Abstract
Purpose
The carcinoembryonic antigen (CEA) is extensively expressed on the vast majority of colorectal, gastric, and pancreatic carcinomas, and, therefore, is a good target for tumor immunotherapy. CD4+ T-helper (Th) cells play a critical role in initiation, regulation, and maintenance of immune responses. In this study, we sought to identify Th epitopes derived from CEA which can induce CEA-specific Th responses. The combined application with cytotoxic T lymphocyte (CTL) epitopes would be more potent than tumor vaccines that primarily activate CTL alone.
Methods
We utilized a combined approach of using a computer-based algorithm analysis TEPITOPE and in vitro biological analysis to identify Th epitopes in CEA.
Results
Initial screening of healthy donors showed that all five predicted peptides derived from CEA could induce peptide-specific T-cell proliferation in vitro. We characterized these CEA epitopes by establishing and analyzing peptide-specific T-cell clones. It was shown that CD4+ T-cells specific for the CEA116 epitope can recognize and respond to naturally processed CEA protein and CEA116 epitope can be promiscuously presented by commonly found major histocompatibility complex (MHC) alleles. Furthermore, it was demonstrated that immunization of human leukocyte antigen (HLA)-DR4 transgenic mice with CEA116 peptide elicited antigen-specific Th responses which can recognize the antigenic peptides derived from CEA protein and CEA-positive tumors.
Conclusion
The MHC class II-restricted epitope CEA116 could be used in the design of peptide-based tumor vaccine against several common cancers expressing CEA.
Keywords: CEA, CD4+, Epitope, Tumor immunotherapy
Introduction
The carcinoembryonic antigen (CEA) is one of the most well-characterized tumor antigens regarding its tissue distribution, biochemistry, and molecular structure. The CEA is a Mr180-kDa membrane intercellular adhesion glycoprotein that is extensively expressed in the vast majority of colorectal, gastric, and pancreatic carcinomas [15, 32, 50]. In addition, CEA is found in 50% of breast cancers and 70% of non-small cell lung carcinomas [15, 32, 55]. The CEA is also present in some normal tissues including colon epithelium and in some fetal tissues, but at much lower levels. Circulating CEA can be detected in the great majority of patients with metastatic CEA-positive tumors. These CEA-associated malignancies represent the most common cancers and leading causes of cancer death in the United States and worldwide. The relative tumor tissue specificity of this antigen makes CEA a potential target for tumor immunotherapy [17].
Tumor vaccination has used many immunization forms, including tumor cells-, peptides-, proteins-, dendritic cells (DC)-, and DNA-based vaccines. Peptide vaccines remain an attractive immunotherapy approach, since peptides are easily manufactured, chemically stable, and free of contaminating substances such pathogens and oncogenic components. Recently, immunization with peptide-pulsed DCs has been tested in the clinics and appears to be an effective way of inducing CTLs and antitumor immunity both in human trials [30, 34, 46] and animal tumor models [39, 43].
Numerous peptides corresponding to CTL epitopes from tumor-associated antigens have already been identified and used for immunotherapy in clinical studies in cancer patients [19, 29, 34, 46, 60, 61]. In order to use CEA as a target for tumor immunotherapy, several CEA CTL epitopes restricted by HLA-A2, HLA-A3, HLA-A24, and B7 have been successfully identified [24, 25, 35, 41]. The ability of CTLs to recognize epitopes derived from CEA has been demonstrated both in cancer patients [58, 59] and in normal individuals whose cells have been immunized in vitro with MHC-binding peptides from CEA [24, 25, 27, 35].
Although immunization with CTL peptide vaccines led to some positive clinical responses, peptide vaccination often resulted in no responses or only low-level responses [18, 38, 45, 46]. CD4+ T-helper (Th) cells that recognize antigenic peptides in the context of MHC class-II molecules play critical roles in initiating, regulating, and maintaining antitumor immune responses [38, 44]. They exert helper activity for the induction and maintenance of CD8+ CTLs [3, 5, 37, 38] in addition to their ability to modulate other immune cells and to directly kill tumor cells [1, 21, 23, 33, 36, 38]. However, the main focus of current tumor vaccination effort has been directed to induce CTL responses [38, 45]; hence, a major limitation of such CTL peptide-based approaches might be that CTLs elicited by the CTL peptide reach the tumor but fail to be stimulated properly at the tumor site by tumor-specific CD4+ T-cells.
Here we utilized a combined approach of using a computer-based algorithm program TEPITOPE [10, 16, 28] to select class-II epitope candidates and in vitro T-cell biological analysis to identify MHC class-II restricted Th epitope(s) in CEA. We found several Th epitopes in CEA, including the CEA116 epitope that is a naturally processed Th epitope, which could be presented by several commonly found HLA alleles, DR4, DR1, DR13, and DR14.
Materials and methods
Blood donors, cell lines, monoclonal antibodies, and tissue culture reagents
Heparinized peripheral blood was obtained from adult healthy donors with their consent (donor BCM02: DRB1*01, 04; BCM04: DRB1*13, 14; BCM19: DRB1*04, 15; BCM26: DRB1*04, 08; BCM28: DRB1*11, 13; BCM36: DRB1*04, 04; BCM39: DRB1*04, 14; BCM40: DRB1*04, 13; BCM44: DRB1*01, 15). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Lymphoprep, Nycomed, Oslo, Norway) gradient centrifugation. The Institutional Review Board on Human Subjects (Baylor College of Medicine) approved this study. The HLA typing of peripheral blood donors was performed by the polymerase chain reaction using sequence-specific primers (PCR-SSP) and DNA-based procedures in the HLA, Flow and Diagnostic Immunology Laboratory of the Methodist Hospital (Houston, Texas). Colon cancer cell line (HT-29) and melanoma cell line (NA-6-MEL) were from American Type Culture Collection.
The following hybridomas were used to produce monoclonal antibodies: HB55 (L243, anti-human HLA-DR, ATCC); and HB95 (W6/32, anti-human HLA-ABC, ATCC). Anti-human CD4, anti-human CD8, anti-mouse CD4, anti-mouse CD8a, anti-human CD4 (PE labeled), anti-human HLA-DR (FITC labeled), and anti-mouse CD4 (FITC labeled) were all purchased from BD PharMingen (San Diego, Calif.). Media used for cell culture were AIM-V serum-free medium (Life Technologies, Grand Island, N.Y.), RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Life Technologies, Grand Island, N.Y.) and L-glutamine/penicillin/streptomycin, and CellGenix DC serum-free medium (CellGenix, Germany). Recombinant human IL-2 was purchased from Boehringer Roche (Indianapolis, Ind.).
Epitope prediction and peptide synthesis
TEPITOPE is a Windows (Microsoft, Redman, Wash.) application that enables the identification of HLA class-II ligand-binding epitopes [10, 16, 28]. The prediction threshold was set at 1% and peptides were selected on the basis of their ability to bind to at least three of the following HLA-DR molecules: DRB1*0101; DRB1*0301; DRB1*0401; DRB1*0701; DRB1*0801; DRB1*1101; DRB1*1501; and DRB5*0101. Five peptides, CEA116 (DTGFYTLHVIKSDLVNEEATGQFRV, aa116-aa140), CEA176 (YLWWVNNQSLPVSPR, aa176-aa190), CEA588 (DVLYGPDTPIISPPD, aa588-aa602), CEA624 (QYSWRINGIPQQHTQ, aa624-aa638), and CEA665 (NNSIVKSITVSASGTS, aa665-aa680), from human carcinoembryonic antigen (Table 1) were synthesized and purified by Genemed Synthesis (San Francisco, Calif.). The purity of the peptides was greater than 95% by high-performance liquid chromatography (HPLC). Synthetic peptides were reconstituted in distilled water or dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml.
Table 1.
Synthetic peptide sequences derived from carcinoembryonicantigen (CEA)
| Peptide | AA position | AA sequence |
|---|---|---|
| CEA116 | 116–140 | DTGFYTLHVIKSDLVNEEATGQFRV |
| CEA176 | 176–190 | YLWWVNNQSLPVSPR |
| CEA588 | 588–602 | DVLYGPDTPIISPPD |
| CEA624 | 624–638 | QYSWRINGIPQQHTQ |
| CEA665 | 665–680 | NNSIVKSITVSASGTS |
Peptide T-cell proliferation assay and T-cell clone establishment
Donor’s PBMCs were plated in U-bottom 96-well plates (Costar) at 200,000 cells/well in AIM-V media. Peptides were added into each well at the concentration of 20 μg/ml. A total of 48 wells were prepared for analysis of each peptide. After 1 week of incubation, the culture medium was removed and cells were resuspended in AIM-V media, and tested for specific proliferative responses to corresponding peptides (20 μg/ml) in the presence of 105 autologous irradiated (6000 rad) PBMC as a source of antigen presenting cells (APC). In cell proliferation assays, cells were incubated at 37°C in a 5% CO2 incubator for 72 h, and cultures were pulsed with 1 μCi [3H]-thymidine per well during the last 16 h. The incorporation of radioactivity into DNA, which correlates with cell proliferation, was measured in a beta scintillation counter (TopCount NXT, Packard) after automated cell harvesting (Packard). The results were presented either as stimulation indices (SI; the mean cpm of peptide-pulsed PBMC/the mean cpm of PBMC not exposed to peptides) or cpm [31, 40]. A T-cell line/well was considered to be positively reactive to peptide if the cpm were >1000 and SI>3 [48, 64]. The frequency of peptide-specific T-cells was determined by dividing the number of positive wells by the total number of PBMC seeded in the initial culture [64]. Overall positive events were defined if there was significant difference at a 95% confidence level between the numbers of positive wells of the peptide-containing wells compared with that of the non-peptide containing wells. Because these low-frequency events were in accordance with Poisson distribution, ≥3 of 48 peptide-containing wells were defined as a positive event (p<0.05).
