Abstract
Purpose: Dendritic cells (DCs) are considered potential candidates for cancer immunotherapy due to their ability to process and present antigens to T cells and stimulate immune responses. However, DC-based vaccines have exhibited minimal effectiveness against established tumors in mice and human cancer patients. The use of appropriate adjuvants can enhance the efficacy of DC-based cancer vaccines in treating established tumors. Methods: In this study we have employed α-tocopheryl succinate (α-TOS), a nontoxic esterified analogue of vitamin E, as an adjuvant to enhance the effectiveness of DC vaccines in treating established murine Lewis lung (3LL) carcinomas. Results: We demonstrate that locally or systemically administered α-TOS in combination with nonmatured DCs injected intratumorally (i.t.) or subcutaneously (s.c.) significantly inhibits the growth of preestablished 10-day tumors (mean tumor volume of 77.5 ± 17.8 mm3 on day 30 post–tumor injection) as compared to α-TOS alone (mean tumor volume of 471 ± 68 mm3 on day 30 post–tumor injection). Additionally, the adjuvant effect of α-TOS was superior to that of cyclophosphamide (CTX). The mean tumor volume on day 28 post–tumor injection in mice treated with CTX+DCs was 611 ± 94 mm3 as compared to 105 ± 36 mm3 in mice treated with α-TOS+DCs. Analysis of purified T lymphocytes from mice treated with α-TOS+DC revealed significantly increased secretion of IFN-γ as compared to T cells from the various control groups. Conclusion: This study demonstrates the potential usefulness of α-tocopheryl succinate, an agent nontoxic to normal cell types, as an adjuvant to augment the effectiveness of DC-based vaccines in treating established tumors.
Keywords: Antitumor, Dendritic cell, Immunotherapy, Lung cancer, α-Tocopheryl succinate, Vaccine
Introduction
The ability of dendritic cells (DCs) to efficiently prime naïve and memory T lymphocytes in an MHC-restricted fashion has been exploited in the design of cell-based vaccines for cancer immunotherapy [1, 12]. For these applications, DCs have been pulsed with defined peptides [3, 32, 35, 39, 50], tumor lysates [7, 10, 16, 34, 35], apoptotic tumor cells [17, 27, 41], tumor RNA [2, 33, 42], and cocultured [8, 23] or fused [38, 43, 45] with intact tumor cells. Whereas antigen-pulsed DCs have been shown to be capable of suppressing tumor growth or conferring resistance to secondary tumor challenge [8, 10, 15], they have been less effective in treating established tumors [8, 10, 14, 15]. Efforts to improve the effectiveness of DC-based vaccines in treating established disease have included the transfer of cytokine [6, 22, 29, 37] or chemokine [5, 13, 24] genes to stimulate Th1-specific responses or promote recruitment of T lymphocytes and DC [5, 22, 29], respectively. Taken together, these studies suggest that the use of adjuvants to improve the effectiveness of DC-based vaccines is a viable strategy for treating established primary or metastatic tumors.
In this study we employed α-tocopheryl succinate (α-TOS), an esterified analogue of vitamin E, as an adjuvant to increase the effectiveness of DC-based vaccines to treat established murine Lewis lung (3LL) carcinomas. Unlike other chemotherapeutic agents that are toxic to the host while causing tumor cell death [18, 21, 36], α-TOS has been reported to induce apoptosis of a wide variety of malignant cells while showing limited or no toxicity toward normal cells [20, 25, 36, 40, 48, 49]. In experimental tumor models, α-TOS has been demonstrated to inhibit the growth of melanoma [31], breast tumor [30], and colon cancer [46]. Our results demonstrate that systemic or local (intratumoral) administration of α-TOS in combination with DCs significantly inhibits growth of established tumors and causes tumor regression in a few cases. Furthermore, subcutaneously (s.c.)-injected DCs were just as effective as intratumorally (i.t.)-injected DCs at mediating tumor growth inhibition. Analysis of cytokine production by splenic lymphocytes from treated animals revealed that in contrast to mice injected only with α-TOS, combination treatment with α-TOS and DCs resulted in significantly higher levels of IFN-γ secretion. Finally, the antitumor response generated by the combination of DCs plus α-TOS was superior to that elicited by a combination of cyclophosphamide (CTX) plus DCs.
Materials and methods
Chemicals and reagents
Alpha-tocopheryl succinate (α-TOS), sodium succinate (NaS), cyclophosphamide (CTX), and ethanol were all purchased from Sigma (St Louis, MO, USA). Murine IL-4, GM-CSF, and TNF-α were purchased from Peprotech (Rocky Hill, NJ, USA). The Annexin-V Flous staining kit and the APO-Direct TUNEL assay kit were purchased from Roche Applied Sciences (Indianapolis, IN, USA) and BD Pharmingen (San Diego, CA, USA), respectively. The magnetic microbeads used for positive selection of CD4+ and CD8+ T cells from splenic lymphocytes were purchased from Miltenyi Biotec (Auburn, CA, USA). The mouse IFN-γ ELISA kit was purchased from R&D systems (Minneapolis, MN, USA). The antibodies for phenotyping DC (anti-IAb, anti-CD40, anti-CD80, anti-CD86) were purchased from Caltag Laboratories (Burlingame, CA, USA). The anti-CD11c antibody was purchased from BD Pharmingen (San Diego, CA, USA).
