Abstract
Previous studies have demonstrated antitumor efficacy of Virulizin in several human tumor xenograft models and a critical role for macrophages in the antitumor mechanism of Virulizin. Although there is growing support for an immune stimulatory mechanism of action for Virulizin, the details remain to be elucidated. The aim of this study was to determine whether infiltration of natural killer (NK) cells into xenografted tumors is altered by Virulizin treatment, and whether such alterations contribute to the antitumor activity of Virulizin. Immunohistochemical analysis demonstrated that xenografted tumors from Virulizin-treated mice had an increase in infiltration of F4/80+ (macrophages) and NK1.1+ (NK) cells. The increase in NK1.1+ cell infiltration occurred at an early stage of Virulizin treatment, which correlated with an early sign of apoptosis. In addition, Virulizin resulted in an increase in the number of NK cells in the spleens, and NK cells isolated from the spleen exhibited increased cytotoxicity to tumor cells in vitro. In NK cell–deficient SCID-beige mice, the antitumor activity of Virulizin was compromised, providing additional support to the hypothesis that NK cells are necessary for inhibition of tumor growth by Virulizin. Finally, depletion of macrophages resulted in the loss of Virulizin-induced increase in NK1.1+ cell infiltration into xenografted tumors, suggesting the involvement of macrophages in NK cell infiltration into tumors. Taken together, these results strongly support a mechanism in which Virulizin stimulates a sustained expansion and infiltration of NK cells and macrophages into tumors with subsequent activation of NK cells that is responsible for the observed antitumor activity.
Keywords: Cancer, Immunotherapy, Macrophages, NK cells, Virulizin
Introduction
Effective tumor surveillance and elimination in mammalian organisms require both innate and acquired immune responses. Innate immunity is the earliest line of host immune defense and is triggered immediately upon recognition of “non-self” or “aberrant-self” entities such as microorganisms and tumor cells [1, 2]. The innate immune response comprises a large number of molecular and cellular components. The cellular components include polymorphonuclear leukocytes, macrophages, dendritic cells, and natural killer (NK) cells. In particular, macrophages can produce cytotoxic agents and acquire the capacity to kill tumor cells upon activation. In addition, macrophages secrete diverse proinflammatory cytokines and chemokines that recruit and activate cytotoxic lymphocytes, such as NK cells. Activated NK cells not only identify and kill tumor cells prior to the development of antigen-specific lymphocytes, but also secrete cytokines to further augment macrophage function resulting in positive feedback activation. The crosstalk that exists between macrophages and NK cells expands the immune response. Furthermore, cytokines produced by macrophages and NK cells can directly or indirectly affect other immune effector cells including T and B cells, thereby inducing an acquired immune response to tumor cells. Therefore, both macrophages and NK cells have pivotal roles in the development of both innate and acquired antitumor immunity.
Activation and expansion of macrophages and/or NK cells has been an attractive approach for the development of anticancer immunotherapy [3–7]. Activated macrophages are capable of lysing tumor cells in vitro [4, 8, 9], and play an important role in the immune-mediated inhibition of tumor growth in vivo [10–12]. In murine models of human tumor xenografts, macrophages and NK cells within the tumor tissue were found to elicit an active immune response, resulting in tumor growth inhibition [10, 13]. NK cells can rapidly recognize and destroy a large variety of tumor cells, without prior sensitization or major histocompatibility complex (MHC)-dependent recognition, the properties of which are required for the activation of cytotoxic T lymphocytes (CTLs) [14, 15]. Moreover, the low-level expression of MHC class I that often accompanies cellular transformation reduces the ability of T cells to detect and eliminate transformed cells, but increases their susceptibility to NK cell recognition [16–19]. The evidence to date suggests that NK cells are an important component of antitumor immunity, in vivo, and contribute to the antitumor efficacy observed in several animal models [20–23].
Virulizin is a novel biological response modifier (BRM) obtained from bovine bile by a standardized extraction process. Previous studies suggest that Virulizin-mediated monocyte/macrophage activation plays a critical role in the mechanism of antitumor activity [24, 25]. In vitro, Virulizin can induce high cytotoxic activity of human blood monocytes, peritoneal macrophages, and alveolar macrophages against human tumor cells [25], and stimulates cytotoxicity of murine peritoneal macrophages against many tumor cell lines and tumor cells isolated from surgical biopsy samples [25]. The level of cytolysis induced by Virulizin is equal to, or greater than, that elicited by other more conventional biological activators including IFN-γ, IL-2, and M-CSF [25]. Recent in vivo studies demonstrated that daily administration of Virulizin suppressed the growth of a wide range of human tumor xenografts including melanoma, pancreatic, breast, ovarian, and prostate cancers [24, 26, 27], and increased survival time in a murine melanoma model [26]. In addition, the antitumor efficacy of Virulizin was significantly compromised in mice depleted of macrophages, indicating substantial involvement of macrophages in the activity of Virulizin [24].
