Abstract
Tumor escape is one major obstacle that has to be addressed prior to designing and delivering successful immunotherapy. There is compelling evidence to support the notion that immunogenic tumors, in murine models and cancer patients, can be rejected by the immune system under optimum conditions for activating adaptive and nonadaptive antitumor immune responses. Despite this capability, a large number of tumors continue to grow and evade recognition and/or destruction by the immune system. The limited success in current immunotherapeutic strategies may be due to a variety of reasons: failure of effector cells to compete with the growing tumor burden, production of humoral factors by tumors that locally block cytotoxicity, antigen/MHC loss, T-cell dysfunction, production of suppressor T cells—to name but a few causes for therapeutic ineffectiveness for the particular malignancy being treated. To optimize immunotherapy strategies, correction of immune-activating signals, eradication of inhibitory factors, and the evasion from newly developed immunoresistant tumor phenotypes need to be simultaneously considered.
Keywords: Vascular Endothelial Growth Factor, Tumor Antigen, Immune Recognition, Immunosuppressive Cytokine, Tumor Escape
Introduction
The immune defense mechanisms are the least effective and final barrier in the natural mechanisms against cancer formation (carcinogenesis) [1]. Both innate and adaptive immunity induce antitumor effects via the activity of NK cells, NKT cells, macrophages, neutrophils, eosinophils, various cytokines, and specific cytotoxic T lymphocytes (CTLs)—to name but a few. However, spontaneous regression is rarely observed in malignant tumors, hence the need for immunotherapeutic intervention to eradicate the tumor.
Advances in both molecular and cellular immunology have improved our understanding of tumor-host interactions, although much still remains elusive despite the progress made in identifying a large number of tumor antigens and new immunotherapeutic strategies. The majority of tumors manage to “escape recognition” and currently there are a number of mechanisms known for tumor escape which have been described: loss/down-regulation of HLA class I; down-regulation, mutation, or loss of tumor antigens; alterations in cell death receptor signaling; production of immunosuppressive cytokines and suppressor T cells; and the involvement of indoleamine 2,3-dioxygenase (IDO) in suppression—all of which will be discussed in detail later.
It is believed that a T-cell response does exist but is ineffective, as the effector phase of the immune response is inadequate. This notion may explain why current immunotherapeutic strategies have limited success. From the immunological perspective, efficient therapeutic intervention should focus on boosting existing antitumor responses and sustaining large numbers of effector T cells at the tumor site.
Recently, this was clearly demonstrated in melanoma patients, where T-cell responses to vaccines occurred primarily in the presence of preactivated T cells prior to vaccination [2]. Analyses of cell-mediated immunity against defined melanoma antigens using tetramer staining and an IFN-γ secretion assay suggested that specific T-cell responses often exist during tumor progression [3]. Adjuvants such as cytokines have been used when immunizing with peptides and proteins [4–7]. Immunization with DNA vaccines leads to the expression of tumor antigens and their processing by antigen-presenting cells (APCs) [8], whereas the direct delivery of antigens as proteins by cell fusion or cDNA to dendritic cells (DCs) represents a more direct attempt to generate antitumor responses [9].
Some antitumor therapeutic interventions have met with limited success which has been attributed mainly to the “tumor-escape” phenomena; i.e., tumor cells have the ability to evade immune recognition and/or destruction, causing major obstacles in using this modality for the treatment of cancer. This was clearly demonstrated in a prostate tumor (TRAMP-C1) model where the injection of Fms-like tyrosine kinase 3 ligand (flt3-L) induced short-term tumor stabilization and regression followed by tumor relapse [10]. Failure of therapy in this model revealed several important immunosuppressive characteristics of the prostate tumor microenvironment including the down-regulation of MHC class II in DCs, profound deficiency in the expression of CD3ε (CD3epsilon) and the β chain of the T-cell receptor (TCR) of tumor-associated T cells. Cancer cells can be detected and destroyed by CTLs in many experimental models and human tumors, but on diagnosis the metastasis of many human tumors can be shown to correlate with unresponsive T cells. Progressive tumor growth occurs, in part, because the cancer cell is capable of escaping immune recognition and not because the immune system is defective [11]. CML patients, for example, possess CTLs detectable by tetramer staining and cytotoxicity that recognize HLA-A3–restricted peptide, although the number of CTLs may be too few to mediate complete rejection of the cancer [12].
Deletion or suppression of activated CTLs can occur as well as their migration away from the tumor site. The precursor frequency can be too low, such that the effector cells fail to compete with the growing tumor burden. However, it is equally possible that the therapies are ineffective for the particular malignancy being treated. To fully appreciate the role of tumor escape in the failure of immunotherapy, an understanding of the basic principles of immunosurveillance and its role in determining tumor phenotypes is necessary.
The hypothesis first proposed by Thomas and Burnet in the 1970s implied that the immune response to tumor occurred at an early stage in tumor development—“eradicating the cancer before it became apparent” [13, 14].
Many studies have evolved in support of this theory, and evidence exists to suggest that in some models and under appropriate conditions, immunosurveillance can play an active role in suppressing the growth of early tumors. Hence in this paradigm, when tumors do successfully grow, they are thought to have escaped from surveillance, and a number of reviews have been written to support this theory [15–17].
The following will focus on the multiple mechanisms that exist leading to “tumor escape” from the immune system; these factors have to be taken into consideration when designing novel therapeutic strategies. The aim of this review is to highlight the required mechanisms of tumor escape and how they influence the failure of immunotherapy.