Cell culture
The murine Lewis lung carcinoma cell line 3LLD122 (metastatic clone) was kindly provided by Dr. Lea Eisenbach (Weizmann Institute of Science, Rehovot, Israel). The cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) with 10% fetal bovine serum. For DC culture, bone marrow (BM) cells were harvested from flushed marrow cavities of femurs and tibiae of C57BL/6 mice under aseptic conditions and cultured with 100 U/ml GM-CSF and 100 U/ml IL-4 at 106 cells/ml in complete media as previously described [26]. On day 6, the nonadherent and loosely adherent cells were collected, washed three times with phosphate-buffered saline (PBS) before injecting 2×106 cells i.t. or s.c. in mice. Dendritic cells were identified by FACS analysis on the basis of their expression of CD11c [43]. These DCs were 40–50% positive for CD11c expression, 70–80% positive for MHC class II (IAb) expression, and 4.5%, 19%, and 7.5% positive for expression of the costimulatory molecules, CD40, CD80, and CD86, respectively.
For in vitro stimulation (i.v.s.) of splenic lymphocytes, on day 6, DCs were pulsed with either 3LL tumor lysate or B16 tumor lysate at a ratio of three tumor cell equivalents per DC for 24 h and then matured with 200 U/ml TNF-α for 48 h [26].
α-TOS treatment and assessment of apoptosis
For the in vitro cell viability and apoptosis assays, the cells were plated at 105 cells/well in 6-well tissue culture dishes. Twenty-four hours later, the medium was replaced with fresh culture medium containing 10, 20, 40, or 80 μg/ml of α-TOS in 0.1% ethanol (final concentration vol/vol). Controls included treated cells, cells treated with 0.1% ethanol or NaS (in 0.1% ethanol). The cells were incubated in a 7% CO2 incubator for 4, 8, or 24 h. Nonadherent and adherent cells were collected, centrifuged at 200 g for 5 min and washed twice with PBS. Cell viability was assessed by staining with acridine orange (AO) and propidium iodide (PI) as previously described [11].
For the apoptosis assay, cells were treated with either 40 μg/ml α-TOS or NaS. After 4, 8, or 24 h, nonadherent and adherent cells were collected and stained with annexin V-FITC/PI following the manufacturer’s protocol (Roche Applied Sciences). Briefly, the cells were resuspended in annexin V–binding buffer and stained with annexin V-FITC and PI for 20 min in the dark. Binding buffer was added to the samples prior to flow cytometric analysis using the FACStarPLUS flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). The cells were gated on forward versus side scatter and bivariate scattergrams of annexin versus PI fluorescence were generated for analysis.
Animal studies
Six-week-old female C57BL/6 mice were purchased from the Harlan Sprague Dawley Laboratory (Indianapolis, IN, USA). All mice were housed at the University of Arizona animal facilities in accordance with the Principles of Animal Care (NIH publication No. 85-23, revised 1985). For establishment of primary tumors, each mouse was injected s.c. with 106 3LLD122 tumor cells in 100 μl PBS on the right hind flank. After tumors were established (~30 mm3), the mice were subjected to different treatment regimens for the various experiments. Unless mentioned otherwise, mice were injected intraperitoneally (i.p.) or i.t. with 200 mg/kg body weight (50 μl in 100% ethanol) of α-TOS on days 10, 14, and 18 after tumor cell injection. The control groups consisted of untreated mice, mice injected with 50-μl vehicle (100% ethanol) or NaS (in 10% ethanol). For the combination treatment, 1×106 DCs were injected i.t. or s.c. (on the opposite flank) on days 12, 16, and 20 in 100 μl of PBS. In experiments in which α-TOS was compared with CTX, 150 mg/kg body weight (50 μl in water) of CTX was injected alone (i.p.) or in combination with DC [44] using the above regimen. Tumor growth was monitored by measuring the tumor length and width with calipers and calculating the tumor volume according to the formula V=(LxW 2)/2 [47].
TUNEL assay
Tumors from mice treated with α-TOS or NaS were resected 48 h after each α-TOS injection, embedded in OCT compound, and frozen using dry ice and 2-methylbutane. Sections of frozen tumor (4 μm thick) were prepared and stained using the APO-Direct kit. Briefly, sections were fixed in 1% paraformaldehyde, washed in PBS, and immersed in 70% ethanol for 30 min. The sections were then reacted with staining solution containing TdT enzyme and FITC-labeled dUTP. After incubating the slides for 1 h at 37°C, the stain was washed off and incubated for 10 min with RNase/PI solution. The slides were rinsed with PBS and sections mounted using Fluoromount G. The presence of apoptosis in the tumor sections was evaluated by fluorescence microscopy (Nikon Eclipse TE2000-S; Nikon, Japan).
