functional properties including the capacity to prime tumor-associated antigen specific CTL.
Keywords: Immunotherapy, Cancer, Type I IFNs DC
Introduction
Dendritic cells (DC) are key players for the initiation of immune responses. The nature of this response is not only determined by the type of DC involved but also by the microenvironment in which these cells reside. Understanding the interactions between DC, cytokines and antigens is pivotal for the development of new vaccines aiming at the triggering of cellular responses against viruses and cancer.
Dendritic cell-based vaccine development has long been hampered by the scarcity of DC in the peripheral blood. The discovery that monocytes can differentiate into DC by culture in the presence of GM-CSF and IL-4 [13] allowed the production of large numbers of myeloid DC. As these cells have been shown to be efficient inducers of Th1 responses and as such responses have long been thought to be responsible for antitumor activity, several clinical trials were initiated using them for the presentation of tumor-associated antigens [10, 17, 20]. Compelling evidence has been recently afforded by murine models that other pathways are involved including the differentiation of Th2 type cells leading to the recruitment of other effectors cells such as eosinophils [5, 8]. In that context, the finding that the functional repertoire of DC generated in the presence of GM-CSF and IL-4 may be incomplete is of interest. Important functions such as migration or the ability to induce natural killer and type 2 helper cell differentiation appear to be impaired [12, 19]. In recent years, it has been shown that type I IFNs promote the rapid differentiation of monocytes into DC endowed with potent helper T-cell stimulatory capacity [2, 15, 11]. These cells can be obtained either by combining type I IFNs with GM-CSF (GM-CSF DC) [15] or IL-3 (IL-3 DC) [2]. These two DC types seem to harbor a semi-mature phenotype. IL-3-DC have been reported to induce strong proliferative responses in mixed lymphocyte reactions (MLR) characterized by both high IFN-γ and IL-5 production by allogeneic helper T cells [2].
Type I IFNs treatment promotes the expression of co-stimulatory and HLA molecules but fails to induce terminal maturation of DC [3]. It is thus assumed that further activation would be susceptible to improve their antigen presentation abilities. Through the expression of toll-like receptors (TLR), monocytes, macrophages and DC recognize pathogen-associated molecular patterns [16]. Among them, TLR-3 is of interest because its interaction with polyriboinosinic polyribocytidilic acid (poly(I:C)), a double-stranded RNA analog that mimics viral infection, has been reported to induce myeloid DC maturation, characterized by an increased expression of MHC and co-stimulatory molecules as well as increased IL-12 production [21]. TLR-3 ligation on myeloid DC also induces the production of type I IFNs that represent essential anti-viral components [3].
In the present work, we address the influence of using either GM-CSF or IL-3 in combination with IFN-β on the nature of the resulting DC assessed by its capacity to mature in response to poly(I:C) and to trigger allogeneic or tumor-antigen specific T-cell activation.
Materials and methods
DC generation and maturation
Dendritic cells were generated in Cell-Factory (Nunc, Roskilde, Denmark) starting from cytapheresis products of HLA-A2 positive cancer patients after approval by the Ethical Committee of the Erasme Hospital. Mononuclear cells were washed in phosphate buffered saline (PBS; Baxter, Lessines, Belgium) supplemented with 5% human serum albumin (HSA) (Belgian Red Cross), resuspended in X-VIVO 20 (Cambrex Europe, Verviers, Belgium) supplemented with 1% autologous serum, transferred to double Cell-Factory and allowed to adhere for 2 h at 37°C. Non-adherent cells were removed and adherent monocytes were washed and cultured in 320 ml of RPMI (Cambrex Europe) containing 100 U/ml of IFN-β (Avonex, Biogen, Cambridge, UK) and either 50 U/ml of IL-3 (Cell Genix, Freiburg, Germany) or 800 U/ml GM-CSF (Leucomax; Schering-Plough, Kenilworth, NJ, USA). On day 2, IFN-β and IL-3 or GM-CSF were added to the cultures. On day 6, non-adherent cells were harvested. The resulting DC preparations were always more than 90% pure as determined by morphological examination and cytometric analysis. Subsequent maturation was induced by addition at day 5 of poly(I:C) (10 μg/ml final concentration) (Sigma, Bornem, Belgium) directly into the Cell-Factory. After 18 h of stimulation, immunophenotypic analysis and cytokines measurements were performed.
