Abstract
Vaccination with established tumour cell lines may circumvent the problem of obtaining autologous tumour cells from patients, but may also need immunological adjuvants. Up-regulation of heat shock proteins within tumour cell vaccines has resulted in increased immunogenicity in some models, but this has yet to be demonstrated for allogeneic (MHC-disparate) cell vaccines. This was investigated here using a rat model for prostate tumour cell vaccination. Heating of tumour cells (42°C, 1 h) elicited significant increases in HSP70 expression. Vaccination with heated autologous PAIII cells elicited protection against PAIII challenge in 60% of rats >50 days compared to 0% with unheated vaccine and was associated with an increased Th1 (IFNγ) immune response. Heated allogeneic MLL cells elicited significant protection against PAIII challenge, in contrast to unheated cells. The principle was confirmed in two mouse models, although the allogeneic melanoma vaccine K1735 elicited the best protection when heated and administered mixed with autologous dendritic cells. Thus, while heating of vaccine cells in some models is highly beneficial, and is a means of enhancing immunogenicity without genetic modification or inclusion of potentially toxic adjuvants, additional immune enhancement may be required.
Keywords: Allogeneic vaccine, Prostate cancer, Heated cells, Adjuvant
Introduction
Despite significant progress in therapy for early-stage prostate cancer (PC), the prognosis for most patients with advanced disease remains little changed over the past 20 years. In particular, the prospects for patients with hormone refractory PC are bleak with only temporary palliative responses to further hormonal manipulation and chemotherapy. This situation has justified the development of novel therapies [8]. The possibility of using immunotherapy to modify the natural history of PC is based on observations of tumour-infiltrating lymphocytes (TIL) within prostate tumours associated with an improved prognosis, the rapid influx of T cells into the prostate gland observed after androgen withdrawl [32] and the identification of T cell epitopes associated with prostate cancer phenotype [6, 28].
This has resulted in non-specific immunotherapy [11] and, more recently, active specific therapy using irradiated tumour cells as the basis of therapeutic ‘vaccines’. Using the Dunning rat model, vaccines comprising irradiated whole prostate cancer cells transfected with either IL-2 or GM-CSF [20, 24, 33] or in combination with M. vaccae [11] have resulted in anti-tumour responses and formed the basis for pilot clinical trials [12]. Later clinical trials have developed these models further and used autologous prostate cancer cells transfected with GM-CSF [26] and most recently dendritic cell strategies using cells pulsed with prostate-associated peptides [30] or tumour RNA [10].
Although it is often argued that whole tumour cell vaccines must be autologous so that they are both HLA matched and contain the full antigen repertoire of the host tumour, this approach is limited in prostate cancer where it is has been extremely difficult to raise autologous cells needed for frequent multiple vaccinations. Allogeneic vaccination using one or a number of established and well characterised tumour cell lines provides an alternative approach to the problem. We have shown in murine melanoma models that allogeneic cell vaccination can protect against subsequent lethal dose challenge with autologous tumour cells [17]. Such an approach has shown promise in the therapy of malignant melanoma and is currently under evaluation in a phase III study [14, 21].
Thus it appears that in certain circumstances class I MHC restriction can be bypassed with allogeneic vaccination [31]. Moreover, the expression of highly immunoreactive allogeneic MHC might enhance the immunogenicity of tumour associated antigens [7, 29, 34], although further immune stimulation with adjuvants may be required. One group of candidates for this adjuvant is the family of heat shock proteins (HSPs). The heat shock proteins are ubiquitously expressed molecules considered essential to the survival of most prokaryotes and eukaryotes. The term HSP follows from observations that their expression is increased during the exposure of cells to heating and other stress factors such as UVB radiation, bacterial and viral infection, chemicals and drugs, and hypoxia. The function of HSPs during stress is to bind to misfolded proteins and thus protect them from denaturation. However, during steady state conditions HSPs facilitate normal protein folding and intracellular transport [9]. Many studies suggest a role of HSP70 in the shuttling of protein antigens from pathologic cells, such as malignant and virally infected cells, to antigen-presenting cells and thus contributing to the process of T cell cross-priming [27]. Therefore, HSP70 extracted from such cells can be used as a vaccine to effect immune destruction of these cells. The recently discovered ability of HSP70 to activate innate immunity [1] may contribute to this vaccination process. We and others have previously shown that HSP70 upregulation in tumour cells, through heat or drug-induced stress or by gene transfection [19, 5, 23, 16] increased tumour immunogencity and protected animals from challenge with wild-type tumour.