IFN-γ production
Mice from the various treatment groups were sacrificed and their spleens removed. Spleens were pooled from three animals from each treatment group. CD4+ and CD8+ T cells were separated from erythrocyte-depleted splenocytes by positive selection using the MiniMACS magnetic separation system (Miltenyi Biotec) according to the manufacturer’s instructions. One million CD4+ or CD8+ T cells were restimulated with 2.5×105 tumor lysate–pulsed TNF-α–matured DCs [26] in a 24-well tissue culture plate for 48 h. In order to determine the specificity of the immune response, DCs were pulsed with lysates derived from 3LL tumor cells or B16 melanoma cells. The supernatant was then collected and assayed for IFN-γ production by ELISA (mouse IFN-γ ELISA kit; R&D Systems), according to the manufacturer’s protocol.
Statistical analysis
For all analyses, Student’s t-tests were performed using Prism software (GraphPad, San Diego, CA, USA). Probability values of p<0.05 were considered indicative of significant differences between data sets.
Results
α-Tocopheryl succinate induces cell death in 3LL tumor cells
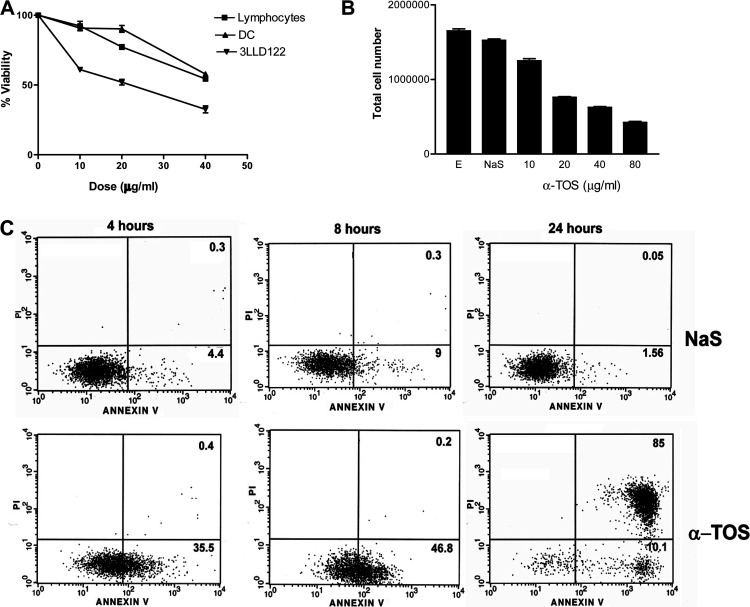
Initially, we evaluated the effect of α-TOS treatment on the viability of 3LL tumor, murine lymphocytes, and dendritic cells in vitro. Tumor cells were more susceptible to α-TOS than either lymphocytes or dendritic cells (Fig. 1A). α-TOS also caused a significant decrease (p<0.0001) in tumor cell yield (Fig. 1B) in a dose-dependent manner. Treatment of tumor cells with either ethanol (vehicle) or NaS had little impact on these parameters. Exposure to α-TOS also induced 3LL cells to undergo cell death as a function of time (Fig. 1C). At 4 h, α-TOS induced phosphatidyl serine translocation to the cell surface (Annexin V positive), which progressively increased with time leading to secondary loss of membrane integrity (Annexin V and PI positive) by 24 h. Transmission electron microscopy did not identify any cells with early (chromatin condensation, chromatin margination, nucleolar segregation) or late (nuclear fragmentation, apoptotic body formation) features of classic apoptosis after treatment with α-TOS (data not shown).
Fig. 1A–C.
