Abstract
Background: Monoclonal antibodies (MAbs) are used for targeting agents to tumours while minimizing normal tissue exposure. Methods: A new anti–prostate cancer MAb, BLCA-38, was radioiodinated (I125) and assessed for its ability to target subcutaneous human prostate cancer (DU-145) xenografts after systemic intraperitoneal administration. For comparison, the profile of J591 MAb (now in clinical trial) against LNCaP-LN3 tumours was examined. Biodistribution profiles were obtained at various times, by assessing injected dose/gram (%ID/g) and xenograft to blood (X/B) ratios. Microautoradiography of xenografts was performed. After conjugation with a melittin peptide toxin, the profiles of BLCA-38 and J591 were compared with that of an irrelevant antibody, DS-1. Results: Xenograft localization by 125I-labeled BLCA-38 and J591 MAbs to their relevant antigen-positive tumors was comparable, and there was no unusual localization in nontumour tissues. F(ab’)2 and Fab fragments gave improved X/B ratios, but the %ID/g xenograft was decreased and they accumulated in kidneys, bladder and stomach. In contrast, the conjugates of irrelevant antibody showed no tumour targeting. Microautoradiography showed more tumour accumulation of MAbs than F(ab’)2s or Fabs. Conclusions: BLCA-38 can target prostate cancer in vivo almost as effectively as J591. Given that J591 is used clinically, BLCA-38, which targets a different antigen, has potential for radioimmunoscintigraphy and for therapeutic targeting of prostate cancer.
Keywords: BLCA-38, J591, Anti–prostate cancer antibodies, Prostate cancer xenografts, Xenograft to blood ratios
Introduction
Prostate cancer is the most common male cancer and second highest cause of cancer mortality in men in Western society. It is a heterogeneous disease that may be indolent and not require intervention, may remain localized to the prostate gland but be clinically significant (>95% 5-year survival), or may be invasive and metastatic, primarily to lymph nodes and bone (5% 5-year survival)[11, 37]. Tumour-specific or prostate tissue–specific monoclonal antibodies (MAbs), may be useful in early detection, imaging and treatment, and are essential for reducing mortality [1, 2, 13, 17]. Antibodies may be radiolabeled for immunodetection of cancer [4, 5, 6] or conjugated with toxins, drugs or radionuclides for immunotherapy [5, 12]. Because the MAb and thus the molecules it can carry are selectively concentrated in tumour tissue, this modality can deliver substantial doses of a killing agent to the cancers while minimizing exposure of normal tissue. Long-term complete remission is achievable in patients treated with high dose radioimmunotherapy (see, e.g., [4]).
Few MAbs against prostate cancer have been produced and studied. Of these, the best known is the 7E11-C5 MAb against the LNCaP cell line [9], and later antibodies against the external domain of the same target, including J591. In this study, we wished to examine the ability of a newly described MAb, BLCA-38, to target prostate cancer. For comparative purposes, we have used the MAb, J591, that has already been successfully used for radioimmunotherapy in phase I/II human trials [30, 31]. J591 targets the external domain of prostate-specific membrane antigen (PSMA; now known as folate hydrogenase 1, FOLH1). This is a cell surface glycoprotein expressed predominantly by prostate cancer cells, with two enzymic activities, folate hydrolase and exocarboxypeptidase [7], that was first identified as a membrane antigen on the prostate cell line, LNCaP [9]. PSMA is expressed at higher levels on prostate cancer cells [26] than in normal prostate, is up-regulated during androgen deprivation therapy [35] and is detected in the neovasculature of malignant neoplasms but not in normal vasculature [3, 16]. J591 MAb is internalized after binding [18], a property that could be important in targeting drugs or toxins to cancer cells.
BLCA-38 is an IgG1 murine MAb [33] raised against the human bladder cancer cell line UCRU-BL-17CL [22, 23]. It recognizes a glycoprotein of around 30 kDa expressed at the cell surface and in the cytoplasm, the nature of which has remained elusive, despite studies using 2-D gel electrophoresis and Western blotting (unpublished). BLCA-38 MAb has previously been shown to effect excellent targeting to human bladder xenografts in nude mice, and a single intraperitoneal (i.p.) dose of 250-μCi BLCA-38 labeled with samarium 153 (153Sm) provided sustained growth delay of well-established subcutaneous (s.c.) bladder cancer xenografts [14]. More recently, we have found that BLCA-38 binds with variable intensity to the surface of most human prostate cancer cell lines, and also to 21/33 biopsy samples of prostate cancer from patients undergoing radical prostatectomy (Russell et al., submitted); it did not bind to any normal tissues, or to 21 biopsy samples of benign hyperplasia of the prostate. Moreover, when conjugated to alpha-labeled bismuth, and used in a cocktail to provide multiple alpha-targeted therapy (MTAT), BLCA-38 resulted in cytotoxicity to DU-145 cells in vitro (Li et al., submitted).
We wished to ascertain the best times and doses for targeting potential therapeutic agents to prostate cancer cells in vivo using BLCA-38 for delivery, and to compare the data with that obtained using J591. The LNCaP-LN3 [19] derivative of LNCaP shows increased binding by J591, and was used as a target in our experiments. BLCA-38 binds strongly to PC-3 and DU-145 cells in vitro (Russell et al., submitted). As DU-145 cells form the more vascular tumours in nude mice, they were used as the target for the BLCA-38 MAb in vivo.
Materials and methods
Cell Lines
LNCaP-LN3 cells were provided by C. Pettaway (MD Anderson Cancer Center, Houston, TX). These cells are androgen-sensitive and express high levels of the antigen PSMA [24]. DU-145 cells were from the American Type Culture Collection (ATCC, Rockville, MD). Cell lines were maintained in RPMI 1640 media (Gibco BRL Life Technologies, Rockville, MD), with 10% fetal bovine serum (Trace Biosciences), penicillin (50 U/ml), streptomycin (0.05 mg/ml) and 0.2-mM l-glutamine (Gibco BRL Life Technologies, Rockville, MD). All cells were maintained at 37°C in 5% CO2 and passaged with trypsin / ethylenediamine tetra-acetic acid (EDTA) (Gibco BRL Life Technologies, Rockville, MD) when confluent.
Mice
Athymic BALB/c nu/nu (nude) mice aged 6–8 weeks were bred at the Biological Resources Centre (BRC), University of New South Wales (UNSW), at Little Bay, NSW, Australia, and maintained in the nude mouse facility at the BRC. They were kept under sterile conditions, housed in cages fitted with filter tops and fed sterile mouse food and water ad libitum. When radioiodinated antibodies were to be injected, their drinking water was supplemented with potassium iodide (0.05%; BDH Chemicals, Aust., Port Fairy, Vic, Australia) to block uptake of radioiodine by the thyroid gland. After injection of a radiolabeled antibody, the mice were kept behind lead shielding, and the cage litter was changed regularly to minimize label ingestion. Experiments were approved by the Animal Care and Ethics Committee, University of New South Wales.
