The long-term survival of patients with distant metastatic (stage IV) melanoma remains worryingly poor: approximately one fifth of patients are alive at 2 years after the occurrence of distant metastases, and the reduction of tumor burden achieved by chemotherapeutic regimens has not yet translated into a significant survival prolongation. The prognosis for those diagnosed with metastases in visceral organs, with the liver as the dominant site, is particularly dismal with a median survival time of 2–5 months, and <10% of patients surviving at 2 years [1].
The immunoactivating cytokine interleukin 2 (IL-2) is employed in the treatment of stage IV melanoma in many European countries and in the United States. IL-2 therapy, which aims at expanding and activating tumoricidal lymphocytes, induces complete regression of melanoma metastases in 3–5% of treated patients, but the toxicity of many IL-2 regimens limits its use [2]. In addition, the tumor-killing cytotoxic T cells and natural killer cells, which are the presumed target cells for IL-2, are frequently inefficient in the tumor environment [3, 4], partly due to suppressive and apoptosis-inducing signals from tumor-infiltrating mononuclear phagocytes (reviewed in [5]). Histamine dihydrochloride (HDC) has been shown to protect tumoricidal lymphocytes from dysfunction and apoptosis induced by mononuclear phagocytes, and thereby to improve the lymphocyte-mediated killing of tumor cells, including melanoma cells, in vitro and in vivo [6, 7].
In 1997, we initiated a phase III trial using an outpatient regimen of IL-2 with or without subcutaneous injections of HDC (1 mg b.i.d.) to determine the effects of HDC on the survival of stage IV melanoma patients. On the basis of the results obtained in smaller phase I/II trials using HDC/IL-2 in advanced melanoma [8], two primary endpoints were selected: the survival of all randomized patients (intention-to-treat overall, ITT-OA; n=305) and that of all randomized patients diagnosed with liver metastases before treatment (ITT liver metastasis, ITT-LM; n=129).
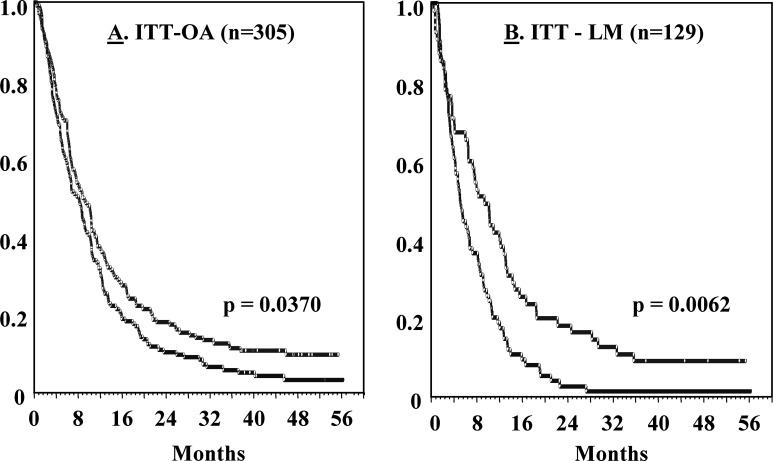
The trial results at ≥1 year after the onset of treatment suggested that the addition of HDC did not significantly impact on the overall survival of patients in the ITT-OA group, but improved the survival of ITT-LM patients [9]. All patients have been followed for at the least >3 year, and the updated survival results are shown in Fig. 1. The addition of HDC prolonged the survival of all patients randomized (p=0.037), and the significance observed for the liver metastases (ITT-LM) population remained at 3 year (p=0.0062; Fig. 1). The survival of patients without liver metastases did not differ significantly between the study groups (p=0.77; data not shown).
Fig. 1A, B.
Kaplan-Meier survival distribution curves for stage IV melanoma patients receiving IL-2 (blue line) or IL-2 + HDC (red line). For details of demography and patient eligibility, see [6]. Overall toxicities of the regimens used were low, as accounted for in detail elsewhere [9]. A Shows results (analyzed using intention-to-treat) obtained in all patients (ITT-OA group). B Shows results obtained in patients with liver metastasis (ITT-LM group) at study entry. P values refer to comparisons between the IL-2 and IL-2/HDC arms using the log-rank test, followed by the Holm-Sidak method to adjust for multiple analyses (for further information about the statistics applied, see [9])
These data suggest that administration of IL-2/HDC may specifically prolong the survival of melanoma patients with liver metastases. A tentative explanation for the apparent liver tropism of this treatment may relate to the proposed mechanism of action for HDC—to protect IL-2’s target lymphocytes by reducing oxidative stress inflicted by mononuclear phagocytes (reviewed in [10]). Such protection may be particularly relevant in liver tissue, which comprises approximately 80% of the host’s mononuclear phagocytic system [11]. A confirmatory trial in melanoma patients with liver metastases is ongoing.
Acknowledgements
We thank the other investigators participating in this study: T. Amatruda, C. Anderson, F. Arena, R. Asbury, N. Bartlett, R. Belt, D. Bodkin, A. Brown, A. Chang, N. Chowhan, S.J. O’Day, A. Deisseroth, M. Ernstoff, L. Fehrenbacher, L. Feun, J. Glaspy, R. Gonzalez, M. Graham, J. Gutheil, E. Hersh, D. Irwin, V. Jones, A. Keller, J. Kirkwood, D. Lawson, J. Lutzky, M. Mitchell, K. McMasters, F. Nathan, R. Oratz, J. Polikoff, W.J. Poo, J. Richards, J. Rosenblatt, F. Sanchez, N. Savaraj, P. Schaefer, L. Sutton, H. Terebelo, M. Thant, R. Weinstein, and E. Whitman. We thank Maxim Pharmaceuticals, San Diego, for trial sponsorship and Omnicare, King of Prussia, Philadelphia, for data management.
References
- 1.Manola J Clin Oncol. 2000;18:3782. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 2.Atkins Semin Oncol. 2002;3S7:12. doi: 10.1053/sonc.2002.33077. [DOI] [PubMed] [Google Scholar]
- 3.Kono Eur J Immunol. 1996;26:1308. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 4.Zea Clin Cancer Res. 1995;1:1327. [PubMed] [Google Scholar]
- 5.Kiessling Cancer Immunol Immunother. 1999;48:353. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellstrand J Immunol. 1994;153:4940. [PubMed] [Google Scholar]
- 7.Hellstrand J Immunol. 1990;145:4365. [PubMed] [Google Scholar]
- 8.Hellstrand Cancer Immunol Immunother. 1994;39:416. doi: 10.1007/BF01534430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwala J Clin Oncol. 2002;20:125. [Google Scholar]
- 10.Hellstrand Semin Oncol. 2002;29:35. doi: 10.1053/sonc.2002.33081. [DOI] [PubMed] [Google Scholar]
- 11.Saba Arch Intern Med. 1970;126:1031. [PubMed] [Google Scholar]