Effect of α-TOS on tumor cell viability in vitro. Lymphocytes, dendritic cells or 3LL cells were plated in 6-well tissue culture plates. A The cells were treated in triplicate with 10, 20 or 40 μg/ml α-TOS or 0.01% ethanol. After 24 h, nonadherent and adherent cells were collected and evaluated for cell viability using AO and PI. B To evaluate cell yield, 3LL cells were treated with 10, 20, 40 or 80 μg/ml α-TOS, sodium succinate (NaS), or 0.01% ethanol (E). At each time point (4 h, 8 h, 24 h), nonadherent and adherent cells were collected and cell yield determined. No significant effect was observed at the 4-h or 8-h time point (data not shown). The results (A, B) depict the mean ± SEM of three independent experiments. C For the apoptosis assay, cells were treated with either 40 μg/ml α-TOS or NaS. At each time point, nonadherent and adherent cells were collected and stained using annexin V and PI. Numbers represent the percentages of cells with phosphatidyl serine externalization (lower right quadrant) and cells with loss of membrane integrity (upper right quadrant), respectively
α-TOS inhibits the growth of established tumors
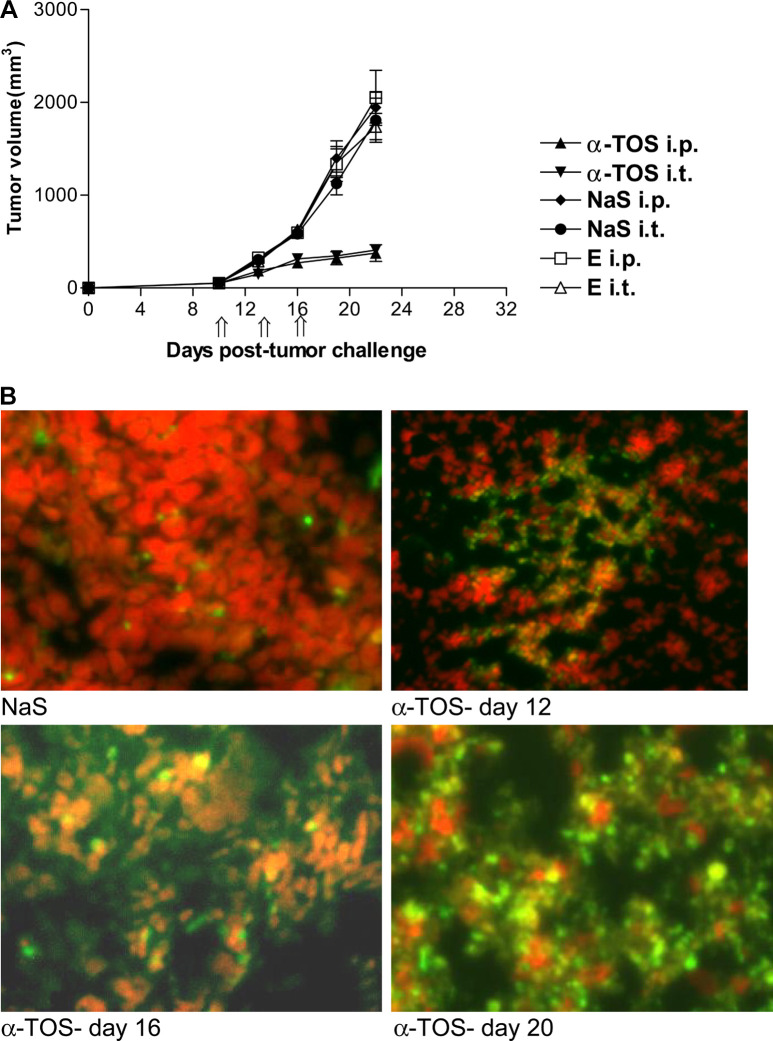
Since α-TOS induced cell death in vitro, we next evaluated its effect on established 3LL tumors in vivo. For this purpose α-TOS was injected i.p. or i.t. into mice bearing 10-day tumors. The data (Fig. 2A) indicate that α-TOS significantly inhibits tumor growth in vivo (p<0.0001). Tumors in the control groups (untreated, succinate i.p./i.t., ethanol i.p./i.t.) grew progressively, ranging from an average volume of 1,800 ± 237 mm3 to 2,000 ± 296 mm3 on day 22 post–tumor inoculation. In contrast, the tumor volume in α-TOS–treated mice ranged between 376.6 ± 39 mm3 (i.p.) and 409.7 ± 22.4 mm3 (i.t.) on day 22. These results demonstrate that α-TOS significantly inhibited growth of established tumors whether injected locally (i.t.) or systemically (i.p.). In order to determine whether tumor growth suppression was correlated with extensive DNA damage or apoptotic cell death, we analyzed tumor sections by TUNEL assay. Established tumors (~30 mm3) were injected with α-TOS or NaS (control) as described in “Materials and methods.” Forty-eight hours after each α-TOS injection, tumors were resected, frozen, sectioned, and evaluated for DNA damage or apoptosis by TUNEL assay. α-TOS caused significant DNA damage/apoptosis in tumors as compared to tumors treated with NaS (Fig. 2B).
Fig. 2A, B.
Effect of α-TOS on established 3LL tumors in vivo. Mice were injected s.c. with 106 3LL tumor cells. On day 10, established tumors (~30 mm3) were injected i.t. or i.p. with α-TOS, NaS, or ethanol. Injections (4-mg α-TOS) were given (depicted by double arrows on x-axis) on days 10, 13, and 16. A Data are mean tumor volumes ± SEM. B For the TUNEL assay, mice with preestablished tumors were injected with α-TOS on days 10, 14, and 18. Forty-eight hours after each α-TOS injection, tumors were resected, frozen, sectioned, and stained with the TUNEL reaction mixture. The orange and green regions in the tumor sections represent TUNEL positive, and red cells depict TUNEL negative cells. Magnification ×400
α-TOS potentiates the antitumor activity of DC vaccines
Since α-TOS inhibited the growth of established tumors, we hypothesized that it would enhance the efficacy of DC vaccines. Animals bearing established tumors (~30 mm3) were treated with a combination of α-TOS (i.p. or i.t.) + DCs (i.t.). The data (Fig. 3A, B) demonstrate that the combination of α-TOS+DCs was more effective than either α-TOS alone or DC alone at inhibiting tumor growth (p<0.0001). Twenty days following initial α-TOS treatment (30 days post–tumor cell injection) the mean tumor volume in mice treated with α-TOS+DCs was 77.5 ± 17.8 mm3 as compared to 471 ± 68 mm3 in mice treated with α-TOS alone (Fig. 3B). The mean tumor volumes in the control groups (untreated, NaS, DCs alone and NaS+DCs) were at least seven times (range of 575 mm3 to 750 mm3 on day 24 post–tumor cell injection) that of the group treated with α-TOS+DCs. These results demonstrate that α-TOS given i.p. or i.t. potentiates the antitumor activity of DCs in a synergistic fashion leading to a sixfold reduction in tumor growth rate. Although α-TOS+DCs treatment was effective at controlling primary tumor growth, it had a limited effect on the formation of visible lung metastases (4±1.1 in control groups vs 2±1.1 in α-TOS+DCs group).