Xenografts
LN3 or DU-145 human prostate cancer cell lines, harvested in 0.02% EDTA/PBS, were resuspended in serum-free media and injected s.c. in male nude mice in the right flank with 200 μl of cell suspension (1.5x106 to 2x106 tumour cells) containing an equal mix of 100 μl of Matrigel (Integrated Sciences, Sydney, Australia) and cells (100 μl) on ice. Tumours were measured twice/week using vernier calipers and their volumes (V, mm3) were determined as described [21], V=π/6(d1.d2)3/2, where d1 and d2 are diameters at right angles (mm).
Monoclonal antibodies, production and purification
Three IgG1 MAbs were used. J591 binds to FOLH1-expressing cells [9], BLCA-38 to human CaP cell lines and biopsy samples (Russell et al., submitted) and DS-1 was used as a negative control. J591 hybridoma was provided by BZL, through N. Bander, New York Presbyterian Hospital/Weill Medical College of Cornell University, NY; BLCA-38 hybridoma and DS-1 were in house. J591 and DS-1 hybridomas were grown serum-free with 0.01% glutamax II, penicillin-streptomycin (51 U/ml, 5 μg/ml) 10 ml/L (each Gibco BRL Life Technologies, Rockville, MD) and glucose 2 g/l (Sigma, St, Louis, MO) in 0.05% CO2 at 37°C, but BLCA 38 hybridoma required 2% ultralow IgG FBS (Gibco BRL). Hybridoma cells (4×107) grown in roller bottles for 9 days yielded MAbs at 0.1–0.2 mg/ml. MAbs were isolated by affinity chromatography using a protein A column (Prosep A; Novachem, Australia), pH 7.4. Bound IgG was eluted with 0.2-M glycine buffer, pH 3.0, neutralized, concentrated to ~5 mg/ml by ultrafiltration using an Amicon 10-kDa cutoff membrane and dialysed into PBS, pH 7.4. Purity was monitored by SDS-PAGE and size exclusion chromatography on a Superose 12 HR10.30 column, pH 7.4. MAb binding was assessed by flow cytometry.
Antibody fragmentation
Each test MAb (at 1.0 mg/ml in 0.5-M sodium acetate, 0.5-M sodium chloride, pH 4.0) was cleaved with pepsin (Sigma Chemical, St. Louis, MO) at 37°C until over 90% of the IgG was converted to the F(ab’)2 fragment. This was monitored hourly by running an aliquot of the mix on a Superose 12 HR10/30 column (Amersham Biociences, Castle Hill, NSW, Australia) to measure the appearance of the F(ab’)2 peaks. The ratios of pepsin to antibody on a w/w basis were 1:30 for J591 IgG, 1:75 for BLCA 38 IgG and 1:100 for DS-1 IgG to obtain maximum cleavage in 7 h of digestion. The digestion was stopped by raising the pH to 7.0 with saturated TRIS (Sigma), and undigested IgG and uncleaved Fc fragments were removed on a Prosep A column (Novochem, Australia), preequilibrated with PBS, pH 7.4. The F(ab’)2 fragment was recovered from the column flow-through, concentrated by ultrafiltration (10-kDa cutoff membrane) to ~1.5–2.0 mg/ml and dialysed into PBS, pH 7.4. The F(ab’)2 fragments were reduced with 0.01-M mercaptoethylamine (37°C, 1 h) and the sulfhydryl groups on the Fab’ fragment were blocked by adding 0.5-M iodoacetate. Excess reagents and unreduced F(ab’)2 were removed by size exclusion chromatography on a Superose 12 column in PBS, pH 7.4. The protein concentration was determined spectrophotometrically at 280 nm using an extinction coefficient of 1.35 for the MAbs and 1.18 for the fragments.
Flow cytometric analysis of antibody binding
Cells (105) harvested in 0.02% EDTA/PBS were pelleted by centrifuging at 1,500 rpm for 5 min and incubated with 100-μl BLCA-38 or J591 MAb supernatant or PBS (negative control) for 60 min at 4°C. Optimal concentrations of purified BLCA-38 and J591 MAbs were 1 μg/ml, for purified BLCA-38 (Fab’)2, 1–5 μg/ml, and for J591 (Fab’)2, 5 μg/ml. Cells were washed in PBS before adding 100 μl 1/50 fluorescein-conjugated sheep antimouse immunoglobin (FITC-SaMIg) (Amrad, Melbourne, Vic, Australia) in PBS, for 30 min at 4°C, washed twice in PBS, and fixed with 500-μl 1% paraformaldehyde (Sigma, St. Louis, MO) in PBS. Surface fluoresescence was assessed in a Fluorescence Activated Cell Scanner (FACScan; Becton Dickinson, Mountain View, CA).
Iodination
Sephadex G-25 (Pharmacia, Uppsala, Sweden) was blocked with 5% BSA/PBS/azide overnight at 4°C, packed in a 10-ml syringe plugged with glass wool, and washed with 100-ml PBS. BLCA-38, J591 or control IgG1 MAbs were labeled with iodine 125 (125I) (2 mCi ml-1; Amersham, Bucks, UK) at 0.2 mCi/100 μg using the Iodogen method [32]. The 125I-labeling efficiency of the antibodies was determined by a trichloracetic acid (TCA) assay; that of 125I to BLCA-38 and J591 antibodies was 80–90% except for J591 Fabs, which labeled with 75% efficiency. One microliter of 125I-antibody was added to 1-ml carrier protein (20% FCS in PBS) and counted on a gamma counter. One milliliter 20% TCA/water was added, and the mixture centrifuged at 3,000 rpm for 10 min. The supernatant and pellet were separated, and both fractions were counted. The percentage of 125I bound to the antibody was assessed as pellet counts per minute (cpm) / pellet cpm + supernatant cpmx100. The same batches of labeled MAbs, (Fab’)2s and Fabs were used for biodistribution and microautoradiographic studies.
Immunoconjugation
Peptide 101 of melittin was labeled with 14C and conjugated via a chelation group to iodinated antibodies, BLCA-38, J591 or DS-1, as described elsewhere [25]. These conjugates were used to test the specificity of binding of test antibodies to their respective tumours.
Animal Studies
Biodistributions
Biodistributions were performed to determine the radiation load on the tumour and other body organs from 125I-MAbs. Nude mice with measurable DU-145 or LN3 s.c. xenografts were injected intraperitoneally (i.p.) with 50, 100 and 200-μg 125I-BLCA-38 or 125I-J591 MAb, respectively. Biodistribution analyses were conducted 5–9 days postadministration of intact MAbs (6 mice / time point) and 1–5 days postadministration of 125I-labeled (Fab’)2s (6 mice / time point) and Fabs (2 mice / time point). Mice were overdosed with enflurane, exsanguinated by cardiac puncture, and blood (BD) and tissues were collected and placed into preweighed tubes: xenograft (X), lungs (LU), liver (LI), spleen (SP), kidney (KD), stomach (ST), bladder (BL), small intestine (SI), large intestine (LI), lymph nodes (LN), gonads (G), muscle (MM), bone (BN), skin (SK) and thyroid (T). Tubes were reweighed to determine tissue mass and counted using a 1470 Wizard gamma counter (Wallac, Wallac Oy, Finland). Data were expressed as tissue to blood ratio (T/B) = cpm/g tissue (counts per minute per gram of tissue or blood), and percentage injected dose corrected for decay per gram (%ID/g): cpm/g injected dose corrected for decay.
