Abstract
The tumour-associated antigen, Ep-CAM, is over-expressed in colorectal carcinoma (CRC). In the present study, a recombinant Ep-CAM protein or a human anti-idiotypic antibody (anti-Id) mimicking Ep-CAM, either alone or in combination, was used for vaccination of CRC patients (n=9). GM-CSF was given as an adjuvant cytokine. A cellular immune response was assessed by measuring anti-Ep-CAM lymphoproliferation, IFN-γ production (ELISPOT) and by analysing the TCR BV gene usage within the CD4+ and CD8+ T-cell subsets followed by CDR3 fragment analysis. A proliferative and/or IFN-γ T-cell response was induced against the Ep-CAM protein in eight out of nine patients, and against Ep-CAM-derived peptides in nine out of nine patients. Analysis of the TCR BV gene usage showed a significantly higher usage of BV12 family in CD4+ T cells of patients both before and after immunisation than in those of healthy control donors (p<0.05). In the CD8+ T-cell subset, a significant (p<0.05) increase in the BV19 usage was noted in patients after immunisation. In individual patients, a number of TCR BV gene families in both CD4+ and CD8+ T cells were over-expressed mainly in post-immunisation samples. Analysis of the CDR3 length polymorphism revealed a higher degree of clonality in post-immunisation samples than in pre-immunisation samples. In vitro stimulation with Ep-CAM protein confirmed the expansion of anti-Ep-CAM T-cell clones. The results indicate that immunisation with the Ep-CAM protein and/or anti-Id entails the induction of an anti-Ep-CAM T-cell response in CRC patients, and suggest that BV19+ CD8+ T cells might be involved in a vaccine-induced immune response.
Keywords: TCR BV genes, Ep-CAM, Anti-Id, Colorectal carcinoma, Vaccination
Introduction
Colorectal carcinoma is the second leading cause of cancer-associated mortality in Western countries. Despite successful surgery, the recurrence rate for stages II–III CRC varies from 20 to 60% [8]. Adjuvant chemotherapy for stage III colon cancer showed an absolute survival benefit of 5–6%. In stage II disease, the survival benefit of adjuvant chemotherapy is controversial. There is therefore a need to develop new treatment approaches. Immunotherapy may be a complementary treatment strategy to standard therapy.
The tumour-associated antigen (TAA), Ep-CAM, has been utilised as a target for immunotherapy [3]. Ep-CAM is a 40-kDa homotypic cell-adhesion glycoprotein. Ep-CAM is expressed at a high level by a large number of epithelial neoplasias, including CRC. Normal epithelial cells also express Ep-CAM but at a lower density and are restricted to the basolateral cell surface [3].
Natural immune responses against Ep-CAM have been described in CRC patients [22, 24]. Treatment with mAb 17-1A (anti-Ep-CAM) may improve survival of patients with resected stage III CRC [12, 27, 29]. Vaccination with Ep-CAM expressed in viral vectors has been shown to induce immune responses against the antigen in animal models and humans [4, 38]. Immunisation with the Ep-CAM protein has been reported to elicit humoral and cellular immunity in CRC patients [35]. Anti-Ids mimicking Ep-CAM have also been used for vaccination and evoked B- as well as T-cell responses against Ep-CAM in cancer patients [4, 20]. Ep-CAM protein or anti-Id has, however, not been used together with the adjuvant cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), which facilitates the development of both humoral and cellular immune responses against weakly immunogenic TAAs [39].
A successful approach for active immunotherapy against cancer entails stimulation of a tumour antigen-specific cellular immune response. Several in vitro assays can be used for monitoring an immune response, including lymphoproliferative assay and enzyme-linked immunospot (ELISPOT) for detection of cytokine-secreting cells [7]. Analysis of the T-cell receptor (TCR) V region gene usage may serve as a complementary in vitro measure of an antigen-specific cellular immune response [7, 41].
Most human mature T cells use α/β TCRs for specific recognition of antigenic peptides in the context of major histocompatibility complex (MHC) molecules [21]. The α/β TCR consists of an α and a β polypeptide chain, each comprising a variable and constant region. Genes encoding the variable regions of these polypeptides are assembled by somatic recombination of one of each of the variable (V), diversity (D, only for β chain) and joining (J) gene segments. Three hypervariable or complementarity-determining regions (CDR1, CDR2 and CDR3) of the TCR have been defined for the variable regions of β (BV) and α (AV) chains. It is primarily the CDR3 loop which is responsible for the specific interaction with the antigenic peptide [21]. Specific recognition of a peptide might induce clonal expansion of T cells exhibiting a particular TCR V gene product with identical CDR3 regions. An antigen-specific immune response may be determined by analysing the expansion of T cells expressing a particular TCR BV region using flow-cytometry or polymerase chain reaction (PCR)-based techniques [14]. Analysis of the CDR3 size distribution pattern defines the degree of clonality of the T cells. Development of mono-/oligoclonality might be a sign of an antigen-driven immune response [14, 26].
In the present study, CRC patients were vaccinated with the Ep-CAM protein or anti-Id, alone or in combination, together with GM-CSF. An anti-Ep-CAM T-cell response was assessed by analysing lymphoproliferation, IFN-γ production and TCR BV gene usage including CDR3 length polymorphism.
Patients and methods
Patients
Nine patients were enrolled in this study. Eligibility criteria included histo-pathological diagnosis of Ep-CAM-positive adenocarcinoma of the colon or rectum, AJCC stage II to III disease and resection of the primary tumour without evidence of remaining macroscopic disease. Patients were required to have a Karnofsky performance status ≥80% and adequate hepatic and renal function. Exclusion criteria were: pregnancy or nursing, HIV seropositivity, alcohol or drug abuse, autoimmune disease, known hypersensitivity to insect cells, baculovirus or GM-CSF, active infection, chronic systemic disorder, chemo- or radiotherapy or other immunosuppressive agent within 30 days prior to study entry. Patients were included before routine adjuvant chemotherapy for stage III colon cancer was introduced in Sweden. The study was approved by the ethics committee of the Karolinska Institute. All patients were treated after informed consent.
Prior to entry into the study, a complete case history was obtained, a physical examination was carried out and blood tests including haemoglobin, WBC with differentials and platelet counts, serum creatinine and electrolytes, serum protein electrophoresis, tests for liver function, thyroid function, serum tumour markers (CEA, CA19-9, CA50) and standard urine analysis were performed. HLA typing was done at the Tissue Typing Laboratory, Huddinge University Hospital. A chest X-ray and CT scan of the abdomen were performed as well. During follow-up, patients were regularly checked for performance status and vital signs, routine blood haematology and chemistry analyses, standard urine analysis, thyroid function tests and serum tumour markers. Adverse events were assessed according to the National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/reporting/ctc.html). Vaccination was discontinued if disease progression occurred, in which event patients received standard therapy (surgery, chemo- or radiotherapy).
Vaccine preparations
The extracellular domain of the Ep-CAM protein was purified using an immuno-affinity column coupled with mAb GA733 (Centocor, Malvern, PA, USA) from supernatants of cultured Spodoptera frugiperda insect cells infected with a recombinant Ep-CAM baculovirus construct as described elsewhere [37]. The only modifications to the previously described method were that serum-free medium SF900II (GIBCO) was used during the whole tissue-culture period and detergent was omitted from the elution buffer in immunoaffinity chromatography.
The human anti-Id (IgG1κ), raised against the murine mAb 17-1A and mimicking Ep-CAM (ab2β), was produced by Epstein–Barr virus-immortalised human lymphoblastoid cell lines of a patient treated with mAb 17-1A [11, 36]. Anti-Id was purified on protein A and mAb Trap G columns (Pharmacia, Uppsala, Sweden) as described [11, 36].
Purity of the Ag preparations was confirmed by SDS-PAGE and electrophoresis. Immunoreactivity of the products was checked by ELISA and Western blot [11, 36, 37]. Tests for sterility, endotoxin and viruses were performed according to a protocol described previously [11].