DC phenotyping
Cells were washed in PBS supplemented with 0.5% bovine serum albumin (BSA; Sigma) and stained with the following monoclonal antibodies (mAbs): anti-CD80, anti-CD86, anti-CD14, anti-HLA-DR, anti-CD123, anti-CCR5 (BD Biosciences, Mountain View, CA, USA), anti-CD83 (Immunotech, Marseille, France) and anti-CCR7 (R&D system, Minneapolis, MN, USA). 2×105 cells were incubated with the relevant mAbs for 20 min at 4°C. After washing, acquisition of fluorescence intensity was performed using a FACS-Calibur (Becton-Dickinson, San Jose, CA, USA) running under the Cell-Quest software.
Cytokines measurements
Commercially available Elisa Kits were used to determine the concentrations of IFN-α, IFN-γ, IL-5 (BioSource International, Fleurus, Belgium) and IL-12 p70 (Endogen, Woburn, MA, USA) in culture supernatants. Results are expressed as means ± SEM (pg/ml).
DC gene profiling
Total RNA of matured DC (5x106) was extracted using a commercially available kit (Qiagen, KJ Venlo, Netherlands). The RNA concentration was determined by absorbance reading. The integrity of the RNA preparation was checked by ethidium bromide-stained agarose gel electrophoresis. After reverse transcription of 1 μg of total cellular RNA, cDNA probes were amplified by Linear Polymerase Reaction (GEArray Ampolabeling-SuperArray Bioscience Corp., Frederick, MD, USA) with incorporation of Biotin-16-dUTP (Roche, Brussels, Belgium). Afterwards, cDNA probes were hybridized to a “Human Dendritic & Antigen Presenting Cell Gene Array” (SuperArray). This membrane contains 192 sequence-verified known marker genes. After hybridization, each gene signal was normalized by the Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) signal on the same membrane, and only gene signals that were well above background (~10% of the GAPDH signal) were considered as specific gene signals. These data were collected, stored and analyzed with the Scanalyse software (Stanford University, Stanford, CA, USA).
Mixed lymphocytes reaction
Unidirectional MLR were performed by coculturing DC with 2x105 allogeneic PBMC (non-adherent fraction) per wells at a 1/10 ratio in 96 well-plates (Nunc, Roskilde, Denmark) in complete medium (RPMI supplemented with 2 μM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1% nonessential amino acids, 1% sodium pyruvate, 5×105 M 2-mercaptoethanol (Gibco, Grand Island, NY, USA) and 5% pooled human serum). After 5 days, cells were pulsed with 1 μCi (methyl-3H) thymidine (Amersham Biosciences Benelux, Netherlands) per well for 18h, harvested and counted. Cytokines (IFN-γ and IL-5) production was also assessed in culture supernatants. Tests were carried out in triplicate, and results are expressed as mean ± SEM (counts/min or pg/ml).
Melan-A CTL generation and characterization
Dendritic cells were pulsed for 2 h with the melan-A 26–35 peptide (ELAGIGILTV, Sigma-Genosys, Woodlands, TX, USA) in serum-free medium. Peptide pulsed DC were thoroughly washed and incubated with autologous lymphocytes (non-adherent fraction) at a 1:10 ratio in complete medium. Human rIL-2 (R&D systems) was added at day 4 and 6 on 10 U/ml final concentration. After day 6, cells were expanded in high IL-2 concentration (500 U/ml) until day 12.
On day 12, peptide specific CD8+ T cells were enumerated by melan-A tetramer (melan-A TTM) staining. Melan-A HLA-A2 tetrameric complexes were kindly provided by Dr Arnaud Marchant. Cells were stained with PE-labeled melan-A TTM at 37°C for 25 min, washed at room temperature and incubated on ice with CD8-APC (Pharmingen, San Diego, CA, USA) or CD8-PerCP (BD Biosciences, Mountain View, CA, USA). The samples were then analyzed on the cytometer after lymphocytes gating according to forward and side scatter. The capacity of melan-A TTM+ cells to produce IFN-γ upon peptide rechallenge was assessed by IFN-γ intracytoplasmic staining. Cells were labeled with the melan-A TTM and subsequently stimulated with 20 μg/ml melan-A peptide in RPMI supplemented with 10% FCS. After 1 h of stimulation, brefeldin A (Sigma) was added at a final concentration of 5 μg/ml. Five hours later, cells were harvested, washed and surface stained for CD8 (anti-CD8-APC, Pharmingen). Afterwards, cells were fixed and permeabilized using a permeabilizing buffer (BD Biosciences, San Jose, CA, USA), Finally, intracytoplasmic staining was performed using a FITC anti-IFN-γ Ab (Pharmingen). Cytolytic activity of peptide-specific CTL was assessed using the chromium release assay. T1 cells, a variant of HLA-A2+ T2 cells, were labeled with 51Cr for 90 min at 37°C and washed twice. Labeled cells were pulsed with 1 μg/ml melan-A for 1 h, washed, and added (5,000 cells/well) to decreasing numbers (30, 10, 3,1) of CD8+ T cells purified by magnetic cell sorting (MACS, Miltenyi Biotec, Germany) from cultures involving IL-3 and GM-CSF peptide pulsed DC. Chromium release was measured in supernatants harvested after 4 h of incubation at 37°C. Total release was determined in the presence of 10% SDS. The percentage of specific lysis was calculated as follows: 100×(experimental − spontaneous release)/(total−spontaneous release). Each value was calculated as the average of triplicates.
