Abstract
Dendritic cells (DCs) are highly effective antigen-presenting cells that, when derived from cancer patients, seem to be functionally deficient. Herein, we show that vaccination with allogeneic DC–autologous tumor cell hybrids affects the phenotype and improves the function of monocyte-derived DCs (Mo-DCs) from cancer patients. Mononuclear cells were isolated from patients’ peripheral blood by density gradient centrifugation, and adherent cells were cultured in medium containing GM-CSF plus IL-4 and, after 5 days, TNF-α. After 2 more days, Mo-DCs were harvested and their CD80, CD86, and CD83 expression was assessed by flow cytometry. They were also used as stimulators in mixed lymphocyte reactions (MLR), where IFN-γ production was measured by ELISA. Mo-DCs from unvaccinated patients expressed significantly lower levels of CD86, and tended to express lower levels of CD83 than Mo-DCs from healthy donors. However, Mo-DCs generated after hybrid cell vaccination presented increased expression of the same markers and induced significantly higher levels of IFN-γ in MLR. These results indicate that the use of allogeneic DC–based cancer vaccines induces recovery of DC function in metastatic cancer patients and, therefore, could precede the use of autologous DCs for vaccine preparation. Such an approach could be relevant and should be investigated in clinical trials.
Keywords: Cancer, Dendritic cells, Vaccines
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs), able of inducing primary and boosting secondary T-cell responses [2, 3, 11]. Not surprisingly, since the immune system fails to eradicate most antigenic cancers [6], several groups have described the defective function of DCs in tumor-bearing mice and cancer patients [10, 13, 23, 32], leading to the inappropriate tumor-antigen presentation by DCs in such instances [9, 17, 18]. Though DCs are found at trace levels in tissues or in circulation, the identification of culture conditions enabling DC generation in vitro [7, 25, 33] has furthered the study of their biology and allowed the design of immunotherapeutic protocols based on their use [22, 24, 28]. In an attempt to circumvent tumor effects upon DC maturation, and considering that mature DCs seem to be resistant to such immunosuppressive factors [1, 27], many antitumor vaccination strategies aim toward the development and maturation of dendritic cells in vitro, in the absence of tumor-derived factors, followed by their readministration to the patients [5, 6, 14]. Indeed, clinical protocols based on this hypothesis have been developed and are currently underway, but their results, though promising, are still not as good as one could expect. A reason for this relative inefficiency could be the functional impairment also of DC precursors in cancer patients [10, 15]. Though the use of healthy donors for the generation of DCs does bypass this problem, it creates others, mainly of HLA compatibility and therefore of antigen presentation and recognition. A strategy that considers both sides of this question is the fusion of tumor cells with DCs obtained from healthy donors [4, 29, 30, ]. This generates, theoretically, cells that present MHC from the DC donor and from the tumor-bearing individual, therefore being able to present tumor antigens in the context of both.
We have conducted a clinical trial for the vaccination of metastatic renal cell carcinoma or melanoma patients with tumor-DC hybrid cells [4]. Though in this trial, most patients had an improvement of immune function and clinical benefit, the latter was limited mostly to disease stabilization, suggesting that this approach also should be modified if one is to expect more consistent and effective clinical results.
We show here that among the immune function parameters that were modified by the vaccination protocol of the metastatic cancer patients is the differentiation and maturation potential of their monocytes into DCs in vitro. This phenomenon highlights the immune function enhancement gained by the hybrid cell vaccination and could be of relevance in the design of new anticancer vaccination protocols.
Materials and methods
Sample collection
Peripheral blood mononuclear cells (PBMCs) were obtained from 8 healthy individuals and 11 patients with metastatic melanoma or renal cell carcinoma, before or after four doses or more of hybrid cell vaccination, performed at Hospital Sirio-Libanês [4]. The protocol was approved by the Institutional Ethics Committee and was conducted in the Oncology Center of the Hospital Sírio-Libanês, São Paulo, Brazil. Samples were collected only after informed consent of donors.