Activation versus suppression during tumor progression
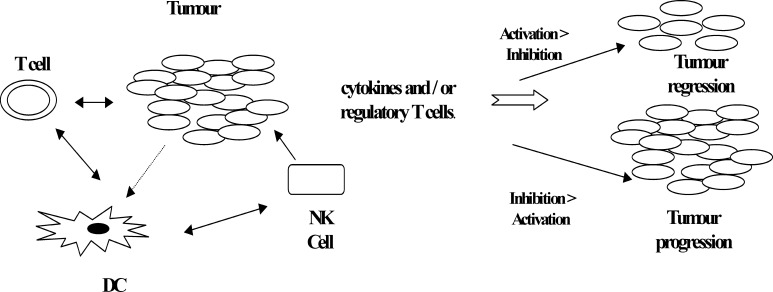
Escape from immune surveillance is a major mechanism for tumors to grow progressively. Tumors may grow undetected by the immune system, being seen as “normal tissue” thus benefiting from any mechanism that makes them appear “healthy” and less dangerous—i.e., exhibiting no danger signals for immune activation [15, 18]. It is believed that even during progressive growth, the tumor has the ability to activate the immune system, and that a fine balance between activation and suppression exists, which determines the fate of the tumor. Tumor vaccines may induce activation and expansion of specific CD8+ T cells and destruction of tumor cells in cancer patients; this was observed in approximately 5–20% of vaccinated melanoma patients. However, this activation can be fraught with the lack of appropriate costimulation or the presence of immunosuppressive cytokines such as IL-10 and TGF-β. The eventual fate of the tumor is therefore determined by the net effect of immune activation and inhibition [15].
Killing of tumor cells in situ by suicide gene transfer to induce cell death through a nonapoptotic pathway was shown to be associated with enhanced immunogenicity [19]. Similar observations were also reported by our group where immunization with irradiated RENCA cells (murine renal carcinoma) infected with DISC-HSV (disabled infectious single cycle–herpes simplex virus) that enhanced immunity to tumor challenge with live parental tumor cells that induced cell death by necrosis as opposed to apoptosis [20] providing the immune system with additional activation as the cells were undergoing necrotic cell lysis enhanced tumor antigen processing and presentation by APCs and hence an increase in T-cell activation [21, 22].
The mechanisms of tumor escape from immune recognition/destruction are likely to be multifactorial, including down-regulation of MHC class I molecules [23–26], loss of tumor antigens [27, 28], defective death receptor signaling [29–33], lack of costimulation [34], and production of immunosuppressive cytokines [35] and suppressive cells [36–40], as shown in Fig. 1.
Fig. 1.
Summary of the processes of activation and suppression during tumor progression
Involvement of indoleamine 2,3-dioxygenase in immunosuppression
The mechanisms that mediate immune tolerance to cancer are not well understood, but recent findings have implicated the tryptophan/IDO metabolic pathway as one of many mechanisms involved. Indoleamine 2,3-dioxygenase (IDO) is an enzyme ubiquitously distributed in mammalian tissues and cells converting tryptophan to N-formylkynurenine [41]. This cytosolic enzyme catalyzes the initial and rate-limiting step in the catabolism of tryptophan in the kynurenine pathway [42]. Low levels of tryptophan at the tumor site causes T cells to arrest in the G1 phase of the cell cycle [43]. Initially the enzyme was recognized for its antimicrobial activity by allowing cells to deplete tryptophan from intracellular pool or local microenvironment [44]; however, recently, IDO was reported to be constitutively expressed by most human tumors, and its expression by immunogenic mouse tumor cells prevents their rejection in preimmunized mice, correlating with the lack of specific T-cell accumulation at the tumor site [43].
IDO expression is an inducible feature of splenic DC subsets, and provides a potential explanation for their ability to regulate T cells. Induction of IDO completely blocked the clonal expansion of T cells from TCR transgenic mice following the administration of the immunomodulatory reagent CTLA4-Ig, whereas the same treatment did not block T-cell clonal expansion in IDO-deficient recipients. Suppression of allogeneic T-cell responses in vitro and in vivo by interstitial APCs was also shown to be IDO-dependent, and blockade of IDO activity with 1-MT (1-methyl-DL-tryptophan [45]) or addition of exogenous tryptophan allowed the recovery of T-cell proliferation in MLR assays [46]. In humans, only a discrete subset of APCs that coexpress the cell surface markers CD123 and CCR6 was shown to express IDO and inhibit T-cell proliferation in vitro [47]. These results clearly demonstrated that the IDO pathway might represent a potential mechanism for DCs to regulate the immune response to tumor antigens.
Loss/down-regulation of HLA class I
Altered MHC class I antigen expression in tumors is a well-known phenomenon. The selective loss of MHC class I alleles was subsequently described in a variety of mouse models including the TL leukemic cell line, MCA-induced tumors, and murine leukemia virus–induced tumors. The altered phenotypes of MHC class I antigen expression permits tumor cells to avoid recognition or survive attack by CD8+ T cells capable of mediating cytotoxicity. This phenomenon has been studied in a series of tumor samples by immunohistochemical techniques and has been shown to be widespread [48].
HLA class I molecule down-regulation occurs frequently in many cancers, and this abnormality might adversely affect the clinical course of disease and the outcome of T cell–based immunotherapies. Over the past few years, HLA class I expression has been characterized in human tumors. Changes in HLA class I expression can occur through various mechanisms—namely, mutations in genes and abnormalities in the regulation and/or defects in HLA class I–dependent antigen processing. These mutations modulate the susceptibility of tumor cells to in vitro lysis by CTLs and natural killer (NK) cells. Immune selection of tumor-resistant CTLs and/or NK cells may explain the rapid progression and poor prognosis of cancers exhibiting HLA class I antigen down-regulation [49].
To date, a number of investigators have classified the types of HLA class I loss into different phenotypes, and Table 1 summarizes the HLA phenotypes that are recognized.
Table 1.