Fig. 3A, B.
Effect of combination treatment with α-TOS and DCs on established 3LL tumors. Mice were injected s.c. with 106 3LL tumor cells. Established tumors (~30 mm3) were injected (i.p. or i.t.) with NaS or α-TOS on days 10, 14, and 18 (indicated by double arrows on x-axis). The mice were next injected with 106 immature DCs (i.t.) on days 12, 16, and 20 (indicated by single arrows on x-axis). A The tumor volumes of six individual mice per group. Untreated mice, mice injected with NaS alone, DCs alone, or with NaS+DCs were euthanized on day 24 due to high tumor burden. Mice injected with either α-TOS or α-TOS+DCs were euthanized on day 30. Down arrow indicates death of a mouse in the group due to high tumor burden. B Data are mean tumor volumes ± SEM
α-TOS is a more potent adjuvant for DC vaccines than cyclophosphamide
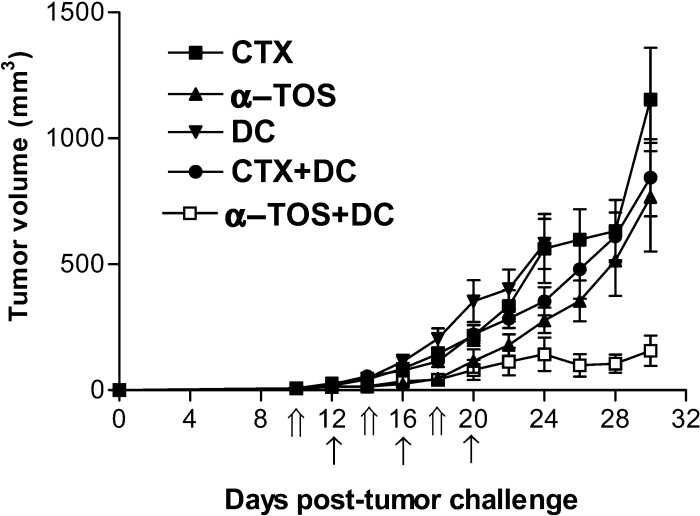
Recently Tong et al. [44] showed that the use of i.p.-administered cyclophosphamide (CTX) in combination with i.t.-injected DCs resulted in inhibition of tumor growth. We compared the effect of α-TOS and CTX on 3LL cells in vitro and observed that CTX causes tumor cell death (cell viability 62% after 24-h treatment) but is not as effective as α-TOS (cell viability 48% after 24-h treatment) (data not shown). Unlike α-TOS which caused DNA damage/apoptosis of tumors in vivo, TUNEL assay did not reveal significant apoptotic death induced by CTX treatment (data not shown). We next evaluated the ability of both drugs to improve the effectiveness of DC vaccines. The data (Fig. 4) show that α-TOS (i.p.) in conjunction with DCs (i.t.) resulted in a more significant inhibition (p<0.0001) of tumor growth than CTX+DCs. On day 28 post–tumor cell injection, the mean tumor volumes in mice treated with α-TOS+DCs and CTX+DCs were 105.2 ± 36 mm3 and 611 ± 95 mm3, respectively. Also, two of the seven mice in the α-TOS+DC–treated group showed complete tumor regression by day 20. These results clearly demonstrate that in this tumor model, the combination of α-TOS+DCs is more effective than CTX+DCs in treating established tumors.
Fig. 4.
