Abstract
Tumor-induced blood vessel formation is a key process for the growth and spread of solid tumors, traditionally attributed to activated host endothelial cells (angiogenesis). Recently, highly aggressive cancer cells have been shown to form vascular channels in the absence of endothelial cells (vasculogenic mimicry). In this work, we have focused on the common dependence of both processes in their interactions with the surrounding extracellular matrix. We had previously described a human recombinant anti-laminin antibody that blocked the capillary morphogenesis of human endothelial cells. Here, we demonstrate that the purified antibody is capable of inhibiting channel formation by human cancer cells, suggesting a common morphogenic pathway in both processes. Moreover, matrix-embedded cells producing antibody fragments may render the surrounding matrix non-permissive for aggressive tumor cells. These results open the way for the development of new therapeutic strategies for cancer.
Keywords: Extracellular matrix, Laminin, Capillary morphogenesis, Tumor plasticity, Single-chain antibody fragments
Introduction
It has been known for 3 decades that the growth and spread of malignant cells from primary tumor deposits depends on their ability to form new blood vessels [12], with the sprouting from preexisting capillaries being the most accepted mechanism of tumoral angiogenesis [7]. However, recent findings have considerably widened this view, including reports of tumor lymphangiogenesis [2], mosaic vessels contributed by both endothelial and tumor cells [8], and vessels lined exclusively by cancer cells mimicking endothelial cells (vasculogenic mimicry) [11, 18]. The term vasculogenic mimicry describes the ability of highly aggressive, but not nonaggressive, tumor cells to form a pattern of vasculogenic-like networks in three-dimensional cultures. Although still controversial [19, 20], this concept has gained credibility from recent evidence [22]. Tumor plasticity is reflected by the expression of endothelial-cell-associated genes, such as VE-cadherin and CD34 [6, 14, 16]. Moreover, the biological relevance of vasculogenic mimicry has been demonstrated in an ischemic mouse model, where metastatic melanoma cells participate in reperfusion [15]. Initially described in uveal and cutaneous melanomas, vasculogenic mimicry has been reported to occur in ovarian carcinoma [29], breast cancer [17], and prostate cancer cell lines [28], and could represent a general component of tumor development [5].
Vasculogenic mimicry, as well as angiogenesis, is strongly influenced by the tumoral microenvironment [5, 15] and implies complex interactions between the extracellular matrix (ECM) and both proteolytic enzymes and adhesion molecules on the surface of the involved cells. Basement membranes (BM) are highly specialized ECMs, usually found underlying epithelial and endothelial cells [30]. BM deposition is known to be a key step in the organization and maturation of newly formed vessels [4], and the main functionally active component of BM, laminin, has recently proved to play an essential role in vasculogenic mimicry by aggressive melanoma cells [27].
We previously reported the generation and characterization of human recombinant antibodies specific for native laminin epitopes [23]. One of these antibodies (L36) blocked the formation of capillary-like structures (CLS) by human endothelial cells (EC) in vitro [24]. Furthermore, L36 inhibited angiogenesis in vivo and prevented the establishment and growth of subcutaneous tumors in mice [24]. In this work, we show that L36 also inhibits patterned network development by human cancer cell lines, selected from a panel for their ability to form tubular structures in three-dimensional culture.
Materials and methods
Reagents
Solubilized BM preparation (Matrigel) extracted from the Engelbretch-Holm-Swarm (EHS) mouse tumor was from Becton Dickinson Labware (Bedford, MA, USA). Tissue culture materials were from Invitrogen Life Technologies (Gaithersburg, MD, USA). Epidermal growth factor (EGF), heparin, trypsin, and hydrocortisone were from Sigma Chemical (St. Louis, MO, USA).
Cells and culture conditions
The human microvascular endothelial cell line CDC/EU.HMEC-1 (HMEC-1) [1] was kindly supplied by Dr E. Ades (Centers for Disease Control, Atlanta, GA, USA) and was cultured in MCBD 131 medium supplemented with 10% FCS, 10-ng/ml EGF, and 1-mg/ml hydrocortisone. Human cervix carcinoma HeLa cells (CCL-2), human colon adenocarcinoma cell lines LS 174T (CL-188) and COLO205 (CCL 222), and human fibrosarcoma HT-1080 cells (CCL-121) were obtained from the ATCC (Rockville, MD, USA). HeLa, LS 174T, and HT-1080 cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 10% FCS, and COLO205 cells were grown in RPMI medium supplemented with 10% FCS. The human metastatic cutaneous melanoma cell lines WM1361C [26] and C8161 [18] were kindly provided by Dr. M. Bunce (Oxford Transplant Centre, Oxford, UK) and Dr. M. Hendrix (University of Iowa Cancer Center, Iowa City, USA), respectively, and were cultured in DMEM supplemented with 10% FCS. The gastric cancer cell line MKN45 [31] was cultured in RPMI medium supplemented with 10% FCS. The human erythroleukemic cell line K-562 (CCL-243) was obtained from the ATCC and cultured in DMEM supplemented with 10% FCS.