The CEA-specific T-cell lines were cloned by limiting dilution at 2 cell/well in the presence of 105 irradiated allogeneic PBMC as accessory cells and 5 μg/ml of phytohemagglutinin protein (PHA-P, Sigma, St. Louis, Mo.). Cultures were refreshed with fresh RPMI 1640 medium containing 10 IU/ml of rhIL-2 every 3–4 days. After approximately 12–14 days, growth-positive wells became visible and were tested for specific responses to CEA peptides in a proliferation assay as described above.
PBMC-derived DC culture
Human dendritic cells (DC) were prepared as recently described by Schroers et al. [47]. Briefly, PBMCs were isolated by Ficoll gradient centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in serum-free DC medium. After adherence to plastic for 2 h, the adherent cell fraction was cultured in serum-free DC medium with 1000 IU/ml recombinant human GM-CSF (rhGM-CSF; R&D Systems) and 1000 IU/ml rhIL-4 (R&D Systems). On day 5, DC were matured by stimulating with a cytokine cocktail consisting of recombinant human tumor necrosis factor alpha (rhTNF-α; 10 ng/ml, R&D Systems), rhIL-1β (1,000 ng/ml, R&D Systems), rhIL-6 (10 ng/ml, R&D Systems), and prostaglandin E2 (PGE2; 1 μg/ml, Sigma, St. Louis, Mo.) as previously described [22].
Production and purification of recombinant CEA protein
Recombinant CEA-Fc baculovirus, which expresses CEA-Fc from the polyhedrin promoter, was constructed by using the pFastBac system (Life Technologies, Grand Island, N.Y.) with the pFB1 donor plasmid. The human CEA DNA fragment was PCR amplified from plasmid pCMV CEA (from Prud Homme-McGill University Montreal, Quebec, Canada) [4] by using the 5′ primer 5′-ATTACGCGTACTAGTAAGCTTACCATGGAGTCTCCCTCGGCCCCT-3′ and the 3′ primer 5′-TATGCGGCCGCTATCAGAGCAACCCCAAC-3′ to introduce SpeI and NotI restriction sites to the 5′ and 3′ ends, respectively. The PCR of human IgG Fc fragment was carried out with two primers, one (5′-ATAAGCGGCCGCTAAAAC TCACACATGCCCA-3′) containing the Not I site and the other (5′-AAAGGTA CCTCTAGATTAATTAACTATCATTTACCCGGAGACAGG-3′) containing the XbaI site. The PCR products were then gel purified, digested, and ligated into SpeI/XbaI cut pFB1 donor plasmid. The resultant vector (pFB1-CEA-Fc) was identified by restriction enzyme analysis and confirmed by DNA sequencing. Site-specific transposition of the CEA-Fc expression cassette from the donor plasmid into the baculovirus genome was performed by transforming DH10Bac E. coli with the pFB1-CEA-Fc donor plasmid. Recombinant baculovirus were identified by X-gal selection, as transposition into the bacmid disrupts expression of the lacZα peptide. Recombinant bacmid DNA was isolated by mini-prep and used to transfect Sf9 insect cells according to the manufacturers’ instructions.
The viral stock obtained from the initial transfection was amplified by repeated infecting a 50 ml suspension culture of Sf9 cells at 2×106 cells/ml. For large-scale expression of CEA, suspension culture of Sf9 cells were infected with the amplified stock and incubated at 27°C for 48 h post-infection. Cells were pelleted by centrifugation at 500×g for 5 min at 4°C, resuspended with ten packed cell volumes of TNT-lysis buffer (25 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100), and allowed to incubate for 1 h on ice. The cell lysate was subjected to centrifugation at 16,000×g for 15 min at 4°C to remove insoluble material.
Recombinant CEA-Fc protein was purified by affinity binding to Protein-A agarose beads (Sigma, St. Louis, Mo.). The cleared cell lysate was mixed with 1 ml of protein-A agarose. After rotating overnight at 4°C, the cell lysate-protein-A agarose slurry was washed by centrifugation at 1000×g for 10 min by suspension in 30 bead volumes of 100 mM Tris-HCl (pH8.0), followed by washes with 10 mM Tris-HCl (pH 8.0). Bound material, including CEA-Fc, was eluted by 100 mM Glycine (pH 2.7) and then was neutralized by 1 M Tris-HCl (pH8.0). Protein containing fractions were determined by A280 and then pooled together for dialysis with PBS. The purity was assessed by 7.5% SDS-PAGE gel stained by coomassie blue. Concentration of protein was determined using the Bradford assay (Biorad, Hercules, Calif.).
Western blot analysis
Fifteen micrograms of the major protein containing eluted fraction was diluted into an equal volume of SDS-sample buffer, boiled 3 min, loaded onto 7.5% SDS-PAGE, and electrophoresed for 1 h at 150 V. Separated proteins were transferred onto nitrocellulose membrane in transfer buffer for 1 h at 100 V. After transfer, membrane was blocked by 5% nonfat dry milk in PBS for 1 h at RT. The rabbit-anti-human CEA polyclonal antibody (Accurate Chemical and Scientific Corp, N.Y.) was used as the primary antibody, and the secondary was peroxidase labeled goat-anti-rabbit IgG (Sigma, St. Louis, Mo.). The membranes were developed using ECL Western blotting detection reagents.
Analysis of antigen-specific proliferative responses of T-cell clones
T-cells (3×104 cells/well) were cocultured with irradiated (4000 rad) DC (1.5×103 cells/well) in complete RPMI 1640 in the presence of various concentrations of antigen (peptides and recombinant protein) in U-bottom 96-well plates. In some cases, recombinant protein (10 μg/ml) was pulsed on DC at day 4 during DC culture before the addition of DC maturation cocktail. In order to identify the MHC restriction molecules involved in antigen presentation, inhibition of antigen-induced T-cell proliferation was analyzed by the addition of various antibodies against MHC class-I and MHC class-II molecules at a final concentration of 20 μg/ml. Antigen-specific T-cell responses were measured by [3H]-thymidine incorporation during the last 16 h of 72-h culture.
Peptide immunization in HLA-DR4 transgenic mice
Human HLA DR4 transgenic mice (HLA-DRB1*0401), which are murine class-II deficient and transduced with human CD4 molecule, were generated by G. Sonderstrup of the Department of Microbiology and Immunology of Stanford University [8, 51, 52]. The transgenic mice have been successfully used to identify human class-II-restricted epitopes and to study immune responses [8, 13, 14, 51, 52]. Founders of the transgenic mouse line were kindly provided by Dr. Sonderstrup, maintained and bred in a barrier facility at Baylor College of Medicine. The HLA DR4 expression on the transgenic mice was analyzed by flow cytometry. Male DR4 transgenic mice 6–10 weeks old were used for the experiment. The transgenic mice were immunized twice at 1-week intervals with 100 μg of CEA116 peptide emulsified in complete Freunds adjuvant (CFA; final volume, 100 μl) and administered subcutaneously (s.c.) into the rear back. Control-group mice were injected with phosphate-buffered saline (PBS) emulsified in CFA.