Comparison of the effects of α-TOS and CTX as adjuvants of DC vaccines. Mice were injected s.c. with 106 3LL tumor cells. They were injected with 4-mg α-TOS or 3-mg CTX i.p. on days 10, 14, and 18 (indicated by double arrows on x-axis). DCs (106 cells) were injected i.t. on days 12, 16, and 20 (indicated by single arrows on x-axis). Data are mean tumor volume ± SEM of seven mice per group. Mice injected with DCs alone were euthanized on day 24 due to high tumor burden
Subcutaneous administration of DCs is as effective as intratumoral administration of DCs in inhibiting tumor growth
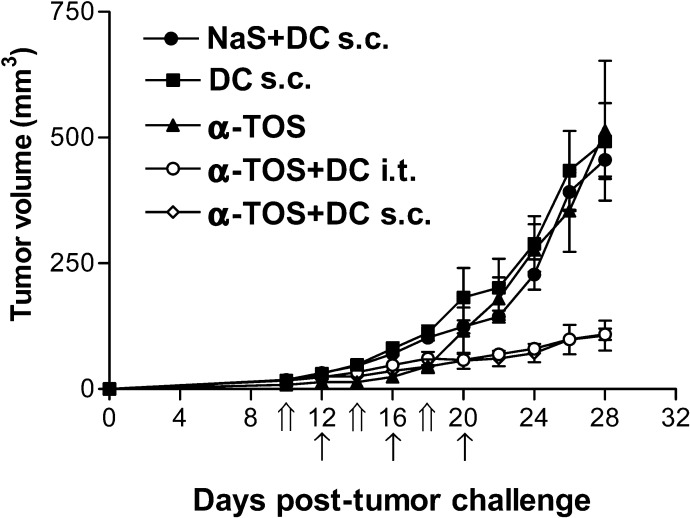
Since s.c. injection of DCs is the usual route of DC vaccinations, we compared the effectiveness of s.c. versus i.t. injection of DCs in treating established tumors. Mice with established tumors were injected with α-TOS (i.p.) followed by i.t. or s.c. injection of DCs similar to previous regimens. On day 28 post–tumor cell injection, the mean tumor volumes of mice receiving s.c. and i.t. injection of DCs were 98.3 ± 29.3 mm3 and 98.8 ± 9.8 mm3, respectively, compared with controls, which ranged between 493 ± 75 mm3 (DCs alone) and 514 ± 138 mm3 (α-TOS alone) (Fig. 5). The results demonstrate that when used in conjunction with α-TOS, s.c. and i.t. routes of DC injection are equally effective at inhibiting the growth of preestablished 3LL tumors. The antitumor effect in animals vaccinated with α-TOS+DCs was significantly (p=0.0003) superior to that observed in mice injected with DCs or α-TOS alone.
Fig. 5.
Antitumor activity of s.c. versus i.t. DC vaccines. 106 3LL tumor cells were injected s.c. in mice. After development of palpable tumors on day 10, mice were given injections of α-TOS, DCs alone, α-TOS+DCs (i.t.), or α-TOS+DCs (s.c.). α-TOS injections were given on days 10, 14, and 18 (indicated by double arrows on the x-axis) followed by DC injections on days 12,16, and 20 (indicated by single arrows on the x-axis). Data are mean tumor volumes ± SEM of tumor volumes of seven mice per group
Combination treatment with α-TOS+DCs elicits increased IFN-γ production by CD4+ and CD8+ T lymphocytes
To determine if the superior antitumor effect of α-TOS+DCs treatment is associated with enhanced T-lymphocyte responses, CD4+ and CD8+ T cells were isolated from spleens by immunomagnetic bead separation, restimulated in vitro with tumor lysate–pulsed, TNF-α–matured DCs, and evaluated for IFN-γ production (Fig. 6). CD4+ T cells from mice injected with α-TOS (i.p.) + DCs (s.c.) produced significantly (p<0.05) higher amounts of IFN-γ (2,223.6 ± 38.5 pg/ml) than those from mice injected with α-TOS (585.9 ± 4.3 pg/ml) alone or DCs alone (204.3 ± 2.4 pg/ml). The amount of IFN-γ produced by CD4+ T cells from mice treated with ethanol only (79.7 ± 1.4 pg/ml) was significantly (p<0.05) lower than that produced by cells from mice treated with α-TOS alone, DCs alone, or α-TOS+DCs. CD8+ T cells from mice injected with α-TOS (i.p.) + DCs (s.c.) also produced significantly higher amounts of IFN-γ (319 ± 3.8 pg/ml) than those from mice treated with α-TOS alone (188.7 ± 2.1 pg/ml), DCs (160.7 ± 3.4 pg/ml) alone, or ethanol alone (79.7 ± 1.1 pg/ml). However, CD4+ T cells secreted significantly more IFN-γ than did CD8+ T cells, in all groups tested. The results demonstrate that a combination of α-TOS+DCs causes immune activation, which may play a role in inhibiting tumor growth. Most importantly, the response was tumor specific since T cells restimulated with DCs pulsed with irrelevant B16 (H-2b) tumor lysate produced only minimal amounts of IFN-γ.
Fig. 6.

