Abstract
Allogeneic stem cell transplantation (SCT) is the treatment of choice for a large number of hematologic malignancies. Its major advantage over conventional chemotherapy lies in the graft-versus-leukemia (GVL) effects mediated by allo- or tumor-reactive donor lymphocytes given in the course of SCT or post transplantation as donor lymphocyte infusions (DLI). The benefits of cell-mediated immunotherapy over myeloablative radiochemotherapy have also made it possible to reduce the intensity of conditioning regimens. Mobilized peripheral blood has proved preferable to bone marrow (BM) as a source of stem cells for transplantation, since it provides a larger number of stem cells on the one hand and immunologically competent lymphocytes on the other. The use of granulocyte colony stimulating factor (G-CSF), which is necessary to mobilize and increase the number of stem cells, may down-regulate the GVL effect by suppression of donor effector T lymphocytes by inducing Th1→Th2 cytokine switch. It has previously been shown that GVL effects may be amplified by both in vivo and in vitro activation of donor lymphocytes with human recombinant interleukin-2 (rIL-2). Our studies using a leukemic murine model prepared for transplantation with low intensity conditioning prior to infusion of G-CSF-mobilized peripheral blood stem cells (PBSC) have demonstrated that mobilization of blood cells with G-CSF and in vivo treatment with rIL-2 following low-intensity conditioning enhances the GVL effects and prolongs survival of recipients inoculated with BCL1. Activation of donor lymphocytes with rIL-2 may thus be useful for amplifying GVL effects following mobilization with G-CSF.
Keywords: SCT, PBSC, GVL, G-CSF, Murine leukemia
Introduction
Granulocyte colony stimulating factor (G-CSF) plays a vital role in the proliferation and differentiation of hematopoietic cells, including the homing of multipotent progenitor cells into the circulation [4, 9]. G-CSF mobilized peripheral blood progenitor cells (PBSC) are increasingly being used to replace bone marrow cells (BMC) as a source of hematopoietic stem cells in both autologous and allogeneic stem cell transplantation [1, 6, 10, 11]. Reports from single institutions [2] and the European Group for Blood and Marrow Transplantations [12] suggest that the transplantation of allogeneic PBSC does not cause devastating graft-versus-host disease (GVHD). Moreover, the high numbers of T and NK cells contained in a typical PBSC inoculum reduce the risk of relapse and thus improve disease-free survival after transplantation. G-CSF mobilized PBSC is preferred by most transplant centers to BMC because stem cell harvesting from the blood avoids discomfort to the donor and the risks connected with general or local anesthesia, and can be done on an outpatient basis. In addition, it provides a larger number of both stem cells and immunologically competent lymphocytes. However, although the blood provides more stem cells, thus improving engraftment [3, 7] and more lymphocytes, thus enhancing allograft-induced GVL effects, the use of G-CSF has been shown to result in Th1→Th2 shift, a process that could result in the down-regulation of GVL effects by suppression of effector T lymphocytes by regulatory Th2 cells [16]. As previously shown in preclinical models [17] and in clinical practice [15], the efficacy of GVL effects may be amplified by activation of donor lymphocytes with human recombinant interleukin-2 (rIL-2) either by concomitant administration of rIL-2 and DLI, or by the use of donor lymphocytes activated in vitro with rIL-2.
The goal of the present investigation was to compare the efficacy of GVL effects induced by bone marrow and mobilized blood stem cell allografts, using unstimulated or rIL-2-stimulated donor lymphocytes in a preclinical model of murine leukemia/lymphoma (BCL1). Our results demonstrate the advantages of stem cells mobilized by G-CSF both in terms of the number of stem cells harvested and as a source of GVL effector cells that can be amplified with rIL-2.
