Abstract
Purpose: Dendritic cells (DCs) play an important role in the host’s immunosurveillance against cancer. It has been shown that the function of DCs is impaired and their population decreased in a cancer-bearing host. In the present study, we investigated the mechanism of down-regulation of DCs in a cancer-bearing host. Methods: We evaluated the relationship between DC infiltration and production of vascular endothelial growth factor (VEGF) in carcinoma tissue by immunohistochemistry. Furthermore, functional and phenotypical alterations of DCs were evaluated when monocyte-derived, mature DCs were treated with VEGF in vitro. Monocyte-derived DCs were generated in a culture of monocyte with interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor, and the maturation of DCs was induced by either lipopolysaccharide (LPS) or a proinflammatory cytokine cocktail: tumor-necrosis factor α, prostaglandin E2, IL-6, and IL-1β. Results: A significant inverse correlation was found between the density of DCs and the quantity of VEGF production in gastric carcinoma tissue (r=−0.39, p<0.05). In LPS-induced maturation, the ability of mature DCs to stimulate allogenic T cells and produce IL-12 (p70 heterodimer) was suppressed by the addition of VEGF in a dose-dependent manner. A lesser expression of costimulatory molecules (CD80 and CD86) was seen in DCs treated with exogenous VEGF than in DCs not treated with VEGF. The population of dead DCs (early and late apoptosis) treated with VEGF increased more than that without VEGF treatment, using the annexin V and propidium iodide evaluation in DCs matured by LPS. In contrast, in DCs matured by the proinflammatory cytokine cocktail, the down-regulation of costimulatory molecules and induction of DC apoptosis was not seen. Conclusions: These findings show that the inhibition of DC maturation by VEGF differs depending on the maturation status of the DCs.
Keywords: Vascular endothelial growth factor, Dendritic cells, Apoptosis, Gastric carcinoma, Costimulatory molecule
Introduction
Dendritic cells (DCs), which are the most potent APCs, play an important role in the host’s immunosurveillance against cancer. The impaired function of DCs in cancer-bearing hosts has been reported by many investigators. DCs failed to present tumor-specific antigen [1, 2] and transfer it to the naïve T cells in patients with advanced stage cancer [2]. Simultaneously, a reduced expression of B7 molecules in tumor-infiltrating DCs was shown [1]. Enk et al. [3] showed that DCs acted as the mediator for tumor progression in a metastatic melanoma patient, resulting from the decreased function of DCs. Moreover, not only the defective function of DCs but also their population was related to prognosis in cancer patients. Almand et al. [4] demonstrated that the number of peripheral blood DCs in a tumor-bearing patient significantly decreased. Tsujitani et al. [5] showed that the population of infiltrating DCs was significantly related to poor prognosis in stage III gastric cancer patients. Similar conclusions were seen in patients with lung cancer [6], colon cancer [7], esophageal cancer [8], and carcinoma of the uterine cervix [9]. Thus, the function of DCs was impaired and their populations were down-regulated in cancer patients.
VEGF, which serves as a mitogen for the endothelium in vitro [10] and an angiogenic factor in vivo [11], is produced by most cancer cells. VEGF produced by tumors was reported to be associated with poor prognosis in osteosarcoma [12], gastric cancer [13], and lung cancer [14]. Saito et al. [13] demonstrated that there was an inverse correlation between the density of DCs and the expression of VEGF, suggesting that VEGF inhibits DC function in the tumor microenvironment. It was reported that VEGF inhibited the functional maturation of immature DCs [15, 16, 17]. Ishida et al. [18] showed that the blockade of VEGF by anti-VEGF neutralizing antibody recovered the function of DCs. Thus, the inhibition of DC maturation by VEGF is one of the important events when the tumor burden alters the function of DCs, but the mechanism which down-regulates the DC function remains unclear.
In the present study, firstly, we performed immunohistochemical staining for DCs and VEGF in serial sections of gastric cancer tissue. We showed that there was a significant inverse correlation between the density of DCs and the expression of VEGF, suggesting that the tumor burden affected the infiltration of DCs through the production of VEGF. Secondly, to assess the effect of VEGF against DCs, monocyte-derived DCs were treated with VEGF in vitro. We demonstrated that VEGF inhibited LPS-induced maturation of DCs with down-regulation of costimulatory molecules and induction of apoptosis, but did not inhibit the DC maturation induced by a proinflammatory cytokine cocktail (TNF-α, PGE2, IL-6, and IL-1β).
Materials and methods
Patients
Sixty-five constitutive patients were enrolled in this study. All the patients underwent gastrectomy for pathological diagnosis of adenocarcinoma of the stomach in our University Hospital from 1993 to 1994. Informed consent was obtained from all the enrolled patients. Clinicopathological characteristics were described as follows. The patients were 56.8 ± 24.3 years old (mean ± SD). Of the patients, 44 were men, and 21 were women. The mean tumor size was 5.0 ± 5.7 cm. Lymph node metastasis was seen in 19 patients and there was peritoneal dissemination in 3 patients. Twenty tumors had lymphatic vessel invasion, and 8 tumors had blood vessel invasion. The tumor-nodes-metastasis (TNM) classification of the International Union Against Cancer was used to classify these tumors. Forty-six tumors belonged to stage I, three were stage II, ten were stage III, and six tumors were stage IV.