Effect of combination treatment with α-TOS+DCs on IFN-γ secretion by splenic lymphocytes. Spleens were isolated on day 30 post–tumor injection from each of three mice in each treatment group and pooled. CD4+ and CD8+ T cells were isolated from spleens by immunomagnetic bead separation, restimulated in vitro with tumor lysate–pulsed, TNF-α–matured DCs in 24-well tissue culture plates for 48 h. The supernatants were collected and assayed by ELISA for the production of IFN-γ. Data are mean ± SEM of triplicate samples
Discussion
In this study, we investigated the effectiveness of α-TOS and DCs in treating preestablished Lewis lung (3LL) tumors. Our results demonstrate that α-TOS by itself, whether administered locally (i.t.) or systemically (i.p.), significantly inhibits the growth of 3LL tumors. This finding corroborates published reports using other tumor models [30, 31, 46]. The TUNEL analysis demonstrated that α-TOS was capable of causing DNA damage/apoptosis of tumor cells in vivo. Most importantly, we show for the first time, that α-TOS synergizes with non-antigen-pulsed, nonmatured DCs in vivo to mediate significant tumor growth inhibition resulting in complete tumor regression in some cases. Nonmatured DCs were just as effective as matured, nonpulsed or tumor lysate–pulsed DCs in controlling tumor growth. Preestablished tumors that were treated with α-TOS plus DCs were one quarter the size of tumors in animals treated with α-TOS or DCs alone by day 28. The superior effect of the combination therapy was correlated with increased IFN-γ production by CD4+ and CD8+ T lymphocytes compared with controls, suggesting a polarization toward a Th1 cell–mediated immune response. Our studies support those of Candido et al. [4] using the MT-901 murine breast cancer cell line that exhibits a significant baseline level of apoptosis in vivo. They demonstrated that i.t.-injected, nonpulsed DCs mediated tumor growth inhibition of MT-901 tumors, and that this effect was enhanced by intravenous injection of TNF-α, an apoptosis-inducing agent. Our studies also corroborate and extend the work of Tong et al. [44] who demonstrated enhanced antitumor activity against established CT26 colon tumors following combination therapy with i.t.-injected DCs plus i.p.-injected cyclophosphamide (CTX).
Unlike the majority of DC-based vaccine strategies, our approach eliminates the requirement for additional ex-vivo manipulations such as maturation and/or loading of DCs with tumor antigens in order to generate DCs capable of mediating antitumor activity in vivo. It is conceivable that the injected DCs migrate to the tumor site and ingest apoptotic bodies resulting in their maturation [27]. Our recent findings (Ramanathapuram et al., manuscript in preparation) that: (1) the antitumor activity of immature DCs plus α-TOS was comparable to that of mature tumor lysate–pulsed DCs plus α-TOS, and (2) coincubation of soluble components of α-TOS–treated tumor cells with immature DCs causes up-regulation of the costimulatory molecules, CD80 and CD86, on DCs, suggest that α-TOS may induce DC maturation in vivo. Such mature DCs are able to migrate to secondary lymphoid organs where they initiate antitumor T-cell responses [1]. Another important feature of this study is that the DCs, whether administered i.t. or s.c., were equally effective at suppressing the growth of preestablished tumors. This observation suggests that the transferred DCs are able to localize at the tumor site [24] and migrate to secondary lymphoid organs where they stimulate antitumor effector T cells [9, 19, 28, 37].
Comparison of the adjuvant effects of α-TOS with that of CTX demonstrated that α-TOS was more effective than CTX in enhancing the effectiveness of the adoptively transferred DCs. In our studies, statistically significant suppression of tumor growth by CTX treatment alone or in conjunction with DCs did not occur. These findings are in contrast to the study by Tong et al. [44] who reported that CTX potentiated the antitumor effect of DC-based vaccines in treating experimental CT26 colon tumors. The discrepant findings may be due, in part, to the differences in the tumor models as well as the numbers and dosing regimens of DCs used in both studies.
In summary, this study demonstrates the adjuvant effect of a chemotherapeutic agent that is selectively toxic to tumor cells, on DC-based vaccines in controlling the growth of preestablished tumors. Since α-TOS preferentially destroys tumor cells [36], it is potentially less likely to induce adverse effects compared with conventional apoptosis-inducing chemotherapeutic drugs [18, 21, 36] and may therefore be clinically useful for enhancing antitumor immune responses. The use of α-TOS plus DCs represents a potentially novel chemoimmunotherapy approach that could be readily translated to the clinic for treating cancer patients.
Acknowledgements
We would like to thank Barbara Carolus for flow cytometric analysis and Meghan Kreeger for technical assistance.
Abbreviations
- AO
acridine orange
- CTX
cyclophosphamide
- DC
dendritic cell
- dUTP
deoxyuridine triphosphate
- FACS
fluorescence-activated cell sorter
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IFN-γ
interferon-gamma
- IL-4
interleukin-4
- NaS
sodium succinate
- OCT
optimal cutting temperature
- PBS
phosphate-buffered saline
- PI
propidium iodide
- Tdt
terminal deoxynucleotidyl transferase
- TNF-α
tumor necrosis factor alpha
- α-TOS
α-tocopheryl succinate
Footnotes
Supported by grants 1 RO1 CA94111-02 from the NIH and DAMD 17010126 from the DOD.