Materials and methods
Mice
Eight- to 12-week-old C57BL/6 (H-2b)(B6), BALB/c (H-2d) (BALB/c × C57BL/6)F1 (H-2d/b) (F1) mice were purchased from the Harlan Breeding Facility (Jerusalem, Israel). Mice were kept under standard animal facility care; cages, sawdust, food and acidified water were all autoclaved. All experimental protocols were approved by the Institutional Committee for Animal Experimentation.
Conditioning by radiation
F1 mice were positioned in radiation chambers and exposed to a sublethal dose of 700 cGy total body irradiation (TBI), delivered by a linear accelerator (Varian Clinac 6X) at a dose rate of 179 cGy/min, at a source-to-skin distance of 80 cm.
Bone marrow cells
The mice were killed by exposure to CO2; the long bones (femura, tibiae and humeri) of B6 donor mice were aseptically removed and flushed with RPMI 1640 medium (Biological Industries, Beth Haemek, Israel) using a 25-gauge needle. Bone marrow cells were resuspended in RPMI-1640 medium, and aliquots of 0.2–0.3 ml medium containing 107 nucleated cells were injected intravenously (IV) into the lateral tail vein of recipient mice 24 h after conditioning. As described, 0.5 ml of blood from untreated mice or PBSC from G-CSF treated mice was injected IV.
Human recombinant interleukin 2 (rIL-2)
rIL-2 provided as 1 mg Proleukin, which corresponds to 18×106 international units (IU), was kindly supplied by Chiron, Amsterdam, The Netherlands. For injection, rIL-2 was initially dissolved in water and subsequently diluted with 5% dextrose.
Granulocyte colony stimulating factor (G-CSF)
Filgrastrim (Amgen Inc., Thousand Oaks, Calif.) 300 μg/ml was diluted 1:5 and 100 μl was injected subcutaneously (SC) into each B6 donor mouse.
Murine B cell leukemia (BCL1)
BCL1 cells were maintained in vivo in BALB/c mice by IV passage of 106-107 peripheral blood lymphocytes (PBL) obtained from tumor-bearing mice as previously described. All F1 recipients of BCL1 ≥104 cells developed splenomegaly followed by marked lymphocytosis in the blood. PBL counts of all experimental groups were carried out weekly. Onset of leukemia was defined as PBL counts ≥20,000/mm3 [13].
Assay for chimerism
Chimerism was confirmed by typing F1 peripheral blood lymphocytes with B6- or BALB/c-specific antisera, using in vitro complement-dependent microcytotoxicity assay with specific alloantisera and rabbit complement to determine the percentage of host or donor type cells. Specific alloantisera (BALB/c-anti-B6 and B6-anti-BALB/c) were prepared by cross-immunization with full-thickness skin allografts followed by six intraperitoneal (IP) injections of 30×106 donor spleen cells at intervals of 1–2 weeks. Mice were bled and the sera stored at −70°C until used. Lymphocytes obtained from F1 recipients showed 100% cytotoxicity with both BALB/c-anti-B6 and B6-anti-BALB/c antisera, whereas lymphocytes obtained from chimeras were lysed by BALB/c-anti-B6 and not by B6-anti-BALB/c antisera. Percent chimerism was therefore calculated by the formula: % donor type B6 cells = cells lysed following treatment with BALB/c-anti-B6 antiserum, minus % cells lysed with B6-anti-BALB/c antiserum, minus % cells lysed with complement only.
Colony-forming cells (CFU-C) assay
Cultured BMC were plated at 100,000 murine cells/ml in murine methylcellulose media (Stem Cell Technologies Inc., Vancouver, Canada) containing 3 U/ml human erythropoietin (Amgen, Thousand Oaks, Calif.) and 10% WEHI-3B-conditioned media. Colonies of more than 50 cells were counted after incubation at 37°C in a humidified 5% CO2 incubator for 14 days.