Although earlier studies demonstrated an essential role for macrophages in Virulizin-mediated antitumor activity, the activation pathway(s) were not elucidated. One of the main pathways leading to innate antitumor immunity is from macrophage activation to NK cell activation and recruitment to the site of tumor growth. In the present study, the effects of Virulizin on NK cell activation and recruitment were investigated and the relationship between macrophage and NK cell involvement was assessed. The results demonstrate that Virulizin treatment significantly increases infiltration of NK cells and macrophages into tumors and furthermore activates the cytotoxic function of NK cells. In addition, the data suggest the prerequisite involvement of macrophages in NK cell infiltration into tumors. These results demonstrate Virulizin’s capability to modulate expansion and activation of NK cells and macrophages, and underscore the therapeutic potential of Virulizin to enhance innate immune responses to tumors.
Materials and methods
Drugs
The active moieties of Virulizin (Lorus Therapeutics, Toronto, ON, Canada) are obtained from bovine bile (derived from US cattle approximately 24 months of age; USDA inspected) by a standardized process including ethanol precipitation, column purification, heat reduction, ether extraction, and tyndallization, which remove most bile salts and large peptides. The drug contains 5% (w/v) solid material and comprises inorganic (95–99% of the dry weight) and organic compounds of molecular weights of <3,000 Da (1–5% of the dry weight). Virulizin is formulated as a sterile injectable solution, buffered with monobasic and dibasic sodium phosphate. The product is sterile filtered as a 3-ml solution into glass vials with non-latex rubber closures and aluminum tear-off caps. GTI-2040 (Lorus Therapeutics) is an antisense drug that specifically targets the R2 component of ribonucleotide reductase [28, 29] and is used as a non-BRM control in in vivo experiments.
Cells and animals
Natural killer sensitive YAC-1 lymphoma cells (TIB 160) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Human melanoma cell line C8161 was a gift from Dr D.R. Welch (Pennsylvania State University, Hershey, PA, USA). Cells were grown in RPMI 1640 medium (Wisent, St Bruno, QC, Canada) with 10% fetal bovine serum, penicillin (100 μ/ml), and streptomycin (100 mg/ml) (Wisent) at 37°C under 95% air and 5% CO2 and maintained with routine media changes. Adherent C8161 cells were passaged by trypsinization with 0.025% trypsin. CD-1 athymic nude, SCID/beige, and C57BL/6 mice (6–8 weeks old, 20–25 g, female) were purchased from Charles River (Montreal, QC, Canada). The mice were maintained in the animal facility at Lorus Therapeutics. Animal protocols were in compliance with the “Guide for the Care and Use of Laboratory Animals in Canada.”
Evaluation of antitumor activity in a murine model of human tumor xenograft
Human tumor xenografts were established in mice as described previously [27]. Briefly, human tumor cells were harvested at approximately 80% confluence in cell culture medium and resuspended in sterile PBS. A total of 10 million tumor cells in 100 μl were subcutaneously implanted into the right flank of CD-1 athymic nude or SCID/beige mice. When the tumors reached a volume of 50–100 mm3, mice were randomly separated into groups of ten animals and treated with Virulizin, GTI-2040, or saline until the endpoint of each experiment. The doses and treatment schedules were as described in the text. Antitumor activity was evaluated as previously described [30]. Tumor volume was estimated by caliper measurements, using the formula: (length × width × height)/2. Tumor weight was determined from tumor tissue surgically excised from the animal on the last day of the experiment. The percentage of inhibition (%) = [(mean tumor weight of controls) − (mean tumor weight of drug-treated group)] / (mean tumor weight of controls)×100. Statistical analyses were carried out by the Biostatistical Consulting Unit of the Department of Community Health Sciences at the University of Manitoba (Winnipeg, Canada). A p value ≤0.05 was considered to be statistically significant.
Immunohistochemistry
The excised tumors and spleens were fixed in periodate-lysine-paraformaldehyde (PLP) fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate solution) overnight at 4°C. The samples were then dehydrated in graded alcohols, embedded in low-melting-point paraffin and 5-μm sections were cut on a rotary microtome. Paraffin sections were stained for F4/80 and NK1.1 using the avidin-biotin-peroxidase complex technique as described previously [31]. Briefly, purified rat antimouse F4/80 antiserum diluted 1:100 (C1/A3-1; SeroTec, Raleigh, NC, USA) or antimouse NK1.1 monoclonal antibody diluted 1:100 (CALTAG Laboratories, Burlingame, CA, USA) was applied to sections overnight at room temperature. As a negative control, preimmune serum was substituted for the primary antibody. After washing with high salt buffer (50 mM Tris-HCl, 2.5% NaCl, 0.05% Tween 20, pH 7.6) for 10 min at room temperature followed by two 10-min washes with TBS (50 mM Tris-HCl, 150 mM NaCl, 0.01% Tween 20, pH 7.6), sections were incubated with secondary antibodies (biotinylated goat anti-rat or mouse IgG), washed as before and processed using the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA, USA). Red pigmentation to demarcate regions of immunostaining was produced by a 10–15-min treatment with Fast Red TR/Naphthol AS-MX phosphate (Sigma), containing 1 mM levamisole as endogenous alkaline phosphatase inhibitor. The sections were then washed with distilled water, counterstained with methyl green, and mounted with Kaiser’s glycerol jelly.