Evaluation of DCs density and VEGF production in gastric carcinoma
To detect tumor-infiltrating DCs and VEGF produced by tumor cells, polyclonal anti-S100 protein antibody (Nichirei, Tokyo, Japan) and anti-VEGF monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used for immunohistochemistry as previously described [5, 13, 19]. Polyclonal anti-S100 protein antibody was diluted 1:100 in PBS, and anti-VEGF monoclonal antibody, which reacts with the 165, 189, and 121 amino acid splice variants of VEGF of human origin, was diluted 1:100 in PBS. Four-micrometer serial sections of formalin-fixed, paraffin-embedded tumor block were cut, and the tissues were deparaffinized with xylene and treated with concentrated ethanol several times. Thereafter, the serial sections were heated by microwave oven for the purpose of antigen retrieval. After washing in PBS, the sections were incubated with hydrogen peroxide to block the endogenous peroxidase activity. The serial sections were then incubated overnight at 4°C with either polyclonal anti-S100 protein antibody or anti-VEGF monoclonal antibody. As a negative control, normal mouse serum was used without primary antibody. The sections were incubated with EnVision labeled polymer reagent (Dako, Copenhagen, Denmark) according to the manufacturer’s recommendation. This polymer reagent is a peroxidase-labeled polymer conjugated to goat antirabbit and goat antimouse immunoglobulins in a Tris-HCl buffer containing carrier protein and an antimicrobial agent. Diaminobenzidine was then used as a chromogen, and the sections were counterstained with hematoxylin. The positive cells were counted by light microscopy (Olympus, Tokyo, Japan).
The population of DCs positive for S100 protein in each cancer cell nest and adjacent mucosa were counted as described previously [5, 13]. The density of DCs was divided into two groups by the average number of DCs in five randomly selected areas at ×200 magnification: the high-density group (more than 20 positive cells) and the low-density group (0–19 positive cells). The population of VEGF-producing cells in the carcinoma cells was counted in five randomly selected areas at ×200 magnification and also divided into two groups: the expressed group (more than 10 positive cells) and the nonexpressed group (0–9 positive cells).
Generation of monocyte-derived DCs
Peripheral blood monocytes were isolated from the peripheral whole blood of healthy donors by means of centrifugation by Ficoll-Paque density gradient (Pharmacia, Uppsala, Sweden) after being incubated with RosetteSep antibody cocktail for monocytes (StemCell Technologies, Vancouver, Canada). RosetteSep antibody cocktail purified from mouse ascites fluid or hybridoma culture supernatant was bound in bispecific antibody complexes which are directed against cell surface antigens on human hematopoietic cells (CD2, CD3, CD8, CD19, CD56, and CD66b) and glycophorin A on red blood cells. Unwanted cells which adhered to red blood cells and the desired cells were separated on a Ficoll-Paque density gradient. Monocytes (1.0×106 cells/ml), resulting in more than 90% monocyte purity confirmed by flow cytometry using anti-CD14 monoclonal antibodies (Dako) were incubated in 6-well plates (Nunc, Roskilde, Denmark) with 500 U/ml GM-CSF and 500 U/ml IL-4 (Peprotech EC, London, UK) in complete culture medium, which contained RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS, 29.2 mg/ml glutamine, 10,000 U/ml penicillin and 10,000 μg/ml streptomycin (Life Technologies) for 5 days. To mature the monocyte-derived DCs, we used LPS or a proinflammatory cytokine cocktail: TNF-α, PGE2, IL-6, and IL-1βas described in a previous report [20, 21] with some modifications. Either 50 ng/ml of LPS (Sigma, St. Louis, MO) or proinflammatory cytokine cocktail (10 ng/ml IL-1β, 1,000 U/ml IL-6, 10 ng/ml TNF-α, and 1 μg/ml PGE2) was added to the wells on day 5 of culture and incubated for 2 days in the presence or absence of VEGF. The cells were then collected and subjected to experiments.
Quantitation of IL-12 secretion from DCs by ELISA
To evaluate the ability of DCs to produce bioactive IL-12 (p70 heterodimer) in the presence of various concentrations of VEGF ligands, the DC-culture medium at day 7 was determined using the standard sandwich ELISA technique with the Quantikine human IL-12 immunoassay (R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendation.
Allogenic mixed lymphocyte reaction (MLR)
To evaluate the ability of DCs to stimulate the proliferation of allogenic T cells, 1×105 allogenic T cells were coincubated in triplicate with irradiated (20 Gy) DCs at various ratios in 96-well U-bottom plates (Nunc). After a 60-h incubation, 1 μCi of [3H]thymidine (Moravek Biochemicals, Brea, CA) was added to each well. The cells were harvested after 16 h and thymidine incorporation was measured in counts per minute (cpm).
Phenotypical analysis of DCs
Phenotypical analysis of DCs was performed using mAbs by FACS Calibur (Becton Dickinson). We used mAbs against CD83 (R-PE conjugated), CD80 (FITC conjugated; both from BD PharMingen); CD86 (FITC conjugated), MHC class I (HLA-ABC), HLA-DR (all three from Dako, Glostrup, Denmark); and VEGF receptor 2, Flk-1 (SantaCruz). Unlabeled mAbs were visualized using FITC-conjugated immunoglobulins (Dako).
Detection of apoptosis in DCs
To evaluate the influence of VEGF with regard to apoptosis in DCs, we measured the rate of apoptosis in DCs under various concentrations of VEGF ligand by flow cytometry. The procedure to measure apoptosis was described in our previous report [22]. In brief, apoptosis in DCs was measured by staining with FITC-conjugated annexin V and propidium iodide (PI) using a MEBCYTO Apoptosis Kit (MBL, Nagoya, Japan) according to the manufacturer’s recommendations.