Conjugates of melittin-peptide 101 with 125I-test and control antibodies (150 μg) were injected i.p. into mice bearing s.c. tumours of DU-145 (BLCA-38, DS-1) or LNCaP-LN3 (J591, DS-1) for comparison of uptake by the tumours. Eight mice/group were sacrificed 8 days (DU-145-bearing mice) or 7 days (LNCaP-LN3-bearing mice) later, for biodistribution analysis based on gamma counting as described above.
Microautoradiography
Tumour-bearing mice were injected i.p. with 100 μg 125I-MAbs, F(ab’)2s and Fabs. Controls were xenograft tissues from uninjected mice. Xenografts were excised from 2 mice/group 7 days after injecting MAbs or 2 days after F(ab’)2s and Fabs, snap frozen in Crymolds (Tissue-Tek, Torrance, CA) with OCT compound (Tissue-Tek, Torrance, CA) and kept at −20°C. Frozen tissues were serially sectioned at 6 μm using a Cryostat (Zeiss HMSOSE Microm, Walldorf, Germany) at −20°C. Tissues on Superfrost slides (Menzel-Glaser, Germany) were stained with hematoxylin and eosin (H&E) directly; for microautoradiography, tissues on SuperfrostPlus slides (Menzel-Glaser, Germany) were stained for H&E after processing. Slides were stored at −20°C until required. Microautoradiography was performed under an Ilford 915 red light filter. Slides were thawed at room temperature (RT) for 3 h or until dry, fixed in 1% paraformaldehyde (Sigma, St. Louis, MO) in PBS at RT for 15 min, dipped in warm water, washed in 70% ethanol for 2 min, and washed twice in 100% ethanol for 5 min. They were dried in air then in a slide box with desiccant overnight at 4°C. Slides were dipped in emulsion (Kodak NTB-3, Integrated Sciences) in glycerol (1:50 glycerol in water) at 1 g emulsion per ml of glycerol solution, that had been melted at 42°C for 1 h, and mixed by gentle inversion, dried overnight at RT with dessicant in darkness, then exposed for 7–14 days at 4°C. They were developed in freshly prepared developer (1:4 with water) for 6 min, washed in Milli Q water for 30 s, dipped in fixer solution (1:3 with water) for 5 min, washed briefly in Milli Q water, rinsed under running tap water for 20 min, dried and stained with H&E.
Immunoperoxidase staining
DU-145 xenografts were formalin-fixed, paraffin-embedded, and LN3 xenografts were frozen in OCT compound (Tissue Tek); 6-μm sections were stained for expression of BLCA-38 and J591, respectively, by indirect immunoperoxidase staining. A mouse-on-mouse (MOM) kit (Vector Labs, Burlingame, CA) prevented nonspecific binding. Negative controls were treated with irrelevant isotype-matched primary antibody or no primary antibody. Paraffin-embedded DU-145 xenografts were deparaffinized, rehydrated in a graded series of ethanol, immersed in 0.01-M citrate, pH 6.0, then pretreated with high-temperature microwave irradiation to demask BLCA-38 antigen expression by heating to boiling in a 1,200-W microwave oven at maximum power for 3 min then at 95°C for 10 min, followed by cooling at RT for 15 min. Endogenous avidin/biotin and peroxidase were quenched using the avidin/biotin kit (Dakopatts, Glostrup, Denmark) and 0.3% H2O2/PBS, respectively. Nonspecific protein binding was blocked with 2% normal goat or 10% normal sheep sera in PBS for 30 min at RT. Slides were incubated with primary antibodies, BLCA-38 at 15 μg/ml or J591 at 10 μg/ml in 2% BSA/PBS in a humidifier overnight at 4°C, washed with PBS plus 0.05% Tween 20 (PBS/Tween), incubated with secondary linked antibody, 1:200 biotinylated goat antimouse IgG (Vector Lab, Burlingame, CA) for 30 min at RT, washed with PBS/Tween, and incubated with a 1:200 biotin-HRP complex (Vector Lab, Burlingame, CA) for 30 min. The brown reaction was visualized with a 1:50 diaminobenzoate (DAB) kit (Dakopatts, Glostrup, Denmark). Slides were counterstained lightly in Harris hematoxylin, dehydrated, cleared in xylene and mounted in Eukitt.
Results
Antigen expression
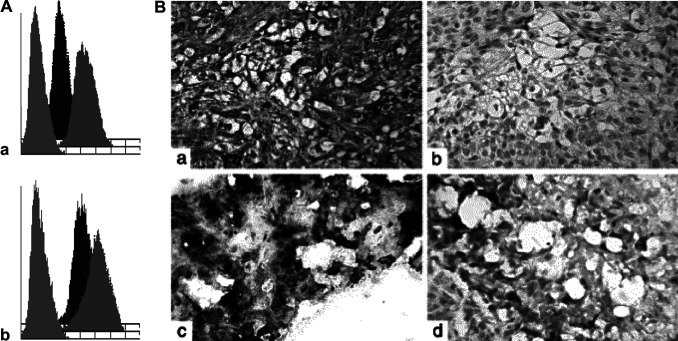
Several prostate cancer cell lines were analysed by flow cytometry for staining with BLCA-38 and J591 MAbs; none expressed both antigens. DU-145 prostate cells highly express BLCA-38 antigen (mean fluorescence intensity [MFI], 186), while LNCaP-LN3 cells highly express FOLH1 (MFI, 804) (Fig. 1A); both antigens were trypsin sensitive, and cells were preferentially harvested using EDTA. For in vivo studies, J591 was tested against LNCaP-LN3 xenografts, and BLCA-38, against DU-145 xenografts grown s.c. in nude mice. Before injection, cells for implantation were monitored for surface expression of target antigens by flow cytometry. In each case, DU-145 expressed BLCA-38 antigen with an MFI of 150–170 and LN3 cells expressed FOLH1 with an MFI between 660 and 804. F(ab’)2s and Fabs also detected the appropriate antigens. DU-145 cells showed consistent tumour growth in vivo. The LN3 cells grow with ~70% take rate, and show irregular growth patterns; some are engorged with blood while others are solid. Both xenografted lines were well vascularized, and maintained antigen expression in vivo (Fig. 1B).
Fig. 1.