We have recently described rat prostate tumour models that allow the investigation of allogeneic vaccines in prostate cancer [13]. The aim of the current study was to extend our previous separate studies that used allogeneic vaccines and autologous HSP-expressing vaccines by attempting to enhance allogeneic vaccine immunogenicity through increased HSP expression. Although, in some cases, effective as the sole adjuvant and generating protective Th1 cytokine responses, heated tumour cells expressing inducible HSP as whole cell vaccines may require further components, such as dendritic cells, to achieve optimal anti-tumour efficacy.
Materials and methods
Animals
Eight-week-old male Copenhagen rats (Cop) (RT1a/h) were obtained from B&K (Hull, UK) and 8-week-old male Lobund-Wistar rats (L-W) (RT1c) were obtained from Harlan (USA). C57 BL/6 (H-2b) mice were purchased from Harlan UK and used between 6 and 12 weeks of age. All procedures were carried out under mild anaesthesia and in accordance with the UK Home Office and ethical guidelines.
Cell lines
The MAT-LyLu (MLL) (RT1a/h) subline of the Dunning rat prostate cancer was obtained from ECACC (European Collection of Cell Cultures) and was cultured in RPMI 1640 (Sigma, Poole, UK), 10% heat inactivated foetal calf serum (Sigma), 2 mM glutamine (Sigma), penicillin 100 U/ml (Life Technologies, Paisley, UK), streptomycin 100 μg/ml (Life Technologies) in 5% CO2. The PAIII (RT1c) rat prostate cancer cell line was kindly provided by Dr. Morris Pollard (Lobund Laboratory, Indiana) and was cultured in DMEM Dulbecco’s Modified Eagles Medium (Sigma), 10% heat inactivated foetal calf serum (Sigma), 2 mM glutamine (Sigma), penicillin 100 U/ml, streptomycin 100 μg/ml, in 5% CO2. CMT93 (H-2b) murine colorectal tumour, B16-F10 (H-2b) and K1735 (H-2k) murine melanoma lines have been described in our previous studies [17, 19], and were grown in DMEM as above. The adherent cells were passaged with 0.05% trypsin and 0.02% EDTA (Sigma) and were free from mycoplasma as assessed by the gene probe method (Gen-Probe, San Diego, Calif.).
Western blotting
MLL and PAIII cells were grown until 60% confluent. The flasks were placed into a water bath at 42°C, after 60 min flasks were returned to the incubator (37°C, 5% CO2). Following 18-h recovery, the cells were harvested with trypsin-EDTA, washed twice in PBS. Control cells were harvested as above without the heating step. Cells were lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, pH 8.0, with 0.1% Triton-X100, 0.01 mg/ml aprotinin and 0.05 mg/ml phenylmethyl sulfonyl fluoride (PMSF), (Sigma, Poole, U.K), centrifuged at 25,000 rpm for 30 min at 4°C to obtain cell lysates. Samples were boiled for 5 min with loading buffer. The lysates were run on 10% SDS-PAGE gels at 40 mA for 1 h. The proteins were transferred to nitrocellulose for 1 h at 100 V. Blots were blocked in PBS/5% skimmed milk powder and incubated with anti-HSP70 Ab-3 (NeoMarkers, Fremont, Calif.) for the constitutive form of HSP70 at 1:200 dilution for 2 h followed by incubation with anti-rabbit horse radish peroxidase congugate. For the inducible form of HSP 70 incubation was performed using anti-HSP70 Clone C92F3A-5 (Stressgen, Victoria, Canada) at 1 μg/ml for 1 h, followed by incubation with rabbit anti-mouse HRP conjugate (Dako, Glostrup, Denmark). Bands were revealed using the enchanced chemiluminescence ket (Amersham, Aylesbury, UK.), following the manufacturer’s instructions, by exposing the blot to Kodak film (Eastman Kodak, Rochester, N.Y.).