Major HLA phenotypes in tumors and the associated molecular mechanisms (adapted from Cabrera et al., 2003 [11])
| HLA phenotype | Mechanisms | References |
|---|---|---|
| I. HLA class I total loss | β2m mutations | [50–52] |
| Alterations in antigen-processing machinery | [50, 53] | |
| II. HLA haplotype loss | LOH in chromosome 6 | [50, 54–56] |
| III. HLA allelic loss | Mutations of HLA class I genes | [50, 57] |
| IV. HLA-A, HLA-B, HLA-C locus down-regulation | Alteration of transcriptional factors | [50, 58, 59] |
| V. Compound phenotype | 2 or more independent mechanisms | [50, 60, 61] |
A number of studies have been carried out to assess HLA class I expression in tumors. In bladder carcinomas, 72% of the tumor studies showed at least one alteration in HLA expression, and these were classified into different phenotypes depending on the type of down-regulation/loss [62]; expression was correlated to the degree of differentiation and tumor recurrence. To determine the type of HLA loss occurring during tumor progression, tissue samples from breast, colorectal, and laryngeal carcinomas were analyzed [63]. HLA-B44 allele loss was observed more frequently than any other HLA class I allele—suggesting an important role for this allele in tumor escape.
Furthermore, mechanisms of MHC class I loss were also investigated in colorectal carcinoma [64]. It was shown that β2m mutation and LMP7/TAP2 down-regulation were responsible for the total loss of MHC class I expression—which contributes to the failure of T-cell recognition during the immune response.
In murine models, the B9 primary tumor clone (H-2 negative) and its metastatic colonies were studied in immunocompetent BALB/c mice [50]. They showed that 83% of the metastases obtained in different syngeneic BALB/c mice repeatedly exhibited a phenotype different from the original B9 clone. The alterations were identical in different colonies of different syngeneic BALB/c mice.
Variation in the MHC class I expression following immunotherapy has been observed in a murine colon carcinoma model (M. Ahmad, S. Ali, and R.C. Rees, unpublished observation). The CT26 (colon carcinoma) murine tumor model was used to investigate the expression of MHC class I antigens in mice failing to regress their tumors following immunotherapy (progressor) and tumor bearer (no therapy) animals [65]. The DISC-HSV/mGM-CSF vector used in this study is an attractive tool for the delivery of cytokines to the tumors [66–68]. This vector was developed by genetically inactivating the HSV-2 for use as a vaccine, by the deletion of the essential glycoprotein H (gH) gene—restricting it to one cycle of replication [66]. On infection, the virus would release noninfectious particles together with the transduced cytokine, e.g. GM-CSF. In our model the DISC therapy was administered intratumorally to mice with established subcutaneous (s.c.) tumors, and approximately 40% of the treated animals failed therapy, and the tumors continued to grow progressively [45, 65]. MHC class I (H-2Ld) expression was investigated in both tumor-bearing and progressor mice. The results (Table 2) clearly demonstrated that a high proportion (55%) of tumors from progressor mice showed loss of class I MHC antigen, unlike the control tumors (treated with PBS) all of which expressed MHC I antigen. Interestingly, the tumor cells from the progressor animals reexpressed class I following overnight in vitro culture, suggesting that the tumor microenvironment may be responsible for the transient down-regulation of class I expression as opposed to a mutation in the β2m gene [69] or a down-regulation in LMP7/TAP 2 as reported in other studies [64].
Table 2.
A tumor study for the expression of H2Ld. A total of 18 tumors were studied (11 progressors, 7 PBS controls) for the expression of H2Ld . The results have been combined for the immunohistochemistry and FACS analysis, such that 45% of the therapy failures completely lost H2Ld surface expression, whereas the tumor bearer animals retained a degree of expression (low, partial, or full expression) (A.Talasila, MSc Thesis, Nottingham Trent University, UK)
| Treatment | No expression (%) | Low expression (%) | Partial expression (%) | Full expression (%) |
|---|---|---|---|---|
| Tumor bearer | 0 | 29 | 29 | 42 |
| DISC/mGM-CSF | 45 | 28 | 27 | 0 |
Recently, a number of studies have investigated the expression of class II in patients. Antigen-presenting cells are crucial for the induction of an antigen-specific immune response, and down-regulation/loss of expression in cell surface molecules such as HLA class II may contribute to an impaired immune response. This phenomenon was observed in PBMs of melanoma patients [70]. A number of different carcinomas (breast, ovarian, and prostate) have been investigated for the expression of MHC class I and II antigens [71–73] where the absence/down-regulation of these recognition molecules correlated with the tumor’s ability to avoid immune surveillance and development of metastatic dissemination of the malignancy.
Down-regulation, mutation, or loss of tumor antigens
Alterations in tumor-associated antigen (TAA) expression is one of the mechanisms by which tumor cells can escape CTL detection. Modifications in the TAA expression can range from down-modulation to complete loss, and loss of antigen expression can occur independently of the deregulation of HLA class I expression. Tumor antigen expression is known to be heterogeneous, even within the same tumor [15]; and a decrease in the expression of gp100 and MART-1 was associated with disease progression [27, 28]. In spite of the presence of TAA-specific CTLs, immunogenic tumors can eventually grow and kill the host in animal models. Tumor escape in the murine mastocytoma P815 tumor was shown to be due to the emergence of stable antigen-loss variants [74].
Antigenic drift, a mechanism used by viruses to escape immune recognition, has recently been described for tumors. Transgenic mice expressing TCR for a single antigenic epitope have been used extensively in establishing antigenic mutation(s) as a mechanism for viral escape of T-cell recognition [75, 76]. A transgenic mouse line expressing TCR specific for tumor antigen P1A35-43 presented by H-2Ld was also developed and used to study tumor escape mechanisms. The recurrence of tumors in mice that have responded favorably to transgenic T-cell adoptive therapy was found to correlate with the presence of tumor variants with mutations within the PIA epitope [77]. These mutations severely diminish T-cell recognition of the tumor antigen by a variety of mechanisms, including modulation of MHC-peptide interaction and TCR binding to MHC-peptide complex. In another study, using a SCID mouse model and a MART-1/Melan-A–specific CD8+ T-cell clone against autologous melanoma, it was found that the in vivo immunoselection of antigen-loss variants was dependent on the presence of suboptimal levels of antigen expression [78]; and loss of the immunodominant T cell–defined MART-1/Melan-A antigen and down-regulation of the TAP-1 gene has been identified in a recurrent metastatic melanoma. Restoring the “antigen loss” in the variant tumor cell line by simultaneously providing both the MART-1/Melan-A gene (by retroviral transfer) and the TAP-1 gene (by a bioballistic approach) resulted in tumor cell sensitivity to MART-1/Melan-A–specific cytotoxic T lymphocytes, suggesting antigen loss and down-regulation of the peptide-transporter protein TAP-1 expression [79]. The emergence of tumor escape variants is more likely to result after effective immunotherapies.