Matrigel tube formation assay
Vascular channel formation assays were developed in a miniaturized format [25]. For microcapillary-like structure (CLS) formation assays, the bottom of each Terasaki (72-well) plate well (Nunc, Roskilde, Denmark) was coated with 2 μl of 1:2 diluted Matrigel (7 mg/ml) and then allowed to solidify at 37°C for 30 min. Between 103 and 5×103 of either endothelial or tumor cells in 10 μl of the appropriate medium supplemented with 0.5% FCS were plated onto the gellified matrix and incubated as above. The total volume of medium in the well was adjusted to 25 μl at the time of plating the cells, and the plates were incubated for 12–16 h at 37°C in a 5% CO2 humidified atmosphere. The formation of CLS was assessed under the microscope. The inhibitory effect of L36 was assessed by adding 15 μl purified antibody, diluted in the appropriate medium at different concentrations, to the Matrigel-coated plates at the time of plating the cells. Experiments were performed in triplicate.
Confocal microscopy
Cells were tripsinized and labeled with Phaseolus vulgaris lectin alexa-488 conjugated (Molecular Probes, Eugene, OR, USA), 15 min at room temperature, washed, and seeded on Matrigel at a density of 5×105 cells/ml. At 16 h the cultures were fixed with 0.25% glutaraldehide (Sigma Chemical), and washed three times with PBS. A series of 34 optical sections was captured on a confocal microscope Bio-Rad Radiance 2000 (Bio-Rad, Hercules, CA, USA) with Olympus optical system using the ×40 1.0 oil objective. Excitation wavelength was 488 and fluorescence signal was recorded through a 530/30-nm band-pass filter. Series of 34 optical sections were scanned using two-fold accumulation. Image stacks projection and sections on different axes were obtained using Imaris software (Bitplane, Zürich, Switzerland).
K-562 transfection and modified Matrigel tube formation assay
K-562 cells were transfected with either pCEP4-L36 or pCEP4-B1.8 plasmids [24] using Lipofectamine, and selected in complete medium with 250 μg/ml Hygromycin B (Invitrogen Life Technologies). For the modified tube formation assay, 5×103 gene-modified K562 cells were embedded in 5 μl Matrigel, seeded into Terasaki plate wells and incubated at 37°C until Matrigel solidification. The total volume of medium in the well was adjusted to 25 μl. After 96 h, endothelial or tumor cells were each plated onto the gellified matrix and incubated as above. Cells were photographed under phase-contrast and images were imported into Adobe Photoshop.
Results and discussion
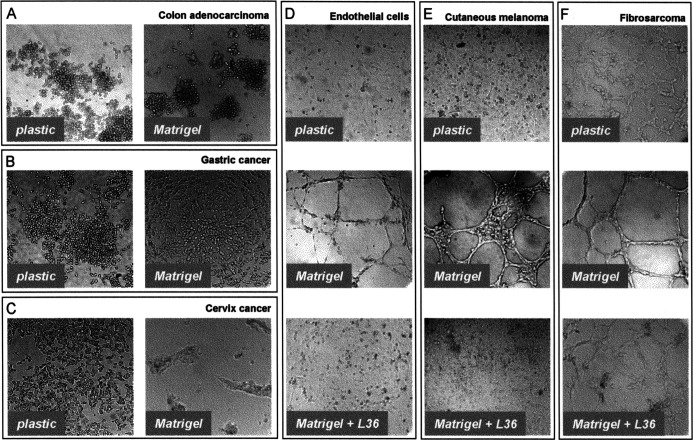
We analyzed the potential of different human tumor cell types (colon adenocarcinoma, gastric cancer, cervix cancer, fibrosarcoma, and melanoma cell lines) for generating tube-like structures in short-term Matrigel assays (Fig. 1). Under these culture conditions, colon adenocarcinoma cell lines (LS 174T and COLO205) and gastric cancer cells (MKN45) did not generate tubular structures (Fig. 1A and B; and data not shown), whereas cervix carcinoma cells (HeLa) showed limited elongation and anastomosis network formation (Fig. 1C). However fibrosarcoma (HT-1080) and metastatic cutaneous melanoma (WM1361C and C8161) cell lines did form visible cords of cells (Fig. 1E and F; and data not shown) comparable to those developed by human microvascular ECs (HMEC-1) (Fig. 1D). The presence of a channel lumen inside the tube-like structures formed by tumor cells was assessed by confocal laser scanning microscopy. Image analysis of a series of optical sections obtained from Phaseolus vulgaris–labeled HT-1080 cells, plated for 16 h on Matrigel substratum, unequivocally show a channel lumen (Fig. 2). Furthermore, the presence of different populations of vacuoled cells and dead cells is a consequence of the morphologic changes which occur during the formation and remodeling of a lumen in capillary tubes [21].