Statistics
The correlation between the density of DCs and the expression of VEGF was analyzed by Spearman rank correlation coefficient. Differences between the two groups were compared by Student’s t-test. Significant differences were established when p<0.05.
Results
In vivo analysis of the density of DCs and the expression of VEGF in gastric carcinoma tissue
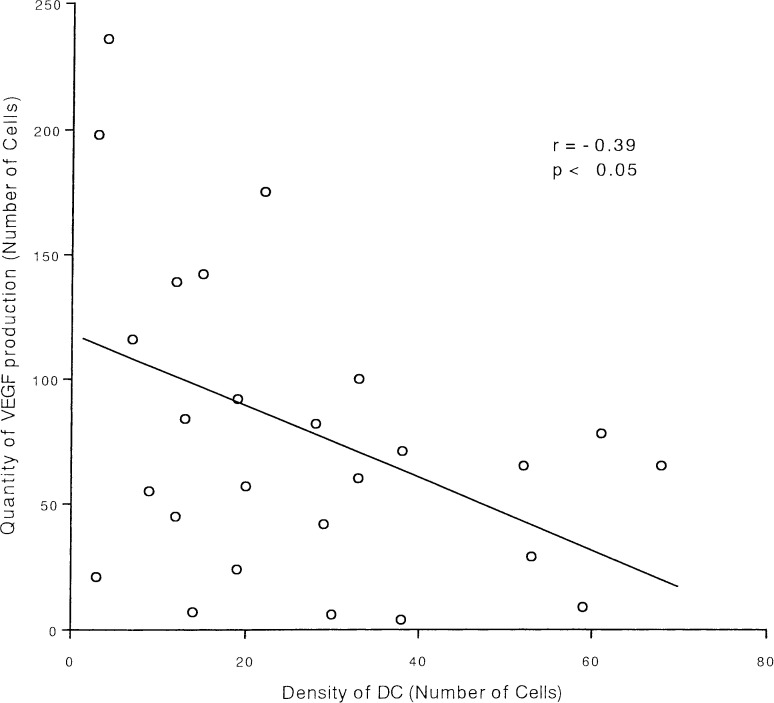
To evaluate the density of DCs and the expression of VEGF in gastric cancer tissue, we performed immunohistochemical staining using the polyclonal anti-S100 protein antibody for DCs, and the anti-VEGF monoclonal antibody for VEGF. We assessed the correlation between the density of DCs and the quantity of VEGF production by Spearman rank correlation coefficient. A significant inverse correlation was found between the density of DCs and the quantity of VEGF production (r=−0.39, p<0.05; Fig. 1), suggesting that VEGF produced by carcinoma tissue may affect the infiltration of DCs.
Fig. 1.
Correlation between DC density and VEGF expression. The density of DCs and the quantity of VEGF expression were immunohistochemically evaluated. A significant inverse correlation was found between the density of DCs and the quantity of VEGF production analyzed by Spearman correlation coefficient (r=−0.39, p<0.05)
Effect of VEGF on the functioning of maturated DCs
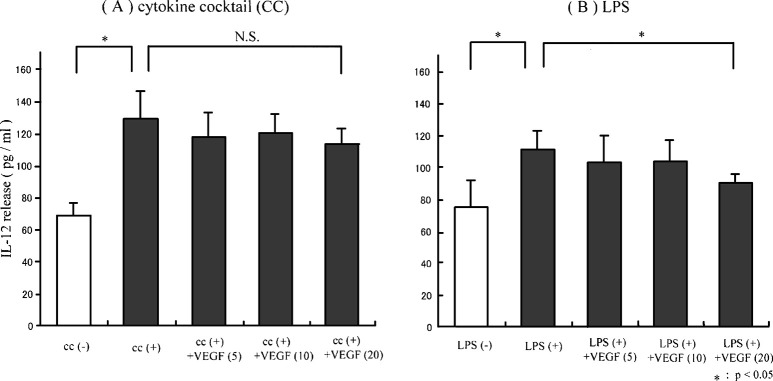
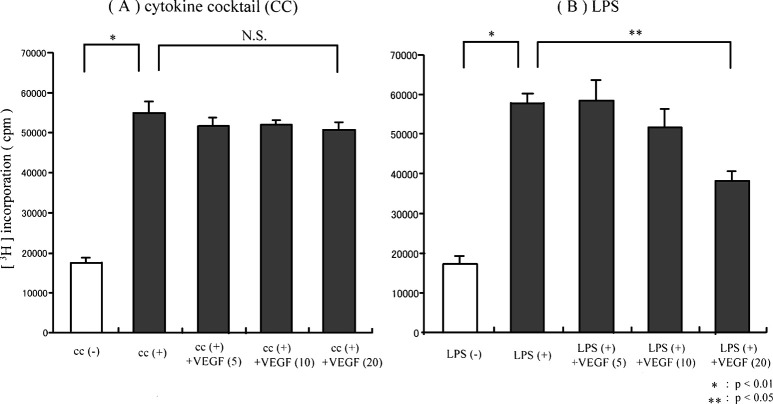
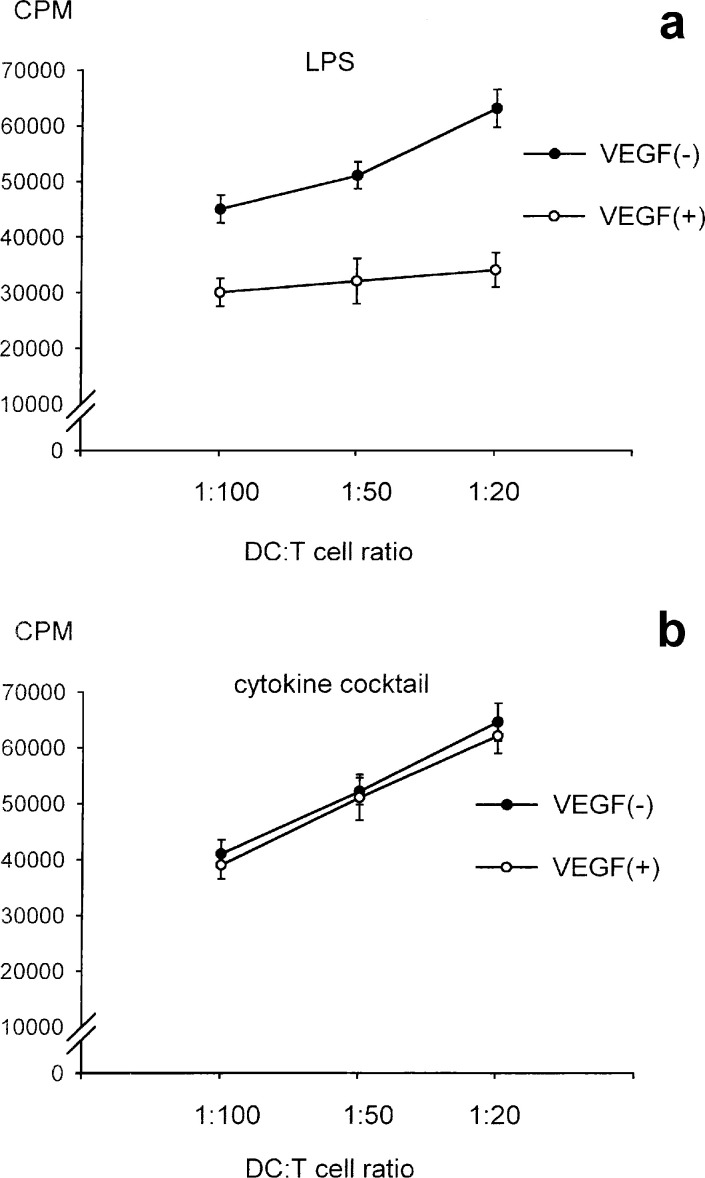
To evaluate the effect of VEGF on the functioning of DCs, monocyte-derived DCs were matured in either LPS or proinflammatory cytokine cocktail in the presence of VEGF in vitro. The ability of DCs to produce IL-12 (p70 heterodimer) and to stimulate allogenic T cells was evaluated. The production of IL-12 by DCs untreated with LPS was clearly lower than that by DCs treated with LPS (Fig. 2B). Treatment with VEGF down-regulated the production of IL-12 from LPS-induced DCs in a dose-dependent manner (5 ng/ml, 10 ng/ml, and 20 ng/ml; Fig. 2B). It