Abstract
Central deletion of “self-reactive” T cells has been the textbook paradigm for inducing “self-tolerance” in the periphery and the concept of a role of T cell-mediated suppression in this process has long been controversial. A decisive shift in the opinion on suppressor T cells has lately occurred with the observations of Sakaguchi’s group that linked a class of CD4+CD25+ T cells to the prevention of autoimmunity from neonatal thymectomy in mice. These CD4+CD25+ T cells have been named T regulatory (Treg) cells. They are believed to be selected in the thymus as an anti-self repertoire. Hence they were referred to as natural T regulatory (nTreg) cells. Presently, in addition to their role in autoimmunity, they are believed to exert regulatory function in infection, in transplantation immunity as well as in tumor immunity. In contrast to these nTreg cells, another class of CD4+ Treg cells also exercises regulatory function in the periphery. These Treg cells are also CD4+ T cells and after activation they also become phenotypically CD4+CD25+. They are, however induced in the periphery as Treg cells. Hence, they are termed as induced Treg (iTreg) cells. There are major differences in the biology of these two types of Treg cells. They differ in their requirements for activation and in their mode of action. Nonetheless, evidence indicates that both nTreg cells and iTreg cells are involved in the control of tumor immunity. The question of how to circumvent their regulatory constraints, therefore, has become a major challenge for tumor immunologists.
Keywords: Treg, Tumor immunity
Introduction
A role of T suppressor (Ts) cells [1], presently renamed as T regulatory (Treg) cells, in the regulation of immune responses in the periphery – once a highly controversial topic [2] – has now gained wide acceptance [3, 4]. This remarkable reversal can be primarily traced to a set of observations by Sakaguchi et al. [5–8] who established a role of a class of CD4+/CD25+ T cells in preventing autoimmune pathologies following neonatal thymectomy in mice . T cells were initially subdivided into twobroad classes—helper T cells and cytolytic/suppressor T cells—depending on the expression pattern of certain cell surface molecules. T cells bearing CD4 molecules (Lyt 1+2-3- in mice in early days) were classified as T helper (Th) cells where as CD8+ T cells (Lyt 2+ in mice in early days) were classified as cytolytic/suppressor T cells. Thus, suppressor T cells were initially thought to be mostly CD8+. North’s group first showed that T cells bearing the helper phenotype (Lyt 1+2-3-) can function as suppressor T cells in a mouse tumor model [9, 10]. That human CD4+ T cells can also function as “suppressor” or “regulatory” T cells were shortly demonstrated in the human tumor system [11–13]. However, the topic of Ts cells fell into controversy and although many investigators (including our group) continued to publish on suppressor cells with the more politically correct nomenclature, “regulatory” T cells, the topic of T cell-mediated suppression of immune responses essentially continued to languish in the periphery. Seminal observations by Sakaguchi’s group identifying CD4+/CD25+ T cells serving as “key controllers of immunologic self-tolerance” [14] removed skepticism on suppressor (or regulatory) T cells and led to a remarkable resurgence of interest in the field (Over 12,000 entries on the subject in Pubmed over the years and over 1,000 papers in 2004 alone!).
Presently, a role of Treg cell is not restricted to maintaining self-tolerance. Tregs are thought to exert regulatory function in the entire immune response system, i.e., Tregs are believed to regulate immune responses against self-antigen, infectious agents, tumor antigens and transplantation antigens. Indeed, the literature on Treg cells in the entire immune response system is substantial. Yet, many important issues on Tregs (their ontogeny, mechanism of activation, mode of action, specificity, etc.) remain to be fully settled. Nonetheless, a consensus seems to have emerged on the following points:
Regulatory T (Treg) cells are primarily CD4+ T cells and although several types of CD4+ T cells are capable of exerting regulatory function, a class of Tregs, bearing both CD4+ and CD25+ markers and seemingly selected by the thymus, serves as natural T regulatory cells (nTregs) as opposed to inducible Tregs (iTregs) that can also be generated from CD4+/CD25- precursors.
The exact mechanism underlying thymic selection of nTregs is unclear. They appear to either escape thymic deletion (leaky deletion) or they may indeed be positively selected as a part of an “antiself” repertoire.
Apart from expressing CD25 molecule, nTregs express Cytolytic T lymphocytes (CTL)A-4, GITR (a glucocorticoid inducible TNF receptor family), and FoxP3 (a forkhead family transcriptional regulator). Although none of them is a distinct marker of the lineage, FoxP3 has turned out to be useful as a marker as well as a critical factor for their “differentiation”.
nTreg cells need to be activated via their receptors (TCR) but they do not need simultaneous TCR signaling and costimulation—two obligate signals needed for naive T cells to be activated. They function in a contact- dependent manner and suppress nonspecifically in a bystander fashion.