Alterations in cell death receptor signaling
The expression of Fas ligand (FasL), a well-known cell death receptor ligand, on tumor cells has been implicated in their evasion of immune surveillance [29, 32, 33, 80, 81]. Apoptosis mediated via CD95 (Fas/Apo-1) is a key regulator for the biology of normal and malignant cells. However, Fas surface expression does not necessarily render cells susceptible to FasL-induced death signals, suggesting a role for inhibitors of the apoptosis-signaling pathway. Phosphatidylinositol 3′-kinase (PI3 K) and Akt (protein kinase B) mediate the survival signal and allow cells to escape from apoptosis in various human cancers. PI3 K inhibitors, such as LY294002, inhibit cell proliferation and increase apoptosis in the human gastric carcinoma cell line by the down-regulation of Mcl-2 and phosphorylated Bad proteins, which are antiapoptotic factors and belong to the Bcl-2 family [82]. Acquisition of apoptosis resistance is a typical mechanism of chemotherapy failure in small cell lung cancer, which was correlated with the overexpression of Bcl-2 [83].
Defects in receptors/signaling can facilitate tumor cell survival and proliferation. The Fas/FasL complex forms and engages caspase-8 which autoactivates itself and cleaves caspase-3, caspase-6, and caspase-7 [84]. The caspase-8 inhibitor, cellular FLICE-inhibitory protein (cFLIP), is expressed in many tumors making them resistant to death receptor–mediated apoptosis [30]. FLICE/caspase-8–inhibitory protein (cFLIP) is a recently identified intracellular inhibitor of caspase-8 activation that potently inhibits death signaling mediated by all known death receptors, including Fas, TNF-receptor (TNF-R), and TNF-related apoptosis-inducing ligand receptors (TRAIL-Rs), The increased expression of cFLIP in tumor cells is thought to contribute to immunoresistance to T cells in vivo [31] while the down-regulation/loss of Fas expression resists apoptosis. In patients with B-cell chronic lymphocytic leukemia (B-CLL cells), an increase in cell surface CD95 expression on T cells was associated with reduced progression-free probability and poorer survival [85]. In TRAIL-mediated apoptosis, the loss of expression of all TRAIL receptors can occur by many mechanisms, including the loss of caspase-8 by chromosomal loss or mutation [86].
Cells expressing low levels of Fas (Faslow) in the B16F10 tumor model grew more slowly in comparison to the cells expressing high levels of Fas. Faslow-cells when injected showed enhanced tumor growth in mice depleted of neutrophils, suggesting that inactivation of neutrophils was an important mechanism by which tumors could escape destruction [78]. The expression of FasL has also been studied in cervix adenocarcinomas, where FasL appears to play an important role in immune evasion, progression, and metastases of the tumor [87]. In contrast, the intracellular expression of FasL in breast cancer and normal tissue, as determined by immunohistochemistry, is unlikely to be an important marker for immune evasion [81]. The expression of membrane-bound FasL (mFasL) on colon cancer cells as a potential mechanism to inhibit host immune function by inducing apoptosis of host lymphocytes was investigated [88]. mFasL can be cleaved to release a soluble FasL (sFasL) to spread the apoptosis induction effect within the environment. These findings have suggested sFasL as a mechanism by which tumor cells can avoid immune attack.
Immunosuppressive cytokines
Tumor cells produce a number of cytokines and chemokines that can have a suppressive effect on immune cells. In non–small cell lung cancer (NSCLC) patients, the mRNA expression of IL-4, IL-10, TGF-α, and TGF-β1 was significantly higher than that of IL-2, IL-12, IL-18, and INF-γ as determined in pleural effusion and tumor tissue [89]. This predominant expression of type II (immunosuppressive) cytokines mirrors an immunosuppressive state in the immunological microenvironment. Vascular endothelial growth factor (VEGF) is secreted by many tumors [90], and in the past few years VEGF has been required as a contributory factor in tumor escape. VEGF is not only important for tumor vascularization but is also a key factor produced by solid tumors to inhibit immune recognition [91], and prevent DC differentiation and maturation by suppressing the transcription factor NF-κB in hemotopoietic stem cells [35]. Blocking NF-κB activation in hemotopoietic cells by tumor-derived factors is thought to be a mechanism by which tumor cells can directly down-regulate the ability of the immune system to generate an antitumor response [35]. Elevated VEGF blood concentrations have been correlated with poor prognosis in human neoplasms [92], possibly as a result of its angiogenic properties and/or its ability to suppress DC maturation [92, 93]. VEGF was negatively related to DC infilteration in immunohistochemical studies on resected lung cancer samples [93], and activation status of DCs and the concentration of VEGF in the peripheral blood have been shown to reflect the malignancy of NSCLC [94].
Supernatants collected from tumor cells of AML patients were shown to inhibit T-cell activation, Th1 cytokine production, and to prevent activated T cells from entering the cell cycle [95]; however, no TGF-β, IL-10, or VEGF was detected. The T-cell immunosuppression induced by AML cells provides a mechanism by which leukemic clones could evade T cell–mediated lysis by inhibiting the NF-κB, c-myc, and pRb pathways [95]. The production of soluble factors such as VEGF, IL-10, TNF, and TGF-β is a proposed mechanism for tumor cells to avoid immune recognition, and the effects of these factors appear to be twofold: to inhibit (a) the effector function (b) the development of the immune cells by acting in the early stages of immunopoiesis [91].