was suggested that the ability of DCs to produce IL-12 (p70 heterodimer) was inhibited by the addition of VEGF ligand when DCs were matured with LPS. In contrast, no significant effect of the addition of VEGF was seen in the IL-12 production from DCs matured by proinflammatory cytokine cocktail (Fig. 2A). Furthermore in MLR at a DC to T cell ratio of 1:50, the proliferation of allogenic T cells by DCs untreated with LPS was significantly lower than that of DCs treated with LPS (p<0.01; Fig. 3B). Moreover, the ability of LPS-matured DCs to stimulate allogenic T cells was inhibited by the addition of VEGF in a dose-dependent manner (5 ng/ml, 10 ng/ml, and 20 ng/ml; Fig. 3B), while the ability of DCs matured by proinflammatory cytokine cocktail was not inhibited (Fig. 3A). Furthermore, the VEGF-induced DC inability to stimulate allogenic T cells was observed at various DC to T cell ratios in LPS-matured DCs (Fig. 4A), but not in cytokine cocktail–induced DCs (Fig. 4B). These observations indicated that VEGF inhibited the functional maturation of DCs in the case of maturation by LPS, but not in the case of maturation by proinflammatory cytokine cocktail. Since the function of DCs was obviously suppressed by the addition of 20 ng/ml of VEGF ligand in vitro, we performed the following study under this condition (VEGF concentration 20 ng/ml).
Fig. 2A,B.
IL-12 (p70 heterodimer) production in the cultured supernatant of DCs. Maturation of monocyte-derived DCs was induced by either LPS or proinflammatory cytokine cocktail in the presence or absence of VEGF. The contents of IL-12 (p70 heterodimer) in the cultured supernatant of DCs were determined by ELISA and expressed as pg/ml. The ability of DCs cultured with LPS to produce IL-12 was inhibited by the addition of VEGF ligand in a dose-dependent manner (5 ng/ml, 10 ng/ml, and 20 ng/ml; B). In contrast, the effect of the addition of VEGF was not significantly seen in the IL-12 production from DCs matured by proinflammatory cytokine cocktails (A)
Fig. 3A,B.