Detection of apoptotic cells in situ
Dewaxed paraffin sections were stained with an in situ cell death detection kit (Boehringer Mannheim, Burlington, ON, Canada) as previously described [32]. Briefly, following treatment with 3 μg/ml of proteinase K for 20 min at room temperature, the sections were incubated with a reaction mixture for terminal deoxynucleotidyltransferase-mediated nick end-labeling (TUNEL) of DNA strand breaks for 60 min at 37°C. Sections were then incubated with Converter-AP for 30 min at 37°C and alkaline phosphatase was visualized after 10–15 min of treatment with Fast Red TR/Naphthol AS-MX phosphate (Sigma, St Louis, MO, USA), containing 1 mM levamisole as an endogenous alkaline phosphatase inhibitor. Sections were counterstained with methyl green and mounted with Kaiser’s glycerol jelly.
Computer-assisted image analysis
After immunohistochemical staining or TUNEL assay of sections from six saline- or Virulizin-treated samples, multiple images of fields were photographed with a Sony digital camera. Images of micrographs from single sections were digitally recorded using a rectangular template, and recordings were processed using Northern Eclipse image analysis software (High Tech Services, North Tonawanda, NY, USA). Thresholds were set using green and red channels. The thresholds were determined as described previously [31].
NK cell isolation and cytotoxicity assay
C56BL/6 mice were injected intraperitoneally with 200 μl of Virulizin or saline, daily for 5 days. An hour after the last injection, spleen from each mouse was removed and ground in RMPI 1640 medium. The cell suspension was passed through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA), and NK cells were isolated from splenocytes according to manufacturer’s protocol using the SpinSep Murine NK Cell Enrichment kit (StemCell Technologies, Vancouver, BC, Canada). The purity of NK cells was determined by flow cytometry. Briefly, cells were resuspended in culture medium supplemented with 2% FBS. Cells (1×106 cells/sample) were stained with anti-DX5 (pan-NK specific) antibody conjugated with PE (BD Bioscience, Mississauga, ON, Canada) on ice for 30 min. The cells were subsequently washed once with medium, once with PBS, fixed with 1% paraformaldehyde in PBS and analyzed by flow cytometry using CellQuest software (FACSCalibur; Becton Dickinson, San Jose, CA, USA).
The NK cell density was adjusted to 7.8×105/ml and 50 μl/well of NK cells was added onto a 96-well round-bottom plate. A range of effector to target ratios (from 0.65:1 to 5.2:1) was obtained by four successive twofold dilutions of the effector cells. YAC-1 cells, used as target cells, were adjusted to a density of 1.5×105/ml, 50 μl added to each well, and the resultant cocultures were incubated for 4 h at 37°C with 5% CO2. The NK cell–mediated cytotoxicity to human tumor cells was also evaluated in which splenic NK cells were cocultured with C8161 cells (1.5×105/ml) in an effector to target ratio of 10:1. The NK cell cytotoxicity was evaluated by lactate dehydrogenase (LDH) release assay using the CytoTox96 Non-Radioactive kit (Promega, Madison, WI, USA), performed in triplicates. Spontaneous release and maximum release were determined by incubating target cells without effectors in medium alone or in 5% Triton X-100, respectively. The percentage cytotoxicity was calculated as [(experimental release) − (target spontaneous release) − (effector spontaneous release)] / [(total release) − (target spontaneous)]×100. In addition, the number of NK cells required to achieve 20% net lysis (lytic unit 20 or LU20) was calculated, and the data presented as LU20/107 NK cells [33].
Macrophage depletion in CD-1 nude mice
Selective depletion of macrophages in vivo was performed essentially as described previously [34], using the liposome/C12MDP technique. A volume of 200 μl of liposome/C12MDP suspension per mouse were injected intravenously the day before transplantation of tumor cells. Additional intraperitoneal injections, once every 3 days, at the same dosage, followed tumor implantation until the end of the experiment.