Statistical analysis
The data obtained using IL-3-DC were compared with those obtained using GM-CSF-DC using a non-parametric test (Mann–Whitney or Wilcoxon). A P value <0.05 was considered as significant.
Results
Immunophenotype of IL-3-DC and GM-CSF-DC
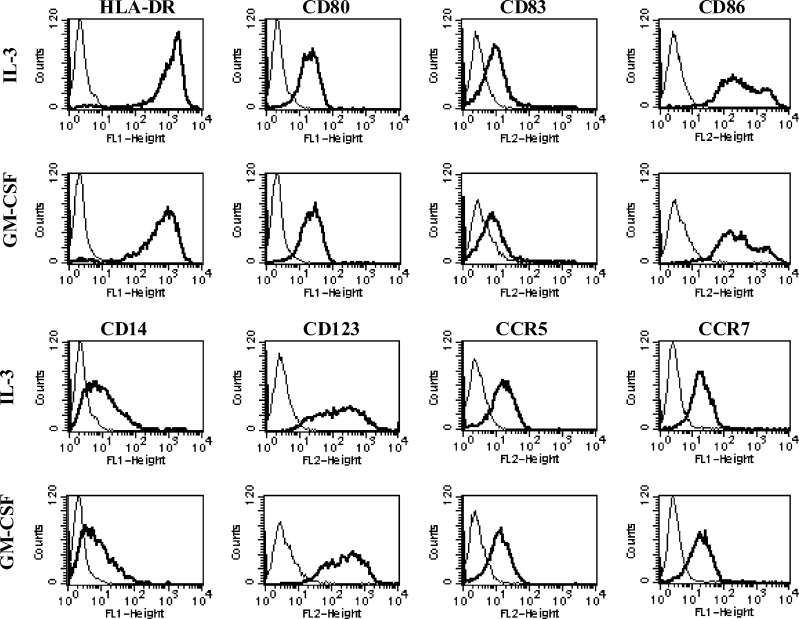
We first studied the influence of GM-CSF as compared to IL-3 on DC phenotype. This first set of experiments did not disclose any difference for all markers tested (Fig. 1). As previously shown [2, 15], GM-CSF DC and IL-3 DC displayed an already intermediate phenotype including the expression of high levels of HLA Class II and co-stimulatory (CD86, CD80) molecules, as well as detectable levels of CD83. IL-3 receptor alpha-chain, CD123 and CD14 were highly expressed by the two cell types confirming a previous observation showing that the loss of CD123 was prevented by the addition of IFN-β [2]. Both DC types also expressed high levels of CD40 and CD11c and low levels of CD1a (data not shown). IL-3-DC clearly expressed the β-chemokine receptors CCR5 and CCR7. These markers were similarly expressed by GM-CSF-DC. These results are consistent with the differentiation of monocytes into DC with a semimature phenotype in the presence of type I IFNs.
Fig. 1.