A Flow cytometric profiles of human prostate cancer cell lines grown in vitro and stained with BLCA-38 or J591 (10 μg). (a) BLCA-38 MAb expression on DU-145 cell (mean fluorescence intensity [MFI]: trypsin, 28.4; MFI EDTA, 185.8); (b) J591 expression on LNCaP-LN3 cells (MFI trypsin, 180.5; MFI EDTA, 496.3). Negative control (second antibody only), cells harvested in trypsin (MFI DU-145, 4.6; MFI LNCaP-LN3, 5.4), and cells harvested in EDTA/PBS (MFI DU-145, 4.7; MFI LNCaP-LN3, 5.4). B. Tumour xenografts grown s.c. in nude mice. DU-145 xenografts were formalin-fixed and paraffin-embedded, and LNCaP-LN3 tumours were fresh-frozen in OCT by standard methods. Xenografts were stained for expression by indirect immunoperoxidase staining with BLCA-38 MAbs (for DU-145 xenografts) or J591 MAbs (for LNCaP-LN3 xenografts) . DU-145 xenografts first underwent antigen retrieval in a 1,200 W microwave oven at maximum power for 3 min then at 95°C for another 10 min, followed by cooling at room temperature for 15 min. (a) Photomicrograph showing BLCA-38 expression in DU-145 xenograft; (b) Photomicrograph of DU-145 xenograft stained with isotype control antibody showing negative staining; (c) Photomicrograph showing positive J591 staining of LNCaP-LN3 xenograft; (d) Photomicrograph of LNCaP-LN3 tissue stained with isotype control antibody
Biodistributions using intact MAbs
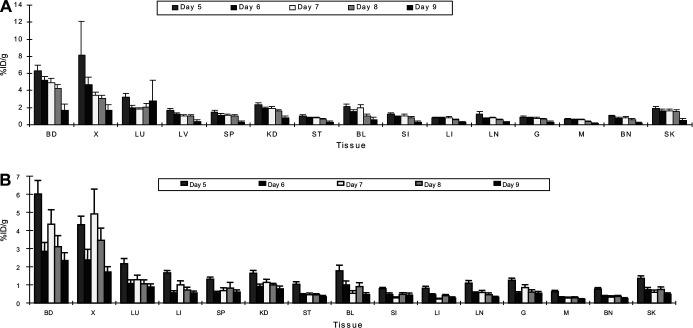
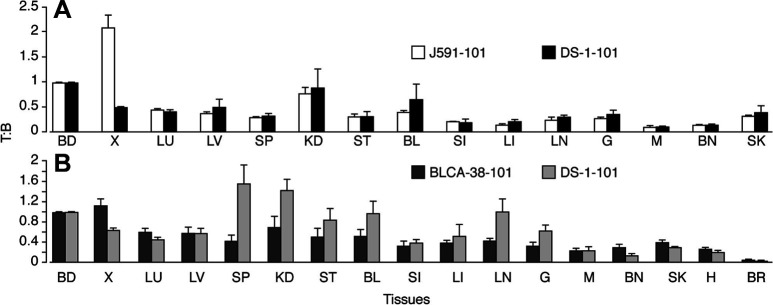
Biodistribution studies were performed 5–9 days after injecting xenograft-bearing mice with 100 or 200 μg 125I-BLCA-38 and J591 MAbs to determine when optimal specific localization occurred to DU-145 or LN3 xenografts, respectively. Good localization of both MAbs to the xenografts was seen, while label uptake by tissues other than blood and xenograft tissue was low (Fig. 2). Tissue to blood (T:B) ratios for 125I-labeled BLCA38 and J591 MAbs are shown in Table 1. T/B ratios in stomach, small intestine, large intestine, lymph nodes, gonads, muscle, and bone were consistently <0.20, and those for liver and spleen <0.28, at each time point studied with both MAbs. For the BLCA-38 MAbs, the highest xenograft to blood (X/B) ratio was 1.12, 5 days after injection of 200 μg into DU-145 xenografts (Table 1), correlating with an uptake of 8.11%ID/g. For J591 MAbs, the highest X/B ratios obtained were 1.09 on day 9 (post 100 μg) and 1.15 on day 8 (after 200 μg) (Table 1); these X/B ratios correlated with xenograft uptakes of 4.41 and 3.45%ID/g, respectively. The best time points postadministration for the localization of 200 μg 125I-labeled MAbs were on days 5, 6 and 7 for BLCA-38 and days 7 and 8 for J591. At useful time points, DU-145-tumour bearing mice (8 days) received 150-μg conjugated 125I-BLCA-38 BLCA-38 or DS-1 antibodies i.p., and LNCaP-LN3-bearing mice (7 days) received 150-μg conjugated 125I-J591 or DS-1 antibodies i.p. Conjugates of the irrelevant MAb, DS-1, showed no tumour tropism to either tumour, compared with conjugates of the test MAbs (Fig. 3).
Fig. 2A, B.
Percentage of injected dose per gram results of injecting 200 μg of A 125I-labeled BLCA-38 MAbs, B 125I-labeled J591 MAbs, 5–9 days postadministration into nude mice bearing DU-145 or LNCaP-LN3 human prostate cancer xenografts, respectively. Vertical bars represent SEM (6 mice/group). BD blood, X xenograft, LU lung, LV liver, SP spleen, KD kidney, ST stomach, BL bladder, SI small intestine, LI large intestine, LN lymph node, G gonad, M muscle, BN bone, SK skin
Table 1.
Tissue to blood ratios ± SEM, obtained in nude mice bearing human prostate cancer xenografts. Results in other tissues are described in the text
| Organ/tissue | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|
| DU-145 (6 mice / time point) | |||||
| 100-μg 125I-BLCA-38 MAbs | |||||
| Xenograft | 0.70±0.05 | 0.79 ±0.03 | 0.83±0.03 | 0.88±0.05 | 0.81±0.07 |
| Lung | 0.32±0.02 | 0.35±0.01 | 0.36±0.03 | 0.33±0.02 | 0.31±0.02 |
| Kidney | 0.33±0.01 | 0.36±0.01 | 0.40±0.02 | 0.42±0.05 | 0.40±0.03 |
| Bladder | 0.22±0.02 | 0.29±0.02 | 0.28±0.01 | 0.40±0.07 | 0.26±0.03 |
| Skin | 0.30±0.02 | 0.36±0.02 | 0.34±0.02 | 0.32±0.01 | 0.31±0.02 |
| 200-μg 125I-BLCA-38 MAbs | |||||
| Xenograft | 1.12±0.40 | 0.87±0.12 | 0.71±0.04 | 0.73±0.04 | 1.11±0.10 |
| Lung | 0.52±0.07 | 0.37±0.04 | 0.36±0.02 | 0.48±0.02 | 1.04±0.65 |
| Kidney | 0.37±0.01 | 0.37±0.01 | 0.39±0.02 | 0.39±0.02 | 2.16±1.62 |
| Bladder | 0.36±0.07 | 0.30±0.02 | 0.38±0.06 | 0.26±0.05 | 0.38±0.12 |
| Skin | 0312±0.03 | 0.31±0.02 | 032±0.02 | 0.36±0.02 | 0.37±0.09 |
| LNCaP-LN3 (6 mice / time point) | |||||
| 100-μg 125I-J591 MAbs | |||||
| Xenograft | 0.71±0.06 | 0.79±0.07 | 0.94±0.18 | 0.92±0.10 | 1.09±0.26 |
| Lung | 0.37±0.03 | 0.37±0.03 | 0.30±0.02 | 0.35±0.02 | 0.37±0.03 |
| Kidney | 0.31±0.01 | 0.39±0.01 | 0.27±0.03 | 0.29±0.01 | 0.33±0.06 |
| Bladder | 0.25±0.03 | 0.37±0.07 | 0.13±0.01 | 0.30±0.02 | 0.23±0.04 |
| Skin | 0.32±0.10 | 0.20±0.03 | 0.16±0.02 | 0.24±0.02 | 0.23±0.02 |
| 200-μg 125I-J591 MAbs | |||||
| Xenograft | 0.73±0.05 | 0.83±0.09 | 1.05±0.21 | 1.15±0.14 | 0.75±0.03 |
| Lung | 0.36±0.01 | 0.38±0.02 | 0.29±0.01 | 0.34±0.02 | 0.38±0.01 |
| Kidney | 0.28±0.02 | 0.35±0.03 | 0.28± 0.02 | 0.36±0.05 | 0.37±0.07 |
| Bladder | 0.31±0.02 | 0.34±0.03 | 0.12±0.01 | 0.28±0.02 | 0.20±0.02 |
| Skin | 0.23±0.02 | 0.26±0.01 | 0.15±0.02 | 0.28±0.01 | 0.21±0.02 |
Fig. 3A, B.