Expression of heat shock proteins and immunosuppressive molecules
Total RNA was isolated from tumour cell pellets by homogenisation with TRIZOL solution (Sigma), according to the manufacturer’s instructions. As estimated by the absorbance ratio for 260:280 nm, 2 μg of RNA were reverse transcribed at 37°C for 1 h using the “first strand cDNA synthesis kit” (Novagen, USA), following the manufacturer’s instructions. An equivalent of 100 ng of resulting cDNA was added to reaction mixtures containing 2 μl 10× buffer (10 mM Tris-HCl, 50 mM KCl, 0.01% gelatine), 160 μM dNTPs (Amersham Pharmarcia-Biotech, St. Albans, UK), 2.5 mM MgCl2, 0.25 U Taq Polymerase (Perkin Elmer, Norwalk, USA) and 0.5 μM of each primer (sequences in Table 1). Amplification was performed on a PTC-100 DNA thermal cycler for 35–40 cycles. Products were visualised on 1.5% (wt/vol) agarose gels with appropriately sized markers (Amersham Pharmacia-Biotech). All samples were initially subjected to PCR with primers for the housekeeping gene beta-actin in order to confirm successful RNA extraction and reverse transcription, and also to verify that the amounts of input cDNA was constant for each reaction. RNA was shown to be free of genomic DNA contamination by directly amplifying the equivalent amount of RNA as was used in the subsequent RT-PCR reactions (not shown).
Table 1.
Primer sequences
| 5’ | 3’ | Size (bp) | |
|---|---|---|---|
| HSP70 | ATGGCCAAGAAAACAGCGATC | CTAATCCACCTCCTCGATGGT | 1,900 |
| TGFβ | ACCCGCGACCGGGTGGCA | GAGTTCTACGTGTTGCTCCAC | 260 |
| IL-10 | TTCCCAGTCAGCCAGACCCCACATGC | TTTCCAAGGAGTTGCTCCCGTTAGC | 530 |
| Fas | CTGCAGATATGCTGTGGATCA | TTTGGTGTTGCTGGTTGGT | 491 |
| FasL | AAAGACCACAAGGTCCAACA | AGTCTCTAGCTTATCCATGA | 341 |
Tumour growth in vivo
The tumour cells were prepared for tumour challenge by detachment with trypsin/EDTA, washed three times in PBS and resuspended at the specified density in PBS. Prostatic tumours were induced ectopically by the injection of 1×106 PAIII cells in 100 μl PBS subcutaneously into the shaven thigh of each Lobund-Wistar rat. In the case of Copenhagen rats the tumorigenic dose of MLL cells was 1×105 cells. Tumour growth was assessed every 2–3 days using microcalipers to measure perpendicular diameters. Rats were killed when the tumour reached 2.5 cm in one of the diameters or when ulceration or bleeding occurred. Mice were challenged by subcutaneous injection of 2×106 CMT93 tumour cells or 2×105 B16 tumour cells, were considered to posses tumour when a palpable tumour of >2 mm could be measured, and were killed when tumour reached 15 mm in any diameter.
Vaccine preparation and immunisation
Cells were prepared for vaccine preparation as for challenge, with or without heating. The cells were irradiated with 50 Gy. The required dose of cells was injected subcutaneously into the left thigh of the rat in a volume of 200 μl per rat. For heated cell vaccine, cells within flasks were placed in a water bath at 42°C; after 60 min flasks were returned to the incubator (37°C, 5% CO2). Following 18 h recovery, the cells were harvested with trypsin-EDTA, washed twice in PBS. In the B16 challenge experiments mice received 5×105 tumour cells with or without 5×105 DCs on days 1, 8 and 15, followed by challenge on day 29. For CMT93 challenge experiments mice received a single vaccination with 2×106 tumour cells followed 2 weeks later by a challenge with 2×106 cells.
Immune responses
Spleens were taken from killed rats that had previously received vaccination but no tumour challenge. The spleens were passed through 100-μm filters to obtain a single-cell suspension and red blood cells were lysed with 0.87% ammonium chloride. The cells were washed and resuspended in RPMI 1640 supplemented as above plus 50 μM 2-mercaptoethanol. Cells at 1×106/ml were cultured with irradiated (50 Gy) tumour cells at a ratio of 20 lymphocytes to 1 tumour cell. PMA and ionomycin (Sigma) were used as a positive control stimulus. After 48 h of incubation supernatants were collected and tested for cytokines (IFNγ and IL-10) using kits from BD-Pharmingen, following the manufacturer’s instructions.
Statistical analysis
Survival data were compared with the log-rank test using the GraphPad Prism statistical package (San Diego, Calif.), or by Fisher Exact test, whilst mean tumour volume was compared using the Student’s t-test.