Finally, although a role of nTregs has mostly been ascribed to maintaining “self-tolerance” in the periphery and although other types of Treg cells can be induced (iTregs) from CD4+/CD25- precursors to suppress and/or dampen immune responses to antigens—“self or nonself”—the regulatory role of nTregs has now been extended to the control of immune responses against infectious agents, tumors, and transplants.
These issues have been amply covered in many authoritative reviews [15–19]. As such, this paper will not be another “general review” of the subject. Instead, we will examine the role of Tregs in tumor immunity from the viewpoint that “tumor immunity” and “autoimmunity” can be viewed as essentially the same process since the idea of generating an immune response against tumor antigens amounts to breaking “tolerance” for self-antigens as most tumor associated antigens (TAAs) have turned out to be self- antigens.
A short sketch of tumor immunity
The idea of immunity against cancer had been, and to an extent remains, controversial. The historic demonstrations of Ehrlich [20] that hosts can mount an immune response against cancer were made in outbred mice. The rejection responses elicited in this model could therefore be viewed as “allo-responses”. Later, using transplantation biology as a tool, researchers more convincingly proved the existence of host immune responses to syngeneic and even autochthonous tumors in chemically induced tumor models [21]. These observations, also, could not vouch for the existence of bonafide immune responses against spontaneously occurring tumors. Interestingly, convincing proof that hosts have the tools to immunologically respond to and can indeed mount immune responses against spontaneously arising tumors—with fine specificity—emerged in human systems as autologous tumor reactive T cell lines and molecular definition of “tumor antigens” recognized by such T cells became available [22, 23]. It is now abundantly clear that many cancer patients can mount serologic as well as cellular immune responses against their own tumor cells and that hosts can respond to a large compendium of TAAs and epitopes, self or mutated (for a list of tumor associated epitopes recognized by T cells (see 24). More importantly, it is also equally clear that vaccination with some of these epitopes, administered with or without an adjuvant or presented by ex vivo cultured antigen-presenting cells (APCs), can induce serologic and CTL responses as well as antitumor responses in some cases [25]. This, therefore, raises the question of how then their activation/expansion is controlled in the periphery?
At this juncture, it should be pointed out that tumor cells can use multiple ways to escape a host immune response and the issue of immune escape has been reviewed [26–31]. This topic will not be discussed. Instead, we will examine the role of Treg cells in controlling host immune responses to tumors. This review will be restricted to Treg-mediated regulation of antitumor T cell responses not because serologic responses are not important but because the topic of cell-mediated antitumor T cell response and its regulation have been most extensively studied. Further, the topic of Treg cells and tumor immunity in human systems will be critically examined.
T regulatory cells in suppression of antitumor immune response: the old and the new
The old paradigm on T cell-mediated suppression in the periphery has been that Ts cells are generated essentially as a brake against an unwanted immune response or to abort an ongoing immune response. In this equation, Ts cells, as all T cells, need to be activated or “induced” from their precursors in an antigen specific (or in some studies, antigen nonspecific) manner. The early literature dealt mostly with CD8+ T cells as suppressor cells. Soon, however, CD4+ T cells were also found capable of suppressing T cell responses to tumor antigens. As mentioned earlier, North’s group first provided the evidence of CD4+ T cells functioning as suppressor cells in an antitumor response in murine model in vivo [9, 10]. They demonstrated that mice bearing progressive Meth A fibrosarcoma, Ly1+2− T cells suppress the generation of Ly1−2+ effector cells resulting in the decay of the antitumor immune response.
Subsequently, peripheral regulation of immune response by CD4+ T cells was also extended in human tumor model in vitro [11–13, 32–35]. In a human melanoma model, CD4+ regulatory T cells were isolated from lymph nodes, tumor tissues as well as from blood. These CD4+ suppressor/regulatory T cells suppressed the generation of CD8+ CTL response from in vitro CTL generation assays. The CD4+ Treg cells were generated from in vitro cultures and as would be expected after activation, they expressed CD25 and they also upregulated CD25 upon subsequent stimulation and, they functioned in Major Histocopatibility Complex (MHC) class II restricted fashion mostly by elaborating Interleukin(IL)-10 [32–35]. These CD4+ “regulatory” T cells, therefore, behaved much like Tr1 or Th3 type regulatory T cells that have been strongly implicated in the prevention of inflammatory bowel disease [36, 37]. These types of studies of CD4+ T cells suppressing antitumor immune responses in humans, however, could not establish the biological significance of the observations mostly for two reasons. First, these were exclusively in vitro studies; and second, the specificity of these CD4+ Treg cells could not be clarified. In this context, it should mentioned that Chakraborty et al. [38] have subsequently shown that while immunization of melanoma patients with synthetic peptide or tumor lysate loaded APC-based vaccines could lead to the expansion of epitope specific CD8+ T cells, in vivo, repetitive vaccinations induced IL-10 producing CD4+ T cell-mediated regulatory responses. Finally, Wang et al. [39] have recently shown that this type of CD4+ Treg T cells exhibit specificity for an epitope derived from the tumor associated but self-antigen LAGE-1.