Vaccination schedule
Patients were enrolled into one of the three vaccination schedules receiving i.d./s.c. injections of (1) Ep-CAM protein (400 μg/dose) at weeks 0, 2 and 6 (n=3); (2) Ep-CAM protein (400 μg/dose) at weeks 0 and 2 followed by anti-Id (500 μg) at week 6 (n=4); (3) anti-Id (500 μg/dose) at weeks 0, 2 and 6 (n=2). Patients received concomitant GM-CSF (Leucomax; Schering-Plough, Kenilworth, NJ, USA; 75 μg/day) for four consecutive days at each immunisation. The first dose of GM-CSF was given the day before the vaccine. Antigens and GM-CSF were injected to the same site in the deltoid region.
Healthy controls
Peripheral blood mononuclear cells (PBMC) from 11 healthy blood donors (10 females and 1 male) were used as controls. The median age was 58 years (range 32–67 years).
Antigens for in vitro testing
As controls for the baculovirus recombinant Ep-CAM protein, a baculovirus control protein (BCP) and baculovirus recombinant carcinoembryonic antigen (CEA; Protein Sciences Corporation, Meriden, CT, USA) were used. Production of BCP and CEA has been described elsewhere [30].
Lymphoproliferative assay
Peripheral blood mononuclear cells before and at a minimum of two time-points after vaccination (between week 12 and 34) were isolated from fresh heparinised venous blood by Ficoll-gradient centrifugation. Proliferative T-cell response was assessed in a standard [3H] thymidine incorporation assay as described [36]. Protein antigens were added at concentrations 1, 10 and 100 ng/ml. Twenty-three Ep-CAM-derived 18-mer peptides (with six amino acid N- and C-terminal overlap) [23] were also used as stimulators (1 and 10 μg/ml). Phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO, USA; 10 μg/ml) and purified protein derivative of tuberculin (PPD; Statens Seruminstitut, Copenhagen, Denmark; 2.5 μg/ml) were used as positive controls. A stimulation index (SI) was calculated for each triplicate by dividing mean cpm in experimental wells by that of the background value (cells in medium alone).
In healthy control donors, the highest SI values induced by Ep-CAM (n=34) was 1.7±1.2 (mean + 2SD). The corresponding value (n=21) for the 18-mer Ep-CAM-derived peptides was 2.1+1.4. Based on this result, the threshold level for a positive proliferative T-cell response was set to >3.0 for protein Ags and >3.5 for peptides.
Enzyme-linked immunospot assay (ELISPOT) for detection of IFN-γ producing cells
Cryopreserved PBMC were thawed and allowed to recover overnight at 37°C in humidified air with 5% CO2. Cells were tested in ELISPOT as previously described in [38] before and at a minimum of two time-points after vaccination (between week 12 and 34). Briefly, IFN-γ secretion was assessed on nitrocellulose membrane-bottomed plates (Millipore, Bedford, MA, USA) using a mouse anti-human IFN-γ mAb pair, clone 1-D1K (10 μg/ml) and 7-B6-1 (1 μg/ml; Mabtech AB, Stockholm, Sweden). The PBMC (105 cells/well) were incubated (20 h) in the presence of Ep-CAM, BCP and CEA proteins, respectively (100 and 1,000 ng/ml). Ep-CAM-derived and HIV RT control peptides [23] were added at 10 μg/ml concentration. Cells stimulated with PHA (10 μg/ml) and PPD (2.5 μg/ml) served as positive controls. Streptavidin-AP (1:1,000; Mabtech), followed by BCIP/NBT (Sigma), was used for development of the colour reaction. Results were quantified using an automated computer-assisted video imaging analysis system (Axioplan 2; Carl Zeiss Vision, Germany).
Results were expressed as the mean number of spot-forming units (SFU) per 106 cells in six experimental wells after subtraction of the background value (cells in medium alone). In some tests, the number of replicates was reduced due to lack of cells. Results were considered positive if the number of SFU in experimental wells was significantly (p<0.05) greater than the background value and was at least twice that of the background. SFU of healthy control donors against Ep-CAM (n=24), BCP (n=14) and CEA (n=14) were 11±3, 6±3 and 11±5 per 106 PBMC (mean ± SEM), respectively. No positive response against any of these proteins was detected in healthy controls.
Isolation of CD4+ and CD8+ T cell for TCR BV analysis
Positive selection of CD4+ and CD8+ T cells from PBMC obtained before and 18 weeks after vaccination was performed using anti-CD4 and anti-CD8 mAb coupled to magnetic beads according to the manufacturer’s instruction (Dynal AS, Oslo, Norway). The purification procedure yielded >95% purity of the respective subset as determined by flow cytometry analysis (FACScan, Becton-Dickinson, Mountain View, CA, USA).
In vitro stimulation of PBMC for TCR BV analysis
Peripheral blood mononuclear cells obtained 18 weeks after vaccination were incubated at 2×106 cells/ml in 24-well sterile tissue culture plates (Nunc, Denmark) in the presence of Ep-CAM (1 μg/ml), BCP (1 μg/ml) and medium alone, respectively, for 4 days at 37°C in humidified air with 5% CO2. CD4+ and CD8+ cells were isolated and analysed for TCR BV gene usage and CDR3 size distribution pattern.
RNA extraction and cDNA synthesis
Total RNA was extracted from 1×106 CD4+ or CD8+ T cells, using standard guanidine isothiocyanate-phenol-chloroform extraction method [6]. First-strand cDNA was synthesised from 3 μg of total RNA using RT Superscript II (Life Technologies, Inc., Gaithersburg, MD, USA) according to the manufacturer’s instructions.
Normalisation of TCR β chain-specific cDNA concentration
The TCR β chain-specific cDNA concentration was normalised using a TCR β chain constant region (BC)-specific PCR [10]. Briefly, a 5′ sense (5′ BC) and a 3′ anti-sense (3′ BC) primer (Table 1), both specific for TCR BC1 and BC2 genes, were used in a 30-cycle PCR with serial dilutions of cDNA. The PCR products were then electrophoresed on 1.5% ethidium bromide-stained agarose gels and photographed using a GDS 7500 UVP gel documentation system (UVP, Upland, CA, USA). TCR BC-specific bands (140 bp) were quantified by gel scanning (2400 Gel Scan XL; Amersham Biosciences, Uppsala, Sweden) and based on the scanned data, equal amounts of TCR β chain-specific cDNA were estimated and used in the subsequent TCR BV PCR amplifications.
Table 1.