Evaluation of T-cell responses by IFN-γ ELISPOT assay
A high resolution of ELISPOT assay for IFN-γ was used to analyze peptide-specific T-cell responses by determining the frequency of Th precursors specific for the peptide. Mice were killed 10 days after the last immunization and splenocytes were obtained for assessing IFN-γ production. Briefly, 96-well MultiScreen-IP plates (Millipore Corporation, Bedford, Mass.) were coated with 100 μl/well capture mAb against mouse IFN-γ (AN-18, Mabtech Inc, Cincinnati, Ohio) at a concentration of 10 μg/ml and incubated overnight at 4°C. The plates were washed four times with PBS, then blocked with RPMI 1640 plus 10% FBS for 2 h at 37°C. After washing, freshly isolated splenocytes were plated at 2×105 cells/well in RPMI 1640 with 10% FBS, in the presence or absence of peptide CEA116 (20 μg/ml), recombinant CEA proteins (20 μg/ml), or CEA-positive HT-29 (ATCC) tumor lysate (50 μl/well). Tumor cell lysates were prepared by three freeze–thaw cycles of 5×107 tumor cells resuspended in 5 ml of RPMI 1640 with 10% FBS. Then the cells were centrifuged at 15,000 g for 30 min at 4°C. Supernatant was recovered, aliquoted, and stored at −80°C for later use, as described previously [48]. After 20 h of cell culture in the incubator, the cells were removed by washing three times with PBS and four times with PBS/Tween20 (0.05%). Biotinylated anti-mouse IFN-γ antibody (R4-6A2, Mabtech, Cincinnati, Ohio), diluted to 1 μg/ml in PBS/Tween20 containing 0.5% bovine serum albumin, was added and incubated for 2 h at 37°C. The plates were then washed six times with PBS/Tween20 (0.05%) and subsequently avidin–peroxidase complex (Vector Laboratories, Burlingame, Calif.) was added and incubated for 1 h at room temperature, and removed by washing three times with PBS and PBS/Tween20 (0.05%). The color of the plates was developed by adding HRP substrate 3-amino-9-ethylcarbozole (Sigma, St. Louis, Mo.). The plates were then washed with tap water and air dried in dark. The plates were evaluated using an automated ELISPOT reader (Zellnet Consulting, New York, N.Y.).
Statistical analysis
Differences in the T-cell proliferation and ELISPOT assay among different groups were analyzed using the two-sided Student’s t test. The overall significance level was set at 5%.
Results
Primary screening of human T-cell responses to peptides derived from CEA
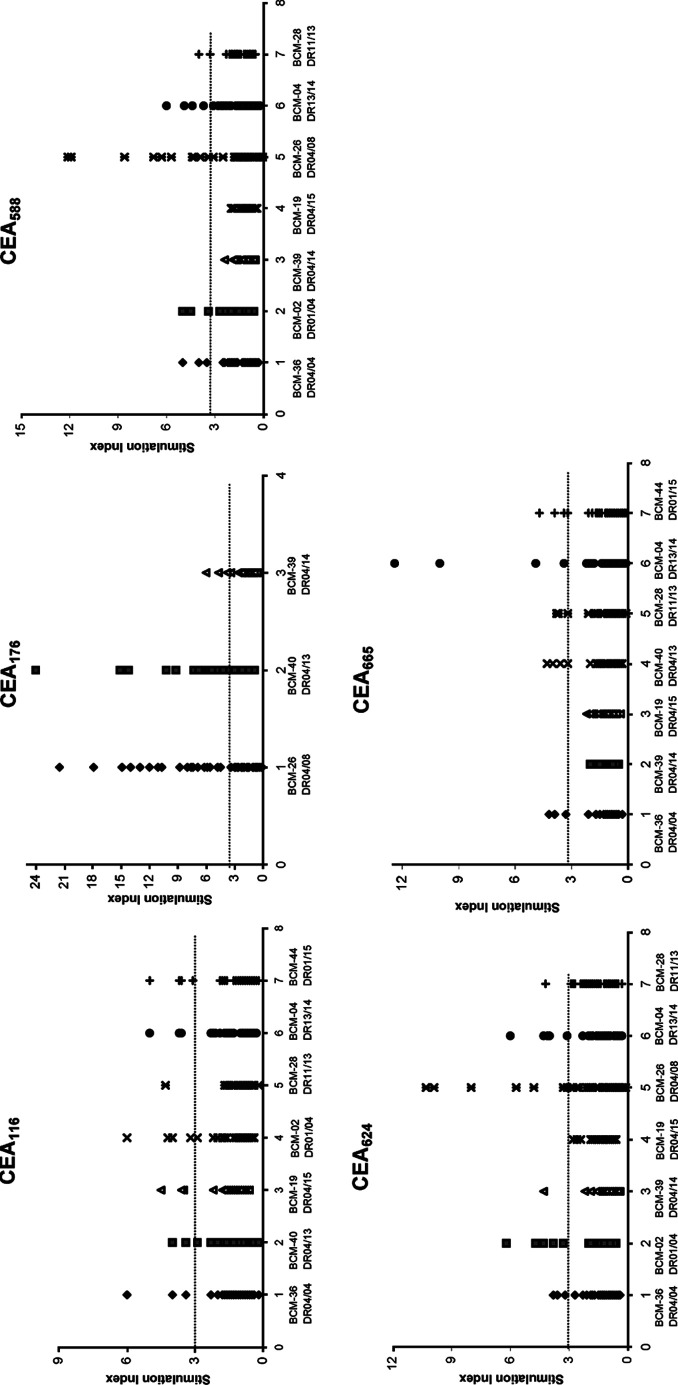
TEPITOPE, a computer-based algorithm analysis program [10, 16, 28], has been successfully used to predict the binding motifs for HLA-DR. In order to generate peptide-based vaccines that can be used in the majority of patients, it is important to know whether the epitopes can be promiscuously presented by commonly found MHC class-II alleles. By using TEPITOPE at the most stringent prediction threshold (1%), peptides were selected on the basis of their ability to bind to at least three of the following eight HLA-DR molecules: DRB1*0101; DRB1*0301; DRB1*0401; DRB1*0701; DRB1*0801; DRB1*1101; DRB1*1501; and DRB5*0101. Based on the prediction, five peptides were synthesized and purified (Table 1). To initially screen human T-cell responses to these peptides, PBMCs from healthy donors were seeded on 96-well plates and stimulated with each peptide. After 1 week of stimulation, the cell cultures were tested for their capacity to respond to the peptides presented by autologous PBMCs. Those cultures that exhibited SI>3 were considered positive [31, 40, 48, 64]. The stimulation indices (SI) of each well from a donor’s PBMCs to each peptide are shown in Fig. 1. According to the statistical analysis described in “Materials and methods,” all five peptides elicited proliferative responses in some or all of the donors, indicating that these five peptides are Th epitope candidates.
Fig. 1.
Initial screening of T-cell responses to predicted peptides from carcinoembryonic antigen (CEA). Peripheral blood mononuclear cells (PBMCs) from healthy donors with HLA-DR4 and other HLA-DR genotypes were seeded on 96-well plates and stimulated with each peptide (20 μg/ml) for 1 week. [3H]-thymidine incorporation of the primed T-cells (2×105 cells/well) was measured after restimulation with autologous PBMCs (1×105 cells/well) as antigen-presenting cells with or without the corresponding peptides (20 μg/ml). Responses to each peptide were tested in total 48-wells. Wells were scored positive if the counts per minute of T-cells stimulated with peptides was >1000 and stimulation indices (SI) >3 [31, 40, 48, 64]. The results are reported as SI of each tested well of different donors
Promiscuous binding of peptides and precursor frequencies
To assess if these peptides can be promiscuously presented by commonly found MHC class-II alleles, PBMCs from donors with different HLA-DR genotypes were stimulated with these peptides, respectively. As shown in Fig. 1, most of the donors with HLA-DR4 (DR04/04, DR01/04, DR04/08, DR04/13 and DR04/15) responded to all five peptides. T-cells from donors with genotypes of DR13/14 responded to the peptide CEA116, CEA588, CEA624, and CEA665. T-cells from donors with DR01/15 also responded to the peptides CEA116 and CEA665. This result indicates that CEA116, CEA588, CEA624, and CEA665 peptides can be presented by HLA-DR4 and other MHC class-II alleles, representing promiscuous Th epitope candidates.
We also primarily assessed the frequency of CEA-specific CD4+ T-cell precursors in human, since CD4+ T-cells for the self-CEA antigen might be largely clonally deleted during T-cell negative selection in thymus. The precursor frequency was calculated as the numbers of positive wells/total numbers of T-cells in all wells tested, since it was demonstrated that an antigen-specific T-cell line derived from a 96-plate well (2×105 cells/well) is most likely to originate from a single precursor T-cell [48, 64]. The primary estimated precursor frequencies of T-cells specific for each peptide in the different DR donors are shown in Table 2, in a range of precursor frequencies for other self antigens [64]. These results indicate precursors exist in normal human T-cell repertoires and that the T-cell precursors are readily activated to respond to peptides when properly stimulated.
Table 2.