Detection of residual BCL-1 cells in treated mice by adoptive transfer experiments
In order to detect the presence of minimal residual clonogenic BCL1 cells in the spleens of treated F1 mice, 105 spleen cells obtained from a pool of three animals were adoptively transferred into secondary untreated BALB/c recipients. Recipients were monitored by weekly PBL counts. It was previously documented that even 10–100 BCL-1 cells would cause leukemia in all adoptive recipients [14].
Experimental design
F1 mice were given sub-lethal TBI of 700 cGy and divided into five major groups: The first group received 107 B6 BMC and was then divided into two subgroups, one of which received rIL-2 120,000 IU × 2/day for 5 days, and the other none. The second group received allogeneic peripheral blood (0.5 ml) obtained from untreated B6 donor mice and was then subdivided into two groups, one of which was injected IP with rIL-2 in vivo (120,000 IU × 2/day for 5 days,), while the other group was not. The thrid group of F1 mice received PBSC from B6 donor mice that had been injected SC with G-CSF twice daily for 4 days; the blood was collected 3 h after the last injection. This group was divided into two subgroups, one of which received rIL-2, 20,000 IU × 2/day for 5 days, while the other group received none. The fourth group, which served as a control group, received F1 BMC. All mice were injected with 105 BCL1 cells together with BMC, blood or PBSC, 1 day after TBI. A fifth group (irradiation controls) received only TBI 700 cGy, without addition of hematopoietic or BCL1 cells.
Six to 17 days later, spleen cells (105) from a pool of three mice from each experimental group were adoptively transferred to naïve BALB/c mice to examine the presence of residual tumor cells. Each experiment was repeated three times.
Statistical analysis
Long rank, Breslow was used for comparison of the experimental groups.
Results
F1 mice were irradiated, transplanted with B6 hematopoietic cells, and inoculated with BCL1 cells. Six to 17 days later, spleen cells from a pool of mice from each experimental group were adoptively transferred to naïve BALB/c mice in order to test for residual tumor cells.
As shown in Fig. 1, which represents a summary of three experiments, 18/19 (94.7%) adoptive transfer recipients that had received spleen cells from F1 mice transplanted with syngeneic (F1) BMC and BCL1 cells, developed leukemia and died. Seven out of 26 (27%) adoptive recipient mice injected with spleen cells from F1 mice transplanted with allogeneic B6 blood developed leukemia and died. In vivo treatment with rIL-2 augmented the GVL effect and only 5/31 adoptive recipient mice (16%) developed leukemia.
Fig. 1.
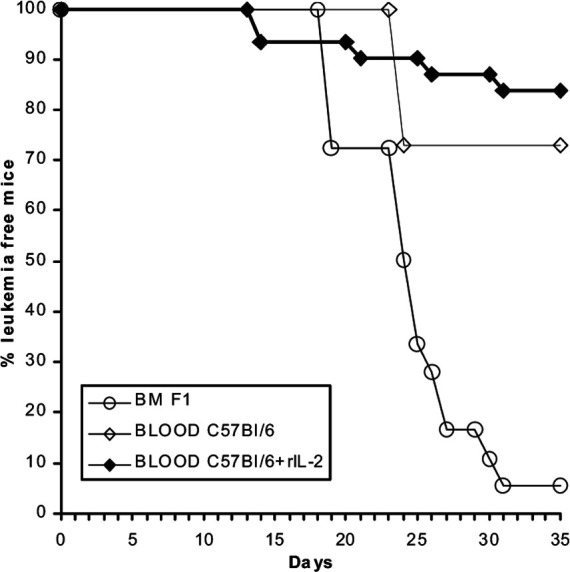
(BALB/c × C57BL/6)F1 mice were irradiated (total body 700 cGy), injected with BCL1 and transplanted with either F1 bone marrow cells (BMC) or C57BL/6 blood; half of them received additional rIL-2 treatment in vivo. Six–17 days post transplantation, 105 spleen cells from three mice of each group were adoptively transferred to secondary naïve BALB/c mice to test for residual leukemia cells
A comparison of the GVL effects in mice transplanted with PBSC and mice transplanted with BMC, both obtained from B6 donors (three experiments), is summarized in Fig. 2. Three out of 25 (12%) adoptive recipient mice transplanted with PBSC developed leukemia. Additional treatment with rIL-2 in vivo augmented the GVL effect, and only one out of 24 mice developed leukemia (4.5%). On the other hand, all 26 adoptive recipient mice that received spleen cells from F1 mice transplanted with B6 BMC, with or without additional rIL-2 in vivo treatment, developed leukemia within 35 days and died.