Results
Characterisation of tumour cells for HSP expression and inhibitory factors
In our previous studies we showed that the PAIII and MLL cell lines expressed MHC class I, class II and ICAM-1 [13]. In this study the expression of immunological factors that may potentially affect immunogenicity were evaluated by RT-PCR. Whilst both lines expressed TGFβ, FasL and Fas, only MLL expressed IL-10 (Fig. 1a).
Fig. 1.
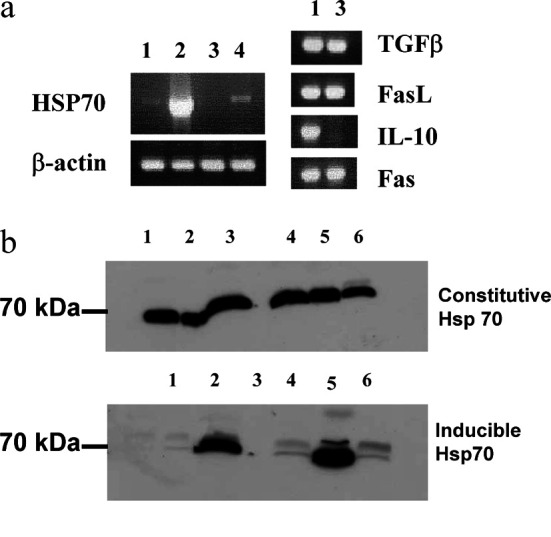
Characterisation of rat prostate cancer cell lines for expression of heat shock protein 70 and immune-related molecules. A Cell lines were examined by RT-PCR for HSP70 expression and for expression of TGFβ, FasL, IL-10 or Fas. Lane 1 is MLL; 2 MLL heated; 3 PAIII; 4 PAIII heated. B Cell lines were examined for constitutive and inducible HSP70 expression using Western blotting with specific antibodies. Lane 1 PAIII; 2 PAIII heated; 3 PAIII-transf. ; 4 MLL; 5 MLL heated; 6 MLL-transf.. Transf. transfection with HSP70
Inducible HSP70 expression (as a result of heating) was examined in tumour cells that were subjected to heat shock at 42°C for 1 h. Following overnight recovery in normal culture conditions, cells were evaluated for HSP70 upregulation by RT-PCR (Fig. 1a) and for protein expression by Western blotting (Fig. 1b). An upregulation of mRNA encoding inducible HSP70 was seen by RT-PCR in both cell lines, though more weakly in PAIII. Protein upregulation was observed by Western blotting in both cell lines at similar levels, whilst constitutive HSP70 expression remained constant.
Vaccination in rat prostate models, protection from challenge and associated immune responses