T-cell precursor frequencies of donors with different human leukocyte antigen (HLA)-DR genotypes
| Peptide | Donor | HLA-DR | Positive wells/total wells | Estimated frequency |
|---|---|---|---|---|
| CEA116 | BCM-02 | DR01/04 | 4/48 | 0.08–0.33×10−6 |
| BCM-04 | DR13/14 | 3/48 | ||
| BCM-19 | DR04/15 | 3/48 | ||
| BCM-28 | DR11/13 | 1/48 | ||
| BCM-36 | DR04/04 | 3/48 | ||
| BCM-40 | DR04/13 | 2/48 | ||
| BCM-44 | DR01/15 | 4/48 | ||
| CEA176 | BCM-26 | DR04/08 | 26/48 | 0.33–2.41×10−6 |
| BCM-39 | DR04/14 | 4/48 | ||
| BCM-40 | DR04/13 | 29/48 | ||
| CEA588 | BCM-02 | DR01/04 | 3/48 | 0–1.17×10−6 |
| BCM-04 | DR13/14 | 5/48 | ||
| BCM-19 | DR04/15 | 0/48 | ||
| BCM-26 | DR04/08 | 14/48 | ||
| BCM-28 | DR11/13 | 2/48 | ||
| BCM-36 | DR04/04 | 3/48 | ||
| BCM-39 | DR04/14 | 0/48 | ||
| CEA624 | BCM-02 | DR01/04 | 5/48 | 0–1.08×10−6 |
| BCM-04 | DR13/14 | 4/48 | ||
| BCM-19 | DR04/15 | 0/48 | ||
| BCM-26 | DR04/08 | 13/48 | ||
| BCM-28 | DR11/13 | 1/48 | ||
| BCM-36 | DR04/04 | 3/48 | ||
| BCM-39 | DR04/14 | 1/48 | ||
| CEA665 | BCM-04 | DR13/14 | 4/48 | 0–0.33×10−6 |
| BCM-19 | DR04/15 | 0/48 | ||
| BCM-28 | DR11/13 | 3/48 | ||
| BCM-36 | DR04/04 | 3/48 | ||
| BCM-39 | DR04/14 | 0/48 | ||
| BCM-40 | DR04/13 | 4/48 | ||
| BCM-44 | DR01/15 | 4/48 |
Establishment and analysis of peptide-specific CD4+ T-cell clones
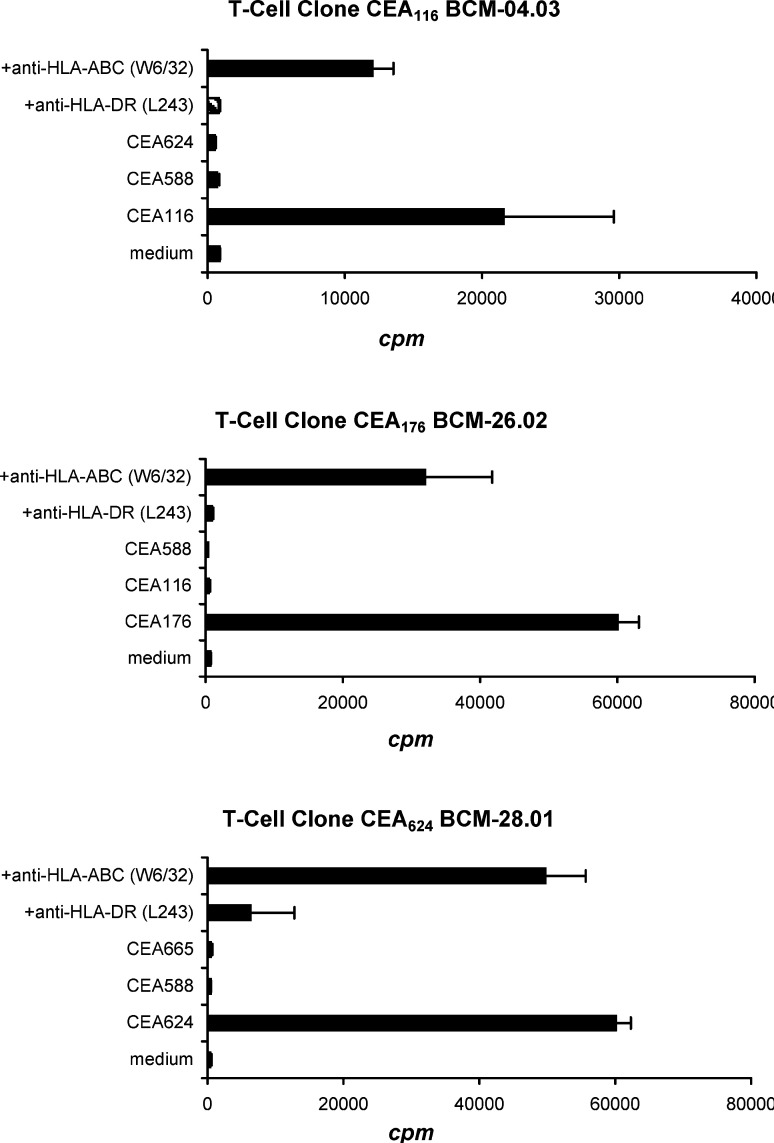
To characterize these T-cell reactive peptides, we tried to generate peptide-specific T-cell clones for each of these epitopes. Individual T-cell clones specific for three (CEA116, CEA176, and CEA624) out of the five peptides tested were established from peptide-reactive T-cell lines. T-cell clone specific for CEA588 and CEA665 failed to expand to sufficient numbers for further analysis despite repeated attempts with different donors’ blood. The specificity of CEA116, CEA176, and CEA624 T-cell clones was further tested. As shown in Fig. 2, these T-cells responded strongly to the stimulation of their corresponding peptides but did not respond to stimulation with irrelevant, non-corresponding CEA peptides. Furthermore, the responses of these T-cell clones to the corresponding peptides were inhibited by an anti-HLA-DR antibody (P<0.01), but not inhibited by an anti-HLA-ABC antibody, indicating that the peptide-reactive T-cell responses are peptide-specific and HLA-DR restricted.
Fig. 2.
Establishment and specificity of T-cell clones. Individual T-cell clones were established from peptide-reactive T-cell lines by limiting dilution. T-cell clones (3×104 cells/well) from CEA116-, CEA176-, and CEA624-positive T-cell lines were restimulated in triplicate with autologous PBMC-derived DCs (1.5×103 cells/well) pulsed with the same concentration (20 μg/ml) of corresponding peptides or irrelevant peptide control in the presence of anti-HLA-DR or anti-HLA-ABC (20 μg/ml). T-cell proliferation was measured by [3H]-thymidine incorporation assays. The proliferation of each T-cell clone cocultured with corresponding peptide was significantly higher than the proliferation with medium control and with the addition of HLA-DR antibody (p<0.01). Mean and standard deviation (SD) are shown for triplicate wells. The data shown are representative of three repeated experiments
Avidity of CD4+ T-cell clones
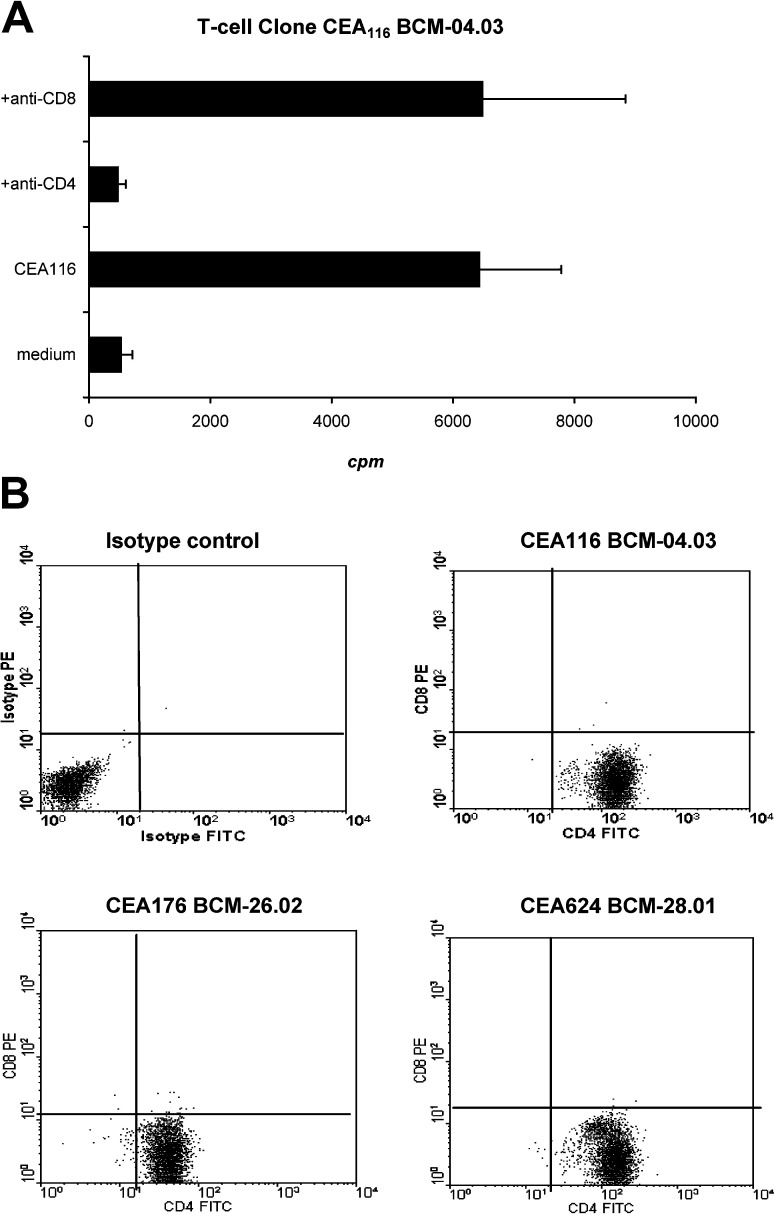
Since the T-cell clones were derived from peptide-stimulated PBMCs, not from isolated CD4+ T-cells, we used an antibody blocking assay to determine if the T-cell responses are mediated by CD4+ T-cells. Flow cytometric analysis was used to test the phenotype of the T-cell clones. As shown in Fig. 3A, the T-cell clone response to the corresponding peptide CEA116 was inhibited by an anti-CD4 antibody (P<0.05) but not inhibited by an anti-CD8 antibody. The phenotype of the T-cell clones was exclusively CD4-positive and CD8-negative (Fig. 3B). The results indicate that the culture and stimulation conditions described in this study selectively stimulated and expanded CD4+ T-cells.