Fig. 1A–F.
Light microscope photos of the human microvascular endothelial cell line HMEC-1 (D) and different human tumor cell lines, plated for 16 h on either plastic or Matrigel substratum in the presence of medium (A, B, C, E, F) or L36 anti-laminin antibody (D, E, F). A Colon adenocarcinoma (LS 174T); B gastric cancer (MKN45); C cervix cancer (HeLa); E cutaneous melanoma (C8161); F fibrosarcoma (HT-1080)
Fig. 2.
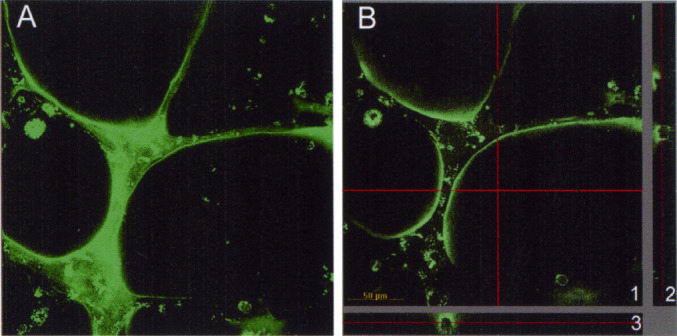
A Capillary-like structures formed by HT-1080 cells labeled with Phaseolus vulgaris lectin alexa-488 conjugated, maximum projection of 34 confocal images. B A section of the data set (all confocal slices together) in the X-Y (1), Z-X (2), and Z-Y (3) axes. The cross red line in X-Y section indicates the area of the section in the axes ZX (horizontal) or ZY (vertical). The red line in the ZX and ZY images indicates the optical section represented in the image XY
To analyze the effect of the anti-laminin scFv antibody L36 on tube-forming tumor cell lines (HT-1080, WM1361C, and C8161), purified scFv was added to the Matrigel-coated wells at the time of plating the cells. Cells were incubated in the continuous presence of the antibody, and the formation of CLS was assessed under the microscope 12–16 h later [25]. L36 treatment strongly inhibited assembly into tube-like structures, with tumor cells remaining dispersed and exhibiting a monolayer-like pattern similar to that observed in cultures seeded on plastic surfaces (Fig. 1E and F; and data not shown). This effect was similar to that observed in L36-treated HMEC-1 cells cultured on Matrigel (Fig. 1D).
The blocking of a sufficient number of laminin-active sites to generate a non-permissive BM is dependent on accessibility and local concentration of antibody fragments. One attractive approach to achieve this would be the use of gene-modified producer cells which localize to tumors as a consequence of their normal functions and the biological properties of tumors [3, 9]. To explore this possibility, human hematopoietic K-562 cells were stably transfected with episomal vectors encoding either L36 or B1.8 [13] (a control scFv specific for the hapten NIP) genes. As expected [24], genetically modified K-562 cells secreted the antibody fragment into the cell culture supernatant in a functional state (data not shown).