IL-10 is a cytokine often quoted as being suppressive, and high levels of IL-10 have been reported in patients with melanoma [96] and pancreatic cancer [97, 98]. IL-10 has the ability to exert its effects in many ways: it inhibits antigen presentation, IL-12 production, and the induction of Th1 responses in vivo [99, 100].
High concentrations of TGF-β are also found in cancer patients [101] and are usually associated with “tumor progression” [102] and poor responses to immunotherapy [103]. The levels of TGF-β are higher in patients with disseminated melanoma when compared to those with localized disease [104]. TGF-β has also been shown to induce the overproduction of IL-10 in tumors, leading to immunosuppression of antitumor responses. This suppression was reversed and Th1 responses reconstituted on the administration of anti-TGF-β antibodies in vivo [105].
Alterations in the expression of signal transduction molecules
Many different mechanisms may contribute to immune evasion. In pancreatic cancer, the loss of signal-transducing CD3 ζ chain (CD3 ζ) of tumor-infilterating lymphocytes (TILs) has been attributed to immune escape together with the production of immunosuppressive cytokines and local impairment of TILs [106]; CD3 ζ loss in many cases has also been correlated with elevated levels of IL-10 and TGF-β. The loss of the CD3 ζ chain has been studied extensively and has been proposed as a mechanism by which tumors are able to escape. The ζ chain is present as a large intracytoplasmic homodimer in the TCR forming part of the TCR-CD3 complex. It functions as a single transducer upon antigen binding [107]. Hence any alterations in this chain could result in changes in the signaling pathway. In pancreatic cancer, the loss of the signal-transducing CD3 ζ chain of TILs has also been attributed to immune escape together with the production of immunosuppressive cytokines and local inactivation of TILs [106]. Alterations in the expression of CD3 ζ chain has also been correlated with elevated levels of IL-10 alone or in association with the down-regulation of IFN-γ [108]. A general down-regulation/decreased expression the CD3 ζ chain has been reported in tumors such as cervical and colorectal carcinomas [109–111]. In cervical cancer, the reduced expression of the ζ chain was associated with a reduction in cellular functions such as the production of TNF [109]. In contrast, PBMCs from breast cancer and colorectal carcinoma patients do not show a decrease in TCR ζ expression, yet the former exhibit an impairment in T-cell function [108].
Lack of costimulation
Most tumors do not regress but continue to grow in spite of the presence of spontaneous or antigen (vaccine)-induced immune responses. The existence of systemic immune responses may not by itself be sufficient to cope with the complex nature of tumor-host interactions because factors such as insufficient costimulation to induce T-cell response may further contribute to the lack of effective immunity. There are a number of molecules that normally perform costimulatory functions by interacting with their counter ligand/receptor on T cells to provide the critical second signal for T-cell and/or APC activation. These include CD80 (B7-1)/CD86 (B7-2) binding to CD28 and CTLA4, respectively; CD40L on activated CD4 helper cells binding to CD40 receptors on APCs; human CD58 and mouse CD48 (LFA-3) binding to CD2; and CD54 (ICAM-1) binding to LFA-1. Members of the TNFR superfamily including CD27, CD30, 4-1BB and OX40 have also been shown to transmit a costimulation signals to leukocytes [112, 113].
It is a recognized phenomenon that T cells are rendered anergic due to the lack of costimulatory molecule(s) expression by tumor cells [34]. Tumor cells are able to induce antigen-specific tolerance or anergy on the basis of MHC-1–restricted antigen presentation without the expression of costimulatory ligand(s) [114]. This unresponsiveness, however, can be reversed when tumor cells are genetically modified to express costimulation molecules. Chen et al. [115] showed that the insertion of genes encoding B7.1 and/or B7.2 molecules into tumors generally increases their immunogenicity.
Recently, fusagene vectors were developed to encode multiple gene products as fusion proteins from a single cistron to increase the immunogenenicity of target tumor cells [116]. Analysis of over 100 individual clones derived from human and murine tumor cell lines demonstrated efficient expression and biological activity of each of the proteins. Tricistronic viral vectors coexpressing IL-12 and B7.1 have been used in the immunotherapy of cancer [117]; the vectors generated could be used in immunotherapy for the treatment of multiple myeloma and other cancers, as they were shown to stimulate allogeneic mixed lymphocyte proliferation and provoke increases in CTL responses and IFN-γ release from normal donor lymphocytes exposed to the parental U266 cells.
In addition, costimulation through molecules like 4-1BB was also found to be critical in the expansion and differentiation of CTLs. Systemic administration of an agonistic mAb against 4-1BB enhanced the CD8 T-cell response, leading to the eradication of established AG104A sarcomas and P815 mastocytomas in vivo [118]. However, resistance to this treatment modality by a number of poorly immunogenic tumors, including the TC-1 lung carcinoma and B16-F10 melanoma, was shown to be due to tumor antigen–specific CTLs’ “ignorance” rather than anergy or deletion; breaking CTL ignorance by peptide immunization was necessary for the anti-4-1BB to function to enhance T-cell responses [119]. Given the importance of costimulation in the regulation of immune responses against cancer, the manipulation of this pathway to increase immunity represents a promising therapeutic approach. Costimulation through OX40L, for example, would be advantageous, since its expression is primarily detected on recently stimulated antigen-specific CD4+ T cells [120], which is considered advantageous for generating CD8+ T-cell responses and antitumor immunity.