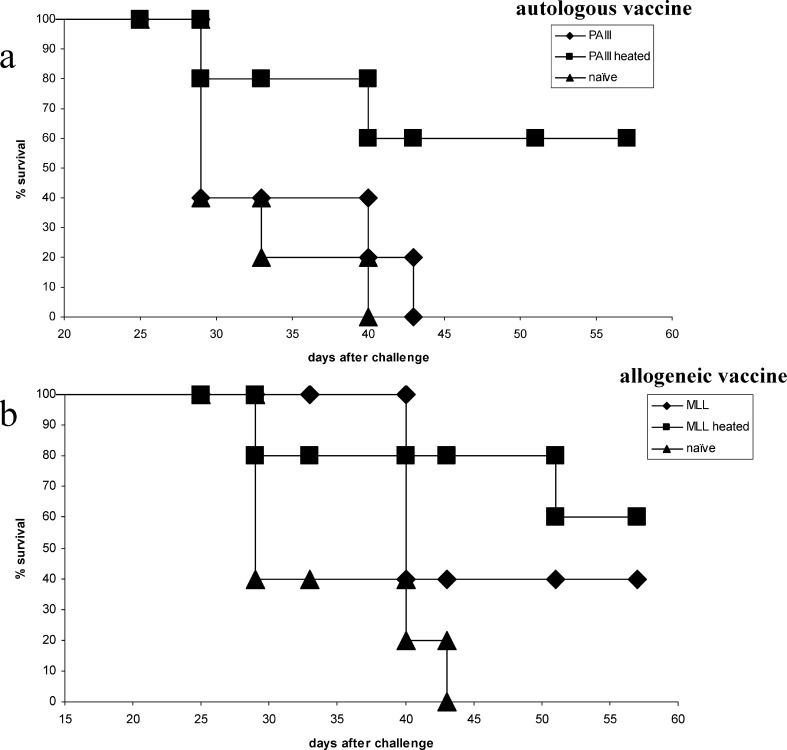
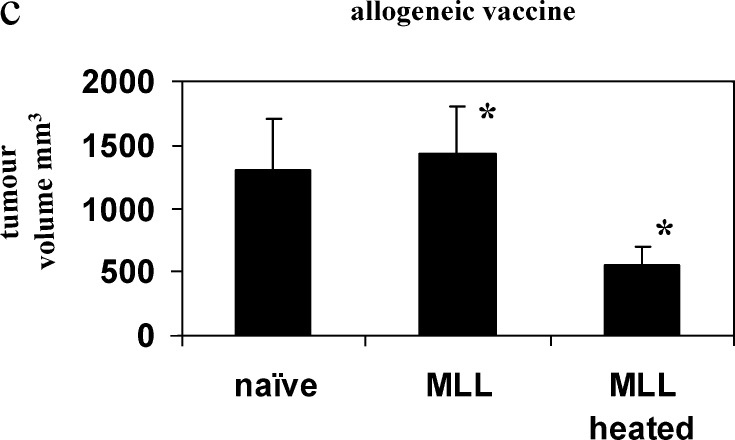
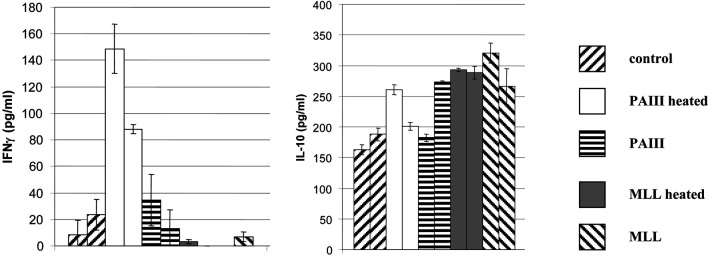
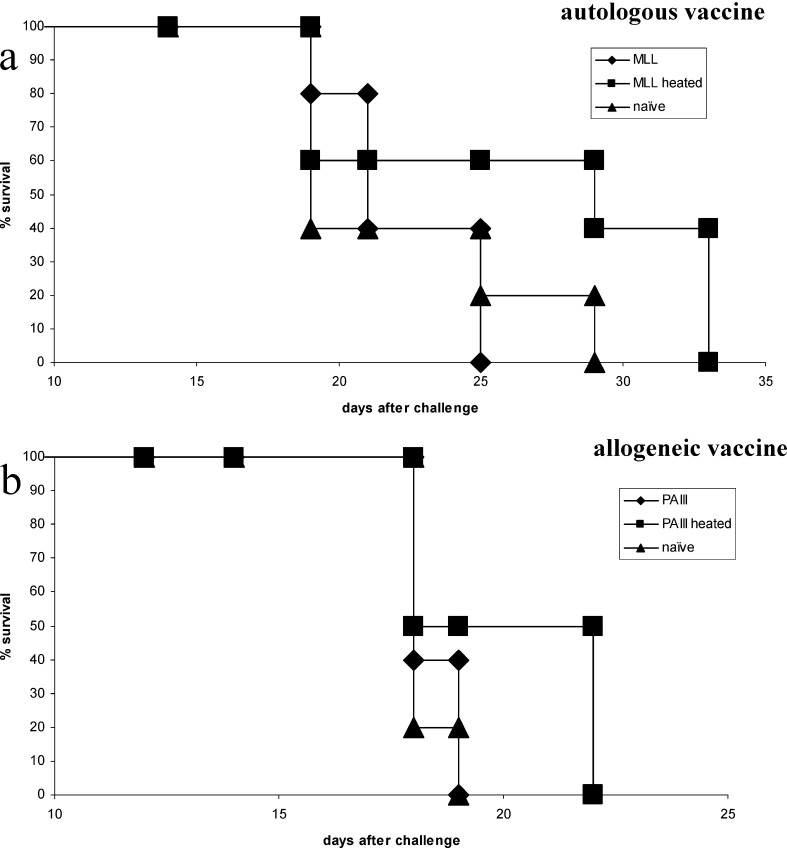
Rats were immunised either with autologous or allogeneic irradiated tumour cells with or without prior heating of the vaccine cells. PAIII were ineffective as an autologous vaccine in L-W rats, but heating of the cells elicited significant protection above unheated cells (P=0.039), affording protection in 60% of rats at day 57 (Fig. 2a). Allogeneic MLL vaccine in L-W rats was moderately immunogenic, but heating of the cells elicited protection in 60% of rats at day 57 and significant survival increase over naïve (P=0.016) in contrast to the unheated vaccine (Fig. 2b), which was not significant over naïve (P=0.066). Moreover, tumour volume shown for day 25 after PAIII challenge (Fig. 2c) was significantly reduced (P=0.034) following heated MLL vaccine compared to unheated. In the Cop rats the autologous heated MLL vaccine was only able to extend survival (Fig. 3a), and the heated PAIII allogeneic vaccine afforded only a modest survival extension over unheated cells (Fig. 3b) (ns by log-rank test). Immune responses were examined in spleen cells of L-W rats 7 days following vaccination with tumour cells. Stimulation of spleen cells with the positive control stimulus (PMA/ionomycin) induced secretion of both IFNγ and IL-10 at high levels (>10 ng and 1 ng/ml, respectively; not shown). The levels of IL-10 were mainly increased above the naïve levels in groups that had received MLL vaccines, whether they were restimulated with tumour cells (Fig. 4) or not (not shown). In contrast, significant IFNγ secretion in response to tumour cells was seen in rats that received heated PAIII vaccine (Fig. 4).