Immunophenotype of IL-3-DC and GM-CSF-DC. After 6 days of culture, DC were harvested and analyzed by flow cytometry for the expression of indicated surface molecules. Results are those from one representative experiment out of five
Response of IL-3-DC and GM-CSF-DC to poly(I:C)
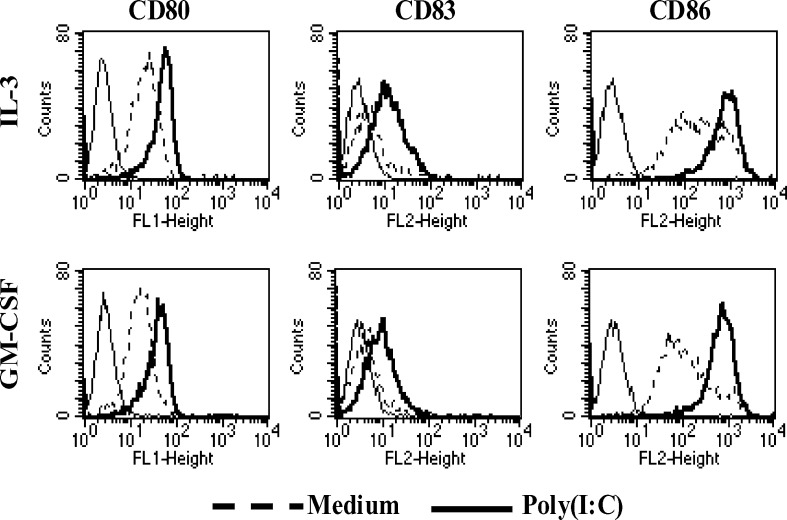
In order to optimize the immunostimulatory properties of type I IFNs DC, we evaluated the action of different maturation agents on their phenotype and production of cytokines. Preliminary experiments led us to identify poly(I:C) as the best maturation agent (10 μg/ml) for IL-3 DC (data not shown). We then tested whether the addition of poly(I:C) differentially affected the phenotype of IL-3-DC as compared to GM-CSF-DC. As shown in Fig. 2, a clear and similar upregulation of CD80, CD86 and CD83 was observed on both DC types.
Fig. 2.
Immunophenotype of IL-3-DC and GM-CSF-DC after poly(I:C) stimulation. Maturation was induced by addition on day 5 of poly(I:C) directly into the Cell-Factory. After 18 h of stimulation, immunophenotypic analysis was performed using the indicated surface molecules. Results are those from one representative experiment out of four
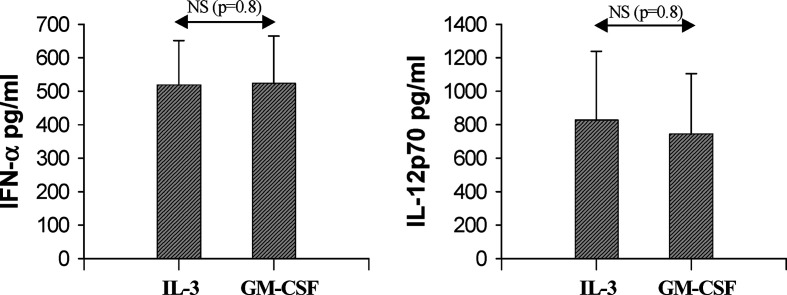
In terms of cytokines production, we paid peculiar attention to IL-12p70 and IFN-α two important Th1 orienting cytokines. Comparable levels of IL-12p70 and IFN-α were detected in supernatants from both DC type after poly (I:C) stimulation (Fig. 3).
Fig. 3.
Cytokines secretion by IL-3-DC and GM-CSF-DC after poly(I:C) stimulation. IL-3-DCs and GM-CSF-DC were matured with poly(I:C) for 18 h. Cytokines production was assessed in culture supernatants by ELISA. Results are expressed as mean ± SEM pg per ml from five independent experiments. NS statistically not significant
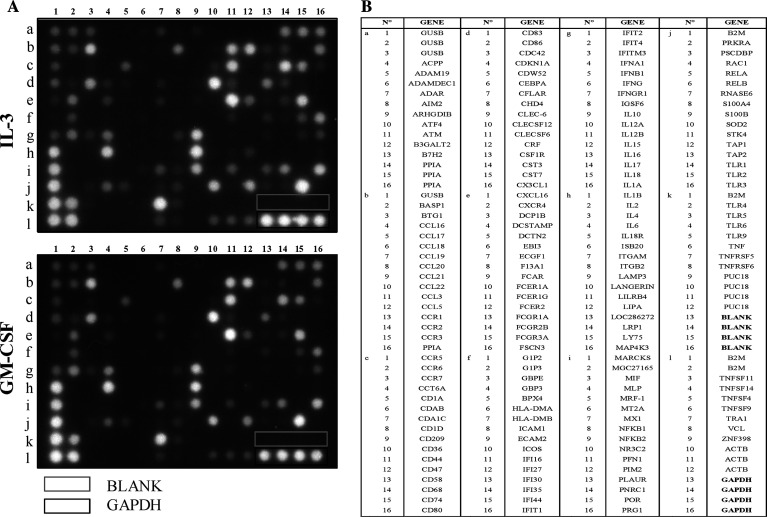
In order to perform a more extensive comparative evaluation of type I IFNs DC after poly(I:C) stimulation, we analyzed these cells at the mRNA level focusing on DC-specific genes encoding other proteins. Gene expression profiling was performed using the “GEArray S Series Human Dendritic and Antigen Presenting Cell Gene Array,” which is designed for studying the expression profile of genes involved in DC activation and maturation. This GEArray includes genes