Immunosuppressive cells
There are at least two types of cells that when produced can impose a suppressive effect on the host’s immune system (CD4+CD25+ T cells and Gr1+CD11b+ myeloid cells), hence providing the tumor with the opportunity to escape immune recognition. Elucidating the mechanism(s) of T-cell unresponsiveness in cancer is critical for the design and application of an effective cancer immunotherapy. Inhibition of a number of T lymphocyte functions in tumor-bearing hosts has been extensively investigated [121, 122], and suppressor cells have been shown to play a role in the progression of cancer [123]. CD4+CD25+ T cells are known to be immunoregulatory and are important in immunological tolerance to self-antigens [39] and inhibition of T-cell proliferation [124]. They constitute approximately 5–10% of the CD4+ T cells in both humans and rodents, and their removal induces autoimmune diseases in various locations in the body. Sutmuller and colleagues [40] demonstrated that the depletion of CD4+CD25+ T cells followed by an injection of an antibody which blocks CTLA-4 enhanced T-cell reactivity to a known tumor-associated antigen. However, it is thought that the mere depletion or blocking of T-regulatory cells is not sufficient to successfully treat established tumors [125].
The existence of anergic and functionally suppressive CD4+CD25+ T cells was demonstrated in patients with melanoma undergoing immunization with known melanoma antigens. The degree of inhibition of T-cell proliferation was proportional to the CD4+CD25+ T cells present; the addition of IL-2 reversed their hyporesponsiveness and abrogated their suppressive function [126]. An increase in CD4+CD25+ regulatory T cells was shown to be correlated with immunosuppression and tumor progression in patients with gastrointestinal malignancies [127]. Increased numbers of CD4+CD25+ T cells secreting TGF-β were detected in tumor infiltrates from patients with early and late stage epithelial tumors [128]. These observations provide clear evidence for the contribution of CD4+CD25+ T cells to immune dysfunction in cancer patients. Immunotherapy aimed at decreasing the role of regulatory T cells would be advantageous to successfully treating cancer.
In addition, immune suppression in tumor-bearing mice has been attributed to the presence of cells with an immature myeloid phenotype that express the granulocyte-monocyte markers Gr1+CD11b+ [36], and accumulate in the spleens and lymph nodes [129] and blood of tumor-bearing mice (Ahmad et al.). They are capable of inhibiting antibody production, CTL generation, T-cell function, and lymphocytic proliferation [38, 129]. Accumulation of Gr1+CD11b+ cells and their ability to inhibit the T-cell function has been reported in cancer patients [130]. The Gr1+ cells are also able to decrease CD3ζ molecule expression significantly [131], and inhibit MHC class I–dependent antigen-specific CD8+ T cells [37].
Conclusion
This review has highlighted the main mechanisms by which tumors are known to escape from immune recognition. Given the many different potential mechanisms that tumors can acquire to avoid or subvert adaptive immunity, future generation immunotherapeutic strategies will need to consider not only how to promote antigen-driven T- and B-lymphocyte responses and their effective targeting to residual tumor, but also to understand how the mechanism(s) of tumor escape can be dealt with. Current research is exploring the application of combination therapy that utilizes several treatment modalities, which may include sequential chemotherapy, radiotherapy, and immunotherapy protocols.
Footnotes
This article forms part of the Symposium in Writing on “Tumor escape from the immune response,” published in vol. 53
References
- 1.Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90(2–3):103–22. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Speiser Cancer Immunol. 2003;3:12. [Google Scholar]
- 3.Romero Pigment Cell Res. 2003;16:588. doi: 10.1034/j.1600-0749.2003.08353.x. [DOI] [Google Scholar]
- 4.Jager E, Jager D, Knuth A (2000) CTL-defined cancer vaccines in melanoma and other epithelial cancer. In: Stern PL, Beverely PCL, Carroll MW (eds) Cancer vaccines and immunotherapy. Cambridge University Press, Cambridge, pp 207
- 5.Kaufmann HL, Schlorn J (2000) Vaccines in colon cancer. In: Stern PL, Beverely PCL, Carroll MW (eds) Cancer Vaccines and Immunotherapy. Cambridge University Press, Cambridge, p 107
- 6.Rudolf MP, Small LA, Velders MP, DaSilva DM, Weijzens S, Kast MW (2000) Vaccine delivery and immunosuppression in cervical cancer. In: Stern PL, Beverely PCL, Carroll MW (eds) Cancer vaccines and immunotherapy. Cambridge University Press, Cambridge, p 82
- 7.Taylor Papadimitriou J, Burchell J, Miles DW (2000) MUC1 vaccines and breast cancer. In: Cancer vaccines and immunotherapy. Stern PL, Beverely PCL, Carroll MW (eds) Cambridge University Press, Cambridge, p 135
- 8.Stevenson FK, Zhu D, Spellerberg, M, King CA, Sahota SS, Rice J, Thompsett AR, Hamblin TJ (2000) DNA vaccines against B-cell tumors. In: Stern PL, Beverely PCL, Carroll MW (eds) Cancer vaccines and immunotherapy. Cambridge University Press, Cambridge, p 218
- 9.Adams M, Jasani B, Colaco CS, Mason MD (2000) Dendritic cell approaches to immunotherapy. In: Stern PL, Beverely PCL, Carroll MW (eds) Cancer vaccines and immunotherapy. Cambridge University Press, Cambridge, p 237
- 10.Ciavarra Cancer Immunol Immunother. 2003;52:535. doi: 10.1007/s00262-003-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera T, Lopez-Nevot MA, Gaforio JJ, Ruiz-Cabello F, Garrido F. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother. 2003;52:1–9. doi: 10.1007/s00262-002-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark Blood. 2001;98:2887. doi: 10.1182/blood.V98.10.2887. [DOI] [PubMed] [Google Scholar]