Fig. 3 A.
Anti-human CD4 or CD8 antibody blocking of T-cell responses. The CEA116 T-cell clones (3×104 cells/well) were stimulated in triplicate with irradiated autologous PBMC-derived DCs (1.5×103 cells/well) pulsed with the concentration (20 μg/ml) of the CEA116 peptide in the presence of anti-human-CD4 or anti-human-CD8 antibody (20 μg/ml). T-cell proliferation was measured by [3H]-thymidine incorporation assays. The proliferation of T-cells was significantly decreased by the anti-CD4 antibody (p<0.05) but not by anti-CD8 antibody which indicates that these T-cell clones are CD4+ T-cells. Mean and SD are shown for triplicate wells. The data shown are representative of three repeated experiments. B Fluorescence-activated cell sorter analysis of T-cell clones. The CEA-reactive T-cell clones were double stained with anti-human CD4-FITC and CD8-PE antibodies or isotype controls (mouse IgG-FITC and IgG-PE). Human PBMCs were used as positive control for CD8 staining. The cells were then examined by flow cytometric analyses. More than 95% of the T-cell population was CD4-positive and CD8-negative
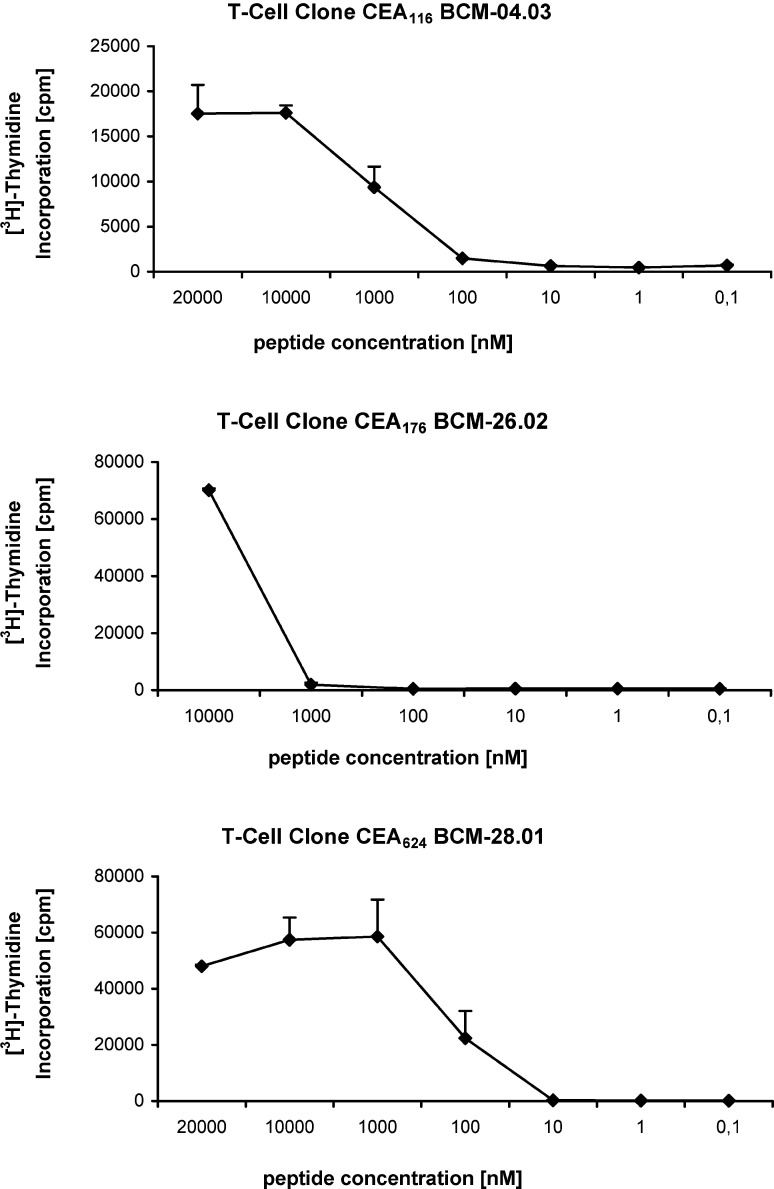
The avidity of peptide binding with MHC/T-cell receptors (TCR) is the important parameter to identify potential epitopes from tumor-associated antigens [24, 25, 35]. Accordingly, we evaluated the avidity of these peptide-specific T-cell clones for their ligands. Peptide titration curves of these T-cell clones were generated with stimulation with autologous peptide-pulsed PBMCs. As shown in Fig. 4, the half-maximal cell proliferation of the CEA116 T-cell clone was obtained at a peptide concentration of approximately 1 μM. The half-maximal cell proliferation of the CEA176 T-cell clone was approximately 5 μM; however, the CEA624 T-cell clone was shown to have a higher avidity with the half-maximal cell proliferation of the CEA624 at a peptide concentration of approximately 0.1 μM (Fig. 4).
Fig. 4.
Peptide titration of T-cell clones. Each of these three CD4+ T-cell clones (CEA 116, CEA 176, and CEA 624) were cultured with irradiated autologous DC (T: DC=20:1) in the presence of various concentrations of peptides for 48 h. Each data point represents the mean and SD (bars) of triplicate samples of [3H]-thymidine incorporation of the T-cells. The data are from one experiment that is representative of three performed
Recognition of natively processed epitopes by CD4+ T-cell clones
A critical feature of functional CD4+ T-cells is their ability to recognize naturally processed antigen; thus, we tested whether the T-cells from different HLA-DR+ donors could recognize and respond to naturally processed and presented epitopes of CEA. A recombinant CEA-Fc protein was produced from a baculovirus expression system and purified by affinity binding to protein-A agarose beads. The purity and authenticity of the recombinant proteins were evaluated by SDS-PAGE/Coomassie blue staining and Western blotting assay with the anti-CEA (Fig. 5).
Fig. 5.