Oligonucleotide sequences of primers and probes used for PCR amplification and detection of TCR β chains
| TCR BV 5′ | |
| TCR BV1 | 5′-GCA CAA CAG TTC CCT GAC TTG CAC-3′ |
| TCR BV2 | 5′-TCA TCA ACC ATG CAA GCC TGA CCT-3′ |
| TCR BV3 | 5′-GGG GTA CAG TGT CTC TAG AGA GA-3′ |
| TCR BV4 | 5′-ACA TAT GAG AGT GGA TTT GTC ATT-3′ |
| TCR BV5S1 | 5′-ATA CTT CAG TGA GAC ACA GAG AAA C-3′ |
| TCR BV5S2-3 | 5′-TTC CCT AAC TAT AGC TCT GAG CTG-3′ |
| TCR BV6S1-3 | 5′-AGG CCT GAG GGA TCC GTC TC-3′ |
| TCR BV7 | 5′-CCT GAA TGC CCC AAC AGC TCT C-3′ |
| TCR BV8 | 5′-ATT TAC TTT AAC AAC AAC GTT CCG-3′ |
| TCR BV 9 | 5′-CCT AAA TCT CCA GAC AAA GCT CAC-3′ |
| TCR BV10 | 5′-CTC CAA AAA CTC ATC CTG TAC CTT-3′ |
| TCR BV11 | 5′-TCA ACA GTC TCC AGA ATA AGG ACG-3′ |
| TCR BV12 | 5′-AAA GGA GAA GTC TCA GAT-3′ |
| TCR BV13S1 | 5′-CAA GGA GAA GTC CCC AAT-3, |
| TCR BV13S2 | 5′-GGT GAG GGT ACA ACT GCC-3′ |
| TCR BV14 | 5′-GTC TCT CGA AAA GAG AAG AGG AAT-3′ |
| TCR BV15 | 5′-AGT GTC TCT CGA CAG GCA CAG GCT-3′ |
| TCR BV16 | 5′-AAA GAG TCT AAA CAG GAT GAG TCC-3′ |
| TCR BV17 | 5′-CAG ATA GTA AAT GAC TTT CAG-3′ |
| TCR BV18 | 5′-GAT GAG TCA GGA ATG CCA AAG GAA-3′ |
| TCR BV19 | 5′-CAA TGC CCC AAG AAC GCA CCC TGC-3′ |
| TCR BV20 | 5′-AGC TCT GAG GTG CCC CAG AAT CTC-3′ |
| TCR BV21S1 | 5′-CTG GTT CAA TTT CAG GAT GAG AGT-3′ |
| TCR BV21S2 | 5′-GAT TCG ATA TGA GAA TGA GGA AGG-3′ |
| TCR BV21S3 | 5′-TCT GAT TCA GTT TCA GAA TAA CGG-3′ |
| TCR BV22 | 5′-AAA GAG GGA AAC AGC CAC TCT G-3′ |
| TCR BV23 | 5′-CGC TGT GTC CCC ATC TCT AAT C-3′ |
| TCR BV24 | 5′-CAG TGA CCC TGA GTT GTT CTC A-3′ |
| TCR constant gene primers | |
| 3′ BC | 5′-GTG CAC CTC CTT CCC ATT –3′ |
| 5′ BC | 5′-GTC GCT GTG TTT GAG CCA TCA GAA-3′ |
| BC primer | 5′-TTC TGA TGG CTC AAA CAC-3′ |
| BC-FITC | 5′FITC-GTG CAC CTC CTT CCC ATT-3′ |
| TCR probe | |
| BC reporter | 5′-CAC AGC GAC CTC GGG TGG GAG CAC-3′ |
Analysis of TCR BV gene usage
Polymerase chain reaction amplifications of BV-specific cDNA were performed using a panel of 28 TCR BV-specific 5′ primers and a TCR BC-specific 3′ primer (BC primer), recognising sequences in both BC1 and BC2 genes [10] (Table 1). PCR mixtures contained 10x concentrated buffer (100 mmol/l Tris-HCl, 15 mmol/l MgCl2, 500 mmol/l KCl, 1 mg/ml gelatin, pH 8.3; Roche, Mannheim, Germany); 0.2 mmol/l final concentration of each dNTPs (Roche); 0.1 μl Taq DNA polymerase (5 U/μl; Roche) and 0.5 μmol/l of each primer. Samples were overlaid with mineral oil and amplified in a thermocycler (Thermo Hybaid, Needham Heights, MA, USA) for 35 cycles with a temperature profile of 94°C for denaturation, 55°C for annealing and 72°C for extension. Each step lasted for 30 s, except for the last extension that continued for an additional 9 min to ensure complete extension of the products. To confirm the expected size of the amplified BV-BC PCR products, 10 μl of each product was electrophoresed on a 1.5% ethidium bromide-stained agarose gel and recorded photographically.
Southern blot analysis of TCR BV-BC products
Polymerase chain reaction products were subjected to electrophoresis on 1.5% agarose gels. The samples were then denatured using a solution containing 0.5 mol/l NaOH and 1.5 mol/l NaCl for 15 min and then neutralised in a solution containing 0.5 mol/l Tris-HCl (pH 7.2) and 1.5 mol/l NaCl for 15 min followed by incubation for 20 min in 10x SSC. The products were transferred onto nylon membranes (Amersham Biosciences) [31]. The membranes were air dried and treated for 2 h at 80°C.
5′-end labelling of BC oligonucleotide probe and hybridisation to PCR products
A TCR BC-specific oligonucleotide probe (BC reporter) (Table 1) recognising both BC1 and BC2 genes [10] was 5′-end labelled [31] using T4 polynucleotide kinase enzyme (Amersham Biosciences) and γ−32P-ATP (Amersham Biosciences). The nylon membranes were first prehybridised in a buffer containing 2x SSPE, 5x Denhardt’s solution and 0.5% sodium dodecyl sulfate (SDS) for 3 h at 42°C followed by hybridisation in the same buffer with 1×106 cpm/ml of 5′-end labelled BC reporter overnight at 42°C. They were then washed twice at 42°C in a washing solution containing 0.2x SSPE and 0.5% SDS and were subsequently exposed to Hyperfilm MP (Amersham Biosciences) for 6 h at −70°C.
Quantification of the relative usage of BV genes
The intensity of the signals shown by specific probing of the TCR BV-BC amplified products on the films was quantified by gel scanning. The relative usage of each BV gene was determined using a standard curve [13].
Analysis of CDR3 length polymorphism
To study the clonal pattern of TCR over-expressions, a number of over-expressed BV genes were selected and CDR3 length polymorphism analysis was performed as described in [26]. Briefly, cDNA samples were amplified in 40 cycles of PCR using the 5′ BV primers and a fluorescein isothiocyanate (FITC)-conjugated 3′ BC-FITC primer (Table 2). The products were then checked on 1.5% ethidium bromide-stained agarose gels. An aliquot was loaded onto 6% denaturing polyacrylamide-sequencing gel, and electrophoresis was performed in the presence of (50–500 bp) fluorescent size markers (Amersham Biosciences) in an ALF-DNA sequencing machine (Amersham Biosciences). The data were collected by a computer and analysed by the “Fragment Manager” software program (Amersham Biosciences). A dominant CDR3 peak was defined as a high-intensity signal with a dramatic reduction of other CDR3 signals within the particular TCR BV family, i.e. when the area under the curve was more than 50% of the total peak area [26].
Table 2.
Patient characteristics and immunisation schedules
| Patients | Age | Sex | AJCC stage | Tumour site | Prior therapya | Immunisationc (weeks) | Clinical status | Survivale (months) |
|---|---|---|---|---|---|---|---|---|
| P1 | 73 | M | III | Colon | No | Ep-CAM (0, 2, 6) | NED | 30+ |
| P2 | 63 | F | II | Colon | No | Ep-CAM (0, 2, 6) | LTR (11 m) | 12.5 |
| P3 | 72 | M | II | Colon | No | Ep-CAM (0, 2, 6) | LTR (7 m) | 10 |
| P4 | 77 | M | II | Rectum | No | Ep-CAM (0, 2) + Anti-Id (6) | NED | 54+ |
| P5 | 70 | M | II | Colon | No | Ep-CAM (0, 2) + Anti-Id (6) | NED | 56+ |
| P6 | 69 | M | II | Colon | No | Ep-CAM (0, 2) + Anti-Id (6) | NED | 56.5+ |
| P7 | 67 | F | II | Colon | No | Ep-CAM (0, 2) + Anti-Id (6) | NED | 26+ |
| P8 | 65 | M | II | Colon | No | Anti-Id (0, 2, 6) | NED | 20+ |
| P9 | 71 | M | II | Rectum | RTb | Anti-Id (0, 2, 6) | NEDd | 39 |
NED no evidence of disease, LTR local tumour recurrence
a Therapy before immunisation except for surgical resection of the tumour
b Pre-operative radiotherapy
c Patients received i.d./s.c. injections of Ep-CAM protein (400 μg/dose) and/or anti-Id (500 μg/dose) in combination with GM-CSF (75 μg/day, for four consecutive days at each immunisation)
d Died of disease (pneumonia) not related to CRC
e Survival is shown from start of immunisation until follow-up in patients alive (+) or until death
Statistical analysis
The non-parametric Wilcoxon–Mann Whitney two-tailed rank sum test was used for comparison of independent variables. For comparison of dependent observations, the two-tailed non-parametric Wilcoxon signed rank test was applied.
Results
Patient characteristics and adverse reactions
Characteristics of the patients and immunisation schedules are summarised in Table 2. The vaccination was well tolerated. Most patients developed local grade 1 or 2 injection-site reactions (pain, redness, heat, swelling, itching, blister or hypoaesthesia), which generally resolved within a week after injection. Systemic adverse events were occasionally reported and mild (grade 1). These consisted primarily of constitutional symptoms, such as fatigue, chills, fever, flu-like symptoms and myalgia. Three patients reported transient chest pain, probably attributed to GM-CSF.