Cells isolation and culture
Mononuclear cells were separated over a Ficoll-Paque gradient (d=1.076), resuspended and seeded in 12-well plates in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) and 2 mM L-glutamine (R10 medium). After 2 h of incubation at 37°C, nonadherent cells were removed, and the adherent cells were cultured in presence of GM-CSF and IL-4 (50 ng/ml; R&D, Minneapolis, MN, USA). On the 5th day, TNF-α (50 ng/ml; R&D, Minneapolis, MN, USA) was added for DC activation. After 2 further days in culture, the cells were harvested and analyzed.
Flow cytometry
Cells (5×105 cells) were labeled with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse monoclonal antibodies (MoAbs) against CD86, CD80, and CD83, or with mouse isotype controls (Caltag Laboratories, Burlingame, CA, USA), and analyzed in a FACSCalibur cytometer (Becton Dickinson, San Jose, CA, USA) with CellQuest software (Becton Dickinson, San Jose, CA, USA). At least 10,000 gated events were acquired per antibody analyzed.
Mixed lymphocyte reactions and IFN-γ assays
Dentritic cells were irradiated (25 Gy) and used as stimulators for allogeneic PBMCs, at a 1:2 ratio. Cells were cultured for 5 days in R10, after which supernatants were collected for interferon γ (IFN-γ) assay by ELISA (DuoSet ELISA; R&D Systems). Assays were performed in duplicate. The lower limit of detection was 10 pg/ml.
Statistical analysis
Analysis of variance was performed to evaluate the differences among groups.
Results
Dendritic cell yield and phenotypic characterization of DCs
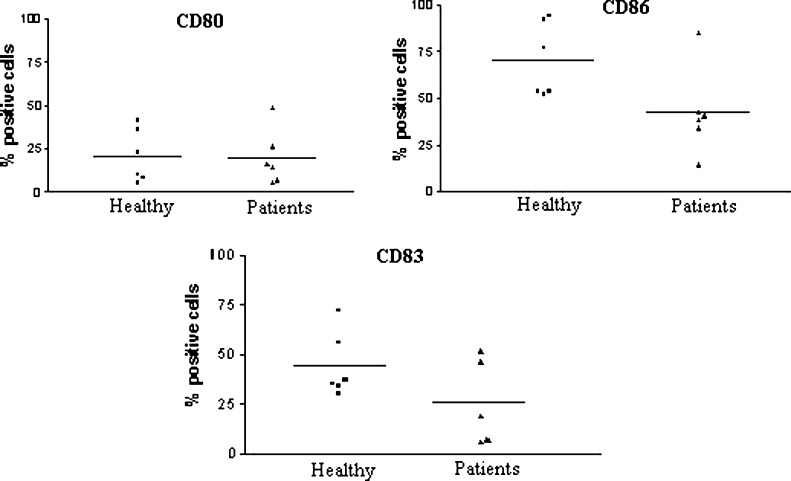
Mononuclear cells from eight healthy individuals and eight metastatic cancer patients were cultured with IL-4 plus GM-CSF and activated with TNF-α. The final yield was calculated as the number of cells obtained at the end of the culture divided by the number of adherent cells. The DC yield from healthy donors was 18% and was not statistically different from that of patients (17.25%). Furthermore, the loosely adherent cell population had a typical veiled DC morphology, and there were no morphological differences between DCs from patients and healthy individuals (data not shown). However, when CD80, CD86, and CD83 expression was assessed by flow cytometry, DCs obtained from cancer patients presented lower levels of the three markers, though this difference reached significance only for CD86 (p<0.05) (Fig. 1). The same pattern of response was observed in melanoma (4) and renal cell cancer patients (4) considered separately, though in this case, the differences were not statistically significant (data not shown).
Fig. 1.
Immunophenotype of monocyte-derived DCs generated in vitro from cells of patients or from healthy individuals. Peripheral blood monocytes from eight cancer patients and eight healthy individuals were cultured in the presence of GM-CSF plus IL-4 and activated with TNF-α on the 5th day of culture. On the 7th day, cells were harvested, and CD80, CD86, and CD83 surface expression was assessed by flow cytometry. Bars represent the mean % of positive cells for each marker; p values (healthy vs patients) were CD86, p=0.04; CD83, p=0.14; CD80, p=0.92.