Allogenic T-cell response by DCs. Maturation of monocyte-derived DCs was induced by either LPS or cytokine cocktail in the presence or absence of VEGF. Allogenic T cells (1×105) were coincubated with irradiated (20 Gy) DCs (2×103) in 96-well U-bottom plates, and thymidine incorporation was expressed in cpm. The ability of DCs matured by LPS to stimulate allo-T cells was inhibited by the addition of VEGF ligand in a dose-dependent manner (5 ng/ml, 10 ng/ml, and 20 ng/ml; B). However, the ability of DCs matured by proinflammatory cytokine cocktail was not suppressed by the addition of VEGF (A)
Fig. 4A,B.
Allogenic T-cell response by DCs at various ratios. Maturation of monocyte-derived DCs was induced by either LPS or cytokine cocktail in the presence or absence of VEGF (20 ng/ml). Irradiated (20 Gy) DCs were added at various ratios to allogenic T cells (1×105) in 96-well U-bottom plates, and thymidine incorporation was expressed in cpm. The ability of DCs matured by LPS to stimulate allo-T cells was inhibited by the addition of VEGF (A), while the ability of DCs matured by proinflammatory cytokine cocktail was not suppressed by the addition of VEGF (B)
Phenotypical analysis of maturated DCs by the addition of VEGF
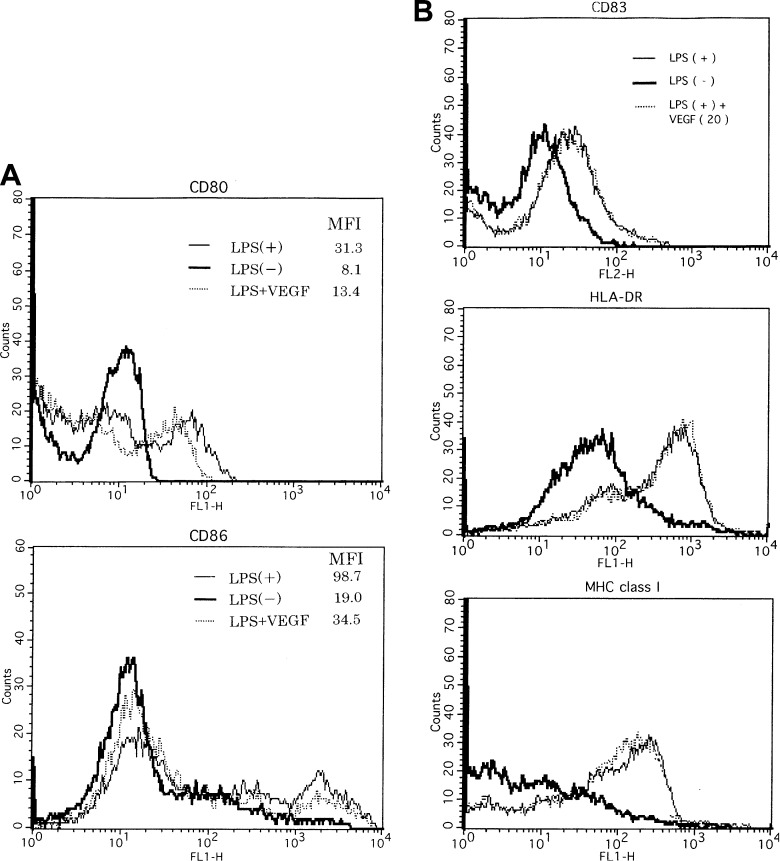
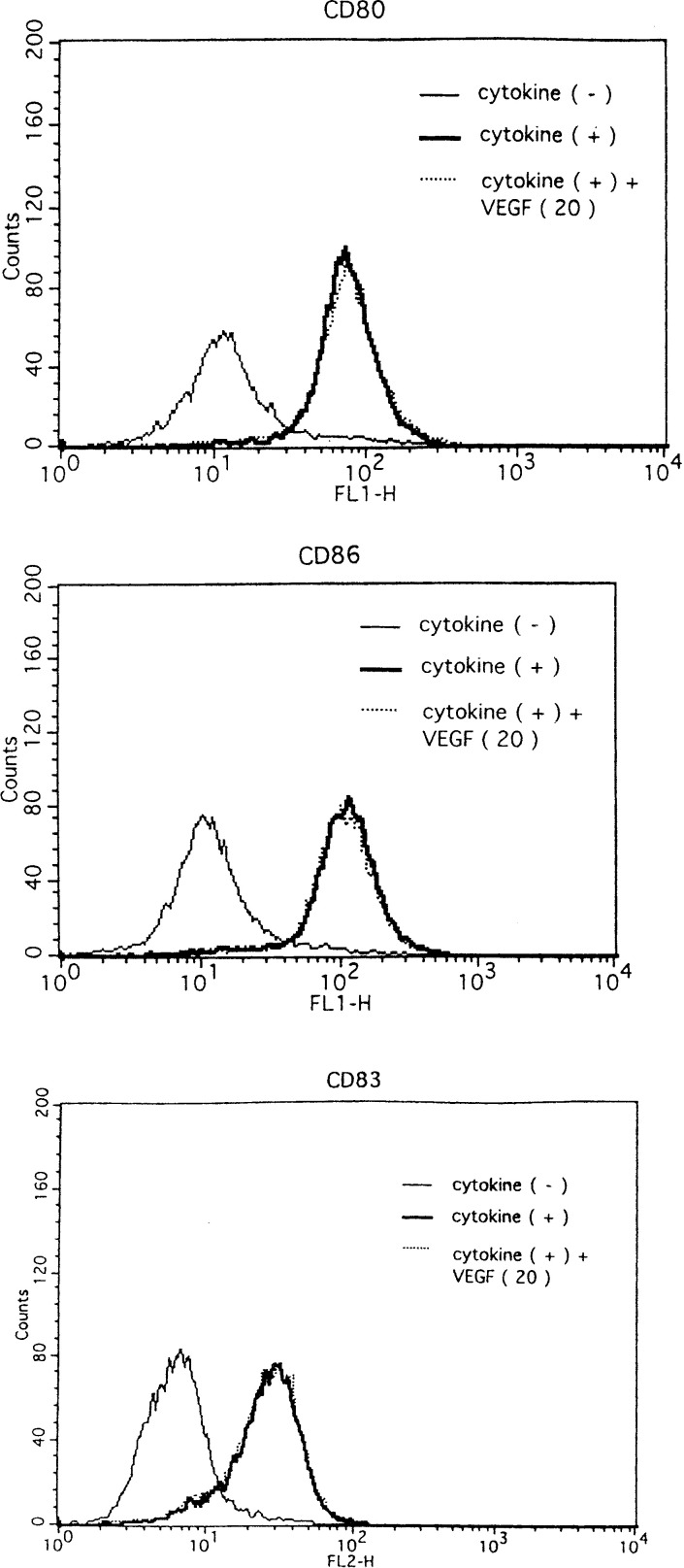
To explain the above-mentioned phenomena, we examined the phenotypical change in DCs by the VEGF treatment. Phenotypical analysis was as follows (Fig. 5A, B): the expressions of CD80, CD83, CD86, HLA-DR, and MHC class I on DCs matured with LPS (50 ng/ml) increased more than those on DCs untreated with LPS (Fig. 5A, B), indicating that LPS phenotypically induced the matured type of DCs. In costimulatory molecules (CD80 and CD86), the expression of these molecules on DCs treated with LPS plus VEGF (20 ng/ml) decreased more than that on DCs treated with LPS. In contrast, the expressions of the other molecules (CD83, HLA-DR, and MHC class I) on DCs treated with LPS were as high as those on DCs treated with LPS plus VEGF. Increased expressions of CD80, CD83, and CD86 on DCs treated with proinflammatory cytokine cocktail were also seen in comparison with DCs untreated by cytokine cocktail (Fig. 6). However, the expression of costimulatory molecules on DCs matured by the proinflammatory cytokine cocktail was not changed by the VEGF addition. In short, these results indicated that VEGF inhibited the LPS-induced maturation of DCs with regards to costimulatory molecules (e.g., CD80 and CD86) but did not inhibit those on DCs matured by proinflammatory cytokine cocktail.