Fig. 2.
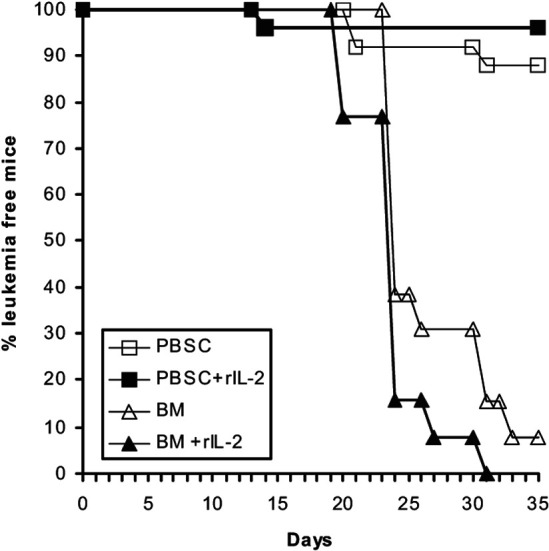
(BALB/c × C57BL/6)F1 mice were irradiated (total body 700 cGy), injected with BCL1 and transplanted with either C57BL/6 bone marrow cells (BM C57) or G-CSF mobilized C57BL/6 blood (PBSC); half of each group received additional rIL-2 treatment in vivo. Six–17 days post transplantation, 105 spleen cells from three mice of each group were adoptively transferred to secondary naïve BALB/c mice to test for residual leukemia cells
The mean percentage of donor type cells in the blood of F1 mice transplanted with B6 BMC was 86%, and additional treatment in vivo with rIL-2 resulted in 95% chimerism. Transplantation with B6 PBSC from mice treated with G-CSF resulted in a high percentage of chimerism, either with or without rIL-2 (77 and 100%, respectively) (Table 1).
Table 1.
Donor type cells in the blood of C57BL/6 → (C57BL/6 × BALB/c) F1 chimeras prepared with G-CSF mobilized blood stem cells (PBSC) or bone marrow cells (BMC)
| Transplanted cells | rIL-2 in vivo | Chimerism |
|---|---|---|
| Average % donor cells | ||
| C57BL/6 BMC | No | 86 |
| C57BL/6 BMC | Yes | 95 |
| C57BL/6 PBSC | No | 77 |
| C57BL/6 PBSC | Yes | 100 |
Table 2 lists the number of CFU-C/105 cells obtained from either BMC, blood or PBSC used for transplantation. BMC contained 47 and 56 CFU-C/105 cells. Blood from B6 mice showed very low numbers of 2 and 4 CFU-C/105, whereas PBSC obtained from mice treated with G-CSF displayed higher colony numbers of 75 and 82 CFU-C/105 cells. All F1 mice that were sub-lethally irradiated (700 cGy), without administration of hematopoietic or BCL1 cells, survived. A comparison between treatment groups showed significant results, as follows: for the PBSC treatment group in comparison to BMC obtained from B6 + rIL-2, P=<0.0001; for the blood treatment group in comparison to BMC obtained from B6 + rIL-2, P=<0.0001
Table 2.