Effects of vaccination upon DC phenotype obtained in culture
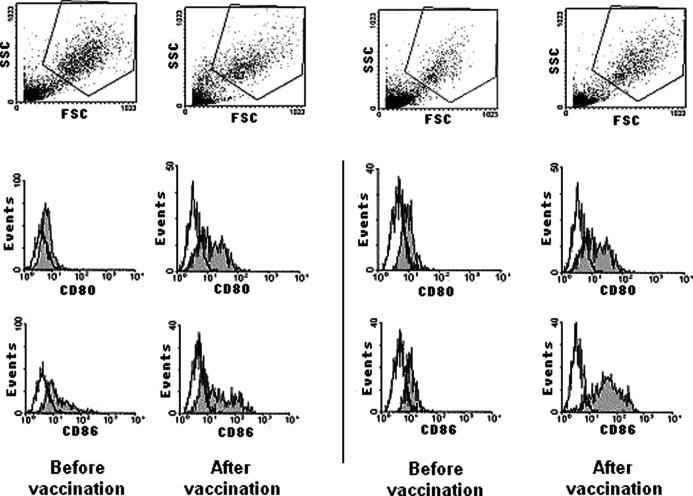
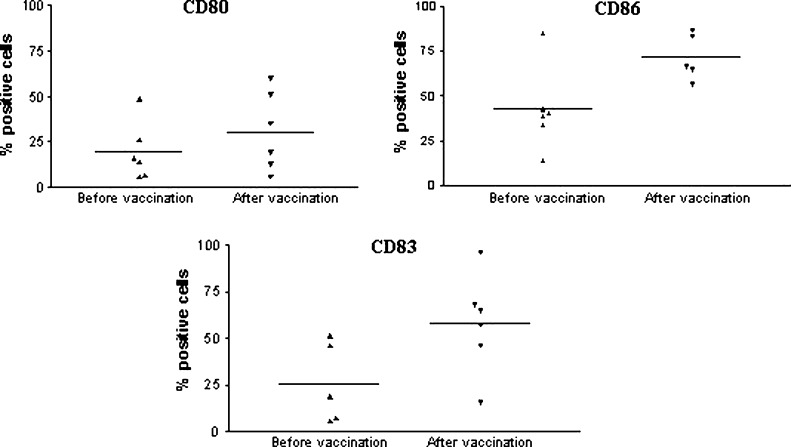
Given the altered cell surface phenotype found on DCs from metastatic cancer patients, we decided to investigate if the hybrid cell vaccination was able to affect the surface expression of the costimulatory molecules, CD80 and CD86, and of the activation marker, CD83. Therefore, DCs which had originated from metastatic cancer patients before any vaccination, were compared with DCs generated from cells of patients who had received at least four doses of the vaccine. Patients received 1–3×107 autologous tumor–dendritic cell hybrids generated by electrofusion with an efficiency of 15–30%, with a 6-week interval (Barbuto et al., submitted). Figure 2 illustrates, for two patients, the recovery of CD80 and CD86 expression observed after vaccination, a general phenomenon that was observed for the three markers in the six different patients tested, though it was only statistically significant for CD86 (p=0.03) (Fig. 3). Interestingly, CD83 expression seemed higher in vaccinated patients than in healthy individuals, but this was not statistically significant (data not shown).
Fig. 2.
Costimulatory molecules expression by monocyte-derived DCs before and after vaccination. Two representative experiments of CD80 and CD86 expression by DCs generated from blood monocytes obtained from metastatic cancer patients before or after vaccination with allogeneic DC–autologous tumor cell hybrids.
Fig. 3.
Immunophenotype of monocyte-derived DCs generated in vitro from cells of cancer patients before or after hybrid cell vaccination. Blood monocytes obtained before or after vaccination of six patients were cultured in the presence of GM-CSF plus IL-4 and activated with TNF-α on the 5th day of culture. On the 7th day, cells were harvested, and CD80, CD86, and CD83 surface expression was assessed by flow cytometry. Bars represent the mean % of positive cells for each marker; p values (before vs after): CD80, p=0.34; CD86, p=0.03; CD83, p=0.06.