Tissue to blood ratios of gamma cpm values following i.p. injection of A 150-μg 14C-peptide 101–125I-J591 and 14C-peptide 101–125I-DS-1 MAbs 8 days postadministration into nude mice bearing human prostate cancer LNCaP-LN3 xenografts; B 150-μg 14C-peptide 101–125I-BLCA-38 and 14C-peptide 101–125I-DS-1 MAb–peptide 101 conjugates 7 days postadministration into nude mice bearing human prostate cancer DU-145 xenografts. Vertical bars represent SEM (8 mice/group). BD blood, X xenograft, LU lung, LV liver, SP spleen, KD kidney, ST stomach, BL bladder, SI small intestine, LI large intestine, LN lymph node, G gonad, M muscle, SK skin, H heart, BR brain
Biodistributions using antibody fragments
Since antibody fragments provide better tumour penetration than intact MAbs [32, 36], biodistribution studies using 125I-labeled F(ab’)2s or Fabs were conducted to compare the results those of intact MAbs. Preliminary data after injecting 60-μg 125I-BLCA-38 F(ab’)2 or 125I-J591 F(ab’)2 into two tumour-bearing mice / time point indicated that higher X/B ratios were seen between days 2 and 4. X/B ratios for 125I-BLCA-38 F(ab’)2 rose from 1.66 (%ID/g=4.96) on day 1, to 3.86 on day 2, and was still 3.57 by day 4, whereas those for 125I-J591 F(ab’)2 increased from 1.20 (%ID/g=2.97) on day 1, to 2.81 by day 3. Biodistributions were therefore performed days 1–5 post-125I-F(ab’)2 administration at 50 μg or 100 μg per mouse (6 mice / point). Results in Table 2 indicate that the best localization of 50-μg 125I-labeled BLCA-38 or J591 F(ab’)2 to the tumour was on days 4 or 5 postadministration. Some uptake (%ID/g) of both labeled F(ab’)2s was seen in the stomach on day 1 (BLCA-38: 1.62 for 50 μg and 1.23 for 100 μg; J591: 3.59 and 5.00 for 50 and 100 μg, respectively), but this fell by day 2, and was negligible by day 3. Uptake of BLCA-38 F(ab’)2 by small intestine, large intestine, lymph nodes, gonads, muscle and bone was always <1 on day 1, and thereafter, <0.16. Similarly that for J-591 F(ab’)2 for other organs, viz, small and large intestine, lymph nodes, gonads, muscle and bone was as high as 3.24 in gonads on day 1, but thereafter, <0.4 for all organs. However, after day 3, activity appeared in the kidney, and this was higher than that in the xenografts after day 4. Activity was also observed in the bladder, but to a lesser extent. This accumulation of label in the kidney could result from excretion of 125I F(ab’)2s. Similar results were seen when 100-μg 125I F(ab’)2s was injected (Table 2). A compromise between X/B ratios and %ID/g suggests that optimal localization of 125I F(ab’)2s in xenografts in the absence of high kidney uptake occurs 2–3 days after administration.
Table 2.
Percentage injected dose per gram of tissue ± SEM, obtained in nude mice bearing human prostate cancer xenografts. Results in other tissues are described in the text
| Organ/Tissue | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| DU-145 (6 mice / time point) | |||||
| 50-μg 125I-BLCA-38 F(ab’)2 | |||||
| Blood | 2.73±0.17 | 0.52±0.03 | 0.16±0.02 | 0.06±0.01 | 0.03±0.01 |
| Xenograft | 2.68±0.22 | 1.19±0.16 | 0.41±0.08 | 0.17±0.04 | 0.10±0.02 |
| Lung | 1.56±0.10 | 0.28±0.01 | 0.11±0.01 | 0.05±0.01 | 0.03±0.01 |
| Liver | 0.50±0.05 | 0.12±0.01 | 0.10±0.01 | 0.05±0.01 | 0.04±0.01 |
| Spleen | 0.62±0.05 | 0.14±0.01 | 0.05±0.01 | 0.03±0.01 | 0.02±0.01 |
| Kidney | 2.04±0.36 | 0.45±0.02 | 0.47±0.09 | 0.34±0.03 | 0.34±0.01 |
| Bladder | 2.44±0.67 | 0.58±0.07 | 0.19±0.04 | 0.06±0.01 | 0.05±0.01 |
| Skin | 0.94±0.07 | 0.25±0.01 | 0.11±0.01 | 0.06±0.01 | 0.04±0.01 |
| 100-μg 125I-BLCA-38 F(ab’)2 | |||||
| Blood | 2.28±0.29 | 0.47±0.03 | 0.15±0.01 | 0.07±0.01 | 0.04±0.01 |
| Xenograft | 2.47±0.28 | 1.09±0.08 | 0.36±0.03 | 0.14±0.01 | 0.07±0.01 |
| Lung | 0.94±0.14 | 0.25±0.02 | 0.10±0.01 | 0.05±0.01 | 0.04±0.01 |
| Liver | 0.41±0.08 | 0.11±0.01 | 0.08±0.01 | 0.06±0.01 | 0.04±0.01 |
| Spleen | 0.55±0.08 | 0.13±0.01 | 0.05±0.01 | 0.03±0.01 | 0.02±0.01 |
| Kidney | 1.44±0.26 | 0.43±0.02 | 0.33±0.01 | 0.30±0.02 | 0.33±0.03 |
| Bladder | 1.94±0.32 | 0.38±0.06 | 0.16±0.03 | 0.10±0.02 | 0.05±0.01 |
| Skin | 0.86±0.11 | 0.23±0.01 | 0.10±0.01 | 0.06±0.01 | 0.05±0.01 |
| LNCaP-LN3 (6 mice / time point) | |||||
| 50-μg 125I-J591 F(ab’)2 | |||||
| Blood | 4.41±0.38 | 0.70±0.19 | 0.18±0.01 | 0.08±0.01 | 0.05±0.01 |