Fig. 5A,B.
Phenotypical analysis of DCs matured by LPS. Maturation of monocyte-derived DCs was induced by LPS in the presence or absence of VEGF. Representative flow cytometric data are shown in the expression of costimulatory molecules (CD86 and CD80; A) and other molecules (CD83, HLA-DR, and MHC class I; B) on DCs. Less expression of costimulatory molecules was seen in DCs treated with LPS plus the exogenous VEGF ligand, but the expression of phenotypical antigens, HLA-DR, MHC class I, and CD83, on DCs treated with LPS plus VEGF ligand was unchanged. Representative data from three independent experiments are shown. DCs untreated with LPS (bold line), DCs treated with LPS (thin line), DCs treated with LPS in the presence of VEGF (dotted line). MFI mean fluorescence intensity
Fig. 6.
Phenotypical analysis of DCs matured by proinflammatory cytokine cocktail. Maturation of monocyte-derived DCs was induced by cytokine cocktail in the presence or absence of VEGF. Representative flow cytometric data are shown in the expression of phenotypical antigens (CD86, CD80, and CD83) on DCs from three independent experiments. DCs untreated with proinflammatory cytokine cocktail (thin line), DCs treated with proinflammatory cytokine cocktail (bold line), DCs treated with proinflammatory cytokine cocktail in the presence of VEGF (dotted line)
Apoptosis of matured DCs induced by VEGF
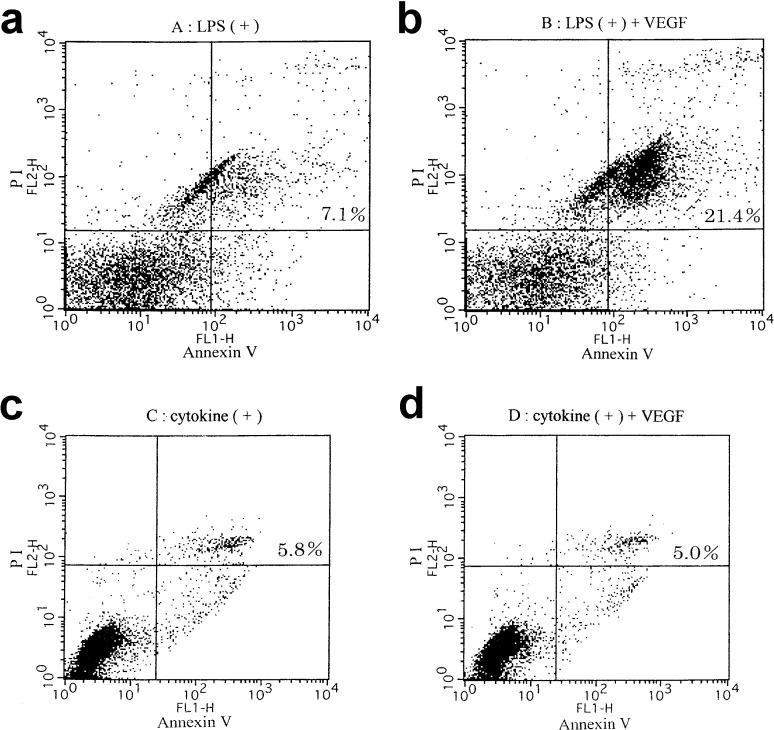
To assess the DC death induced by the addition of VEGF ligand, DC death was evaluated using annexin V and propidium iodide (PI). The population in the right half, of DCs cultured with LPS plus 20 ng/ml of VEGF ligand, which means early apoptosis, late apoptosis, and necrosis, was obviously greater than that of DCs treated with LPS without VEGF ligand (Fig. 7A, B). These results suggested that VEGF induced DC death. As shown in Fig. 7C, D, the population in the right half, of DCs cultured with proinflammatory cytokine cocktail with or without VEGF ligand was almost the same proportion. These results suggested that VEGF did not induce death of DCs matured by proinflammatory cytokine cocktail, in contrast to the case with LPS maturation. Thus, the inhibition of DC maturation by VEGF was different depending on the maturation status of DCs.