encoding proteins important for DC function such as cytokines, chemokines, chemokines receptors, signal transduction molecules and proteins involved in antigen uptake, processing and presentation. IL-3-DC and GM-CSF-DC were generated from three different subjects, and RNA was extracted and hybridized to GEArray membrane. These experiments disclosed that both DC types expressed the same pattern of genes. This pattern of expression is represented in Fig. 4. Cytokines (IL-6, IL-1-β and IL-10) genes were highly expressed. Several chemokines and chemokines-receptors genes were expressed including CCL20 (MIP-3a), CCL3, CCL5, CXCR4, CCR5 and CCR2. Genes encoding molecules involved in T-cell/ DC interactions (CD44), antigen recognition/uptake (TLR2, TLR4 and CLECSF12 (Dectin-1)) processing (PRG1 and TAP1) antigen presentation (β-2 microglobulin, CD74 (invariant chain), CD1a, CD68 and LAMP-3), signal transduction (NF-κB2), protection against oxidative stress (SOD2) and motility (Cdc42, IFIT1) were also transcribed.
Fig. 4.
Gene-expression profiles of IL-3-DC and GM-CSF-DC after poly(I:C) stimulation. IL-3-DCs and GM-CSF-DC were matured for 18 h in the presence of poly(I:C). Total RNA was extracted, reverse transcripted and amplified by PCR before hybridization on GEArray membrane. A Gene array, B list of genes tested. Results are those from one representative experiment out of three
Allostimulatory potential of IL-3-DC and GM-CSF-DC
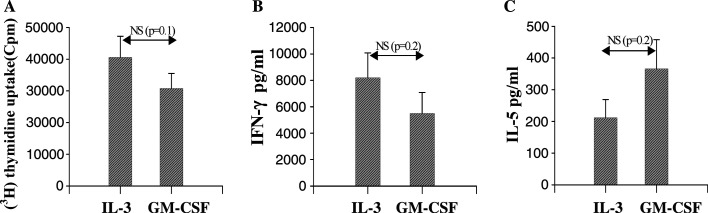
We next performed a series of functional experiments aimed at comparing the ability of poly(I:C)- matured IL-3-DC and GM-CSF-DC to induce MLR. T-cell proliferation and IFN-γand IL-5 release were used as read-out. The proliferative response is illustrated in Fig. 5A. A strong and similar proliferative response, as assessed by [3H] thymidine incorporation, was observed in experiments using both DC types. Assessment of IFN-γ and IL-5 release in MLRs supernatants revealed high secretion of IFN-γ (Fig. 5B) and a clear release of IL-5 (Fig. 5C), suggesting a potential ability of DC generated in the presence of type I IFN to promote both Th1 and Th2 responses. Although IL-3-DC appear to produce more IFN-γ and less IL-5 as compared to GM-CSF-DC, this difference did not reach the level of significance.
Fig. 5.