Fig. 2.
Survival from PAIII challenge following vaccination in L-W rats. Rats were vaccinated with irradiated tumour cells as indicated and challenged 14 days later with live autologous tumour cells. Graphs show survival following challenge; a autologous vaccine, b allogeneic vaccine or c tumour volume (mean +SEM) on day 25 after challenge *P=0.034; n=5 per group
Fig. 3.
Immune responses in L-W rats following vaccination. Seven days following vaccination as indicated rat spleen cells were cultured with irradiated PAIII tumour cells for 48 h. Cytokines secreted into the supernatant were measured by ELISA (mean for triplicate cultures + sd). The results for two individual rats are shown for each vaccine
Fig. 4.
Survival from MLL challenge following vaccination in Cop rats. Rats were vaccinated with irradiated tumour cells as indicated and challenged 14 days later with live autologous tumour cells. a autologous vaccine, b allogeneic vaccine; n=5 per group
Vaccination in mouse models
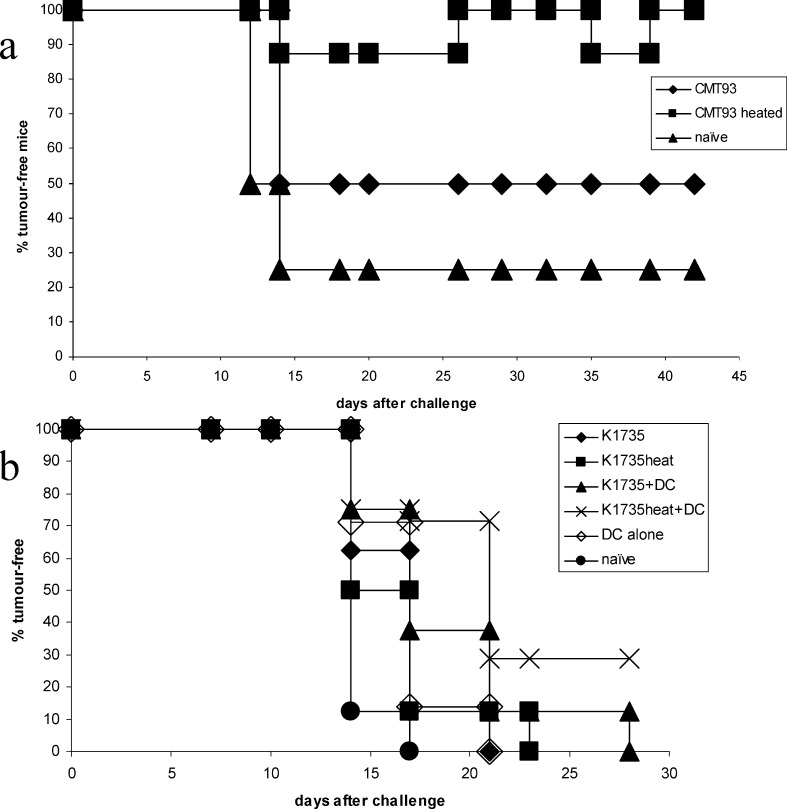
The in vivo studies were extended to other models using the same basic approach as the rat prostate study, as indicated in the Materials and methods section. Two murine models of colorectal cancer and melanoma were used. Following vaccination with CMT93 colorectal tumour cells 50% of C57 BL/6 mice were free of tumour. Heating of the vaccine cells was able to enhance tumour-free protection to 100% for 42 days, the duration of the experiment (Fig. 5a) (P=0.016).