- 13.Dunn Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll Nature. 2001;411:1010. doi: 10.1038/35082676. [DOI] [PubMed] [Google Scholar]
- 15.Khong Nat Immunol. 2002;3:999. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller J Environ Pathol Toxicol Oncol. 2002;21:277. [PubMed] [Google Scholar]
- 17.Rivoltini Immunol Rev. 2002;188:97. doi: 10.1034/j.1600-065X.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 18.Pardoll DM. Cancer vaccines. Nat Med 5[Suppl. 1998;](4):525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 19.Melcher Nat. 1998;Med:581. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 20.Ali Cancer Res. 2000;60:1663. [PubMed] [Google Scholar]
- 21.Kovacsovics-Bankowski Science. 1995;267:243. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 22.Matzinger Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 23.Algarra Int J Clin Lab Res. 1997;27:95. doi: 10.1007/BF02912442. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen Int J Cancer. 2001;91:366. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1056>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Restifo Curr Opin Immunol. 1996;8:658. doi: 10.1016/S0952-7915(96)80082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Cancer Res. 1999;59:3461. [PubMed] [Google Scholar]
- 27.de Cancer Res. 1997;57:3223. [PubMed] [Google Scholar]
- 28.Hofbauer Melanoma Res. 1998;8:337. doi: 10.1097/00008390-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Davidson Exp Med. 1998;187:1825. doi: 10.1084/jem.187.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irmler Inhibition of death receptor signals by cellular FLIP Nature. 1997;388:190. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 31.Medema J Exp Med. 1999;190:1033. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda Cell Immunol. 2001;214:194. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
- 33.Taylor FASEB J. 1991;5:2516. [PubMed] [Google Scholar]
- 34.Schwartz Science. 1990;248:1349. [Google Scholar]
- 35.Oyama J Immunol. 1998;160:1224. [PubMed] [Google Scholar]
- 36.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells Blood. 2000;96(12):3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich J Immunol. 2001;166:5398. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 38.Kusmartsev J Immunol. 2000;165:779. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 39.McHugh Immunity. 2002;16:311. [Google Scholar]
- 40.Sutmuller J Exp Med. 2001;194:823. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frumento J Exp Med. 2002;196:459. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellor Immunol Today. 1999;20:469. doi: 10.1016/S0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 43.Uyttenhove Nat Med. 2003;9:1269. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 44.Swanson Am J Respir Cell Mol Biol. 2004;30:311. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad M, Rees RC, McArdle SEB, Li G, Mian S, Entwisle C, Loudon P, Ali SA (2003) Regulation of CTL responses to a MHC- restricted class I peptide of the gp70 tumor antigen by splenic parenchymal CD4+ T cells in mice failing immunotherapy with DISC-mGM-CSF (Submitted) [DOI] [PubMed]
- 46.Takeda J Exp Med. 2002;195:161. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munn Science. 2002;297:1867. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 48.Koopman J Exp Med. 2000;191:961. doi: 10.1084/jem.191.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hicklin Mol Med Today. 1999;5:178. doi: 10.1016/s1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 50.Garrido Cancer Res. 2001;83:117. [Google Scholar]
- 51.Keating Br J Cancer. 1995;72:405. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez Tissue Antigens. 1999;53:569. doi: 10.1034/j.1399-0039.1999.530607.x. [DOI] [PubMed] [Google Scholar]
- 53.Serrano Int J Cancer. 2001;94:243. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 54.Feenstra Lab Invest. 2000;80:405. doi: 10.1038/labinvest.3780045. [DOI] [PubMed] [Google Scholar]
- 55.Ramal Tissue Antigens. 2000;55:443. doi: 10.1034/j.1399-0039.2000.550507.x. [DOI] [PubMed] [Google Scholar]
- 56.Ramal Hum Immunol. 2000;61:1001. doi: 10.1016/s0198-8859(00)00171-3. [DOI] [PubMed] [Google Scholar]
- 57.Browning J Immunother. 1993;14:163. [Google Scholar]
- 58.Griffioen M, Ouwerkerk IJ, Harten V, Schrier PI. HLA-B locus-specific downregulation in human melanoma requires enhancer A as well as a sequence element located downstream of the transcription initiation site. Immunogenetics. 2000;52(1–2):121–128. doi: 10.1007/s002510000262. [DOI] [PubMed] [Google Scholar]
- 59.Soong Cancer Detect Prev. 1991;15:231. [PubMed] [Google Scholar]
- 60.Ikeda Immunity. 1997;6:199. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 61.Real Cancer Immunol. 2001;Immunother:621. doi: 10.1007/s002620000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabrera Tissue Antigens. 2003;62:324. doi: 10.1034/j.1399-0039.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 63.Cabrera Hum Immunol. 2003;64:941. doi: 10.1016/S0198-8859(03)00164-2. [DOI] [PubMed] [Google Scholar]
- 64.Cabrera Tissue Antigens. 2003;61:211. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 65.Ali J Immunol. 2002;168:3512. doi: 10.4049/jimmunol.168.7.3512. [DOI] [PubMed] [Google Scholar]
- 66.Boursnell Adv Exp Med Biol. 1998;451:379. doi: 10.1007/978-1-4615-5357-1_59. [DOI] [PubMed] [Google Scholar]
- 67.Rees Curr Opin Mol Ther. 2002;4:49. [PubMed] [Google Scholar]
- 68.Todryk S, Ali SA, Dalgleish A, Rees RC (2001) Genetically modified tumor cells for cancer immunisation. In: Robins RA, Rees RC (eds) Cancer immunology. Kluwer, The Netherlands, pp 181–194
- 69.Restifo J Natl Cancer Inst. 1996;88:100. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ugurel Cancer Immunol Immunother. 2004;53:551. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Exp Mol Med. 2002;34:18. doi: 10.1038/emm.2002.3. [DOI] [PubMed] [Google Scholar]
- 72.Maiorana Clin Exp Metastasis. 1995;13:43. doi: 10.1007/BF00144017. [DOI] [PubMed] [Google Scholar]