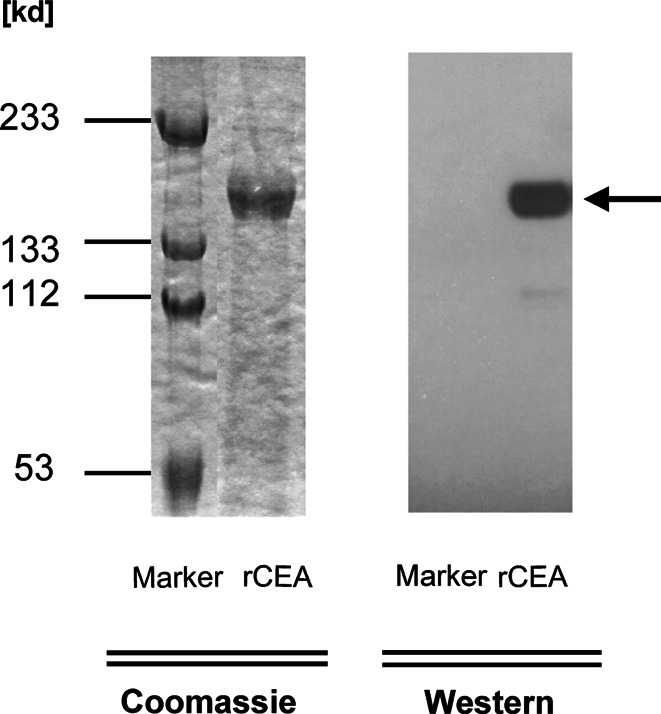
Production and analysis of recombinant CEA-Fc proteins. The recombinant human CEA-Fc protein was produced from a baculovirus expression system and purified by affinity binding to protein-A agarose. The purified protein was then analyzed by a 7.5% SDS-PAGE Coomassie blue staining. The authenticity of the purified CEA-Fc protein was examined by Western blotting (arrow) with the antibody against human CEA
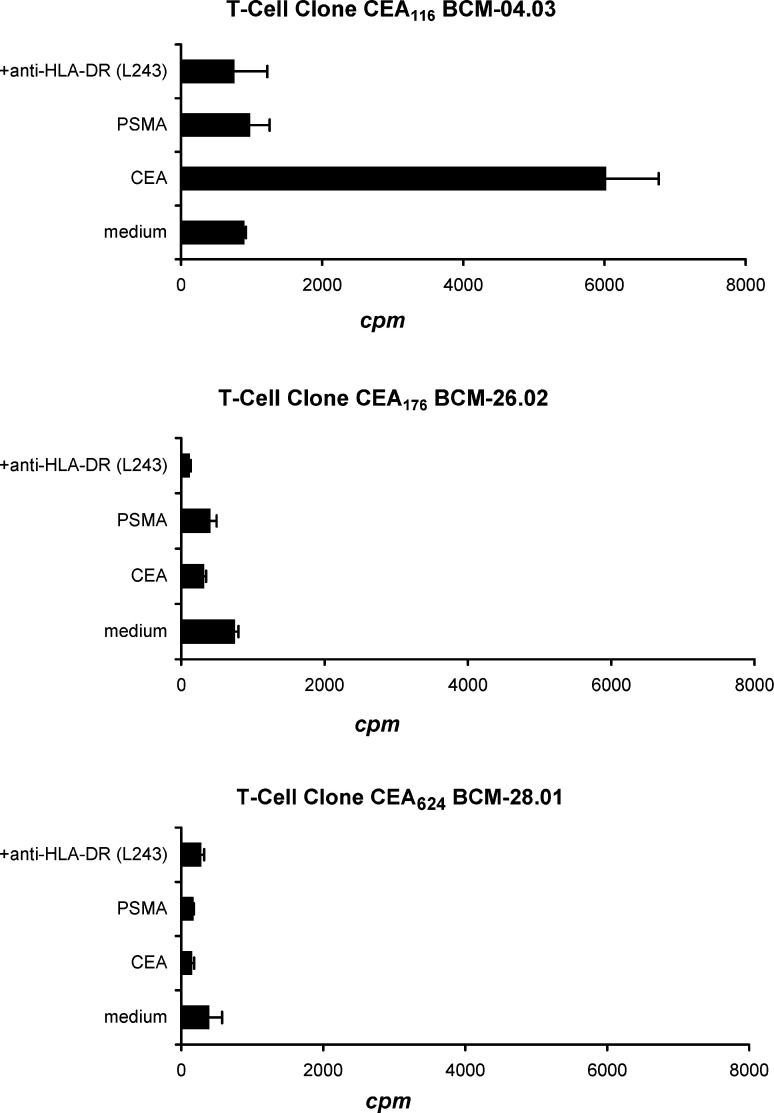
These recombinant proteins were then used to pulse PBMC-derived DC and the ability of the T-cell clones to recognize and respond naturally processed CEA was examined by co-culturing with protein-pulsed autologous DC. As shown in Fig. 6, the CEA116 T-cell clone responded to the CEA protein after processing and presentation by autologous DC, as demonstrated by active T-cell proliferation (p<0.01). Repeated experiments showed the similar results. The T-cell response was specific, since the CEA116 T-cell clone did not respond to the stimulation with an irrelevant prostate-specific membrane antigen (PSMA) protein that was produced from the same baculovirus expression system, and their responses to CEA proteins were inhibited by the anti-HLA-DR antibody. The T-cell clones specific for two other peptides, CEA176, and CEA624, failed to respond to DCs pulsed with various concentrations of CEA proteins with repeated tests, indicating that these two peptides CEA176, and CEA624 are likely cryptic. The failure of these two T-cell clone responses to the recombinant CEA-Fc proteins further ruled out the possibility that the observed CEA116 T-cell clone responses to the CEA-Fc stimulation were due to the stimulation by the possible contaminants or by the Fc fragment in the purified recombinant CEA-Fc proteins. Taken together, we conclude that the peptide sequence CEA116 recognized by the CD4+ T-cells is a naturally processed epitope for CEA.
Fig. 6.
The CD4+ T-cell responses to natively processed CEA. Each of three peptide-reactive T-cell clones (CEA 116, CEA 176, and CEA 624; 3×104/well) were stimulated in triplicate wells with irradiated autologous PBMC-derived DC (1.5×103/well) pulsed with recombinant CEA protein (10 μg/ml) in the presence or absence of the anti-HLA-DR antibody (L243; 20 μg/ml). These T-cell clones were also stimulated with DC pulsed with irrelevant recombinant prostate-specific membrane antigen (PSMA) protein (10 μg/ml). T-cell proliferation was determined by [3H]-thymidine incorporation assay during the last 16 of 72 h of culture. The proliferation of T-cells coculture with CEA-pulsed DC was significantly higher than proliferation of T-cells with non-pulsed DC (medium control) and with addition of anti-HLA DR antibody (p<0.01). Values shown are the means of triplicate determinations (bars: SD). The representative result of one of three repeated experiments is shown
CEA-specific T-cell response induced by CEA116 immunization of HLA-DR transgenic mice
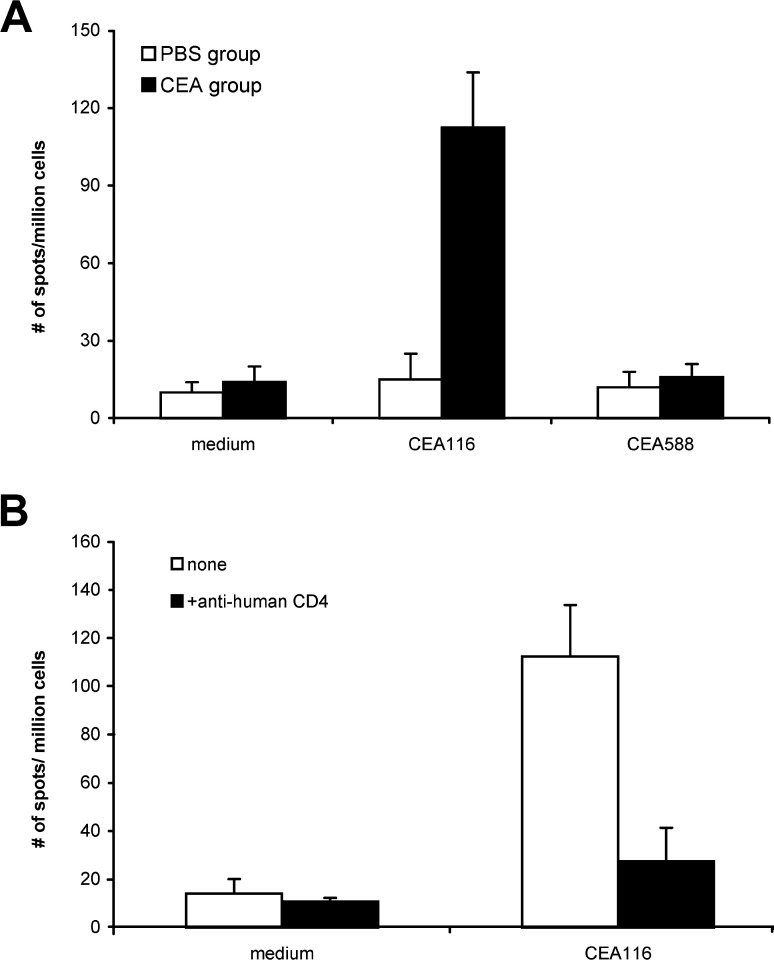
To further assess the therapeutic potential of CEA116, we used HLA-DR4 transgenic mice [8, 51, 52] to determine whether immunization with the peptide can induce a CD4+ Th response specific not only for the peptide, but also for the CEA protein. The HLA-DR molecule expression on transgenic mice was detected by flow cytometric assay. The spleen cells were isolated and stained with FITC-conjugated mouse anti-human HLA-DR, PE-conjugated mouse anti-human CD4, or FITC-conjugated rat anti-mouse CD4 (BD PharMingen, San Diego, Calif.). These transgenic mice are human HLA-DR positive, human CD4 positive and mouse CD4 negative. Ten days after the last immunization with CEA116 in CFA, the transgenic mice were killed and the response of their splenocytes to peptides, recombinant CEA protein, and CEA-positive colon cancer cell were examined by using IFN-γ ELISPOT assays. The splenocytes of CEA116-immunized mice responded strongly to the CEA116 stimulation, producing IFN-γ at a frequency of 113 spots per million of splenocytes (medium control, 14/106). In contrast, the splenocytes of PBS-immunized control mice produced IFN-γ at a background frequency of 15 spots per million splenocytes to the peptide CEA116 (Fig. 7A). The splenocytes of CEA116-immunized mice did not respond to an irrelevant CEA588 stimulation, indicating that T-cells induced by peptide immunization specifically responds to the immunized peptide.
Fig. 7A,B.