As pointed out earlier, a decisive shift in the skepticism on Ts cells took place with the seminal observations of Sakaguchi’s group linking CD4+/CD25+ T cells with a key role in the prevention of autoimmunity in mice [15]. Others confirmed the basic observations and added legitimacy to the basic finding. Collectively, these observations (See [16–19] for reviews) led to a new paradigm on suppression by T cells. It is that a class of CD4+CD25+ Treg cells gains access to the periphery after being generated and instructed in the thymus as “antiself” regulatory T cells. The fundamental phenotypic and functional characteristics of these nTreg cells have been summarized in this paper earlier. Hence, they will not be repeated.
Shortly after their work in autoimmunity, Sakaguchi’s group demonstrated that the removal of CD25+CD4+ T cells as well as injections of anti-CD25 monoclonal antibody can induce antitumor response in mice and enunciated a “common basis” between tumor immunity and autoimmunity [40–42]. Affirmation of their basic observations came from other laboratories [43–47]. It was further shown that these CD4+CD25+ Treg cells interfere with the generation of long-lasting tumor immunity and that their removal results in better results from tumor immunotherapy [48–50]. When collectively taken with the other reports in the literature, it is now fairly clear that a class of CD4+CD25+ T cells do exercise some regulatory role in antitumor immunity in animal models. Of interest, the essence of the original observations of North et al. [9, 10] have been recently repeated in the Houghton laboratory [51] and it appears that the CD4+ suppressor cells of North do qualify as today’s Treg cells. Their work, however, revealed that CD4+CD25+ T cells are not functionally monolithic as they found that CD4+CD25+ T cells include disparate functional types of CD4+ T cells.
A number of studies soon extended the role of Treg cells in human tumor models. Several groups described increased frequencies of CD4+CD25+ Treg cells in blood, malignant effusions, draining lymph nodes, and tumor tissues, implicating impaired immune responses to cancer to a higher frequency and/or hyperactivity of Treg cells [52–56]. Freshly isolated CD4+CD25+ T cells from cancer patients, or from patients receiving immunotherapy, were found to suppress the proliferation of CD4+CD25- T cells in vitro—an assay that has been extensively used to assess Treg cell function in vitro. These studies (For a review, see 57) suggested that a higher frequency of and/or hyperactivity in the CD4+CD25+ T cell population might have a negative effect on antitumor response. Lately, a stronger correlation between Treg activities and impaired tumor immunity in human tumor models has emerged from two groups of investigators [58, 59]. Curiel et al. [58] have reported that CD4+/CD25+/GITR/Fox P3 + T cells preferentially accumulate in ovarian tumors and in malignant ascites (and not in lymph nodes) seemingly attracted by CCL22 elaborated by tumor cells and macrophages in tumor beds. They have also shown that such accumulation of CD4+/CD25+/GITR+/Fox P3 + Treg cells in tumor sites correlates with poor outcome. The authors have referred to these population as “tumor Treg” cells and given that they accumulate mostly in tumor sites and not in lymph nodes, they have suggested that these Treg cells mostly interfere with the function of effector T cells and do not affect their “priming” (i.e., induction). The authors have stated that these “tumor Treg” cells might exhibit specificity for the HER-2/neu derived peptide, an ovarian tumor associated epitope, but this has not been proven. Viguier et al. [59], in contrast, have found higher accumulation of Tregs in draining lymph nodes infiltrated by melanoma cells. They have also found both IL-10 producing Tr1 type Treg cells over and above IL-10 negative Treg cells in tumor infiltrated nodes. As such, they have suggested that both nTregs and iTregs contribute to the local immunosuppressive milieu. They have also shown that these Tregs are polyclonal and suggested that these Treg cells might have been primed by a “larger” panel of antigens [59], the nature of which remains unsettled. Setting the differences in these two studies aside, when taken with the other reports, they provide considerable support to the growing notion that Treg cells exercise control over antitumor immune response in humans in vivo.
Operational framework underlying Treg-based suppression of antitumor immunity
Although the literature on Treg cell activities in antitumor immunity is substantial, the operational framework behind Treg cell-based regulation of antitumor immunity is poorly understood. It appears that both nTregs and iTregs can be involved in the regulation of antitumor immunity. However, these two types of Treg cells differ in the requirements for their activation as well as in their mode of action. They may also differ in efficiency. Thus a critical review of the operational framework (i.e., mechanism of activation and mode of action and relative efficacy) under which Treg cells operate to control antitumor immunity will be useful. Under this broad premise, we will address three issues that we believe are relevant. These are: (1) how does Treg cell-based regulatory arm operate in antitumor immunity; (2) which of the two Treg cells (nTregs vs. iTregs) pose more of a constraint in antitumor immunity; (3) can the regulatory constraints be circumvented?