In order to approximate in vivo conditions and to investigate the ability of locally produced antibody molecules to inhibit cancer channel formation, we used a modified Matrigel assay. In this system, L36 and B1.8 producer K-562 cells were each embedded in Matrigel and maintained 4 days in complete medium. During this period, no cell death was detected after recovery of cells from the Matrigel and trypan blue staining (data not shown). Tumor (HT1080 and WM1361C) and endothelial cells were each plated onto either L36- or B1.8-rich matrices and incubated for 16 h before assessing tube formation (Fig. 2A, see also Fig. 3). In wells containing matrigel-embedded K-562/B1.8 cells, both tumor (Fig. 2B; and data not shown) and endothelial (data not shown) cells developed distinct tube-like networks. By contrast, cells seeded onto Matrigel containing K-562/L36 cells remained dispersed (Fig. 2C; and data not shown).
Fig. 3.
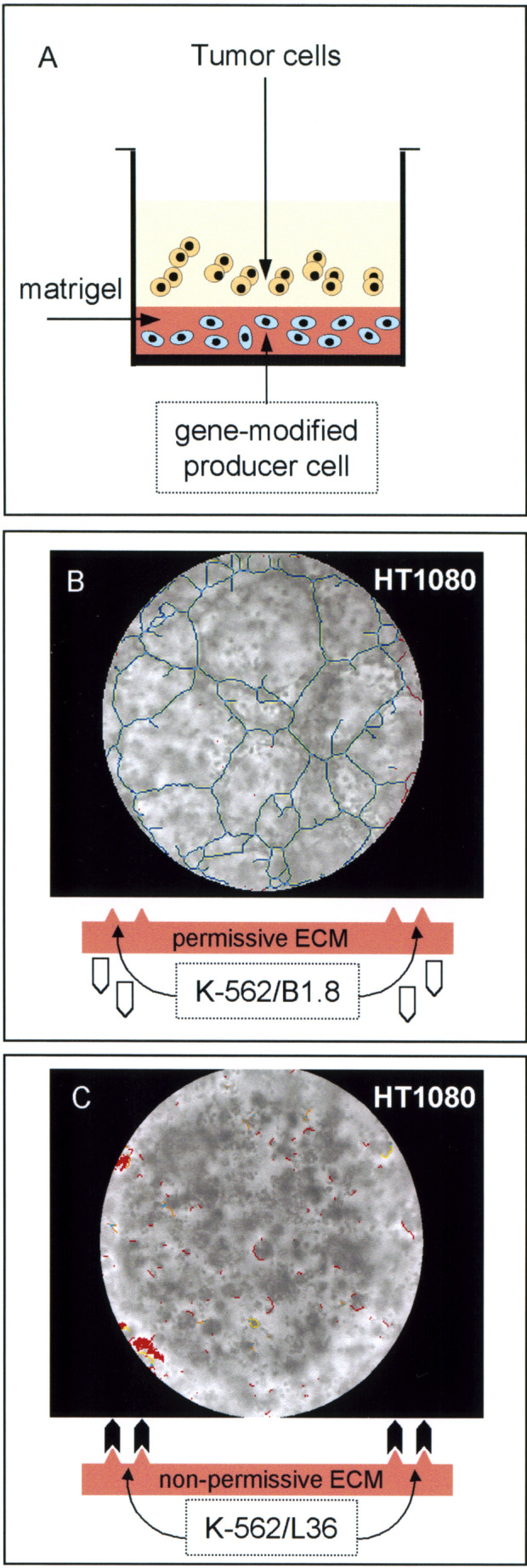
A Scheme of the modified Matrigel tube formation assay. The bottom of each plate well was coated with a diluted Matrigel (7 mg/ml) solution containing 5×103 gene-modified K-562 cells (K-562/B1.8 or K-562/L36) and then allowed to solidify at 37°C for 30 min. After 96 h, between 103 and 5×103 tumor cells were plated onto the gellified matrix and incubated for an additional 16-h period. B and C Light microscope photos of the human fibrosarcoma cell line HT-1080, plated for 16 h on either a B1.8-rich (B) or L36-rich (C) Matrigel substratum. Images were processed using software designed to detect tube formation [25]. One of three similar experiments is shown
Laminins are major constituents of the BM and play key roles in the promotion of cell adhesion, spreading, and growth; neurite outgrowth; tumor metastasis; and protease secretion [10]. Our findings indicate a critical role for the laminin domain recognized by the L36 antibody in both angiogenesis and vasculogenic mimicry, and suggest that the genetic program leading to channel formation by endothelial and nonendothelial cells is regulated by the same ECM-cell interactions. Therefore, this anti-laminin antibody can help us in the elucidation of the cell-matrix interactions implicated in angiogenesis as well as in vasculogenic mimicry.