Fig. 5.
Heated vaccines in murine models. C57 BL/6 mice were vaccinated with irradiated tumour cells (± DC) as indicated (and as in Methods) and challenged 14 days later with live autologous tumour cells. a CMT93 colorectal tumour, b B16 melanoma cells; n=8 per group
Vaccination of C57 BL/6 mice with allogeneic K1735 melanoma cells elicits a limited degree of protection against challenge with autologous B16 melanoma cells. However, heating these cells did not significantly enhance protection. Admixing of K1735 cells with DCs in the vaccine gave significant protection over naïve mice (P=0.01). However, when the allogeneic K1735 vaccine cells were additionally heated prior to vaccination, the level of significance over naïve mice was increased dramatically (P=0.0019) (Fig. 5b).
Discussion
There have been a number of attempts at improving the anti-tumour efficacy of allogeneic whole cell vaccines by the use of adjuvants such as BCG and GM-CSF, and other approaches continue to be evaluated. Two rat prostate tumour models were used in this study that can be used as reciprocal allogeneic vaccines. These show different characteristics from each other that may mirror human disease. PAIII in L-W rats is a slow-growing tumour that may be more akin to early human disease. MLL in Cop rats on the other hand is a fast-growing, aggressive tumour that may have some semblance to hormone refractory disease. We have previously demonstrated the efficacy of the two cell lines as autologous whole cell vaccines when combined with a mycobacterial adjuvant [11], and that the antitumour effects were more marked when used as allogeneic vaccines [13].
The rat prostate tumour cell lines have been characterised previously with respect to surface molecules involved in antigen presentation and alloreactivity [13]. A number of factors may explain relatively poor immunogenicity. This includes low expression of MHC class I molecules in the MAT-LyLu rat prostate cancer cell line and was consistent with the findings reported by others in human prostate cancer [3]. In addition to this, we found here that both cell lines expressed immunosuppressive TGFβ, Fas (a target for cytotoxic lymphocytes) and FasL (a killer of cytotoxic lymphocytes). Only MLL expressed IL-10, which is an immunosuppressive cytokine and so may contribute to its aggressive nature.
In relation to whole cell vaccination, tumour-specific T cell stimulation can theoretically be achieved in one of two ways: by direct presentation when the tumour vaccine cells themselves present the tumour antigens to T cells [22] or by cross-priming when tumour antigens from the vaccine cells are then taken up by professional autologous APC of the host, which then migrate to the regional lymph node and activate tumour-specific T cells [15, 18]. Allogeneic tumour cells are largely unable to present antigen directly to T cells due to their unmatched MHC molecules. However, the expression of allogeneic MHC molecules, even at relatively low levels, may serve as an immunological adjuvant by causing alloreactive T cell activation at the vaccine site as we have shown for murine melanoma [34].
A number of studies involving autologous tumour models have suggested a role of HSPs in the generation of anti-tumour immunity through upregulation of inducible rather than consitutive HSP70. HSP is proposed as a vehicle for antigen cross-priming, shuttling antigens from tumour cells to the MHC class I pathway of APCs [27]. HSPs may also have the ability to activate cells of the innate immune system, providing signals that further promote T cell priming [1, 2].
In this study the vaccination of L-W rats with heated prostate tumour cells of both autologous and allogeneic origin improved survival following tumour challenge. For autologous heated PAIII vaccine this protection was associated with increased spontaneous IFNγ from spleen cells of vaccinated rats, and this was further increased in response to tumour re-stimulation. Since the tumour cells posses MHC class I but not class II, it can be inferred that CD8+ T cells are the main producers of IFNγ, this being the subject of current investigations. Following the allogeneic MLL vaccine, IFNγ responses were considerably reduced compared to autologous vaccination. This may reflect our finding that MLL cells expressed IL-10 and that after vaccination we observed increased secretion of IL-10 from splenocytes spontaneously and in response to PMA/ionomycin. This suggests that although both heated vaccines afforded protection from tumour challenge, the underlying mechanisms involved in this effect may be different. Indeed, both IFNγ and IL-10 responses have been shown to have anti-tumour effect in different models [4, 25]. The finding that heated vaccine was effective in the murine CMT93 colorectal model supported the above finding with autologous vaccine. The data with allogeneic K1735 melanoma vaccine was more intriguing. Whilst K1735 vaccine and its heated counterpart elicited weak immunity, this was enhanced by including autologous DCs into the vaccine. This suggests that heating alone may not be sufficient to enhance allogeneic vaccines in all cases.