MLRs involving poly(I:C) matured IL-3-DC or GM-CSF-DC. Mature DC were co-cultured with allogeneic PBMC for 5 days. A Allogeneic proliferative response induced by IL-3-DC as compared to GM-CSF-DC. Proliferation was measured by 3(H) thymidine incorporation for 18 h. Results are expressed as mean ± SEM CPM from three independent experiments performed in triplicate. B IFN-γ and C IL-5 secretion in MLR supernatants. Results are expressed as mean ± SEM pg per ml from five experiments performed in triplicate
In vitro induction of melan-A specific CD8 T-cell responses by IL-3-DC and GM-CSF-DC
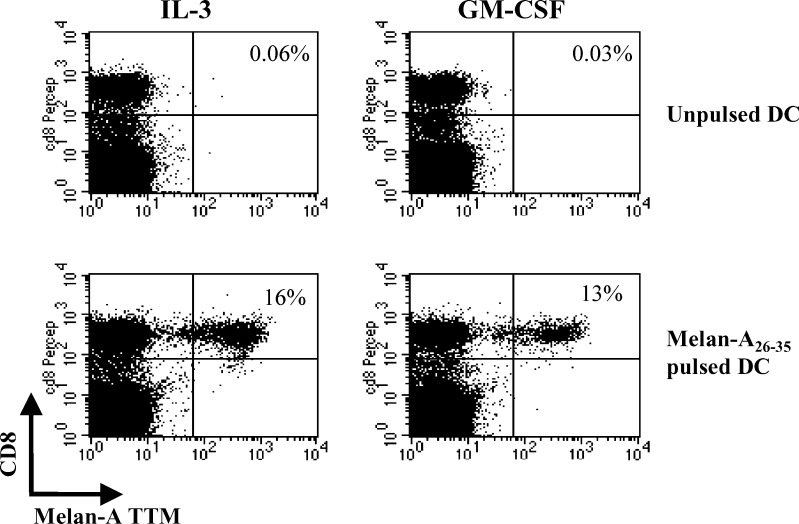
We compared the ability of poly(I:C)-matured IL-3-DC and GM-CSF-DC to prime CD8+ cytotoxic T-cell responses against the HLA-A2 melan-A peptide. A significant expansion of melan-A-specific CTL was obtained after one round of stimulation by using either IL-3-DC (from 0% -unpulsed DC- to 11.75% ±3.8 –pulsed DC-, P=0.01, n=4) or GM-CSF-DC (from 0% to 10.47% ±3.9, P=0.01, n=4) as APC. One representative experiment out of the four is represented in Fig. 6. This expansion was observed at low peptide loading concentrations (100 ng/ml peptide). No expansion of melan-A-specific cells was observed when PBMC were cultured alone (data not shown).
Fig. 6.
Expansion of melan-A specific-CTL by autologous mature IL-3-DC or GM-CSF-DC. PBMC cultured with unpulsed (top) or peptide-pulsed (bottom) poly (I:C) matured DC were stained with PE-conjugated anti-melan-A TTM and PerCP-conjugated anti-CD8 mAb. Numbers in the upper right quadrants represent the percentage of melan-A TTM+ cells among the whole CD8+ population (one representative experiment out of four)
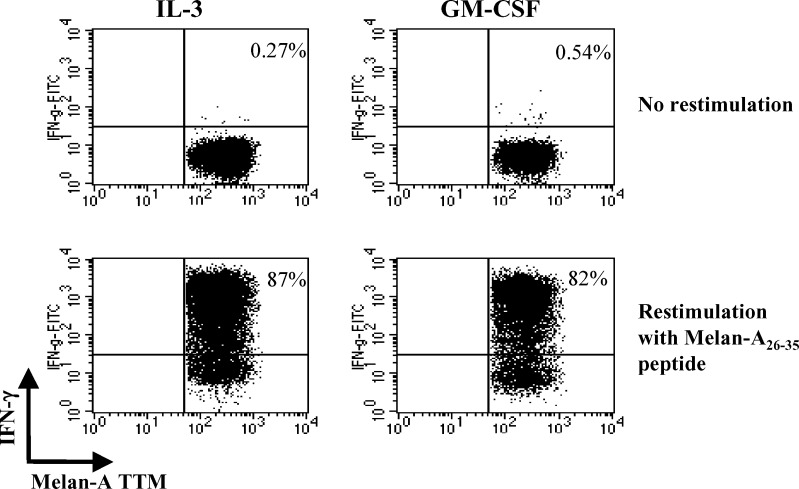
To assess the acquisition of effector function by expanded melan-A-specific T cells, TTM+ cell cultures were re-stimulated or not for 6 h with melan-A26–35 peptide before measuring the production of IFN-γ by intracellular staining (Fig. 7). A high proportion of melan-A TTM+ CD8+ T cells produced IFN-γ. The percentage of IFN-γ-producingCTL was variable from one donor to another, ranging from 11% to 80% but was always comparable in the same patient whatever the DC type used for T-cell stimulation.
Fig. 7.