Fig. 7A–D.
Spontaneous DC apoptosis affected by the addition of VEGF. Apoptosis in DCs was measured by flow cytometry staining with annexin V and PI. The population in the right half (late and early apoptosis), of DCs treated with LPS in the presence of VEGF (20 ng/ml) was obviously greater than that of DCs treated with LPS in the absence of VEGF (A and B). The population in the right half, of DCs cultured with the cytokine cocktail with or without VEGF ligand was almost the same proportion (C and D). Representative data from three independent experiments are shown
Expression of Flk-1 on DCs
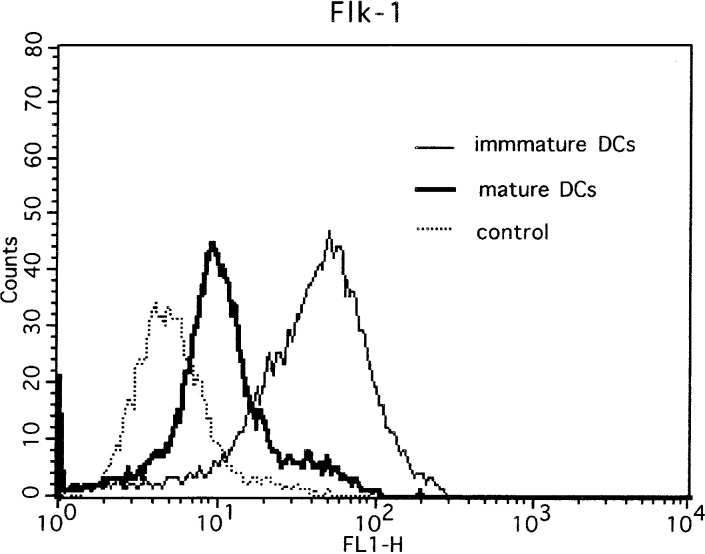
It has been shown that VEGF receptor 1, Flt-1, was abundantly expressed on the surface of immature DCs. We further analyzed the expression of VEGF receptor 2, Flk-1, on DCs. As expected, the VEGF receptor 2, Flk-1, was expressed on immature DCs and the expression was down-regulated on mature DCs (Fig. 8).
Fig. 8.
Flk-1 expression of DCs. The expression of VEGF receptor (Flk-1) on matured DCs was down-regulated in comparison to that on immature DCs
Discussion
In the present study, we showed, firstly, that there was an inverse correlation between the quantity of VEGF production and DC infiltration in gastric cancer tissue. Secondly, VEGF ligand inhibited the maturation of DCs induced by LPS, e.g., in (1) down-regulation of the costimulatory molecules on DCs, (2) decreased IL-12 (p70 heterodimer) production, and (3) impaired ability to induce the proliferation of allogenic T cells. Moreover, DC death (early and late apoptosis) was induced by the addition of VEGF ligand. However, this VEGF-induced phenomenon on DCs was not seen in the case of maturation by the proinflammatory cytokine cocktail.
It has been reported that DCs play a pivotal role in the host’s anticancer immune response. Tsujitani et al. [23] showed that the number of DCs reflected the local immune response. Actually, the population of peripheral blood DCs in breast cancer patients was reduced and the function of DCs in these patients failed [4]. Maehara et al. [24] showed that the expression of PCNA in tumors, which means tumor growth potential, was inversely related to the population of DCs in advanced gastric cancer. We demonstrated that there was significant inverse correlation between the quantity of VEGF production and the population of DC infiltration in patients with gastric cancer. These observations supported the fact that VEGF may affect the distribution of DCs in the tumor microenvironment.
To further investigate the alteration of DCs induced by VEGF, we established an in vitro model, where maturation of monocyte-derived DCs was induced by either LPS or a proinflammatory cytokine cocktail in the presence or absence of VEGF. It was confirmed that mature DCs induced by LPS without VEGF treatment were more dendritic than DCs induced by LPS with VEGF treatment phenotypically and the functioning (e.g., allo-MLR and IL-12 production) of DCs under this condition was also impaired, in line with previous reports that VEGF inhibited the functional maturation of DCs [15, 16, 17]. Oyama et al. [17] showed that the blockade of nuclear factor-κB activation of DCs by VEGF inhibited the maturation of DCs. It was demonstrated that the blockade of VEGF using an anti-VEGF neutralizing antibody recovered the function of DCs [18]. Considering our previous results, VEGF inhibits the LPS-induced maturation of DCs phenotypically and functionally.