Peptide-specific Th response induced by immunization with CEA116. The HLA-DR4 transgenic mice (4 mice/group) were twice immunized with 100 μg CEA116 or phosphate-buffered saline (PBS; 100 μl) emulsified with complete Freunds adjuvant (CFA). Ten days after the second immunization, the splenocytes of each mouse group were pooled and tested for peptide CEA116-induced production of IFN-γ by ELISPOT. A The frequency of peptide-specific T-cells of CEA116-immunized mice was significantly higher than that of PBS control group and irrelevant control peptide CEA588 (p<0.01), and B significantly decreased by monoclonal antibody anti-human CD4 (p<0.01). Data show the mean and SD of spot numbers from triplicate wells from one experiment that is representative of two experiments performed
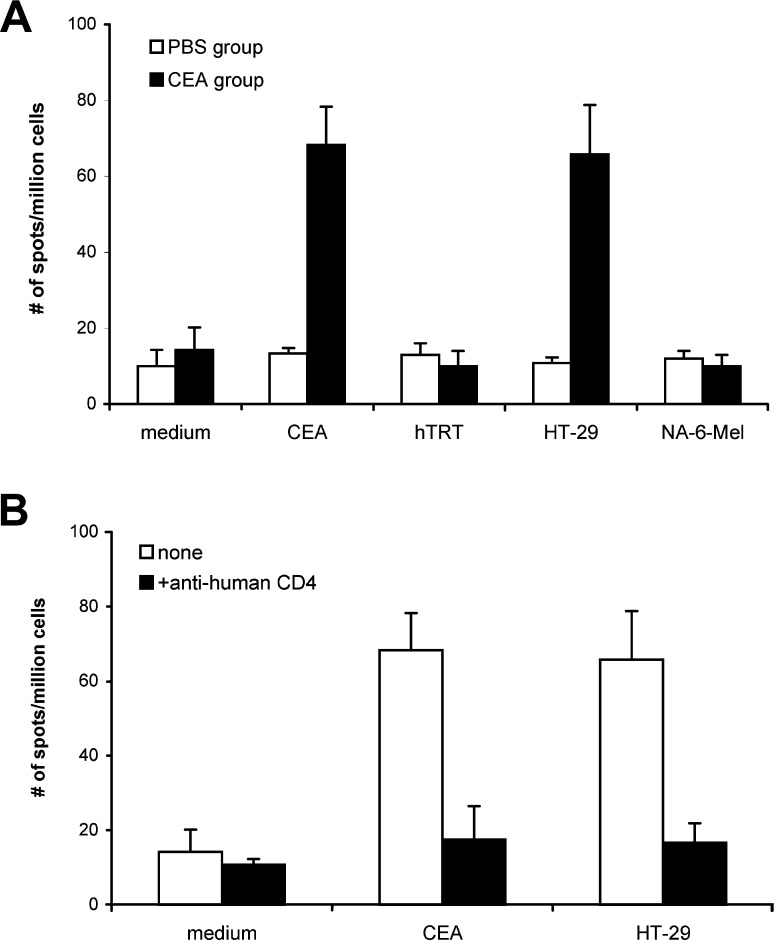
Since most tumor cells are MHC class-II negative, the tumor-specific MHC class II-restricted CD4+ T-cells are not able to recognize these tumor cells directly. CD4+ T-cells induced by peptide immunization can react with antigen presenting cells (APCs) that take up and process the tumor antigen protein; thus, we tested if transgenic mouse T-cells become activated when cocultured with splenocytes containing T-cells and APCs pulsed with the recombinant CEA proteins. As shown in Fig. 8A, when stimulated with the recombinant CEA protein, the splenocytes of CEA116-immunized mice produced IFN-γ at a frequency of 69 spots per million cells(medium control, 14/106), significantly higher than the splenocytes of the PBS-immunized mice (frequency of 13 spots per million cells). Furthermore, the splenocytes of CEA116-immunized mice produced IFN-γ at a background frequency, when stimulated with irrelevant hTRT-Fc proteins (10/106). These results indicate that CEA116 immunization activates T-cells that recognize antigenic peptides processed from CEA proteins.
Fig. 8A,B.
Th response to antigenic peptides derived from CEA proteins and CEA+ tumor lysates. Splenocytes from HLA-DR4 transgenic mice (4 mice/group) immunized with 100 μg CEA116 or PBS (100 μl) emulsified with CFA were assessed for the production of IFN-γ by ELISPOT. Recombinant CEA proteins (20 μg/ml) and CEA-positive HT-29 tumor lysate (50 μl) were used to pulse the splenocytes. A The frequencies of IFN-γ-secreting cells of CEA116-immunized mouse group when stimulated with CEA proteins or tumor lysates were significantly higher than that of PBS control mouse group and irrelevant control protein hTRT-Fc and CEA-negative NA-6-Mel tumor lysates (p<0.05). B Anti-human CD4 antibody inhibited the IFN-γ production induced by CEA protein and HT-29 tumor lysates (p<0.05). Data show the mean and SD of spot numbers from triplicate wells from one experiment that is representative of two experiments performed
Finally, we tested whether activated CD4+ T-cells can recognize APCs that directly take up and process the tumor antigen from tumor cells. Colon cancer cell line (HT-29) that express high levels of CEA [35] were used for this assay. As shown in Fig. 8A, when stimulated with HT-29 cell lysates, the splenocytes of CEA116-immunized mice produced IFN-γ at a frequency of 66 spots per million cells (medium control, 14/106), significantly higher than the splenocytes of the PBS-immunized mice at a frequency of 10 spots per million cells. The splenocytes of CEA116-immunized mice produced IFN-γ at a background frequency, when stimulated with CEA-negative NA-6-Mel tumor lysates (10/106), suggesting that T-cells activated by CEA116 immunization specifically respond to antigenic peptides derived from CEA-positive tumor.
To confirm that the IFN-γ production was from CD4+ Th cells, we tested the blocking effects of anti-human CD4 and CD8 monoclonal antibodies (20 μg/ml) in the antigen-induced IFN-γ ELISPOT assay. The splenocytes produced IFN-γ at a frequency of 27 spots against CEA116, 17 spots against CEA protein, and 17 spots against HT-29 tumor lysates in the presence of anti-human CD4 antibody. As shown in Figs. 7B and 8B, the production of IFN-γ induced by CEA116, CEA protein and HT-29 tumor lysates was all significantly decreased by the anti-human CD4 antibody (p<0.01) but not by anti-human CD8 antibody (data not shown), which indicates that antigen-specific IFN-γ was produced by CD4+ Th cells. The antigen-specific CD4+ Th responses can be induced by immunization with MHC class-II peptide CEA116.
Discussion
In the present study, we identified several MHC class-II-restricted Th epitopes in CEA, including one naturally processed epitope CEA116, using the computer-based algorithm analysis and in vitro T-cell biological analysis. Furthermore, we demonstrated that the T-cell precursors for CEA116 are normal part of T-cell repertoire of human with the frequency of 0.08–0.33×10−6 estimated from seven donors tested and can be readily activated when properly stimulated. A concern for peptide-based therapy is the MHC genotype restriction, since class-I-restricted epitopes are largely restricted by a particular class-I genotype [24, 25, 26]. Here, we found that the CEA116 epitope can be promiscuously presented by commonly found HLA-DR alleles, including some genotypes in HLA-DR4, DR1, DR13, and DR14. Furthermore, CEA116 peptide immunization of HLA-DR4 transgenic mice activates T-cells that specifically responded to antigenic peptides derived from CEA protein and CEA-positive tumor. The identification of the promiscuous, naturally processed epitope CEA116 should facilitate the development of improved tumor vaccines through the simultaneous stimulation of CEA-specific CTL and T-helper responses for treating CEA-associated cancers, including colorectal, gastric, pancreatic, breast, and non-small cell lung carcinomas [17, 50].
The main focus of current tumor vaccination effort has been directed to induce CTL responses [38, 45]; however, CD4+ T-helper (Th) cells play critical roles in initiating, regulating, and maintaining CTL responses [38, 44]; thus, the potency of current tumor vaccines that mainly use CTL epitopes to induce CTL could be enhanced by combined application with CD4+ Th epitopes. Increasing efforts have been made to activate Th to enhance tumor vaccines. Non-specific Th stimuli, such as keyhole limpet hemocyanin (KLH), have been used to enhance CTL responses. The KLH, which can serve as a helper antigen, induces T-cell responses and enhances in vivo antitumor activity [9, 49]. A universal, nonspecific Th epitope, PADRE, was developed and has been used to enhance CTL responses in clinical vaccine trials [2, 42, 60]. Although responses to the PADRE epitopes are induced in vivo, the T-cell responses to antigen epitopes usually have been very limited [42, 60]; thus, Th epitopes from the same antigen may be more effective than non-specific Th stimuli such as PADRE or KLH to enhance antigen-specific CTL responses and anti-tumor activity.