Colony formation (CFU-C/105) by bone marrow and blood cells in mice treated with G-CSF
| Donor cell source | CFU-C/105 cells |
|---|---|
| Untreated C57BL/6 bone marrow | 47.56 |
| Untreated C57BL/6 blood | 2.4 |
| G-CSF mobilized C57BL/6 blood | 75.82 |
Discussion
Data presented in this paper suggest that following engraftment, alloreactive donor lymphocytes can exert effective anti-tumor effects against murine leukemia. Although alloreactive lymphocytes are also present in bone marrow preparations, the number of alloreactive T lymphocytes is much higher in the blood. The alloreactive capacity of donor lymphocytes may be further increased following activation in vivo with rIL-2, which affects not only T lymphocytes, but also NK cells and NK T cells, thus potentially providing such cells with a higher capacity to react against tumor cells of host origin. Considering the fact that nowadays G-CSF mobilized blood stem cells rather than bone marrow aspiration are commonly used in clinical practice, we considered it of interest to compare GVL effects mediated by BMC versus PBSC without and with additional activation of the anti-tumor effector cells with rIL-2. The role of alloreactive lymphocytes obtained from naïve donors has previously been well documented, using the same BCL1 murine model [18]. We have also previously documented our finding that GVL effects may be amplified by rIL-2 activation of lymphocytes in vitro or in vivo [19]. In clinical trials too, as well as in murine studies [5], we have shown that GVL effects may be amplified with rIL-2 as well as IL-12 administration in vivo by activation of donor lymphocytes in vitro [15, 18, 19, 5]. Some patients that did not respond to DLI when naïve donor lymphocytes were administered subsequently responded to a second infusion of rIL-2 activated DLI. Indeed, more recent investigations in another murine model of myelomonocytic leukemia with chromosome 2 deletion in SJL/J mice also confirmed that amplification of GVL effects may be accomplished by rIL-2. Furthermore, we have demonstrated that following rIL-2 activation of lymphocytes, their cytotoxic effect against allogeneic target cells across minor and particularly across major histocompatibility barriers increased, thus suggesting that improved GVL effects following rIL-2 activation may be due to the increased frequency of cytotoxic T lymphocyte precursor cells [8]. Considering the fact that G-CSF mobilized blood stem cells are increasingly being used instead of bone marrow preparations for transplantation of patients with hematologic malignancies following both myeloablative and non-myeloablative conditioning, and considering the fact that under such conditions, larger numbers of alloreactive donor lymphocytes are administered together with the G-CSF mobilized stem cells, GVL effects may be improved even without additional DLI. On the other hand, it has been documented that G-CSF mobilization of blood stem cells may cause a Th1→Th2 cytokine shift, which could explain the relatively low incidence of acute GVHD in recipients of PBSC despite the markedly increased number of T lymphocytes [15]. Considering the fact that GVL effects usually go hand in hand with GVHD, the reduced incidence of GVHD because of the Th1→Th2 cytokine shift and the down-regulation of alloreactivity by regulatory Th2 cells and Th2 cytokines (e.g., IL-10) may attenuate the GVL effects mediated by allogeneic T lymphocytes. In view of the above, it seemed important to compare the efficacy of GVL effects inducible by G-CSF mobilized lymphocytes with and without additional activations in vivo with rIL-2.