Mechanism of activation and mode of action of Treg cells in antitumor immunity
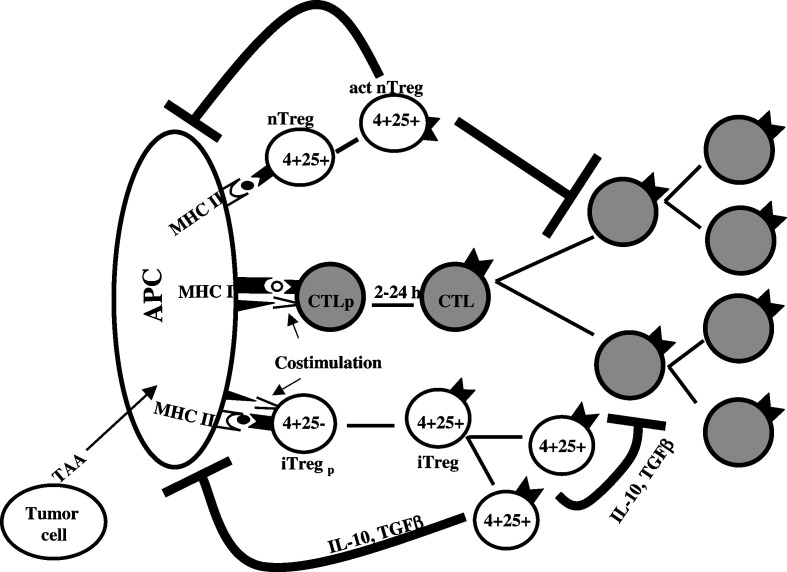
At the outset, it should be pointed out that while the topic of mechanism of activation have been extensively addressed with Tregs involved in controlling autoimmunity, they have not been systematically studied in the tumor immunity model. Nonetheless, considering that a common basis exists in autoimmunity and tumor immunity, information generated in the autoimmunity model may be extrapolated in the tumor immunity model as well. In this context, it is generally understood that being CD4+ T cells, nTregs as well as iTregs need to be activated by MHC class II bound epitopes on APCs. The caveat in this fundamental construct is that it is widely believed that while iTregs need both TCR ligands and costimulation, nTregs need only TCR-driven signal for functional activation. Nonetheless, since only a limited class of cells express MHC class II molecules, nTregs need APCs for their activation. Thus from an operational view point, just as APCs are indispensable in activating naive effector T cells, they are also needed for the activation of Treg cells. Figure.1 attempts to depict the process in a schematic format.
Fig. 1.
Schematic representation of the operational framework of Treg cell-mediated suppression of antitumor CTL response. The cartoon depicts the basic mechanism underlying the activation of CTLs and the two major types of Treg cells. As shown, while nTregs may not require simultaneous TCR and costimulatory signals to undergo activation and clonal expansion, as do all naive T cells to be functional, we propose that iTregs need to be activated and expanded very much the way all effector T cells also get activated and expanded. iTregs primarily suppress by synthesizing suppressive cytokines while nTregs, after activation, act in a contact-independent mechanism, at least in in vitro experiments, although several groups have shown that nTregs also elaborate immunosuppressive cytokines in vivo (See text). Although we have assembled all three cell types (APC, Treg, and CTL) in the act, we do not imply that the CTL precursors and Tregs need to be activated on the same APC
In this schema of things, the induction of iTreg cells (Tr1 or Th3 type regulatory T cells) generated from CD4+CD25- precursors—whether in inflammatory bowel disease or in tumor immunity—can be viewed as a process by which CD4+ T cells get activated and then get differentiated (or polarized) into their functional phenotype (i.e., Tr1 or Th3) by the same process that leads to the activation and polarization of T cells, in general. Thus, iTreg cells require MHC class II-bound ligands for their activation, appropriate microenvironment (e.g., antigen presentation in steady state vs under an inflammatory background, type 2 cytokine synthesis, etc.). Admittedly, the precise nature of the APC and “microenvironment” that induce tolerance is yet to be fully settled. However, whatever the underlying mechanism of antigen presentation that leads to the activation and polarization of Tr1 or Th3 type regulatory T cells might be, the fact remains that the induction of an iTreg response from CD4+CD25- precursors is an APC-based full inductive process.