- 73.Sharpe Br J Urol. 1994;74:609. doi: 10.1111/j.1464-410x.1994.tb09193.x. [DOI] [PubMed] [Google Scholar]
- 74.Uyttenhove Int J Cancer. 1997;70:349. doi: 10.1002/(SICI)1097-0215(19970127)70:3<349::AID-IJC17>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 75.Ciurea Nat Med. 2001;7:795. doi: 10.1038/89915. [DOI] [PubMed] [Google Scholar]
- 76.Rohrlich Int Immunol. 2003;15:765. doi: 10.1093/intimm/dxg073. [DOI] [PubMed] [Google Scholar]
- 77.Bai J Clin Invest. 2003;111:1487. doi: 10.1172/JCI200317656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lozupone Eur J Immunol. 2003;33:556. doi: 10.1002/immu.200310032. [DOI] [PubMed] [Google Scholar]
- 79.Maeurer J Clin Invest. 1996;98:1633. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J Immunol. 2003;171:1183. doi: 10.4049/jimmunol.171.3.1183. [DOI] [PubMed] [Google Scholar]
- 81.Ragnarsson Br J Cancer. 2000;83:1715. doi: 10.1054/bjoc.2000.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osaki J Cancer Res Clin Oncol. 2004;130:8. doi: 10.1007/s00432-003-0505-z. [DOI] [PubMed] [Google Scholar]
- 83.Fennell Clin Lung Cancer. 2003;4:307. doi: 10.3816/clc.2003.n.012. [DOI] [PubMed] [Google Scholar]
- 84.Salvesen Proc Natl Acad Sci U S A. 1999;96:10964. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Groneberg Exp Hematol. 2003;31:682. doi: 10.1016/S0301-472X(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 86.Hersey Nat Rev Cancer. 2001;1:142. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 87.Kase Gynecol Oncol. 2003;90:70. doi: 10.1016/S0090-8258(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 88.Song Br J Cancer. 2001;85:1047. doi: 10.1038/sj.bjc.6692042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Langenbecks Arch Surg. 2003;388:406. doi: 10.1007/s00423-003-0416-0. [DOI] [PubMed] [Google Scholar]
- 90.Toi Eur J Cancer. 1996;32A:2513. doi: 10.1016/s0959-8049(96)00397-8. [DOI] [PubMed] [Google Scholar]
- 91.Ohm JE, Carbone DP. VEGF as a mediator of tumor associated immunodeficiency. Immunol Res. 2001;23(2–3):263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 92.Lissoni J Biol Regul Homeost. 2001;Agents:140. [Google Scholar]
- 93.Inoshima The influence of dendritic cell infilteration and VEGF expression on the prognosis of NSCLC Clin Cancer Res. 2002;8:3480. [PubMed] [Google Scholar]
- 94.Fan Zhonghua Jie He He Xi Za. 2003;Zhi:539. [PubMed] [Google Scholar]
- 95.Buggins J Immunol. 2001;167:6021. doi: 10.4049/jimmunol.167.10.6021. [DOI] [PubMed] [Google Scholar]
- 96.Fortis Cancer Lett. 1996;104:1. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- 97.Bellone Am J Pathol. 1999;155:537. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mukherjee P, Ginardi AR, Madsen CS, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ. MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J. 2001;18(11–12):931–942. doi: 10.1023/a:1022260711583. [DOI] [PubMed] [Google Scholar]
- 99.De Eur J Immunol. 1997;27:1229. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 100.Sharma J Immunol. 1999;163:5020. [PubMed] [Google Scholar]
- 101.Tsushima Gastroenterology. 1996;110:375. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 102.Gorsch Cancer Res. 1992;52:6949. [PubMed] [Google Scholar]
- 103.Doran J Immunother. 1997;20:372. doi: 10.1097/00002371-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 104.Krasagakis Br J Cancer. 1998;77:1492. doi: 10.1038/bjc.1998.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maeda J Immunol. 1996;156:73. [Google Scholar]
- 106.von Clin Cancer Res. 2001;7:925s. [PubMed] [Google Scholar]
- 107.Janeway CA, Travers P, Walport M, Shlomchik M (2002) Immunobiology: the immune system in health and disease, 5th edn. Garland, New York
- 108.Nieland J Immunother. 1998;21:317. doi: 10.1097/00002371-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 109.Kono Eur J Immunol. 1996;26:1308. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 110.Nakagomi Cancer Res. 1993;53:5610. [PubMed] [Google Scholar]
- 111.Schule Breast Cancer Res Treat. 2002;74:33. doi: 10.1023/A:1016009913699. [DOI] [PubMed] [Google Scholar]
- 112.Gruss Blood. 1995;85:3378. [PubMed] [Google Scholar]
- 113.Smith Cell. 1994;76:959. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 114.Abken Trends Immunol. 2002;23:240. doi: 10.1016/S1471-4906(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 115.Chen J Exp Med. 1994;179:523. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaken Gene Ther. 2000;7:1979. doi: 10.1038/sj.gt.3301341. [DOI] [PubMed] [Google Scholar]
- 117.Wen Cancer Gene Ther. 2001;8:361. doi: 10.1038/sj.cgt.7700321. [DOI] [PubMed] [Google Scholar]
- 118.Melero Nat Med. 1997;3:682. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 119.Wilcox J Clin Invest. 2002;109:651. doi: 10.1172/JCI200214184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weinberg J Immunol. 2000;164:2160. [Google Scholar]
- 121.Levey Immunol Today. 1996;17:365. doi: 10.1016/0167-5699(96)10013-X. [DOI] [PubMed] [Google Scholar]
- 122.Plescia Ann N Y Acad Sci. 1976;276:455. doi: 10.1111/j.1749-6632.1976.tb41670.x. [DOI] [PubMed] [Google Scholar]
- 123.Dye J Exp Med. 1981;154:1033. doi: 10.1084/jem.154.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thornton J Immunol. 2000;164:183. [Google Scholar]
- 125.Antony J Immunother. 2002;25:202. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Javia J Immunother. 2003;26:85. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sasada Cancer. 2003;98:1089. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 128.Woo Cancer Res. 2001;61:4766. [PubMed] [Google Scholar]
- 129.Mazzoni J Immunol. 2002;168:689. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 130.Almand J Immunol. 2001;166:678. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 131.Otsuji Proc Natl Acad Sci U S A. 1996;93:13119. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]