Overall, this study suggests that increased expression of HSPs through heating, within whole tumour cell vaccines of autologous or allogeneic origin, is a means of enhancing immunogenicity without genetic modification or inclusion of potentially toxic adjuvants.
Acknowledgements
S. Todryk acknowledges the support of Onyvax, Ltd., and Science Foundation Ireland. J. Eaton and R. Greenhalgh were supported by the Swires Trust. A. Melcher is supported by CRUK and MRC. H.S. Pandha is supported by the Sobel Trust.
References
- 1.Asea Nat Med. 2000;6:435. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 2.Basu Int Immunol. 2000;12:1539. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 3.Blades Urology. 1995;46:681. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 4.Blankenstein Curr Opin Immunol. 2003;15:148. doi: 10.1016/S0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 5.Clark Cell Stress Chaperones. 2001;6:121. doi: 10.1379/1466-1268(2001)006<0121:tihaam>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corman Clin Exp Immunol. 1998;114:166. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish Cancer Surv. 1996;26:289. [PubMed] [Google Scholar]
- 8.Harris Semin Oncol. 1999;26:439. [PubMed] [Google Scholar]
- 9.Hartl Nature. 1996;381:571. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 10.Heiser J Immunol. 2001;166:2953. doi: 10.4049/jimmunol.166.5.2953. [DOI] [PubMed] [Google Scholar]
- 11.Hrouda Br J Urol. 1998;82:870. doi: 10.1046/j.1464-410X.1998.00881.x. [DOI] [PubMed] [Google Scholar]
- 12.Hrouda Br J Urol. 1998;82:568. doi: 10.1046/j.1464-410x.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 13.Hrouda BJU Int. 2000;86:742. doi: 10.1046/j.1464-410x.2000.00887.x. [DOI] [PubMed] [Google Scholar]
- 14.Hsueh J Clin Oncol. 2002;20:4549. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 15.Huang Science. 1994;264:961. [Google Scholar]
- 16.Ito Cancer Immunol Immunother. 2001;50:515. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight Melanoma Res. 1996;6:299. doi: 10.1097/00008390-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Maass Proc Natl Acad Sci USA. 1995;92:5540. [Google Scholar]
- 19.Melcher Nat Med. 1998;4:581. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 20.Moody Prostate. 1994;24:244. [Google Scholar]
- 21.Morton Ann Surg. 2002;236:438. doi: 10.1097/00000658-200210000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochsenbein Nature. 2001;411:1058. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto Int J Hyperthermia. 2000;16:263. doi: 10.1080/026567300285277. [DOI] [PubMed] [Google Scholar]
- 24.Sanda J Urol. 1994;151:622. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 25.Segal J Immunol. 2002;168:1. doi: 10.4049/jimmunol.168.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Simons Cancer Res. 1999;59:5160. [PubMed] [Google Scholar]
- 27.Srivastava Immunity. 1998;8:657. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 28.Terasawa Clin Cancer Res. 2002;8:41. [Google Scholar]
- 29.Thomas Hum Gene Ther. 1998;9:835. doi: 10.1089/hum.1998.9.6-835. [DOI] [PubMed] [Google Scholar]
- 30.Tjoa Prostate. 1997;32:272. doi: 10.1002/(SICI)1097-0045(19970901)32:4<272::AID-PROS7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Toes Cancer Res. 1996;56:3728. [Google Scholar]
- 32.Vesalainen Eur J Cancer. 1994;30A:1797. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 33.Vieweg Cancer Res. 1994;54:1760. [PubMed] [Google Scholar]
- 34.Ward S, Casey D, Labarthe MC, Whelan M, Dalgleish A, Pandha H, Todryk S. Immunotherapeutic potential of whole tumour cells. Cancer Immunol Immunother. 2002;51:351–357. doi: 10.1007/s00262-002-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]