In contrast, the requirements for the activation of nTregs are poorly defined. In fact, the literature on this subject is confusing and, at times, frankly contradictory. For example, although it is widely believed that nTregs are “anergic”, and the anergic state has been thought to be important for their function; they are anergic only to “weak” TCR signals (e.g., to soluble anti-CD3 antibody or to PHA) but not to “strong” stimuli (to plate-bound anti-CD3 antibody or to PHA plus PMA) and it has been shown that they can be expanded in cultures and the in vitro expanded cells function as more potent suppressors; nTregs are believed to be driven by self peptides and require only TCR stimulation for functional activation, they, however, seem to require TCR signals and costimulation for their maintenance; it has been proposed that IL-2 plays a “critical role” for their functional activation although they are unable to synthesize IL-2 and they have been shown to prevent IL-2 synthesis by the effector cells (Thus, if they are dependent on IL-2 from the responding effector cells during their early induction phase, the window of receiving the paracrine IL-2 signal is very narrow indeed); it has been shown that the strength of activation signals for the effector cells is an important determinant as to whether nTregs could block effector cell activation or not, it is not, however, clear if a “strong” signal makes effector cells refractory to the nTregs or the robustness of the effector cell response turns them off by one mechanism or another [60–67]. The last point will be again taken up later. Presently, it is apparent that while the activation requirements for nTregs are not the same as that of naive T cells, the rule that governs their activation in the regulation of tumor immunity is yet to be firmly established.
On mode of action, distinct differences have emerged between the two Treg cell types. The primary mode of action of iTregs is through IL-10 and TGFβ—two powerful immuno-suppressive cytokines with broad inhibitory properties on T cells, APCs, and other immunocytes that express receptors for these cytokines. Hence, they function in a contact-independent manner. Since their action is primarily cytokine mediated, they can suppress priming of the effector cells as well as their effector function. A large body of information also exists on the molecular mechanism underlying the suppressive effects of these cytokines. Thus a great deal is known on the mode of action of iTregs. nTregs, in contrast, are widely believed to function in a contact-dependent manner although major discord has emerged from observations on their mode of action from in vivo and in vitro experiments. Several groups have shown that CD4+CD25+ Treg cells act through cytokine-independent as well as cytokine-dependent manner, in vivo, and the role of CTLA-4 has also been controversial [65, 68–72]. Contrasting results have been reported on this issue in human models. Various investigators have also reported human CD4+CD25+ Treg cells to be functioning via IL-10 or TGF-β in a contact-independent manner, in contact-dependent manner, and via CTLA-4 or not via CTLA-4 [65, 73–75]. nTregs seem to regulate effector cell expansion by blocking IL-2 synthesis and Interferon(IFN)γ synthesis [65, 76]. Finally, the major effect of nTregs is thought to be mediated through a noncognate T–T interaction although they can inhibit APC function and thus interfere with the generation of immune response by making “poor” APCs [77, 78].
Presently, whether nTregs act during induction of a response, at the effector phase, or both, is not fully clear. Available data seem to suggest that nTregs, by virtue of their mode of action, are capable of controlling both induction and effector function. However, the study by Curiel et al. [58] suggests that the Tregs function mostly at the effector phase. The authors have drawn this conclusion as they could not find many Tregs in lymph nodes. The absence of many CD4+CD25+ T cells in lymph nodes, however, does not negate the possibility of nTregs functioning at the induction phase. Indeed, Viguier et al. [59] have reported CD4+CD25+ T cells in draining lymph nodes and have suggested that they might be functional in such nodes at an earlier point in the natural history of tumorigenesis. From an operational standpoint, the question that at what point Treg cells operate has some physiologic as well as logistic connotations. An immune response can be controlled at the induction phase (i.e., by preventing initiation of an immune response) and at the effector phase (i.e., by preventing the function of already activated and expanded effector T cells). For simplicity as well as efficiency in biology, one would imagine that preventing activation and amplification of the effector T cells is preferable than allowing activation and amplification to go ahead and then interfering with their function at the target sites. Given that antigens—self or foreign—are brought to secondary lymphatics by APCs and as nTregs require TCR signaling for functional activation and as their function is contact dependent (especially at T:T level), no major impediment exists for them to get activated and to abort a full blown activation of effector cells right there in the secondary lymphatics. At the same time, being activated in secondary lymphatics is not an impediment for them to exit from lymph nodes and act at the effector phase either. In this context, it should be pointed out that in order to prevent a full blown CTL response generation, nTregs have to operate during the initial 2 h–24 h window of antigen presentation in which CTLp gets activated (See Fig.1) and gain “fitness” to enter into an autonomous expansion phase [79].
Which Tregs (nTregs or iTregs) are more of a constraint in antitumor immunity?