IFN-γ production by melan-A specific CD8 T cells after peptide restimulation. Melan-A specific CD8+ T cells primed by autologous peptide-pulsed mature DC were restimulated (bottom) or not (top) with the melan-A peptide. Intracytoplasmic staining for IFN-γ of TTM+ CD8+ cells is shown. Numbers in the upper right quadrants represent the percentages of IFN-γ-producing cell (one representative experiment out of four)
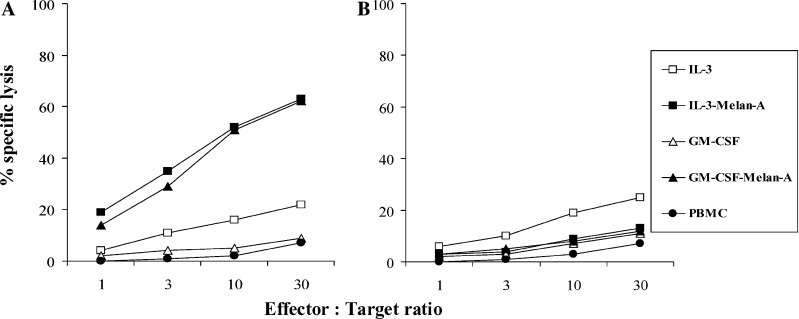
Finally, we assessed the presence of cytotoxic T cells among CD8+ T cells at the end of the first round of stimulation. A strong and comparable specific lysis of melan-A-pulsed HLA-A2+ target cells was observed when using the CD8+ population from cultures involving either IL-3 or GM-CSF peptide-pulsed DC (Fig. 8).
Fig. 8.
Cytotoxic activity of melan-A specific T cells elicited by IL-3-DC or GM-CSF-DC against peptide pulsed (A) or unpulsed (B) T1 targets. T cells expanded by poly(I:C)-matured IL-3-DC or GM-CSF-DC pulsed or not with melan-A peptide were tested in a 51Cr release assay against T1 pulsed or not with 10 μg/ml of melan-A peptide. Results are expressed as percentages of specific lysis (one representative experiment out of three)
Discussion
This study aimed at comparing types I IFNs DC generated in IFN-β combined with either IL-3 or GM-CSF disclosed that the two DC types shared similar immunophenotype and functional properties. They highly expressed CD14 and CD123 and were further characterized by a high expression of molecules involved in DC trafficking (CCR5, CCR7), co-stimulation (CD80 and CD86) and antigen presentation (HLA). Poly(I:C) treatment of both DC types resulted in a marked upregulation of most of these molecules and in the appearance of the maturation marker CD83. Besides CD14 and CD123 expression, the phenotype of type I IFNs DC at the end of the poly (I:C) maturation process is similar to the one of myeloid DC generated in the presence of GM-CSF and IL-4 (GM-CSF/IL-4) (O. Detournay et al., in press). Functionally, maturation of type I IFNs DC was associated with the production or high expression of genes related to both Th1 (IL-12p70, IFN-α) and Th2 (IL-6, IL-10) orienting cytokines. Gene profiling revealed that both type I IFNs DC types expressed the same pattern of genes. Finally, these mature type I IFNs DC were endowed with potent stimulatory properties during the primary MLR and were able to efficiently prime melan-A-specific CTL.
Several studies have investigated the effect of type I IFNs on monocytes differentiation. GM-CSF combined with type I IFNs [15] induce differentiation of monocytes into DC endowed with potent functional activities notably through IL-15 production strongly promoting Th1 responses. IL-3 combined with IFN-β [2] cooperates to induce differentiation of monocytes into DC exhibiting a semimature phenotype. In vitro, these cells induce strong allogeneic response characterized by both Th1 (IFN-γ) and Th2 (IL-5) type cytokine production. Monocytes express both GM-CSF and IL-3 receptors. Our work shows that engagement of these two receptors concomitantly with the ligation of IFN-β receptor results in the same global effect on monocytes differentiation into DC. This observation could be explained by the existence of a common β subunit (βc) shared by the GM-CSF and IL-3 receptors [4, 9]. Indeed, some studies indicate that βc is the major signal-transducing subunit [6], suggesting that overlapping biological activities between IL-3 and GM-CSF could be due to signal transduction mediated by the common β subunit.
The degree of DC maturation is pivotal for the orientation of T-cell responses. Th1 differentiation has long been considered as crucial for antiviral and antitumor immunity. More recently, Th2 type response and other immune cells than CTL, that is, eosinophils, have been recognized as major actors of efficient immune responses in different settings including cancer [5]. In that context, type I IFNs DC are of interest. They express not only IL-12p70, and IFN-α but also IL-6 and IL-10. After poly (I:C) activation, type I IFNs DC release less IL-12 as compared to GM-CSF/IL-4 DC but more IFN-α and IL-6 [2]. Despite this lower expression of IL-12, type I IFNs DC are able to induce an increased secretion of IFN-γ during the MLR as compared to GM-CSF/IL-4 DC associated with the release of IL-5. These functional features not only indicate a more potent immunostimulatory potential but also suggest a broadest range of immune activation as compared to GM-CSF/IL-4 DC by recruiting both the cellular and humoral arms of immunity.