As shown by the data presented, the outcome for mice treated with PBSC was superior to that observed in mice treated with unmodified BMC (Fig. 2). Activation of lymphocytes in the bone marrow with rIL-2 presented no advantage over unstimulated BMC (Fig. 2), whereas additional activation of PBSC by administration of rIL-2 provided significantly better results than unstimulated PBSC. As can be seen in Fig. 2, better survival data were observed in adoptive recipients of spleen cells from F1 mice treated with PBSC collected following administration of rIL-2 (96%) than in adoptive recipients of PBSC alone (88%), confirming better GVL effects in the first group. These results confirmed a better outcome for recipients treated with PBSC following rIL-2 treatment than for recipients of BMC with or without prior treatment with rIL-2. Survival of adoptive recipient mice that were inoculated with spleen cells obtained from F1 mice treated with unstimulated or rIL-2-stimulated BMC were extremely poor, since almost all mice died within 33 days. Allogeneic blood lymphocytes infused with or without prior IL-2 treatment featured improved capacity to eradicate BCL1, with survival of 73 and 85% of adoptive recipients of spleen cells from successfully treated primary recipients, respectively. F1 mice irradiated with 700 cGy TBI with no hematopoietic reconstitution did not die, thus confirming that this conditioning was indeed non-myeloablative, in analogy with recent clinical trials with various reduced intensity conditioning regimens. Thus, our data appear to support the preferential use of G-CSF mobilized blood stem cells, which are indeed much more efficient in inducing GVL effects. It seems reasonable to hypothesize that increased efficacy of GVL effects by rIL-2 activation of donor lymphocytes may be particularly important for the treatment of patients with primary resistant disease, especially for recipients prepared with non-myeloablative, reduced intensity conditioning. Considering the risk of GVHD, the use of rIL-2 for amplification of GVL effects should be considered only for patients with truly resistant and progressive disease, or in conjunction with DLI administered late after transplantation in patients with primary resistant or progressive disease and no evidence of GVHD following discontinuation of cyclosporine. Future clinical studies will indicate whether these assumptions based on pre-clinical animal models and pilot clinical observations are indeed valid as practical guidelines for improving GVL effects in clinical practice.
Acknowledgements
We wish to thank the Danny Cunniff Leukemia Research Laboratory, the Gabrielle Rich Leukemia Research Foundation, the Cancer Treatment Research Foundation, the Novotny Trust and the Fig Tree Foundation for their continuous support of our ongoing basic and clinical research.
References
- 1.Bensinger Blood. 1995;85:1655. [PubMed] [Google Scholar]
- 2.Bensinger Blood. 1996;88:2794. [PubMed] [Google Scholar]
- 3.Dreger Br J Haematol. 1994;87:609. doi: 10.1111/j.1365-2141.1994.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 4.Duhrsen Blood. 1988;72:2074. [PubMed] [Google Scholar]
- 5.Glass B, Uharek L, Hartung G, Zeis M, Steinmann J, Dreger P, Kronke M, Schmitz N (1998) Immunotherapeutic aspects of allogeneic peripheral progenitor cells. Bone Marrow Transplant 21 [Suppl 3]:S3 [PubMed]
- 6.Korbling Blood. 1995;85:1659. [PubMed] [Google Scholar]
- 7.Korbling Blood. 1995;86:2842. [PubMed] [Google Scholar]
- 8.Leshem Cytokines Cell Mol Ther. 2000;6:141. doi: 10.1080/mccm.6.3.141.147. [DOI] [PubMed] [Google Scholar]
- 9.Molineux Blood. 1990;75:563. [PubMed] [Google Scholar]
- 10.Russell Lancet. 1993;341:1482. doi: 10.1016/0140-6736(93)90929-B. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz Blood. 1995;85:1666. [PubMed] [Google Scholar]
- 12.Schmitz Semin. 2002;Hematol:3. [Google Scholar]
- 13.Slavin Nature. 1978;272:624. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- 14.Slavin Cancer Res. 1981;41:4162. [PubMed] [Google Scholar]
- 15.Slavin Blood. 1996;87:2195. [PubMed] [Google Scholar]
- 16.Volpi Blood. 2001;97:2514. doi: 10.1182/blood.V97.8.2514. [DOI] [PubMed] [Google Scholar]
- 17.Weiss Cancer Invest. 1992;10:19. doi: 10.3109/07357909209032785. [DOI] [PubMed] [Google Scholar]
- 18.Weiss J Immunol. 1994;153:2562. [PubMed] [Google Scholar]
- 19.Weiss, Cytokines Cell Mol Ther. 1999;5:153. [PubMed] [Google Scholar]