The literature is essentially silent on this issue. So far, no head to head comparison of these two has been carried out. Considering that a relatively high nTregs: effector cell ratio and a relatively weak effector T cell activation signal are needed to elicit regulation by nTregs and as nTregs are essentially ineffective when the effector cells are stimulated with “strong signals” (such as plate bound anti-CD3 antibody, PHA+PMA, or when IL-2 is added to the culture), nTregs do not appear to pose a major constraint in antitumor immunity in the face of optimal activation signal. Yet, a role of CD4+CD25+ Treg cells on autoimmunity in the animal model is indisputable. We propose that nTregs may also be able to regulate antitumor response particularly at steady state. Tumor associated but self-antigen presentation in steady state is unlikely to be “optimum” and rarely would be viewed as “dangerous” (Invasion by tumor cells and tumor cell growth seldom present with the same “danger” as do invading pathogens.). A suboptimal stimulation may lead to a low-level effector T cell activation that is likely to contract on its own or brought down by nTregs, when needed. nTregs, however, may not be able to abort a full blown effector T cell activation orchestrated by optimal stimulation with all the right ingredients (antigen presentation by fully activated APCs, provision of costimulation, inflammatory backdrop, etc.). Indeed it has been shown that nTregs do not regulate effector T cell activation well, when the TCR signal is robust [61, 63].
It may be argued that iTregs, in contrast, are likely to be more efficient by virtue of their mode of action. IL-10 and TGFb are two formidable immunosuppressive cytokines. The authors group has recently compared the regulatory properties of freshly isolated CD4+CD25+ T cells and CD4+CD4-T cells in an in vitro CTL generation protocol [80] against a tumor associated but essentially self-epitope such as the MART-127-35 peptide presented by fully activated dendritic cells. In these experiments, freshly isolated CD4+CD25+ T cells were found to be quite inefficient—on a per cell basis—compared to their CD4+CD25- cohorts, in preventing activation and amplification of the epitope specific CTLs (Chattopadhyay S, Mehrotra S, Chakraborty NG, Mukherji B in Preparation).
Can Treg cell-based constraints be circumvented?
Given that nTreg cells (CD4+CD25+ T cells) seem to exercise a negative role in tumor immunity, one obvious strategy to circumvent their negative effects will be to physically remove and/or inactivate them by one mechanism or another. Indeed, North [81] originally showed that cyclophosphamide could facilitate adoptive immunotherapy of an established tumor by eliminating tumor-induced suppressor T cells. Subsequently North and Awwad [82] described similar effect of a drug-induced elimination of CD4+ suppressor T cells in the regression of an advanced lymphoma. Eventually, Berd’s group [83] employed this strategy in human cancer vaccine therapy and Jaffee’s group showed that a combination of drugs enhances the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in mice [84]. Jaffee’s group has also employed a combination of drugs and vaccine in cancer patients. Preliminary analyses have shown that such an approach could uncover high avidity anti-tumor T cells by inhibiting Treg cells (Laheru and colleagues, personal communication). Others have also used cyclophosphamide as an “antisuppressor cell” agent in immunotherapy with different forms of cancer vaccines in humans. The results of these studies, however, have not been all that persuasive. Presently, several investigators are pursuing the elimination of Treg cells with the immunotoxin labeled anti-CD25 antibody (ONTAK). Time will answer if such a strategy will work. Another potentially profitable strategy might be to institute an active immunization approach taking advantage of their weakness. As nTregs are less potent when the effector T cell activation signal is “strong”, their regulatory function might be circumvented by: (a) increasing the potency of a vaccine; (b) instituting a “strong” CTL activation/expansion strategy accompanied by the removal/inactivation of the nTregs; or (c) active immunization following adoptive transfers of PBL, depleted of nTregs, in a homeostatic expansion mode.
Similarly, considering the mode of action of iTregs, their action might also be amenable to circumvention. Indeed, Chakraborty et al. [32] have shown that in the presence of anti-IL-10 antibody, a more prolonged antitumor CTL response could be engineered through an in vitro CTL generation assay. Further, we have recently found that in the anti-MART-127-35 epitope specific CTL generation model, the regulatory effect of CD4+/CD25- T cells (iTregs) can be blocked in the presence of antibodies to MHC class II molecules and IL-10R (unpublished data). Since iTregs act primarily through IL-10, TGFβ or CTLA-4, their action can, therefore, be circumvented by appropriate blocking reagents. Further studies will be needed to answer the question and to test the hypotheses posed here.
Conclusion
In 2002, Shevach pointed out that there are “more questions than answers” on the topic of Treg cells [85]. Some of the unanswered questions have been answered. Nonetheless, after several thousand more papers on the subject, since then, essentially the same can be said in the beginning of 2005. Hopefully, many remaining issues will be resolved with time. Presently, although many questions remain unanswered and despite a recent scathing commentary on Treg cells by Cohn [86], it is safe to say that suppressor cells (in a Treg cell camouflage) are in. An impressive body of evidence has emerged to suggest that in addition to having control over autoimmunity, they also have regulatory function over tumor immunity. Useful knowledge has already emerged on their biology. More insight will be gained from the massive amount of ongoing work in the field. Meanwhile, just as optimism has risen that some day Treg cells will be used in the treatment of autoimmune pathologies, there are reasons to be hopeful that the Treg biology can also be exploited to bolster antitumor immunity and to design better therapeutic strategies.