Dendritic cells are migratory cells that travel from bone marrow to various tissues and secondary lymphoid organs [1]. Their ability to migrate is associated with chemokines receptors expression and chemokines secretion. Our work discloses that poly(I:C)-treated type I IFNs DC highly express several chemokines and chemokine-receptor genes including those encoding CCL20 (Mip-3α), CCL5, CCL3, CCR2, CCR5 and CCR7. These molecules are crucial for the attraction and recruitment of DC and effector T cells, for DC migration to inflammatory sites and finally for their homing to lymph nodes. The differential treatment of monocytes with either GM-CSF or IL-3 did not alter this pattern of expression suggesting a common control of chemokines and chemokines receptors systems by GM-CSF and IL-3.
Dendritic cells activation before vaccination in immunotherapy seems to be a sort of double-edged sword. Indeed, this maturation step could potentially lead to an exhaustion of DC with the consequence that these cells would not be able to respond to rechallenge in vivo. Beyond the subsequent inefficiency to trigger the expected immune response, these exhausted DC could even induce tolerance. In that context the use of poly(I:C) as maturation agent is of interest. Indeed, poly(I:C) used for DC maturation has been reported to preserve their ability to secrete bioactive IL-12 upon rechallenge notably with CD40L [ 7, 14]. This capacity to respond to a secondary stimulation is also dependent on the duration of poly(I:C) stimulation [18], the shorter activation step being associated with the best secondary responses. In our model, we observed that a short time of incubation with poly(I:C) is sufficient to allow phenotypic and functional maturation of type I IFNs DC (data not shown). IFN-α production was detected soon as after 1 h whereas IL-12p70 secretion occurred later (9 h).
The results of this study show that type I IFNs can be associated either with GM-CSF or IL-3 to constitute a powerful differentiating combination for the generation of DC endowed with potent immunostimulatory properties. The use of such cytokines combination opens new perspectives for DC-based immunotherapy against viruses and cancer.
Acknowledgements
This work was supported by BruCells SA through the ROBIII Project. OD is a fellow of the Fonds National de la Recherche Scientifique (FNRS Belgium, Grant Télévie). We thank Eric Thille and Corinne Vanhelleputte for their technical expertise.
Footnotes
N. Mazouz and O. Detournay equally contributed to this work.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Buelens C, Bartholome EJ, Amraoui Z, Boutriaux M, Salmon I, Thielemans K, Willems F F, Goldman M. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993. doi: 10.1182/blood.v99.3.993. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebner K, Bandion A, Binder BR, De Martin R, Schmid JA. GMCSF activates NF-kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood. 2003;102:192. doi: 10.1182/blood-2002-12-3753. [DOI] [PubMed] [Google Scholar]
- 5.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh T, Liu R, Yokota T, Arai KI, Watanabe S. Definition of the role of tyrosine residues of the common beta subunit regulating multiple signaling pathways of granulocyte-macrophage colony-stimulating factor receptor. Mol Cell Biol. 1998;18:742. doi: 10.1128/mcb.18.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WjKalinski WJ, Kalinski P. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 8.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyajima A, Mui AL, Ogorochi T, Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993;82:1960. [PubMed] [Google Scholar]
- 10.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, Giammarioli AM, Malorni W, Fais S, Belardelli F. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 12.Rieser C, Ramoner R, Holtl L, Rogatsch H, Papesh C, Stenzl A, Bartsch G, Thurnher M. Mature dendritic cells induce T-helper type-1-dominant immune responses in patients with metastatic renal cell carcinoma. Urol Int. 1999;63:151. doi: 10.1159/000030438. [DOI] [PubMed] [Google Scholar]
- 13.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouas R, Lewalle P, El Ouriaghli F, Nowak B, Duvillier H, Martiat P. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int Immunol. 2004;16:767. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 15.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, DiPucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 17.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spisek R, Bougras G, Ebstein F, Masse D, Meflah K, Mcilroy D, Gregoire M. Transient exposure of dendritic cells to maturation stimuli is sufficient to induce complete phenotypic maturation while preserving their capacity to respond to subsequent restimulation. Cancer Immunol Immunother. 2003;52:445. doi: 10.1007/s00262-002-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurnher M, Zelle-Rieser C, Ramoner R, Bartsch G, Holtl L. The disabled dendritic cell. Faseb J. 2001;15:1054. doi: 10.1096/fj.00-0508hyp. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, Van Beckhoven A, Liles TM, Engleman EG, Levy R. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 21.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57. [PubMed] [Google Scholar]