Proliferative and IFN-γ T-cell responses
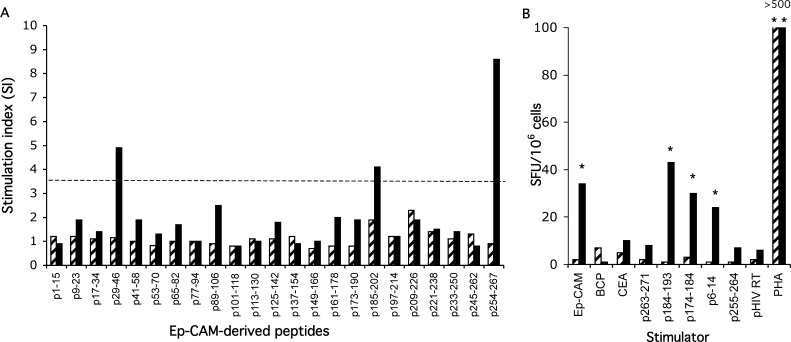
All patients with one exception (8/9) mounted a proliferative and/or IFN-γ T-cell response against the Ep-CAM protein after vaccination (Table 3). Immunisation with the bona fide antigen, either alone or in combination with anti-Id, induced a more potent lymphoproliferative response than anti-Id alone. Three patients receiving Ep-CAM vaccine mounted a lymphoproliferative response against baculovirus contaminants. The magnitude of the response against the Ep-CAM protein was, however, more than twice as high as that against BCP. IFN-γ-producing T cells were induced irrespective of vaccination with Ep-CAM or anti-Id or a combination of the two antigens. Two patients had IFN-γ-producing cells against the Ep-CAM protein prior to vaccination, which was boosted after immunisation. All patients mounted a proliferative T-cell response against 18-mer overlapping Ep-CAM-derived peptides and/or produced IFN-γ against MHC class I or II restricted Ep-CAM-derived peptides. As an example, results of patient 1 after immunisation with the Ep-CAM protein are shown in Fig. 1.
Table 3.
Cellular immune responses in patients
| Patients | Lymphoproliferative response (3H-thymidine incorporation; SI)a | IFN-γ producing cells (ELISPOT; SFU/106 cells)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-vaccine | Post-vaccine | Pre-vaccine | Post-vaccine | |||||||||
| Ep-CAM | BCP | CEA | Ep-CAM | BCP | CEA | Ep-CAM | BCP | CEA | Ep-CAM | BCP | CEA | |
| P1 | 0.8 | 1.2 | 1.7 | 15.2 | 1.9 | 1.6 | 2 | 7 | 5 | 34 | 0 | 10 |
| P2 | 1.2 | 1.8 | 0.8 | 51.3 | 1 | 0.9 | 2 | 0 | 2 | 0 | 2 | 4 |
| P3 | 1.1 | 1.2 | 1.1 | 3.7 | 2.8 | 2.7 | 0 | 0 | 0 | 14 | 15 | 0 |
| P4 | 1.5 | 1.4 | 1.3 | 57.4 | 7.9 | 3.9 | 88 | 14 | 13 | 107 | 16 | 12 |
| P5 | 1.3 | 1.2 | 0.5 | 18.2 | 6.0 | 1.1 | 33 | 15 | 8 | 96 | 20 | 4 |
| P6 | 2.9 | 1.6 | 2.4 | 24.9 | 9.5 | 2.6 | 14 | 16 | 7 | 25 | 12 | 13 |
| P7 | 1.3 | 1.4 | 1.6 | 2.5 | 1.6 | 2.1 | 60 | 14 | 7 | 140 | 14 | 10 |
| P8 | 1.0 | 0.7 | 0.9 | 2.8 | 1.4 | 1.2 | 0 | 14 | 0 | 0 | 11 | 0 |
| P9 | 1.2 | 1.0 | 1.0 | 3.9 | 3.0 | 1.4 | 27 | 5 | 7 | 83 | 17 | 20 |
a The highest SI value induced by 1 or 10 or 100 ng/ml protein antigen is shown. A proliferative T-cell response was considered positive (bold letters) if the SI value was >3.0 (mean+2SD of healthy controls)
b The mean number of spot-forming units (SFU) per 106 cells in experimental wells are shown after subtraction of the background value (cells in medium alone). The highest SFU induced by 100 or 1,000 ng/ml protein antigen is shown. Results were considered positive (bold letters) if the number of SFU in experimental wells was significantly (p<0.05) greater than the background value and was at least twice that of the background
Fig. 1.
Proliferative T-cell response against 18-mer Ep-CAM-derived peptides (a) and IFN-γ producing cells (ELISPOT) recognising the Ep-CAM protein and Ep-CAM-derived HLA A2 restricted peptides (b) before (hatched bar) and after immunisation (solid bar) with Ep-CAM protein (patient 1). a Dashed line represents the threshold level (mean + 2SD of healthy controls) for a positive proliferative T-cell response. b The mean number of spot-forming units (SFU) per 106 cells in experimental wells is shown after subtraction of the background value (cells in medium alone). Asterisks show significantly (p<0.05) greater SFU in experimental wells than in the background.
TCR BV gene usage of CD4+ and CD8+ T cells
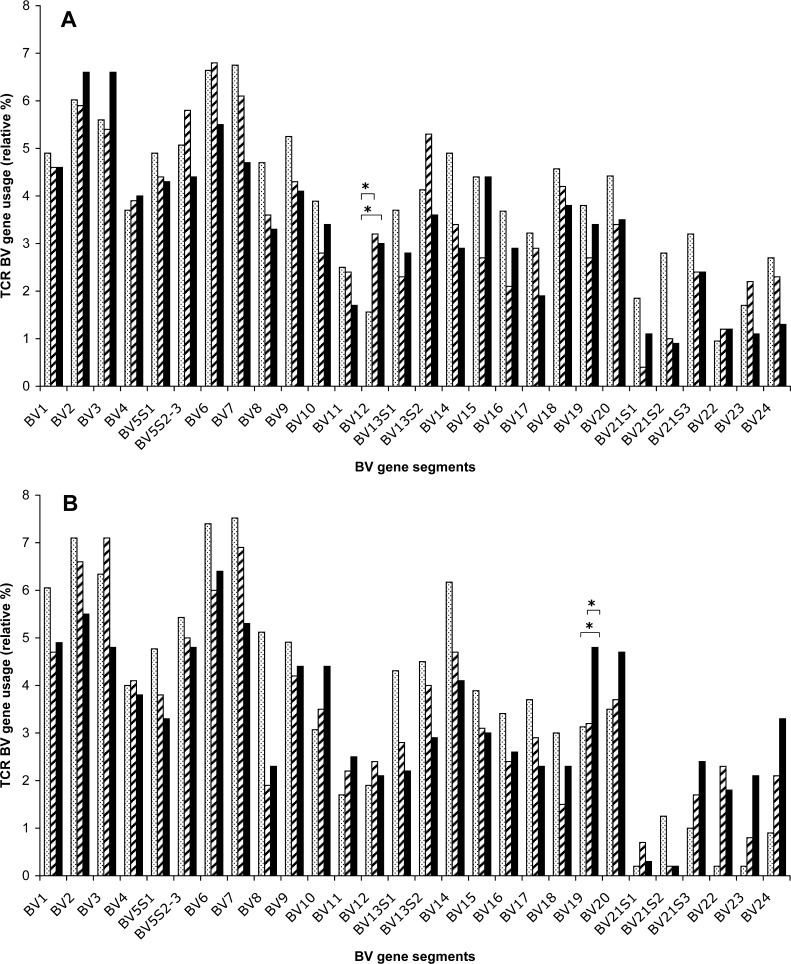
The TCR BV gene usage within the CD4+ T-cell subset of healthy donors and patients before and after immunisation is depicted in Fig. 2a. A statistically significant increase in BV12 usage was seen within CD4+ T cells of patients, both before and after immunisation, compared to controls (p<0.05). Within the CD8+ T-cell subset, a significantly higher usage of BV19 was observed in patients after immunisation than before immunisation (p<0.05) (Fig. 2b), which may reflect a T-cell response induced by vaccination. The BV19 family usage of post-vaccination CD8+ T cells was also significantly higher than that of controls (p<0.05). The BV19 gene usage of CD8+ T cells was similar in controls and patients before immunisation.
Fig. 2.
TCR BV gene usage (relative percentage of the total TCR BV gene usage) of blood-derived CD4+ (a) or CD8+ (b) T cells. The median percentage of TCR BV gene usage in healthy controls (dotted bar) (n=11), pre-vaccine (hatched bar) and post-vaccine (solid bar) samples of CRC patients (n=9) is shown. Asterisks indicate a significant (p<0.05) over-expression of a particular BV gene family in patients compared to controls or in post-vaccine compared to pre-vaccine samples of patients.