| Xenograft | 5.17±1.18 | 2.37±0.66 | 1.04±0.15 | 1.02±0.17 | 0.52±0.14 |
| Lung | 2.04±0.25 | 0.50±0.14 | 0.15±0.01 | 0.08±0.01 | 0.05±0.01 |
| Liver | 2.73±0.28 | 0.51±0.14 | 0.17±0.02 | 0.08±0.01 | 0.06±0.01 |
| Spleen | 4.04±0.68 | 0.88±0.19 | 0.31±0.02 | 0.14±0.03 | 0.11±0.03 |
| Kidney | 5.95±0.70 | 1.41±0.32 | 0.44±0.03 | 0.24±0.02 | 0.13±0.01 |
| Bladder | 6.15±1.09 | 0.94±0.31 | 0.40±0.09 | 0.08±0.02 | 0.06±0.02 |
| Skin | 1.08±0.05 | 0.28±0.07 | 0.12±0.01 | 0.07±0.01 | 0.06±0.01 |
| 100-μg 125I-J591 F(ab’)2 | |||||
| Blood | 3.95±0.64 | 0.64±0.13 | 0.15±0.02 | 0.06±0.02 | 0.03±0.01 |
| Xenograft | 4.01±0.36 | 1.92±0.41 | 0.75±0.16 | 0.41±0.17 | 0.25±0.10 |
| Lung | 1.82±0.25 | 0.43±0.07 | 0.11±0.01 | 0.05±0.01 | 0.03±0.01 |
| Liver | 2.52±0.40 | 0.42±0.09 | 0.12±0.02 | 0.06±0.02 | 0.04±0.01 |
| Spleen | 3.18±0.49 | 0.75±0.17 | 0.18±0.02 | 0.08±0.02 | 0.07±0.02 |
| Kidney | 5.97±0.65 | 1.18±0.17 | 0.40±0.08 | 0.16±0.02 | 0.12±0.02 |
| Bladder | 7.59±2.08 | 1.14±0.43 | 0.23±0.08 | 0.08±0.03 | 0.06±0.01 |
| Skin | 1.20±0.13 | 0.33±0.05 | 0.12±0.01 | 0.06±0.01 | 0.04±0.01 |
Two experiments were performed after i.p. injection of 100-μg 125I-Fabs per appropriate tumour-bearing mouse, with labeling efficiencies of 75 and 85%, respectively. DU-145-bearing mice were examined at days 1–5, and LN3-bearing mice at 3, 4, 18 and 48 h (experiment 1) and 1–5 days (experiment 2). Good localization was observed in xenografts by day 1 (xenograft to blood ratios of 2.13 and 3.44, respectively, correlating with %ID/g of 2.93 in DU-145 tumours and 1.85 in LN3 tumours). The X/B ratio increased to 8.34 in LN3 xenografts by day 2, but the %ID/g had dropped to 0.56. Most activity was cleared from the xenografts by day 2–3, with increasing activity in the kidney and bladder. This may be due to the excess 125I present that is being excreted, or the smaller fragments (Fabs) may show some localization to kidney as has been reported elsewhere [34].
The optimal localizations of MAbs, F(ab’)2s and Fabs obtained are compared in Table 3. Overall, the X/B ratios of the BLCA-38 and J591 F(ab’)2 and Fabs indicated improved localization in the xenograft tissue compared with the MAbs, but there was increased activity in the kidneys and bladder. The %ID/g reduced dramatically between 1 and 2 days postadministration of the F(ab’)2s (Table 2) and had also decreased compared with the MAbs. A compromise between the T/B and %ID/g values suggested that the optimal day postadministration of the BLCA-38 and J591 F(ab’)2 was day 2 as indicated in Table 3. The Fabs showed even higher T/B ratios, but the %ID/g was lower, and the Fabs were cleared rapidly, making it unlikely that they would be suitable for delivery of anything but very rapidly acting radionuclides or toxins.
Table 3.
Tissue to blood ratios (T/B) and percentage of injected dose per gram of tissue corrected for decay (%ID/g) of the DU-145 (BLCA-38) and LNCaP-LN3 (J591) xenografts at the optimal days postadministration
| Antibody | Dose | Optimal days | T/B (mean ± SEM) | %ID/g (mean ± SEM) |
|---|---|---|---|---|
| BLCA-38 MAb | 100 μg | 5 | 0.70±0.05 | 7.21±0.30 |
| 8 | 0.88±0.05 | 3.12±0.40 | ||
| 200 μg | 5 | 1.12±0.40 | 8.11±3.97 | |
| 6 | 0.87±0.12 | 4.66±0.93 | ||
| BLCA-38 F(ab’)2 | 50 μg | 2 | 2.29±0.29 | 1.19±0.16 |
| 60 μg | 2 | 3.86±1.40 | 1.89±0.28 | |
| BLCA-38 F(ab’)2 | 100 μg | 2 | 2.37±0.27 | 1.09±0.08 |
| BLCA-38 Fab | 100 μg | 1 | 2.13±0.37 | 2.93±0.04 |
| J591 MAb | 100 μg | 9 | 1.09±0.26 | 4.41±1.45 |
| 200 μg | 7 | 1.05±0.21 | 4.91±1.37 | |
| 8 | 1.15±0.14 | 3.45±0.68 | ||
| J591 F(ab’)2 | 50 μg | 2 | 3.11±0.62 | 2.37±0.66 |
| 100 μg | 2 | 3.12±0.47 | 1.92±0.41 | |
| J591 Fab | 100 μg | 1 | 3.44±0.21 | 1.85±0.18 |
Microautoradiography
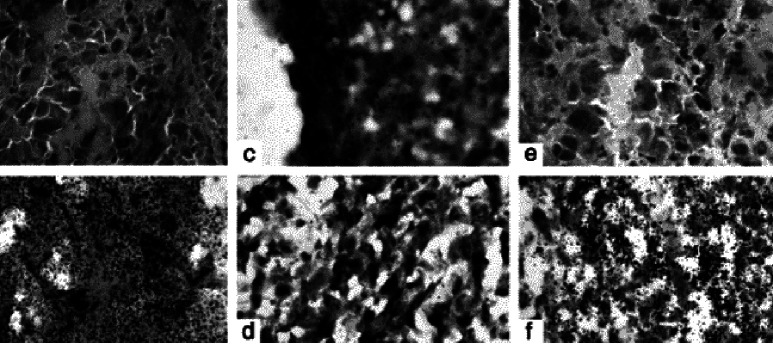
The penetration of labeled MAbs, F(ab’)2s was examined by microautoradiography of frozen xenograft sections (Fig. 4). Two sections/slide were scanned together with the surrounding area for background under a light microscope at ×40 magnification. Both BLCA-38 and J591 Fabs were sparsely located within the DU-145 and LN3 xenograft tissues. Increased silver grains were seen with both the BLCA-38 and J591 F(ab’)2s and MAbs. In each case, the grains were concentrated over tumour epithelial cells, and these were seen in nonnecrotic areas of the tumours.