Thus far, only a few class-II-restricted epitopes on human tumors have been identified, despite the importance of CD4+ Th in the induction of antitumor responses [6, 7, 20, 26, 28, 56]. By comparison with the identification of MHC class-I-restricted CTL epitopes in tumor-associated antigens, it is technically more challenging to identify MHC class-II-restricted Th epitopes, since antigen-specific T-helper cells are usually difficult to generate and the length of MHC class-II-restricted epitopes is invariable. Several recent studies have utilized an algorithm analysis to narrow down the epitope candidates and then use in vitro T-cell analysis to identify class-II-restricted epitopes [6, 7, 20, 26, 28, 57, 62, 63]. In this study, we used a combined approach of using a computer-based algorithm analysis TEPITOPE and in vitro biological analysis to identify Th epitopes in CEA that induce Th responses. An essential feature of functional CD4+ T-cells is their ability to recognize naturally processed antigen; thus, the primary goal of this study is to identify naturally processed epitopes in CEA. Although all of peptides derived from the predicted binding motifs in CEA by TEPITOPE were found to be able to induce human T-cell responses, the challenge lies in the identification of the naturally processed epitopes. The key to identify naturally processed epitopes is to generate peptide-specific human T-cell clones, since in-depth and repeatable analyses would be impossible without a specific T-clone. Here, we established an efficient method to generate peptide-reactive T-cell clones. The peptide-specific T-cell clones for three peptides were established, although T-cell clones for other two other peptides were unable to establish even after repeated attempts for some unknown reasons. By testing these peptide-specific T-cell clones, we found that the T-cell clone specific for CEA116 responded to DCs pulsed with CEA proteins, indicating this peptide CEA116 is a naturally processed epitope. This in vitro Th induction method we established is efficient, since several MHC class-II restricted Th epitopes in other tumor antigens, including the prostate specific membrane antigen (PSMA) and human telomerase reverse transcriptase [48], were also identified by this described method.
The overall affinity/avidity of CD4+ Th cells for a peptide is an important parameter that may affect the recognition of naturally processed epitopes from tumor-associated antigens [24, 25, 26, 27, 35]. By evaluating the avidity of these peptide-specific T-cell clones for their ligands, we found that the CEA624 T-cell clone had a higher avidity than the two other clones but did not respond to DC pulsed with recombinant CEA proteins. In contrast, the CEA116 T-cell clone with a lower avidity vigorously responded to DC pulsed with recombinant CEA proteins. These results suggest that there is no clear correlation between the avidity of peptide binding with MHC molecule/T-cell receptor (TCR) and the T-cell ability to recognize naturally processed antigens. It may be because the synthetic peptide, although binding to MHC/TCR at a high avidity, is not naturally processed from the antigen protein and presented with MHC class-II molecules by antigen-presenting cells (APC); thus, the only reliable means to identify a native epitope, we believe, is to test the responsiveness of a peptide-specific T-cell clone to protein-pulsed APC.
Immunotherapies for the induction of immune responses against CEA are currently being tested in the clinic [17, 58, 59]. It was demonstrated that immunization with CEA CTL peptides induced CEA-specific CTL responses in cancer patients [58, 59]. In a recent clinical trial, immunization with DCs pulsed with a modified CTL peptides derived from CEA induce CD8+ CTL that recognized CEA-positive tumor cells [11]. After vaccination, some colorectal and lung cancer patients experienced dramatic tumor regression, which seems to correlate with the expansion of CD8+ tetramer-positive T-cells, suggesting the therapeutic effect of CEA CTL peptide immunization. Given the potential critical role of antigen-specific Th cells on CTL responses [12, 53, 54, 65], it would be interesting to test whether the combined use of CEA Th peptides with CTL peptides could enhance the potency and duration of anti-CEA CTL responses against these common cancers, including colorectal, gastric, pancreatic, breast cancer, and non-small cell lung carcinomas.
Acknowledgments
Acknowledgements. We thank J. Zhang for his expert advice, L. Rollins for technical assistance, and volunteers for donating their blood. This work was supported by grants from the U.S. Army Breast Cancer Research Program BC990963 (X.F.H.) and NIH AI48711 (S.Y.C.). This work was also supported in part by MithraGen Inc.
References
- 1.Abbas Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Alexander Immunity. 1994;1:751. [Google Scholar]
- 3.Banchereau Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Barnett Genomics. 1988;3:59. [Google Scholar]
- 5.Bennett J Exp Med. 1997;186:65. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaux J Exp Med. 1999;189:767. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochlovius J Immunol. 2000;165:4731. doi: 10.4049/jimmunol.165.8.4731. [DOI] [PubMed] [Google Scholar]
- 8.Congia Proc Natl Acad Sci USA. 1998;95:3833. doi: 10.1073/pnas.95.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis Clin Exp Immunol. 1972;10:171. [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhard Annu Rev Immunol. 1994;12:181. doi: 10.1146/annurev.immunol.12.1.181. [DOI] [PubMed] [Google Scholar]
- 11.Fong Proc Natl Acad Sci USA. 2001;98:8809. [Google Scholar]
- 12.Gao Cancer Res. 2002;62:6438. [PubMed] [Google Scholar]
- 13.Geluk Biotherapy. 1998;10:191. doi: 10.1007/BF02678296. [DOI] [PubMed] [Google Scholar]
- 14.Geluk Proc Natl Acad Sci USA. 1998;95:10797. doi: 10.1073/pnas.95.18.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarstrom Semin Cancer Biol. 1999;9:67. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 16.Hammer Adv Immunol. 1997;66:67. doi: 10.1016/s0065-2776(08)60596-9. [DOI] [PubMed] [Google Scholar]
- 17.Hodge Cancer Immunol Immunother. 1996;43:127. doi: 10.1007/s002620050313. [DOI] [PubMed] [Google Scholar]
- 18.Jaeger Int J Cancer. 1996;66:162. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.3.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Jager Int J Cancer. 1996;67:54. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Jager J Exp Med. 2000;191:625. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James Immunology. 1991;72:213. [PMC free article] [PubMed] [Google Scholar]
- 22.Jonuleit Eur J Immunol. 1997;27:3135. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 23.Kalams J Exp Med. 1998;188:2199. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawashima Hum Immunol. 1998;59:1. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima Cancer Res. 1999;59:431. [PubMed] [Google Scholar]
- 26.Kobayashi Immunol Invest. 2000;29:105. doi: 10.3109/08820130009062291. [DOI] [PubMed] [Google Scholar]
- 27.Lu Cancer Res. 2000;60:5223. [PubMed] [Google Scholar]
- 28.Manici J Exp Med. 1999;189:871. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchand Int J Cancer. 1995;63:883. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 30.Mayordomo Nat Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 31.McNeel Cancer Res. 2001;61:5161. [PubMed] [Google Scholar]
- 32.Mitchell Semin Oncol. 1998;25:12. [Google Scholar]
- 33.Mumberg Proc Natl Acad Sci USA. 1999;96:8633. [Google Scholar]
- 34.Nestle Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 35.Nukaya Int J Cancer. 1999;80:92. doi: 10.1002/(sici)1097-0215(19990105)80:1<92::aid-ijc18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Ossendorp J Exp Med. 1998;187:693. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardoll Proc Natl Acad Sci USA. 1999;96:5340. [Google Scholar]
- 38.Pardoll Curr Opin Immunol. 1998;10:588. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 39.Porgador J Immunol. 1996;156:2918. [PubMed] [Google Scholar]
- 40.Raju J Immunol. 2001;167:1118. doi: 10.4049/jimmunol.167.2.1118. [DOI] [PubMed] [Google Scholar]
- 41.Ras Hum Immunol. 1997;53:81. doi: 10.1016/S0198-8859(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 42.Ressing J Immunother. 2000;23:255. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Ribas J Immunother. 2000;23:59. doi: 10.1097/00002371-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg Immunity. 1999;10:281. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg Nat Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroers Gene Ther. 2002;9:889. doi: 10.1038/sj.gt.3301711. [DOI] [PubMed] [Google Scholar]
- 48.Schroers Cancer Res. 2002;62:2600. [PubMed] [Google Scholar]
- 49.Shimizu Cancer Res. 2001;61:2618. [PubMed] [Google Scholar]
- 50.Shively Crit Rev Oncol Hematol. 1985;2:355. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- 51.Sonderstrup Immunol Rev. 1998;164:129. doi: 10.1111/j.1600-065x.1998.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 52.Sonderstrup Immunol Rev. 1999;172:335. doi: 10.1111/j.1600-065x.1999.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 53.Stober J Immunol. 2003;170:2540. [Google Scholar]
- 54.Tatsumi J Exp Med. 2002;196:619. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson J Clin Lab Anal. 1991;5:344. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 56.Topalian Proc Natl Acad Sci USA. 1994;91:9461. [Google Scholar]
- 57.Touloukian J Immunol. 2000;164:3535. [Google Scholar]
- 58.Tsang J Natl Cancer Inst. 1995;87:982. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 59.Tsang Clin Cancer Res. 1997;3:2439. [PubMed] [Google Scholar]
- 60.Weber J Immunother. 1999;22:431. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Zaks Cancer Res. 1998;58:4902. [PubMed] [Google Scholar]
- 62.Zarour Proc Natl Acad Sci USA. 2000;97:400. doi: 10.1073/pnas.97.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Proc Natl Acad Sci USA. 2001;98:3964. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J Exp Med. 1994;179:973. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zwaveling J Immunol. 2002;169:350. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]