References
- 1.Banchereau Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Boczkowski Cancer Res. 2000;60:1028. [PubMed] [Google Scholar]
- 3.Burdin Cell Biol Toxicol. 2001;17:67. doi: 10.1023/A:1010944003649. [DOI] [PubMed] [Google Scholar]
- 4.Candido Cancer Res. 2001;61:228. [PubMed] [Google Scholar]
- 5.Cao J Immunol. 1998;161:6238. [PubMed] [Google Scholar]
- 6.Cao Immunology. 1999;97:616. doi: 10.1046/j.1365-2567.1999.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Clin Cancer Res. 2002;8:1021. [Google Scholar]
- 8.Coveney Surgery. 1997;122:228. doi: 10.1016/s0039-6060(97)90013-1. [DOI] [PubMed] [Google Scholar]
- 9.Eggert Cancer Res. 1999;59:3340. [PubMed] [Google Scholar]
- 10.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foglieni Histochem Cell Biol. 2001;115:223. doi: 10.1007/s004180100249. [DOI] [PubMed] [Google Scholar]
- 12.Fukao Trends Immunol. 2002;23:231. doi: 10.1016/S1471-4906(02)02198-1. [DOI] [PubMed] [Google Scholar]
- 13.Fushimi J Clin Invest. 2000;105:1383. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich Cell Immunol. 1996;170:101. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich Cell Immunol. 1996;170:111. doi: 10.1006/cimm.1996.0140. [DOI] [PubMed] [Google Scholar]
- 16.Gatza J Immunol. 2002;169:5227. doi: 10.4049/jimmunol.169.9.5227. [DOI] [PubMed] [Google Scholar]
- 17.Gregoire Vaccine. 2003;21:791. doi: 10.1016/S0264-410X(02)00600-X. [DOI] [PubMed] [Google Scholar]
- 18.Henderson Am Heart J. 1980;99:671. doi: 10.1016/0002-8703(80)90743-7. [DOI] [PubMed] [Google Scholar]
- 19.Hirao Cancer Res. 2000;60:2209. [PubMed] [Google Scholar]
- 20.Israel Nutr Cancer. 2000;36:90. doi: 10.1207/S15327914NC3601_13. [DOI] [PubMed] [Google Scholar]
- 21.Jo Nat Med. 2000;6:564. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 22.Ju Cancer Res. 2001;61:3735. [PubMed] [Google Scholar]
- 23.Kim Int Immunopharmacol. 2001;1:1421. doi: 10.1016/S1567-5769(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 24.Kirk Cancer Res. 2001;61:8794. [PubMed] [Google Scholar]
- 25.Kline J Nutr. 2001;131:161S. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]
- 26.Kobie Cancer Res. 2003;63:1860. [PubMed] [Google Scholar]
- 27.Kotera Cancer Res. 2001;61:8105. [PubMed] [Google Scholar]
- 28.Labeur J Immunol. 1999;162:168. [PubMed] [Google Scholar]
- 29.Liu Leuk Res. 2002;26:757. doi: 10.1016/S0145-2126(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 30.Malafa J Surg Res. 2000;93:163. doi: 10.1006/jsre.2000.5948. [DOI] [PubMed] [Google Scholar]
- 31.Malafa Surgery. 2002;131:85. doi: 10.1067/msy.2002.119191. [DOI] [PubMed] [Google Scholar]
- 32.Mayordomo Nat Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell Curr Opin Mol Ther. 2000;2:176. [PubMed] [Google Scholar]
- 34.Moingeon Vaccine. 2001;19:1305. doi: 10.1016/S0264-410X(00)00372-8. [DOI] [PubMed] [Google Scholar]
- 35.Nestle Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 36.Neuzil Br J Cancer. 2001;84:87. doi: 10.1054/bjoc.2000.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishioka Cancer Res. 1999;59:4035. [PubMed] [Google Scholar]
- 38.Orentas Cell Immunol. 2001;213:4. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- 39.Porgador J Exp Med. 1995;182:255. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose J Surg Res. 2001;95:19. doi: 10.1006/jsre.2000.6022. [DOI] [PubMed] [Google Scholar]
- 41.Scheffer Int J Cancer. 2003;103:205. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt J Cancer Res Clin Oncol. 2001;127:203. doi: 10.1007/s004320000201. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Clin Immunol. 2001;101:192. doi: 10.1006/clim.2001.5112. [DOI] [PubMed] [Google Scholar]
- 44.Tong Cancer Res. 2001;61:7530. [PubMed] [Google Scholar]
- 45.Wang J Immunol. 1998;161:5516. [PubMed] [Google Scholar]
- 46.Weber Clin Cancer Res. 2002;8:863. [Google Scholar]
- 47.Wu Cancer Immunol Immunother. 2001;50:229. doi: 10.1007/s002620100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Nutr Cancer. 1997;27:92. doi: 10.1080/01635589709514508. [DOI] [PubMed] [Google Scholar]
- 49.Yu Nutr Cancer. 1999;33:26. doi: 10.1080/01635589909514744. [DOI] [PubMed] [Google Scholar]
- 50.Zitvogel J Exp Med. 1996;183:87. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]