Over-expression of TCR BV gene families in individual patients
T-cell receptor BV gene family expression in individual patients higher than mean + 3SD of that of healthy controls is considered an over-expression [28, 34]. Table 4 shows the pattern of TCR BV gene over-expression in CD4+ and CD8+ T cells of individual patients before and after immunisation. Totally, 20 BV families were over-expressed, of which 13 families (65%) were seen in samples obtained after immunisation. TCR BV gene over-expression in the CD8+ subset was more frequently seen in post-immune than in pre-immune samples (7 vs. 2 families).
Table 4.
TCR BV gene family over-expression in individual patients
| Patients | CD4+ T cells | CD8+ T cells | ||
|---|---|---|---|---|
| Pre-immune | Post-immune | Pre-immune | Post-immune | |
| P1 | BV 2, 6, 7 | BV 7 | – | – |
| P2 | – | – | – | – |
| P3 | BV13S2 | BV11 | – | BV21S2 |
| P4 | – | BV14 | – | – |
| P5 | – | – | – | – |
| P6 | BV12 | – | BV11, 13S1 | BV10 |
| P7 | – | – | – | – |
| P8 | – | BV4, 8 | – | BV1, 5S2-3, 19, 23 |
| P9 | – | BV22 | - | BV20 |
TCR BV gene usage was determined by semi-quantitative RT-PCR and expressed as the relative percentage of the total TCR BV gene usage by CD4+ or CD8+ T cells. TCR BV over-expression in individual patients was defined as the percentage of a particular BV gene usage exceeding the mean+3SD of that of healthy controls
TCR BV gene over-expressions after in vitro stimulation with Ep-CAM
To assess the proportion of the induced immune response directed against baculovirus contaminants, post-immune (18 weeks) PBMC from patients 1 and 4 were stimulated in vitro with Ep-CAM and the control protein (BCP) for 4 days followed by analysis of TCR BV gene usage of the CD4+ and CD8+ T-cell subsets, respectively. A TCR BV over-expression was defined as >50% increase in a particular BV gene usage as compared to unstimulated cells (medium control). The results are summarised in Table 5. Ep-CAM as well as BCP stimulation evoked an increased usage of particular TCR BV genes, suggesting that a response against baculovirus contaminants was also induced. However, four different BV genes in both patients were over-expressed only in Ep-CAM-, but not BCP-stimulated CD4+ T cells. Similarly, four and seven different BV genes were over-expressed in CD8+ T cells solely after Ep-CAM stimulation in patients 1 and 4, respectively. In addition, the degree of over-expression induced by Ep-CAM was greater than that induced by BCP.
Table 5.
Over-expression of TCR BV gene families after in vitro stimulation with Ep-CAM and the control protein (BCP)
| BV gene families | Patient 1 | Patient 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ T cells | CD8+ T cells | CD4+ T cells | CD8+ T cells | |||||
| Ep-CAM | BCP | Ep-CAM | BCP | Ep-CAM | BCP | Ep-CAM | BCP | |
| BV1 | − | − | − | − | − | − | − | − |
| BV2 | + | − | − | − | − | − | − | − |
| BV3 | − | − | − | − | − | − | − | − |
| BV4 | − | − | − | − | − | − | − | − |
| BV5S1 | − | − | − | − | − | − | − | − |
| BV5S2-3 | − | − | − | − | − | − | − | − |
| BV6 | + | − | − | − | − | − | − | + |
| BV7 | + | − | − | − | − | − | − | − |
| BV8 | ++ | − | − | − | − | − | − | − |
| BV9 | − | − | + | − | − | − | − | − |
| BV10 | − | − | − | − | + | + | − | + |
| BV11 | − | − | − | − | − | − | + | − |
| BV12 | − | − | − | − | ++ | + | − | − |
| BV13S1 | − | − | − | − | − | − | − | − |
| BV13S2 | − | − | − | − | ++ | − | ++ | + |
| BV14 | − | − | + | − | − | − | − | − |
| BV15 | − | − | − | − | − | − | ++ | − |
| BV16 | − | − | + | − | − | − | − | − |
| BV17 | + | ++ | ++ | − | − | ++ | + | − |
| BV18 | − | − | − | − | − | − | − | − |
| BV19 | − | − | − | − | ++ | − | + | − |
| BV20 | − | − | − | − | ++ | + | + | − |
| BV21S1 | − | − | − | − | − | − | ++ | − |
| BV21S2 | − | − | ++ | + | − | − | − | + |
| BV21S3 | ++ | ++ | − | − | + | − | − | − |
| BV22 | ++ | + | − | − | − | − | ++ | − |
| BV23 | − | − | − | − | − | − | − | − |
| BV24 | − | − | − | − | ++ | − | − | − |
Post-vaccination PBMCs were cultured in the presence of Ep-CAM protein (1 μg/ml) or BCP (1 μg/ml) or medium alone for 4 days. CD4+ and CD8+ cells were separated by magnetic beads and the relative usage of TCR BV genes determined by semi-quantitative RT PCR. TCR BV gene over-expression was defined as >50% (+) or >100% (++) increase in usage of a particular BV gene compared to an unstimulated sample (medium alone)
CDR3 length polymorphism of CD8+ BV19+ T cells
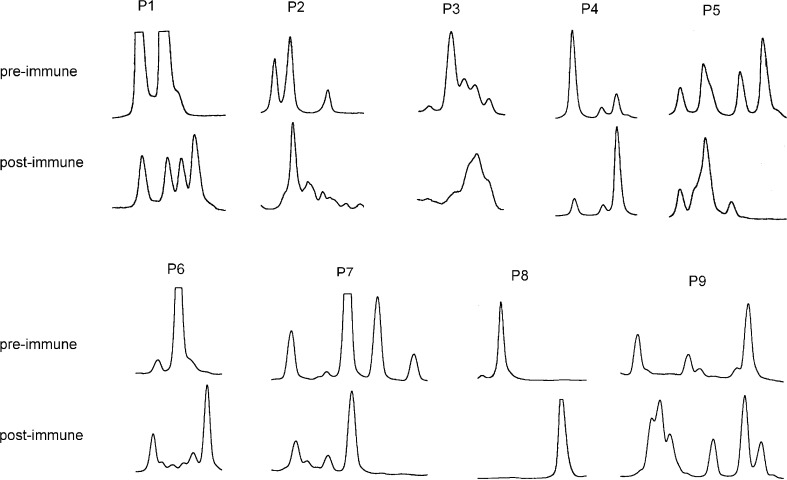
As shown above, BV19 usage of CD8+ T cells was significantly increased after vaccination (Fig. 2b). To study the CDR3 distribution pattern in over-expressed CD8+ BV19+ T cells, BV19-BC-FITC PCR products from pre- and post-immune CD8+ T cells of all patients were subjected to fragment analysis [26, 28]. The results are depicted in Fig. 3. In all patients, either new peaks emerged or an existing peak expanded considerably.
Fig. 3.
CDR3 length distribution pattern of CD8+ BV19+ T cells in CRC patients (n=9) before and after immunisation.