IFN-γ production by cells stimulated with patients’ DCs in mixed leukocyte reaction (MLR)
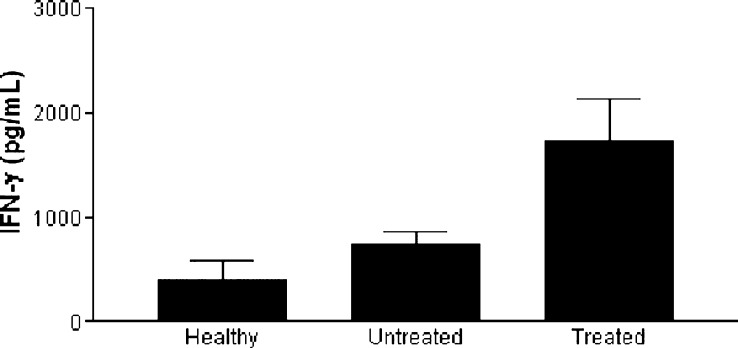
DCs obtained from a randomly selected group of patients before and after vaccination were used as stimulators in MLR. Both DC preparations induced IFN-γ production, but this was significantly higher (p<0.05) in cultures stimulated with DCs obtained after vaccination (Fig. 4). Interestingly, the levels of IFN-γ detected in MLR stimulated with DCs from untreated patients did not differ from those present in cultures stimulated with DCs from healthy donors.
Fig. 4.
IFN-γ production in mixed lymphocyte reactions stimulated with monocyte-derived DCs. DCs generated in vitro from cells of five healthy individuals and from three cancer patients before or after vaccination were used as stimulators for allogeneic lymphocytes in MLR. Cells were cultured for 5 days, after which their supernatants were collected and assayed for the presence of IFN-γ by ELISA. Means ± SE are represented (p<0.05).
Discussion
The recent development of in vitro techniques that allow the differentiation of DCs in vitro has opened new possibilities for immunotherapy [28]. A promising approach uses allogeneic DCs fused to tumor cells [4, 30] in an attempt to bypass the possible deficits of DCs generated from cancer patients [10, 13, 15], while at the same time avoiding the MHC compatibility issues that would occur if nonfused cells were used. Though theoretically attractive, this approach is based on the assumption that MHC antigens, from the patient and from the DC donor are present in the hybrid cell. Even if this occurs, there will be many occasions when tumor cells will be deficient in MHC expression, and consequently so will the hybrid cells. Despite that, clinical protocols based on this strategy have been developed and yielded promising results [4, 30]. Nevertheless, the fact that there could be a deficit in antigen presentation in the context of the patient’s MHC with this strategy justifies the search for alternate approaches.
This report describes phenotypic and functional features of DCs differentiated in vitro from patients with metastatic melanoma or renal cell carcinoma after vaccination with hybrid cells that indicate one such alternate strategies. We show that while monocyte-derived DCs from untreated cancer patients do exhibit lower levels of function and activation markers, this does not occur after vaccination. Furthermore, IFN-γ production in MLR stimulated with patient-derived DCs was significantly improved after vaccination, suggesting that the surface expression of DC markers is indeed a sign of improved antigen-presenting function of these cells.
The interaction of DCs with T cells via binding of MHC-peptide and TCR/CD3 complexes as well as adhesion/costimulatory molecules and their counter ligands induce an effective T-cell activation [12, 26] that seems to depend on both CD80 and CD86 molecules, highly expressed on activated DCs, and the main ligands for CD28 and CTLA-4 on T cells [20]. Several studies have shown that CD80 and CD86 expression are quantitatively and qualitatively different on antigen-presenting cells [12, 20]. The role of each molecule on the initiation or maintenance of immune responses is not completely understood, and the literature shows contrasting reports concerning this issue [12, 19, 21, 31]. Nevetheless, a study performed by Caux et al. [8] showed that monoclonal antibodies against CD86 suppressed the DC-dependent alloreaction by 70%, demonstrating the requirement of this molecule for allostimulatory activity of DCs. This is in agreement with our data, since we noted a significant improvement in CD86 expression by DCs generated after vaccination at the same time as an improvement in IFN-γ induction by these cells in MLR. Furthermore, the significant enhancement of CD83 expression on DCs derived from the vaccinated patients’ monocytes indicate that the phenomenon is not restricted to the expression of one marker, but is very likely a real recovery of DC function in the patients.