Fig. 4a–f.
Autoradiography was done on 6-μm serial sections of xenografts of LNCaP-LN3 and DU-145, fresh-frozen in OCT, 7 days after i.p. injection of 125I-labeled MAbs (BLCA-38 or J591, respectively) or 2 days after injection of appropriate 125I-labeled F(ab’)2s or Fabs. Control slides were xenograft tissue from mice that had not been injected with antibodies. Thawed slides were fixed in 1% paraformaldehyde (Sigma, St. Louis, MO), dipped in Emulsion (Kodak NTB-3, Integrated Sciences) at 1 g emulsion/ml of glycerol solution in a darkroom lit with an Ilford 915 red light filter and then exposed for 7–14 days at 4°C before being developed in freshly prepared developer, dried and stained with H&E. Autoradiographs of fresh-frozen xenografts: a DU-145 xenograft tissue, negative control; b DU-145 xenograft 7 days after injection of 125I-labeled BLCA-38 MAbs; c DU-145 xenograft 2 days after injection of 125I-labeled BLCA-38 F(ab’)2; d DU-145 xenograft 2 days after injection of 125I-labeled BLCA-38 Fab; e LNCaP-LN3 xenograft, negative control; f LNCaP-LN3 xenograft, 7 days after injection of 125I-labeled J591 MAb
Discussion
Given the high incidence of prostate cancer and the fact that it is the second highest cause of cancer mortality in men, procedures that allow earlier detection and therapy of nonresponsive, hormone-refractory tumours are in demand for reducing mortality in patients with this disease. It is likely that MAbs will have potential in these areas, both for imaging, and for delivery of cancer killing agents, such as drugs, toxins or radionuclides [1, 5, 12, 13, 17]. In this study, we have tested a new MAb, BLCA-38, for its ability to target human prostate cancer cell lines grown as xenografts in nude mice, and have determined the optimal timing postadministration, for examining its localization in tumour tissues. The results have been compared with those obtained for J591 that has already shown potential for immunotherapy in clinical trials. While BLCA-38, when labeled with 153Sm has previously been shown to retard the growth of well-established s.c. bladder cancer xenografts [14], it has not previously been tested against prostate cancer in vivo. A recent study reports biodistributions and pharmacokinetics of antibodies to the external domain of FOLH1, including J591, against LNCaP xenografts [27].
Our biodistribution studies indicate that the BLCA-38 MAbs F(ab’)2s and Fabs (Figs. 2 and 3; Tables 1, 2 and 3) localize well within the DU-145 xenografts, indicating that it has excellent properties for targeting prostate cancer tissue in vivo. No unusual localization was seen in any nontumour tissue or organ when either 100 μg or 200 μg 125I-labeled MAbs was administered; the level of radioactivity in the normal tissues and organs was at or well below that seen in the blood. The data were similar to those obtained with J591 MAb, F(ab’)2s and Fabs against the LN3 xenografts. The data we obtained with J591 were comparable to those reported using 131I-J591 against LNCaP xenografts [27]. Taken together, these data demonstrate that BLCA-38, like J591, may be clinically useful for radioimmunoscintigraphy of prostate cancer. Given that the BLCA-38 and J591 target separate antigens, their conjoined use could prove beneficial. While the BLCA-38 antibodies may need to be humanized before clinical trial, this could be achieved, as we have prepared scFv antibodies of BLCA-38 (Dr Shaun O’Mara, Oncology Research Centre, Prince of Wales Hospital, PhD thesis).
In support of this, imaging using indium 111 (111In)–labeled CYT-356 (ProstaScint; Cytogen Corporation, Princeton, NJ), an antibody against the intracellular domain of FOLH1, has been successfully used after treatment of patients with prostate cancer to detect recurrence in soft tissues [20, 28]. The sensitivity and negative predictive value of ProstaScint imaging are better than that of computed tomography and magnetic resonance imaging for detection of soft tissue and nodal metastases from prostate cancer [8]. As recently reported at the AUA conference in 2003, therapy using J591 against the external domain of FOLH1 has already shown promise in phase I [30] and phase II [31] clinical trials.
In our biodistribution studies, the X/B ratio after 200-μg 125I-labeled BLCA-38 MAb was higher at days 5 and 6 but lower at days 7 and 8 than the values seen when 100-μg 125I-labeled BLCA-38 was administered (Table 1), and the %ID/g followed the same trend (DU-145 tumour: %ID/g on day 5 and 7 were 8.11±3.97 and 3.43±0.35, respectively, in mice that received 200 μg, compared with 5.06±0.44 and 3.57±0.43, respectively, in those that received 100-μg 125I-labeled BLCA-38). These data were of the same order as those for J591 in LNCaP-LN3 tumours (Table 1), and while the %ID/g in the xenograft was higher after 200-μg than after 100-μg 125I-labeled J591 on day 5, by day 6 this trend was reversed (LNCaP-LN3 tumour: %ID/g on day 6, 2.38±0.58 in mice that received 200 μg, compared with 3.26±0.40 in those that received 100-μg 125I-labeled-J591). These data suggest that saturation of both BLCA-38 and J591 antigenic sites in the tumours occurred at a dose less than 100 μg. This is consistent with studies of CYT-356 localization in LNCaP tumours in nude mice [17].
Two factors, the specificity for targeting (X/B) and the amount of activity to reach the target (%ID/g) are important in the therapeutic use of immunoconjugates. As antibody fragments have increased ability compared with MAbs to penetrate tumours [36], we explored their use in biodistribution and autoradiography. When F(ab’)2 and Fab fragments were used, improved localization to the xenografts was seen with a substantial increase in tissue to blood ratios (Fig. 3 and Table 2). However, the F(ab’)2s and Fabs accumulated in the kidneys and bladder, and the Fabs also in the stomach (Fig. 2). Moreover, the quantity of F(ab’)2s and Fabs in the xenograft tissue was dramatically decreased compared with the MAbs. This was confirmed by our microautoradiographical studies (Fig. 4) that showed more extensive tissue localization by 125I-labeled-MAbs than by F(ab’)2s, while that of 125I-labeled Fabs was almost negligible. This may have been because the Fab label was already leaching out of the tissue by day 2 when the tissues for microautoradiography were obtained. The MAbs were shown to be taken up in tumour cells, whilst the distribution of F(ab’)2s was more heterogeneous—consistent with studies by Kamigaki et al. [10].