Patterns of clonality in selected BV gene families
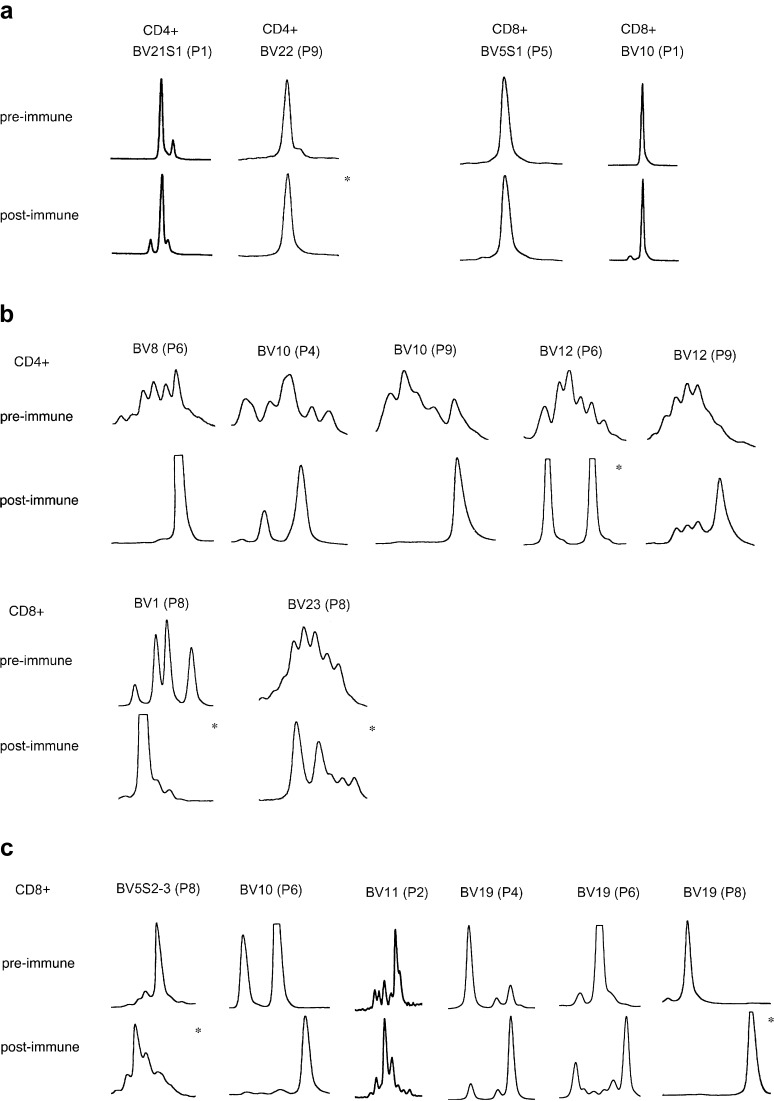
The pattern of clonality before and after immunisation was studied in several over-expressed (see Table 4) and some randomly selected, non-over-expressed BV gene families in individual patients using CDR3 length analysis (Fig. 4). Three patterns of CDR3 size distribution were observed: (1) both pre- and post- immune cells showed a monoclonal pattern with the same CDR3 size; (2) the CDR3 distribution pattern changed from a polyclonal to a more restricted mono-/oligoclonal pattern; (3) the CDR3 distribution pattern changed from a mono-/oligoclonal pattern to another mono-/oligoclonal pattern with a different size of the main peak.
Fig. 4.
CDR3 length polymorphism of several over-expressed (asterisks) and some randomly selected, non-over-expressed TCR BV genes before and after immunisation. a Monoclonal patterns with identical CDR3 size distribution pattern in pre- and post-immune CD4+ and CD8+ T cells. b CD4+ and CD8+ T cells where the CDR3 distribution pattern changed from a polyclonal to a more restricted mono-/oligoclonal pattern after vaccination. c A shift from a mono-/oligoclonal pattern to an alternative mono-/oligoclonal pattern with a different CDR3 size of the main peak after vaccination in CD8+ T cells.
CDR3 size distribution pattern after in vitro stimulation with Ep-CAM
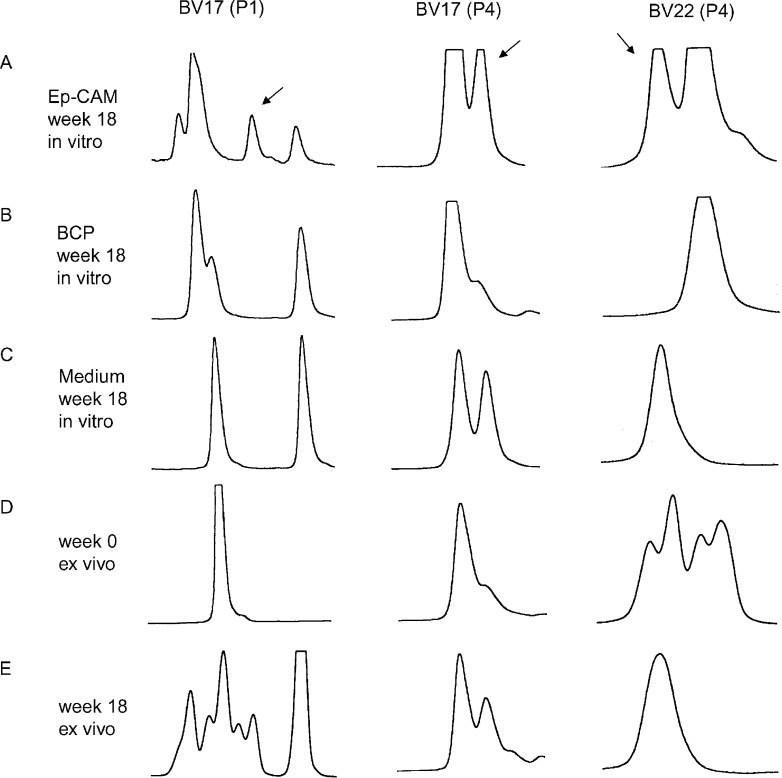
Post-immune (week 18) PBMC from patients 1 and 4 were analysed for TCR BV gene usage after in vitro stimulation with Ep-CAM or the control protein (BCP). Those TCR BV families that were over-expressed (Table 5) were subjected to CDR3 size distribution analysis. Figure 5 shows the results of CDR3 fragment analysis of CD8+ BV17+ cells of patient 1, and CD8+ BV17+ as well as CD8+ BV22+ cells of patient 4. Arrows indicate peaks, representing T-cell clones that were expanded after stimulation with Ep-CAM but not with BCP. For comparison, ex vivo pre- and post-immune samples were included. A new peak emerging in a post-vaccine sample was considered to represent an anti-Ep-CAM response if such a peak was not noted in pre-immune and BCP stimulated samples. Anti-Ep-CAM peaks were also noted in some post-immune samples without in vitro stimulation, when the magnitude of the anti-Ep-CAM T-cell clone in vivo was sufficiently expanded to be detected without further in vitro stimulation. Non-Ep-CAM-specific peaks in post-immune samples could also be seen due to an immune response against baculovirus contaminants.
Fig. 5.
CDR3 length distribution in post-immunisation CD8+ T cells in two patients after in vitro stimulation with Ep-CAM (a), BCP (b) and medium alone (c) compared to that of ex vivo cells obtained before (d) and after (e) immunisation.
Discussion
This study forms a part of a project aiming to develop a vaccination strategy in CRC patients using Ep-CAM as a target structure. In an attempt to induce an immune response against Ep-CAM, three different immunisation regimens were explored: the Ep-CAM protein or anti-Id was used alone or in combination and GM-CSF was given as an adjuvant cytokine. The Ep-CAM protein alone or in combination with anti-Id as compared to anti-Id alone induced a more potent proliferative T-cell response against the Ep-CAM protein. IFN-γ producing T cells were, however, induced irrespective of the immunising antigen. Cellular immune responses against Ep-CAM-derived peptides were generated in all patients vaccinated with the recombinant protein Ags. To further characterise the T-cell response, the TCR repertoire was analysed, as a restricted TCR repertoire in malignant diseases might be an indication of an anti-tumour immune response [14]. The amplification of clonal T-cell populations in regressive melanoma lesions is a well-known phenomenon [14]. CTL derived from lymphocytes infiltrating lung carcinoma showed a restricted TCR usage and was associated with prolonged survival [18]. A restricted TCR BV gene usage of tumour-infiltrating lymphocytes (TIL) has also been noted in cancer patients after immunotherapy [14, 19, 32, 41]. An association between response to IL-2 therapy and over-expression of a limited number of TCR BV families in TIL has also been described [40]. Oligoclonal expansions of blood-derived T cells have also been seen in patients with solid tumours treated with immunotherapy [15, 16, 33].