Acknowledgements
The authors thank Robert B Clark for a critical reading of the manuscript and for helpful suggestions. This work was supported by PHS grants CA 88059 and CA83130
Abbreviations
- Treg
T regulatory
- nTreg
Natural Tregulatory
- iTreg
Inducible T regulatory
- Ts
T suppressor
- APC
Antigen presenting cell
- CTL
Cytolytic T lymphocytes
- MHC
Major histocompatibility complex
- IL
Interleukin
- IFN
Interferon
- TAA
Tumor associated antigen
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723. [PMC free article] [PubMed] [Google Scholar]
- 2.Moller G. Do suppressor T cells exist. Scand J Immunol. 1988;27:247. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41. doi: 10.1084/jem.193.11.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Takahasi T, Nisizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice: I. Requirement of Lyt-1 effector cells for oocyte damage after adoptive transfer. J Exp Med. 1982;156:156. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Takahasi T, Nisizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice: II Requirement of Lyt-1 effector cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Fukuma K, Kuribiashi K, Masuda T. Organ specific autoimmunie diseases induced in mice by elimination of T cell subsets. I. Evidence for the active participation of T cells in natural self-tolerance: deficit of a subset as possible cause of autoimmunity. J Exp Med. 1985;161:72. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self tolerance maintained by activated T cells expressing IL-2 receptor a-chain (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 9.Berendt MJ, North RJ. T-cell-mediated suppression of antitumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980;151:69. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.North RJ. Down-regulation of the antitumor immune response. Adv Cancer Res. 1985;45:1. doi: 10.1016/s0065-230x(08)60265-1. [DOI] [PubMed] [Google Scholar]
- 11.Mukherji B, Wilhelm SA, Guha A, Ergin MT. Regulation of cellular immune response against autologous human melanoma. I. Evidence for cell-mediated suppression of in vitro cytotoxic immune response. J Immunol. 1986;136:1888. [PubMed] [Google Scholar]
- 12.Mukherji B, Nashed AL, Guha A, Ergin MT. Regulation of cellular immune response against autologous human melanoma. II. Mechanism of induction and specificity of suppression. J Immunol. 1986;136:1893. [PubMed] [Google Scholar]
- 13.Mukherji B, Guha A, Chakraborty NG, Sivanandham M, Nashed AL, Sporn JR, Ergin MT. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med. 1989;169:1961. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;26:455. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki Y, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahasi T. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 16.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 18.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bach JF. Regulatory T cells under scrutiny. Nature Rev Immunol. 2003;3:189. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich P. Nederlandsch Tijdschrift voor Geneeskunde: Ueber den jetzigne Stand Der Karzinomforchung. Weekblad Jaargang Eerst helft. 1909;5:273. [Google Scholar]
- 21.Srivastava PK, Old LJ. Individually distinct transplantation antigens of chemically induced mouse tumors. Immunol Today. 1988;9:78. doi: 10.1016/0167-5699(88)91269-8. [DOI] [PubMed] [Google Scholar]
- 22.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175. doi: 10.1016/S0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 24.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2005;54:187. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 26.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 27.Marincola FM, Jaffee EM, Hiclin DJ, Ferrone S. Escape of human solid tumors from T cell-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 28.Pawlec Tumor escape from the immune response. Cancer Immunol Immunother. 2004;53:843. doi: 10.1007/s00262-004-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy; possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anichini A, Vigetti C, Mortini R. The paradox of T cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma. Cancer Immunol Immunother. 2004;53:855. doi: 10.1007/s00262-004-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. Down-regulation of the z-chain expression in T cells: a biomarker of prognosis in cancer. Cancer Immunol Immunother. 2004;53:865. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty NG, Li L, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Emergence of Th2 type CD4+ T cell response to repetitive stimulation with antigen and antigen presenting cells, in vitro: Implications in designing tumor vaccines. J Immunol. 1999;162:5576. [PubMed] [Google Scholar]
- 33.Mukherji B, Guha A, Loomis R, Ergin MT. Cell-mediated amplification and down-regulation of cytotoxic immune response against autologous human cancer. J Immunol. 1987;138:1987. [PubMed] [Google Scholar]
- 34.Chakraborty NG, Twardzik DR, Sivanandham M, Ergin MT, Hellstrom KE, Mukherji B. Autologous melanoma-induced activation of regulatory T cells that suppress cytolytic response. J Immunol. 1990;145:2359. [PubMed] [Google Scholar]
- 35.Mukherji B, Chakraborty NG, Sivanandham M. T cell clones that react against human tumors. Immunol Rev. 1990;116:33. doi: 10.1111/j.1600-065x.1990.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 36.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T cell subset inhibits antigen specific T cell responses and prevents colitis. Nature. 1997;389:737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 37.Roncarolo MG, Bachetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68. doi: 10.1034/j.1600-065X.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty NG, Chattopadhyay S, Mehrotra S, Chhabra A, Mukherji B. Regulatory T cells response and tumor vaccine induced CTL in human melanoma. Hum Immunol. 2004;65:794. doi: 10.1016/j.humimm.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor specific human CD4+ regulatory T cells and their ligands: implications for immunottherapy. Immunity. 2004;20:107. doi: 10.1016/S1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 40.ShimizuJ. Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211. [PubMed] [Google Scholar]