Recently, it has been shown that matrices conditioned by aggressive melanoma cells induce tube formation by poorly aggressive tumor cells, suggesting that instructional information is deposited into the ECM [27]. The functional role of laminin (specifically, the γ2 chain) in vasculogenic mimicry was highlighted by the observation that its expression is enhanced in aggressive melanoma cells, compared with the poorly aggressive ones, and that it colocalized with the patterned tubular networks formed by the aggressive cells [27].
Especially interesting are the therapeutic implications of these findings. We have previously demonstrated that L36 prevents the establishment and growth of tumors in murine models [24], a phenomenon attributed to its ability to disrupt the matrix-dependent morphogenesis of ECs. In the light of the results here presented, we could put forward the hypothesis that the antitumoral effect mediated by L36 is due to its effect not only on ECs, but also on the cancer cells themselves. Furthermore, our results demonstrate that scFv molecules directed against ECM functionally active sites, when produced by gene-modified cells, may diffuse locally through surrounding extracellular matrices, rendering them non-permissive for the specific biological process that is blocked by the antibody. This may provide new perspectives for the development of novel therapeutic strategies directed at the ECM to halt tumor progression.
Acknowledgements
Financial support for this research was provided to L. A-V. from the Fondo de Investigación Sanitaria (grant PI021144) and the Ministerio de Ciencia y Tecnología (grant BIO2001-0385). L. S. was supported by grants from the 5th framework of the European Community to L. A-V. B. B. was supported by a Comunidad Autónoma de Madrid training grant 01/0369/2000.
References
- 1.Ades J Invest Dermatol. 1992;99:683. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo Cancer Cell. 2002;1:219. doi: 10.1016/S1535-6108(02)00051-X. [DOI] [PubMed] [Google Scholar]
- 3.Álvarez-Vallina Curr Gene Ther. 2001;1:385. doi: 10.2174/1566523013348418. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff J Clin Invest. 1997;100:S37. [PubMed] [Google Scholar]
- 5.Bissell Nat Rev Cancer. 2001;1:46. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner Nature. 2000;406:536. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet Nature. 2000;407:249. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Chang Proc Natl Acad Sci USA. 2000;97:14608. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chester Nat Biotechnol. 2002;20:256. doi: 10.1038/nbt0302-256. [DOI] [PubMed] [Google Scholar]
- 10.Colognato Dev Dyn. 2000;218:213. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Folberg Am J Pathol. 2000;156:361. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkman Ann Surg. 1972;175:40930. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins J Mol Biol. 1992;226:889. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix Proc Natl Acad Sci USA. 2001;98:8018. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrix Cancer Res. 2002;62:665. [PubMed] [Google Scholar]
- 16.Hoang J Cutan Pathol. 2001;28:508. doi: 10.1034/j.1600-0560.2001.281003.x. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi Cancer Res. 2002;62:860. [PubMed] [Google Scholar]
- 18.Maniotis Am J Pathol. 1999;155:739. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald Cancer Metastasis Rev. 2000;19:109. doi: 10.1023/a:1026529222845. [DOI] [PubMed] [Google Scholar]
- 20.McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol. 2000;156:383. doi: 10.1016/S0002-9440(10)64740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer Anat Rec. 1997;249:327. doi: 10.1002/(SICI)1097-0185(199711)249:3<327::AID-AR3>3.3.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Rusolahti Nat Rev Cancer. 2002;2:8321. [Google Scholar]
- 23.Sanz Cancer Immunol Immunother. 2001;50:557. doi: 10.1007/s00262-001-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz Gene Ther. 2002;9:1049. doi: 10.1038/sj.gt.3301725. [DOI] [PubMed] [Google Scholar]
- 25.Sanz Microvasc Res. 2002;63:335. doi: 10.1006/mvre.2001.2389. [DOI] [PubMed] [Google Scholar]
- 26.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M (1997) Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res 7 [Suppl 2]:S35 [PubMed]
- 27.Seftor Cancer Res. 2001;61:6322. [PubMed] [Google Scholar]
- 28.Sharma Prostate. 2002;50:189. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- 29.Sood Am J Pathol. 2001;158:1279. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timpl Curr Opin Cell Biol. 1996;8:618. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 31.Watson Int J Cancer. 1990;45:90. doi: 10.1002/ijc.2910450117. [DOI] [PubMed] [Google Scholar]