Therefore, an alternative approach for a DC-based vaccination of cancer patients would be the initial use of allogeneic DC–autologous tumor hybrid cells for vaccination, followed by the use of autologous DCs pulsed with tumor cells/extracts/antigens. This strategy would bypass the defective function of the patient-derived DCs initially and, after the functional recovery of these cells, would eliminate the complexities of cell fusion and/or the requirement of DC donor selection. Due to its simplicity, this strategy seems to us an attractive approach for the further exploration of DC-based therapies for cancer patients and should be pursued in future clinical trials.
Acknowledgements
This work was supported by FAPESP, grants no. 01-02339-8 and no. 01-07751-4 and by Capes.
References
- 1.Allavena Eur J Immunol. 1998;28:359. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Barbuto JAM, Ensina LF, Neves AR, Bergami-Santos PC, Marques R, Leite KRM, Camara-Lopes LH, Buzaid AC (2002) Dendritic-tumor hybrid cell therapeutic vaccination for metastatic melanoma and renal cell carcinoma patients. International symposium on predictive oncology and intervention strategies, Paris, France, 9--12 February 2002. http://www.cancerprev.org/Journal/Issues/26/101/1011/4579 Cited 24 August 2004
- 5.Bedrosian J Clin Oncol. 2003;21:3826. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Brossart J Exp Hematol. 2001;29:1247. doi: 10.1016/S0301-472X(01)00730-5. [DOI] [PubMed] [Google Scholar]
- 7.Caux Nature. 1992;360:258. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 8.Caux J Exp Med. 1994;180:1841. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaux Lab Invest. 1996;74:975. [PubMed] [Google Scholar]
- 10.Della Br J Cancer. 2003;89:1463. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Haematologica. 2000;85:202. [PubMed] [Google Scholar]
- 12.Fields J Immunol. 1998;161:5268. [PubMed] [Google Scholar]
- 13.Gabrilovich Clin Cancer Res. 1997;3:483. [PubMed] [Google Scholar]
- 14.Godelaine J Immunol. 2003;171:4893. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 15.Hasebe Biomed Pharmacother. 2000;54:291. doi: 10.1016/S0753-3322(00)80050-5. [DOI] [PubMed] [Google Scholar]
- 16.Hsu Nat Med. 1996;2:52. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 17.Ishida J Immunol. 1998;161:4842. [PubMed] [Google Scholar]
- 18.Kim J Immunol. 1995;155:2240. [PubMed] [Google Scholar]
- 19.Lang J Immunol. 2002;168:3786. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- 20.Linsley Immunity. 1994;1:793. [Google Scholar]
- 21.Martin-Fontecha J Immunol. 2000;164:698. doi: 10.4049/jimmunol.164.2.698. [DOI] [PubMed] [Google Scholar]
- 22.Nestle Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 23.Orsini Cancer Res. 2003;63:4497. [PubMed] [Google Scholar]
- 24.Piemonti Cancer Immunol Immunother. 2000;49:544. doi: 10.1007/s002620000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani J Immunol Methods. 1996;196:137. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 26.Slavik Immunol Res. 1999;19:1. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrink J Immunol. 1997;159:4772. [PubMed] [Google Scholar]
- 28.Thurner J Immunol Methods. 1999;223:1. doi: 10.1016/S0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 29.Trefzer Mol Biotechnol. 2003;25:63. doi: 10.1385/MB:25:1:63. [DOI] [PubMed] [Google Scholar]
- 30.Trefzer Int J Cancer. 2000;85:618. doi: 10.1002/(SICI)1097-0215(20000301)85:5<618::AID-IJC4>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Vasilevko DNA Cell Biol. 2002;21:137. doi: 10.1089/10445490252925404. [DOI] [PubMed] [Google Scholar]
- 32.Young Int J Immunopharmacol. 1999;21:675. doi: 10.1016/S0192-0561(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Proc Natl Acad Sci U S A. 1996;93:2588. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]