- 41.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukine-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128. [PubMed] [Google Scholar]
- 42.Gallimore A, Sakaguchi S. Regulation of tumor immunity by CD25+ T cells. Immunology. 2002;107:5. doi: 10.1046/j.1365-2567.2002.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotixic T lymphocytes-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocytes responses. J Exp Med. 2001;194:823. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immunol. 2002;2:1. [PubMed] [Google Scholar]
- 45.Wei-Zen W, Morris GP, Kong Yi-chi M. Antitumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother. 2004;53:73. doi: 10.1007/s00262-003-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka H, Tanaka J, Jorgen K, Shu S. Depletion of CD4+CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunolther. 2002;25:207. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine rejection antigens. Eur J Immunol. 2002;32:3267. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients inhibit autologous T cell proliferation. J Immunol. 2002;168:4272. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 49.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+D25+ regulatory T cells suppress tumor immunity sensitive to cyclophosphamide which allows immunotherapy in established tumors to be curative. Eur J Immunol. 2004;34:336. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 50.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diazde Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor primed CD4+ T cells with IFN-γ-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 51.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;20:771. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Hermann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 53.Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, Monos D, Peritt D, Marincola F, Cai D, Birebent B, Bloome E, Kim J, Berencsi K, Mastrangelo M, Herlyn D. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267. [PubMed] [Google Scholar]
- 54.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606. [PubMed] [Google Scholar]
- 55.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells ate abundant in tumor lymphocytes of Hodgkins lymphoma. Blood. 2003;103:1755. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 56.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:167. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 59.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25+ regulatory T cells are overexpressed in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 60.Cozzo C, III, Larkin J, Caton AJ. Cutting Edge: Self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 61.Thronton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317. [PubMed] [Google Scholar]
- 63.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann P, EderR Kunz-Schughart LA, Andreesen R, Edinger R. Large-scale in vitro expansion of polyclonal human CD4(+) CD25high regulatory T cells. Blood. 2004;104:895. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 65.Thronton AM, Picirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;24:366. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 66.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 67.Malek TR. The main function of IL-2 is to promote the development of regulatory T cells. J Leukoc Biol. 2003;74:961. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 68.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J Autoimmun. 2001;16:115. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 69.Asseman C, Mauze S, Leach M, Coffman Rl, Powrie F. An essential role for interleukin-10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sachs DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi T, Tagami T, Yamazuki S, Ueda T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Dominant immunologic self-tolerance by CD4+CD25+ regulatory T cells constitutively expressing CTLA-4. J Exp Med. 2000;192:303. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Read S, Maimstrom S, Powrie F. CTLA-4 plays an essential role in the function of CD4+CD25+ regulatory cells which control intestinal inflammation. J Exp Med. 2000;192:295. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+ CD25+ regulatory T cells is mediated by cell surface bound transformation growth factor beta. J Exp Med. 2001;194:629. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype localization and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med. 2002;196:379. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 76.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 77.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 78.Misra N, Bayry J, Lacroix-demazes S, Kazatcchkine MD, Kaveri SV. Cutting Edge: Human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 79.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 80.Mehrotra S, Chhabra A, Chattopadhyay S, Dorsky DI, Chakraborty NG, Mukherji B. Rescuing melanoma epitope specific cytolytic T lymphocytes from activation-induced cell death, by SP600125, an inhibitor of JNK: implications in cancer immunoterapy. J Immunol. 2004;173:6017. doi: 10.4049/jimmunol.173.10.6017. [DOI] [PubMed] [Google Scholar]
- 81.North RJ. Cyclophosphamode-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. 1990;71:90. [PMC free article] [PubMed] [Google Scholar]
- 83.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T -cells. Cancer Res. 1988;48:1671. [PubMed] [Google Scholar]
- 84.Michiels J-P H, Reilly RT, Emens LA, Ercollini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin and paclitaxel enhance the antitumor immmune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689. [PubMed] [Google Scholar]
- 85.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 86.Cohn M. Whither T-suppressors: if they did not exist would we have to invent them. Cell Immunol. 2004;227:81. doi: 10.1016/j.cellimm.2004.02.004. [DOI] [PubMed] [Google Scholar]