In new findings, we showed that costimulatory molecules (CD80 and CD86) on DCs matured by LPS, but not other molecules (CD83 and HLA-DR), were down-regulated by the treatment with VEGF. It is likely that down-regulation of costimulatory molecules is one of the mechanisms by which functional maturation of DCs was inhibited by VEGF. Also, this observation is consistent with the in vivo phenomenon of reduced expression of B7 molecules in tumor-infiltrating DCs [1]. Moreover, for the first time, we showed that VEGF induced DC death (early and late apoptosis). Recently, it was also reported that tumor culture supernatant inhibited the maturation and function of DCs, and the tumor supernatant induced apoptosis of monocyte-derived DCs [25]. Most cancer cells are well-known for producing VEGF. It is possible that VEGF produced by tumors induces DC apoptosis. This observation may point to one of the mechanisms to explain the in vivo results that there was an inverse correlation between the VEGF expression and the population of DCs in gastric cancer tissue.
Interestingly, we showed that DCs matured by proinflammatory cytokine cocktail did not show the down-regulation of the costimulatory molecules and the increased population of DC death when treated with VEGF, in contrast to the DCs matured by LPS. As an explanation of this discrepancy, it is likely that the maturation status of DCs was different between the stimulation conditions. Regarding the maturation marker, CD83 expression on DCs, DCs matured by proinflammatory cytokine cocktail were more matured than those by LPS. This observation suggested that the difference in sensitivity for VEGF treatment may depend on the difference of maturation status on DCs. The use of ex vivo manipulated DC to overcome the impaired APC function in a cancer-bearing host is the rationale behind immunotherapy using DCs. Since VEGF was produced in most cancer cells, the use of strongly matured DCs may be preferable because of the resistance against VEGF derived from the tumor. Alternatively, blockade of VEGF signaling by novel agents such as antibodies may improve the function of antigen presentation on DCs.
It was reported that the VEGF receptor, Flt-1, was abundantly expressed on the surface of immature DCs and the receptor was down-regulated when DCs matured [26]. In this study, we showed that the expression of Flk-1, which is another type of VEGF receptor, on matured DCs was down-regulated in comparison with immature DCs. The differential effects of VEGF on maturation of DCs between stimulants may contribute to the quantitative and functional difference of the VEGF receptor on DCs.
In conclusion, in the present study, VEGF inhibited the maturation of DCs induced by LPS with down-regulated expression of costimulatory molecules and increased DC apoptosis. However, this phenomenon was not seen in the case of DCs matured by proinflammatory cytokine cocktail. To develop the treatment of cancer, and immunotherapy in particular, it is important to understand VEGF regulation against the immune system in cancer-bearing hosts and to develop a strategy for VEGF production in the tumor microenvironment.
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HLA
human leukocyte antigen
- IL
interleukin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- PCNA
proliferative cell nuclear antigen
- PE
phycoerythrin
- PG
prostaglandin
- PI
propidium iodide
- TNF
tumor-necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1.Choux Int J Cancer. 1997;72:619. doi: 10.1002/(sici)1097-0215(19970807)72:4<619::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich Clin Cancer Res. 1997;3:483. [PubMed] [Google Scholar]
- 3.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Almand Clin Cancer Res. 2000;6:1755. [PubMed] [Google Scholar]
- 5.Tsujitani Cancer. 1987;59:501. doi: 10.1002/1097-0142(19870201)59:3<501::aid-cncr2820590325>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa Cancer. 1985;56:2651. doi: 10.1002/1097-0142(19851201)56:11<2651::aid-cncr2820561120>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Ambe Cancer. 1989;63:496. doi: 10.1002/1097-0142(19890201)63:3<496::aid-cncr2820630318>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda Cancer. 1990;65:2261. doi: 10.1002/1097-0142(19900515)65:10<2261::aid-cncr2820651017>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Nakano Arch Pathol Lab Med. 1989;113:507. [PubMed] [Google Scholar]
- 10.Leung Science. 1989;246:1306. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 11.Connolly J Clin Invest. 1989;84:1470. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaya Clin Cancer Res. 2000;6:572. [Google Scholar]
- 13.Saito Br J Cancer. 1998;78:1573. doi: 10.1038/bjc.1998.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake N Engl J Med. 1992;327:14. [Google Scholar]
- 15.Gabrilovich Nat Med. 1996;2:1096. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich Blood. 1998;92:4150. [PubMed] [Google Scholar]
- 17.Oyama J Immunol. 1998;160:1224. [PubMed] [Google Scholar]
- 18.Ishida J Immunol. 1998;161:4842. [PubMed] [Google Scholar]
- 19.Takahashi Oncology. 2002;62:121. doi: 10.1159/000048257. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Proc Natl Acad Sci U S A. 1996;93:2588. [Google Scholar]
- 21.Dhodapkar J Exp Med. 2002;195:125. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Clin Cancer Res. 2001;7:74. [PubMed] [Google Scholar]
- 23.Tsujitani Cancer. 1990;66:2012. doi: 10.1002/1097-0142(19901101)66:9<2012::aid-cncr2820660928>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Maehara Int J Cancer. 1997;74:224. doi: 10.1002/(sici)1097-0215(19970422)74:2<224::aid-ijc15>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Kiertscher J Immunol. 2000;164:1269. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 26.Ohm Immunol Res. 2001;23:263. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]