Several strategies to increase the antibody uptake at a tumour site, such as the use of engineered dimers and trimers (or diabodies and triabodies) have been devised. For example, stable dimers of scFv of the MAb CC49, against the tumour-associated mucin TAG-72, show rapid clearance from the blood, higher tumour uptake and longer retention than scFv or Fab, reaching a 10% ID/g at 60 min after injection [18]; tetravalent scFv of CC49 showed a binding-affinity constant approximately twice that of the divalent scFv together with a further improved biological half-life [6]. The advantages of the use of diabodies, triabodies and tetrabodies for cancer targeting have been reviewed [29].
In conclusion, we have shown that BLCA-38, an antibody directed against a cell surface glycoprotein, shows comparable localization within DU-145 xenografts to that of J591, a clinically proven MAb, to its target LNCaP-LN3 xenografts. In neither case was there any unusual localization in any nontumour tissues or organs. Antibody fragments showed increased xenograft to blood ratios, but less overall uptake within the tumour than the MAbs. Strategies such as the use of multivalent scFvs derived from these antibodies may improve antibody avidity. As J591 MAbs have proven clinical use for radioimmunoscintigraphy and radioimmunotherapy, the data suggest that the BLCA-38 MAb, against a different antigen, may also have clinical utility, especially if used in combination with other prostate-targeting antibodies. Thus, when conjugated to alpha-labeled bismuth, and used in a cocktail to provide multiple alpha-targeted therapy (MTAT), BLCA-38 resulted in cytotoxicity to DU-145 cells in vitro (Li et al., submitted).
Acknowledgements
The authors thank Dr Neil Bander, Cornell University, New York, USA, and Millennium Pharmaceuticals for the J591 hybridoma. The work was financially supported by an AusIndustry grant and by Commonwealth Serum Laboratories (CSL). We gratefully acknowledge helpful discussions with Drs Peter Molloy and Jerome Werkmeister, CSIRO, Molecular Science, and Dr Simon Green, CSL.
References
- 1.Babaian J Urol. 1987;137:439. doi: 10.1016/s0022-5347(17)44060-2. [DOI] [PubMed] [Google Scholar]
- 2.Bazinet Cancer Res. 1988;48:6938. [PubMed] [Google Scholar]
- 3.Chang Clin Cancer Res. 1999;5:2674. [PubMed] [Google Scholar]
- 4.DeNardo Anticancer Res. 1997;17:1745. [PubMed] [Google Scholar]
- 5.DeNardo Curr Opin Immunol. 1999;11:563. doi: 10.1016/s0952-7915(99)00017-5. [DOI] [PubMed] [Google Scholar]
- 6.Goel Cancer Res. 2000;60:6964. [PubMed] [Google Scholar]
- 7.Heston WD (1997) Characterization and glutaymyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urol 49[Suppl 3A]:104 [DOI] [PubMed]
- 8.Holmes Expert Opin Investig Drugs. 2001;10:511. doi: 10.1517/13543784.10.3.511. [DOI] [PubMed] [Google Scholar]
- 9.Horoszewicz Anticancer Res. 1987;7:927. [PubMed] [Google Scholar]
- 10.Kamigaki Int J Cancer. 1996;66:261. doi: 10.1002/(SICI)1097-0215(19960410)66:2<261::AID-IJC21>3.3.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Kantoff PW, Wishnow KI, Loughlin KR (1997) In: Kantoff PW, Wishnow KI, Loughlin KR (eds) Prostate cancer: a multidisciplinary guide. Blackwell Science, Malden, MA
- 12.Knox SJ (1995) Overview of studies on experimental radioimmunotherapy. Cancer Res 55[Suppl]:5832s [PubMed]
- 13.Leroy Cancer. 1989;64:1. doi: 10.1002/1097-0142(19890701)64:1<1::aid-cncr2820640102>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Lightfoot Antibod Immunoconj Radiopharm. 1991;4:319. [Google Scholar]
- 15.Liu Cancer Res. 1997;57:3629. [PubMed] [Google Scholar]
- 16.Liu Cancer Res. 1998;58:4055. [PubMed] [Google Scholar]
- 17.Lopes Cancer Res. 1990;50:6423. [PubMed] [Google Scholar]
- 18.Pavlinkova J Nucl Med. 1999;40:1536. [Google Scholar]
- 19.Pettaway Clin Cancer Res. 1996;2:1627. [PubMed] [Google Scholar]
- 20.Rosenthal Tech Urol. 2001;7:27. [PubMed] [Google Scholar]
- 21.Russell Cancer Res. 1986;46:2035. [PubMed] [Google Scholar]
- 22.Russell Int J Cancer. 1988;41:74. doi: 10.1002/ijc.2910410115. [DOI] [PubMed] [Google Scholar]
- 23.Russell Urol Res. 1988;16:79. doi: 10.1007/BF00261960. [DOI] [PubMed] [Google Scholar]
- 24.Russell Int J Cancer. 1988;41:74. doi: 10.1002/ijc.2910410115. [DOI] [PubMed] [Google Scholar]
- 25.Russell PJ, Hewish D, Carter T, Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira D, Molloy PL, Werkmeister JA, Kortt AA (2003) Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivo studies. Cancer Immunol Immunopathol (in press) [DOI] [PMC free article] [PubMed]
- 26.Silver Clin Cancer Res. 1997;3:81. [Google Scholar]
- 27.Smith-Jones J Nucl Med. 2003;44:610. [PubMed] [Google Scholar]
- 28.Sodee Prostate. 1998;37:140. doi: 10.1002/(SICI)1097-0045(19981101)37:3<140::AID-PROS3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Todorovska J Immunol Methods. 2001;248:47. doi: 10.1016/S0022-1759(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 30.Trabulsi EJ, Yao D, Joyce MA, Milowksy M, Kostakoglu L, Vallabhajosula D Nanus D, Goldsmith S, Bander NH (2003) Phase I radioimmunotherapy (RIT) trials of monoclonal antibody (MAB) J591 to the extracellular domain of prostate specific membrane antigen (PSMAEXT) radiolabeled with 90Yttrium (90Y) or 177Lutetium (177Lu) J Urol 169[Supp 4l] [DOI] [PubMed]
- 31.Trabulsi J Urol. 2003;170:1717. [Google Scholar]
- 32.Walker Eur J Nucl Med. 1986;12:461. [Google Scholar]
- 33.Walker J Urol. 1989;142:1578. doi: 10.1016/s0022-5347(17)39172-3. [DOI] [PubMed] [Google Scholar]
- 34.Wilbur Bioconjug Chem. 1998;9:322. doi: 10.1021/bc970182v. [DOI] [PubMed] [Google Scholar]
- 35.Wright Urology. 1996;48:326. doi: 10.1016/S0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 36.Yazaki Bioconjug Chem. 2001;12:1220. doi: 10.1021/bc000092h. [DOI] [Google Scholar]
- 37.Yoneda Eur J Cancer. 1998;34:240. doi: 10.1016/S0959-8049(97)10132-0. [DOI] [PubMed] [Google Scholar]