In the present study, significantly higher usage of BV12 within the CD4+ T-cell subset was observed in patients prior to immunotherapy as compared to healthy controls, which might suggest an involvement of this particular TCR BV family in a natural T-cell response against hitherto unidentified CRC antigens. Controls and patients stratified below and above median age showed no significant difference in BV12 usage within CD4+ T cells (data not shown), suggesting that age had no significant influence on the results. A recent study also indicates that gender has no effect on the TCR BV gene usage in CD4+ or CD8+ subpopulations [5]. Natural T-cell responses in CRC patients against MHC class I [24] and MHC class II [23] restricted epitopes have been reported. However, limited information is available on TCR BV gene usage. A restricted TCR repertoire usage by TIL of CRC patients has been described [2, 25]. Oligoclonality of CD8+ blood lymphocytes in CRC patients has also been reported but with large individual variability. However, the TCR repertoire was not analysed in comparison to healthy controls [1]. It has recently been suggested that a natural T-cell response against weakly immunogenic TAAs is not necessarily mono-/oligoclonal, but may exhibit broad diversity [9]. The expanded BV12+ CD4+ cells in the present study displayed a polyclonal pattern determined by CDR3 length analysis (data not shown).
Kumar et al. [16] treated CRC patients with IL-2 and noticed clonal T-cell expansions in TIL and in blood. In our study, a significant over-expression of BV19 was noted in CD8+ T cells after vaccination, which might reflect a T-cell expansion in response to vaccination. Eight of nine patients (89%) were either HLA A2 or HLA A3 positive. Several shared 9- and 10-mer Ep-CAM-derived peptides have been predicted to bind with high affinity to both HLA A2 and A3 molecules (http://SYFPEITHI.de/). We have recently shown that Ep-CAM or anti-Id immunisation induced IFN-γ producing T cells recognising Ep-CAM-derived MHC class I restricted peptides [23]. These findings might support the notion that an appropriate MHC-peptide combination may permit BV19+ T cells to be engaged in antigen recognition. This might drive a clonal expansion of BV19+ T cells, which could be evidenced by analysis of CDR3 size distribution. CD8+ BV19+ T cells expanded after immunisation showed either a more restricted CDR3 distribution pattern or developed another CDR3 peak as compared to the pre-immune sample. Both these patterns are compatible with an antigen-driven clonal expansion. It should be noted that a monoclonal pattern is not necessarily expected as clonal diversity may occur even in response to a single epitope [9]. It cannot be completely ruled out that the difference in the TCR BV patterns comparing pre- and post-immunisation samples was due to exposure to environmental antigens. However, this possibility seems unlikely, as the TCR patterns in three normal donors studied on two occasions at an interval of 18-weeks did not reveal a substantial difference between the two time-points (data not shown).
Individual patients showed a number of BV over-expressions in both CD4+ and CD8+ T cells. These over-expressions may result from a specific response to the antigen. However, it may also represent a phenomenon predominantly observed in elderly individuals [17]. Furthermore, it has recently been shown that non-expanded T cells in tumour patients might also show clonal or oligoclonal CDR3 distribution pattern, indicating a necessity to analyse a TAA-specific response after in vitro stimulation [28]. CDR3 length polymorphism was analysed in pre- and post-immunisation samples in some over-expressed and in randomly selected, non-over-expressed BV genes. The CDR3 distribution patterns after immunisation were similar in over-expressed and non-over-expressed BV families, i.e. they remained unaltered or changed to a more restricted clonal pattern, or a new dominant peak appeared but with a different CDR3 size. To confirm specificity, the TCR BV gene usage in post-vaccination samples of two selected patients, after in vitro stimulation with Ep-CAM, was studied. Several BV genes in both CD4+ and CD8+ T cells showed over-expression only after Ep-CAM, but not BCP stimulation, suggesting that the expansion of T cells using these particular BV genes entailed an anti-Ep-CAM response. According to the CDR3 size distribution analysis, however, among a large number of TCR BV over-expressions only three, all in the CD8+ T-cell subset, showed an anti-Ep-CAM response (BV17 in patient 1 and BV17 as well as BV22 in patient 4).
In conclusion, the Ep-CAM protein and/or anti-Id used for vaccination generated a proliferative T-cell response and/or IFN-γ-producing T cells against the Ep-CAM protein and Ep-CAM-derived peptides in CRC patients. Analysis of TCR BV gene usage suggests that BV12 over-expression in patients might represent a natural CD4+ T-cell response to hitherto unidentified CRC antigens and that BV19+ CD8+ T cells might be involved in a vaccine-induced immune response.
Acknowledgements
This study was supported by grants from the Swedish Research Council, Swedish Cancer Society, Cancer Society in Stockholm, King Gustav V Jubilee Fund, Cancer and Allergy Foundation, Torsten and Ragnar Söderberg Foundation, Gunnar Nilsson Cancer Foundation and Karolinska Institute Foundations. We thank Dr H. Wigzell for the scientific discussion.
Abbreviations
- AJCC
American Joint Committee on Cancer
- Anti-Id
Anti-idiotypic antibody
- BCP
Baculovirus control protein
- CEA
Carcinoembryonic antigen
- CRC
Colorectal carcinoma
- CTL
Cytotoxic T lymphocyte
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immunospot
- Ep-CAM
Epithelial-cell adhesion molecule
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IFN-γ
Gamma interferon
- mAb
Monoclonal antibody
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
- PPD
Purified protein derivative of tuberculin
- SDS
Sodium dodecyl sulfate
- SFU
Spot-forming unit
- SI
Stimulation index
- TAA
Tumour-associated antigen
- TCR
T-cell receptor
- TIL
Tumour-infiltrating lymphocytes
References
- 1.Akolkar PN, Gulwani-Akolkar B, McKinley M, Fisher SE, Silver J. Comparisons of T-cell receptor (TCR) V beta repertoires of lamina propria and peripheral blood lymphocytes with respect to frequency and oligoclonality. Clin Immunol Immunopathol. 1995;76:155–163. doi: 10.1006/clin.1995.1110. [DOI] [PubMed] [Google Scholar]
- 2.Baier PK, Wimmenauer S, Hirsch T, von Specht BU, von Kleist S, Keller H, Farthmann EH. Analysis of the T cell receptor variability of tumor-infiltrating lymphocytes in colorectal carcinomas. Tumour Biol. 1998;19:205–212. doi: 10.1159/000030008. [DOI] [PubMed] [Google Scholar]
- 3.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 4.Basak S, Eck S, Gutzmer R, Smith AJ, Birebent B, Purev E, Staib L, Somasundaram R, Zaloudik J, Li W, Jacob L, Mitchell E, Speicher D, Herlyn D. Colorectal cancer vaccines: antiidiotypic antibody, recombinant protein, and viral vector. Ann N Y Acad Sci. 2000;910:237–252. doi: 10.1111/j.1749-6632.2000.tb06712.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonfigli S, Doro MG, Fozza C, Derudas D, Dore F, Longinotti M. T-cell receptor repertoire in healthy Sardinian subjects. Hum Immunol. 2003;64:689–695. doi: 10.1016/S0198-8859(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res. 2001;7:1127–1135. [PubMed] [Google Scholar]
- 8.Demols A, Van Laethem JL. Adjuvant chemotherapy for colorectal cancer. Curr Gastroenterol Rep. 2002;4:420–426. doi: 10.1007/s11894-002-0013-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich PY, Walker PR, Quiquerez AL, Perrin G, Dutoit V, Lienard D, Guillaume P, Cerottini JC, Romero P, Valmori D. Melanoma patients respond to a cytotoxic T lymphocyte-defined self-peptide with diverse and nonoverlapping T-cell receptor repertoires. Cancer Res. 2001;61:2047–2054. [PubMed] [Google Scholar]
- 10.Doherty PJ, Roifman CM, Pan SH, Cymerman U, Ho SW, Thompson E, Kamel-Reid S, Cohen A. Expression of the human T cell receptor V beta repertoire. Mol Immunol. 1991;28:607–612. doi: 10.1016/0161-5890(91)90129-8. [DOI] [PubMed] [Google Scholar]
- 11.Fagerberg J, Frodin JE, Ragnhammar P, Steinitz M, Wigzell H, Mellstedt H. Induction of an immune network cascade in cancer patients treated with monoclonal antibodies (ab1). II. Is induction of anti-idiotype reactive T cells (T3) of importance for tumor response to mAb therapy? Cancer Immunol Immunother. 1994;38:149–159. doi: 10.1007/s002620050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields Proc Am Soc Clin Oncol. 2002;21:128a. [Google Scholar]
- 13.Grunewald J, Jeddi-Tehrani M, Pisa E, Janson CH, Andersson R, Wigzell H. Analysis of J beta gene segment usage by CD4+ and CD8+ human peripheral blood T lymphocytes. Int Immunol. 1992;4:643–650. doi: 10.1093/intimm/4.6.643. [DOI] [PubMed] [Google Scholar]
- 14.Halapi E, Jeddi-Tehrani M, Osterborg A, Mellstedt H. T cell receptor usage in malignant diseases. Springer Semin Immunopathol. 1999;21:19–35. doi: 10.1007/s002810050050. [DOI] [PubMed] [Google Scholar]
- 15.Jager E, Maeurer M, Hohn H, Karbach J, Jager D, Zidianakis Z, Bakhshandeh-Bath A, Orth J, Neukirch C, Necker A, Reichert TE, Knuth A. Clonal expansion of Melan A-specific cytotoxic T lymphocytes in a melanoma patient responding to continued immunization with melanoma-associated peptides. Int J Cancer. 2000;86:538–547. doi: 10.1002/(sici)1097-0215(20000515)86:4<538::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Farace F, Gaudin C, Triebel F. Clonal T cell expansion induced by interleukin 2 therapy in blood and tumors. J Clin Invest. 1996;97:1219–1226. doi: 10.1172/JCI118536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maini MK, Casorati G, Dellabona P, Wack A, Beverley PC. T-cell clonality in immune responses. Immunol Today. 1999;20:262–266. doi: 10.1016/S0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 18.Mami-Chouaib F, Echchakir H, Dorothee G, Vergnon I, Chouaib S. Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen. Immunol Rev. 2002;188:114–121. doi: 10.1034/j.1600-065X.2002.18810.x. [DOI] [PubMed] [Google Scholar]
- 19.Manne J, Mastrangelo MJ, Sato T, Berd D. TCR rearrangement in lymphocytes infiltrating melanoma metastases after administration of autologous dinitrophenyl-modified vaccine. J Immunol. 2002;169:3407–3412. doi: 10.4049/jimmunol.169.6.3407. [DOI] [PubMed] [Google Scholar]
- 20.Mellstedt H, Fagerberg J, Frodin JE, Hjelm-Skog AL, Liljefors M, Markovic K, Mosolits S, Ragnhammar P. Ga733/EpCAM as a target for passive and active specific immunotherapy in patients with colorectal carcinoma. Ann N Y Acad Sci. 2000;910:254–261. doi: 10.1111/j.1749-6632.2000.tb06713.x. [DOI] [PubMed] [Google Scholar]
- 21.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 22.Mosolits S, Harmenberg U, Ruden U, Ohman L, Nilsson B, Wahren B, Fagerberg J, Mellstedt H. Autoantibodies against the tumour-associated antigen GA733-2 in patients with colorectal carcinoma. Cancer Immunol Immunother. 1999;47:315–320. doi: 10.1007/s002620050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosolits S, Markovic K, Frodin JE, Virving L, Magnusson CG, Steinitz M, Fagerberg J, Mellstedt H. Vaccination with Ep-CAM protein or anti-idiotypic antibody induces Th1-biased response against MHC class I and II restricted Ep-CAM epitopes in colorectal carcinoma patients. Clin Cancer Res. 2004;10:5391–5402. doi: 10.1158/1078-0432.CCR-04-0425. [DOI] [PubMed] [Google Scholar]
- 24.Nagorsen D, Keilholz U, Rivoltini L, Schmittel A, Letsch A, Asemissen AM, Berger G, Buhr HJ, Thiel E, Scheibenbogen C. Natural T-cell response against MHC class I epitopes of epithelial cell adhesion molecule, her-2/neu, and carcinoembryonic antigen in patients with colorectal cancer. Cancer Res. 2000;60:4850–4854. [PubMed] [Google Scholar]
- 25.Ostenstad B, Sioud M, Lea T, Schlichting E, Harboe M. Limited heterogeneity in the T-cell receptor V-gene usage in lymphocytes infiltrating human colorectal tumours. Br J Cancer. 1994;69:1078–1082. doi: 10.1038/bjc.1994.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E, Jones D, Dethling J, Colman J, Coward L, MacGregor S. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002;360:671–677. doi: 10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 28.Rezvany MR, Jeddi-Tehrani M, Wigzell H, Osterborg A, Mellstedt H. Leukemia-associated monoclonal and oligoclonal TCR-BV use in patients with B-cell chronic lymphocytic leukemia. Blood. 2003;101:1063–1070. doi: 10.1182/blood-2002-03-0746. [DOI] [PubMed] [Google Scholar]
- 29.Riethmuller G, Holz E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Funke I, Pichlmaier H, Hirche H, Buggisch P, Witte J, Pichlmayr R. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–1794. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 30.Samanci A, Yi Q, Fagerberg J, Strigard K, Smith G, Ruden U, Wahren B, Mellstedt H. Pharmacological administration of granulocyte/macrophage-colony-stimulating factor is of significant importance for the induction of a strong humoral and cellular response in patients immunized with recombinant carcinoembryonic antigen. Cancer Immunol Immunother. 1998;47:131–142. doi: 10.1007/s002620050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor; 1989. [Google Scholar]
- 32.Schrama D, Fuchs E, Brocker EB, Thor Straten P, Becker JC. Identical T-cell receptor transcripts in multiple melanoma metastases. Cancer Res. 2002;62:5664–5667. [PubMed] [Google Scholar]
- 33.Sensi M, Farina C, Maccalli C, Lupetti R, Nicolini G, Anichini A, Parmiani G, Berd D. Clonal expansion of T lymphocytes in human melanoma metastases after treatment with a hapten-modified autologous tumor vaccine. J Clin Invest. 1997;99:710–717. doi: 10.1172/JCI119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soroosh P, Shokri F, Azizi M, Jeddi-Tehrani M. Analysis of T-cell receptor beta chain variable gene segment usage in healthy adult responders and nonresponders to recombinant hepatitis B vaccine. Scand J Immunol. 2003;57:423–431. doi: 10.1046/j.1365-3083.2003.01256.x. [DOI] [PubMed] [Google Scholar]
- 35.Staib L, Birebent B, Somasundaram R, Purev E, Braumuller H, Leeser C, Kuttner N, Li W, Zhu D, Diao J, Wunner W, Speicher D, Beger HG, Song H, Herlyn D. Immunogenicity of recombinant GA733-2E antigen (CO17-1A, EGP, KS1-4, KSA, Ep-CAM) in gastro-intestinal carcinoma patients. Int J Cancer. 2001;92:79–87. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1164>3.3.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Steinitz M, Tamir S, Frodin JE, Lefvert AK, Mellstedt H. Human monoclonal anti-idiotypic antibodies. I. Establishment of immortalized cell lines from a tumor patient treated with mouse monoclonal antibodies. J Immunol. 1988;141:3516–3522. [PubMed] [Google Scholar]
- 37.Strassburg CP, Kasai Y, Seng BA, Miniou P, Zaloudik J, Herlyn D, Koprowski H, Linnenbach AJ. Baculovirus recombinant expressing a secreted form of a transmembrane carcinoma-associated antigen. Cancer Res. 1992;52:815–821. [PubMed] [Google Scholar]
- 38.Ullenhag GJ, Frodin JE, Mosolits S, Kiaii S, Hassan M, Bonnet MC, Moingeon P, Mellstedt H, Rabbani H. Immunization of colorectal carcinoma patients with a recombinant canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA) and granulocyte macrophage colony-stimulating factor induced a tumor-specific cellular immune response. Clin Cancer Res. 2003;9:2447–2456. [PubMed] [Google Scholar]
- 39.Warren TL, Weiner GJ. Uses of granulocyte-macrophage colony-stimulating factor in vaccine development. Curr Opin Hematol. 2000;7:168–173. doi: 10.1097/00062752-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Willhauck M, Mohler T, Scheibenbogen C, Pawlita M, Brossart P, Schmier JW, Keilholz U. T-cell receptor beta variable region diversity in melanoma metastases after interleukin 2-based immunotherapy. Clin Cancer Res. 1996;2:767–772. [PubMed] [Google Scholar]
- 41.Willhauck M, Scheibenbogen C, Pawlita M, Mohler T, Thiel E, Keilholz U. Restricted T-cell receptor repertoire in melanoma metastases regressing after cytokine therapy. Cancer Res. 2003;63:3483–3485. [PubMed] [Google Scholar]