Flow cytometry analysis
The spleens and human tumor xenografts from mice treated with saline or Virulizin were isolated after perfusion of each mouse with saline. The blood was also collected from saline- or Virulizin-treated mice. Spleen and tumor tissues were homogenized in PBS and filtered with a Cell Strainer (Becton Dickinson, Franklin Lakes, NJ, USA) to produce a cell suspension. The dead cells and red blood cells from tumor suspensions were removed by gradient centrifugation with Histopaque-1077 (Sigma, St Louis, MO, USA), while those from spleen suspensions or blood were removed using 1X RBC Lysis Buffer (eBioscience, San Diego, CA, USA). NK cells from spleen were further enriched by depletion of B cells (CD19+) using EasySep Murine CD19 positive selection kit (StemCell Technologies, Vancouver, BC, Canada). Cells were then washed twice with PBS and resuspended in culture medium supplemented with 2% FBS. Cells (1×106 cells/sample) were stained with anti-DX5 (pan-NK specific) antibody conjugated with FITC (eBioscience, San Diego, CA, USA) on ice for 30 min. The cells were subsequently washed once with medium, once with PBS, fixed with 1% paraformaldehyde in PBS, and analyzed by flow cytometry using CellQuest software (FACSCalibur; Becton Dickinson, San Jose, CA, USA).
Results
Increase in macrophage and NK cell infiltration to tumors by Virulizin treatment
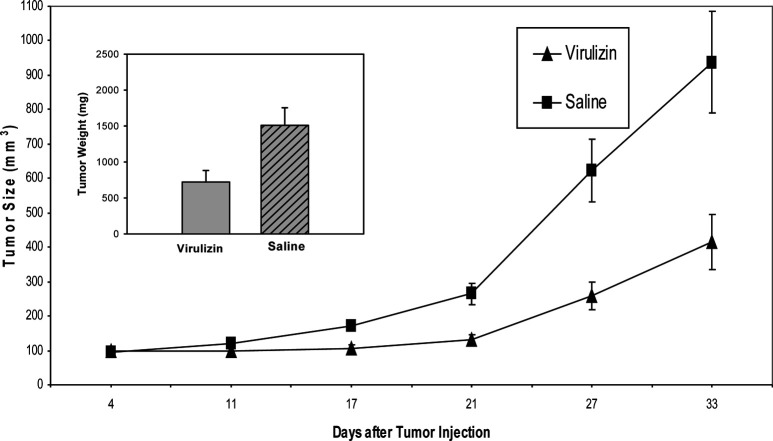
Previous studies demonstrated that Virulizin stimulated the cytolytic activity of splenocytes and macrophages against tumor cells in a dose-dependent manner, and the antitumor efficacy of Virulizin observed in CD-1 nude mice was compromised in mice depleted of macrophages, indicating that macrophages may play a critical role in antitumor activity of Virulizin [24]. The current experiments build upon the previous observations to determine whether macrophage infiltration into tumors could be induced in mice treated with Virulizin and furthermore whether there was a concomitant increase in NK cell infiltration into tumors. Human C8161 melanoma cells were implanted into CD-1 nude mice and treated with Virulizin when the xenografted tumor volume reached 50–100 mm3. Consistent with the previous results [27], Virulizin significantly inhibited the growth of human melanoma xenograft. The average tumor weight in the Virulizin-treated group (721 mg) was significantly lower than that in saline-treated group (1,509 mg) (p=0.0148) (Fig. 1), with an average value of 52% growth inhibition. Tumors were isolated from treated and untreated mice, and immunohistochemistry was used to examine tumor infiltration of macrophages. Macrophage infiltration was detected with antimouse F4/80 macrophage-specific antibodies. As shown in Fig. 2b, there was a dramatic increase in the number of F4/80 positive cells in tumors from mice treated with Virulizin compared to tumors from mice treated with saline (Fig. 2a). A few scattered F4/80 positive macrophages were detected in the subcapsular and focal regions of tumors from the control group, whereas an obvious increase was observed in both the subcapsule of tumors and the regions of inflammatory cell infiltration following the treatment with Virulizin. In the regions of inflammatory cell infiltration, there was a high density of smaller cells that had a much lower cytoplasm to nuclear ratio than tumor cells. Besides F4/80-positive macrophages and NK1.1-positive NK cells, other inflammatory cells may be present.
Fig. 1.
Antitumor activity of Virulizin. C8161 cells (1×107 cells in 100 μl of PBS) were subcutaneously injected into the right flank of CD-1 nude mice. The mice were then administered intraperitoneally with 0.2 ml of saline or Virulizin daily when tumors reached a volume of 50–100 mm3. Tumor dimension was periodically measured using calipers over a 4-week period. Each point represents average volume calculated from ten mice. Bars SE. Inset a day after the last treatment, tumors were excised from the animals, and tumor weight was measured. Antitumor activity was estimated by the reduction of tumor weight. The data are representative of three independent experiments.
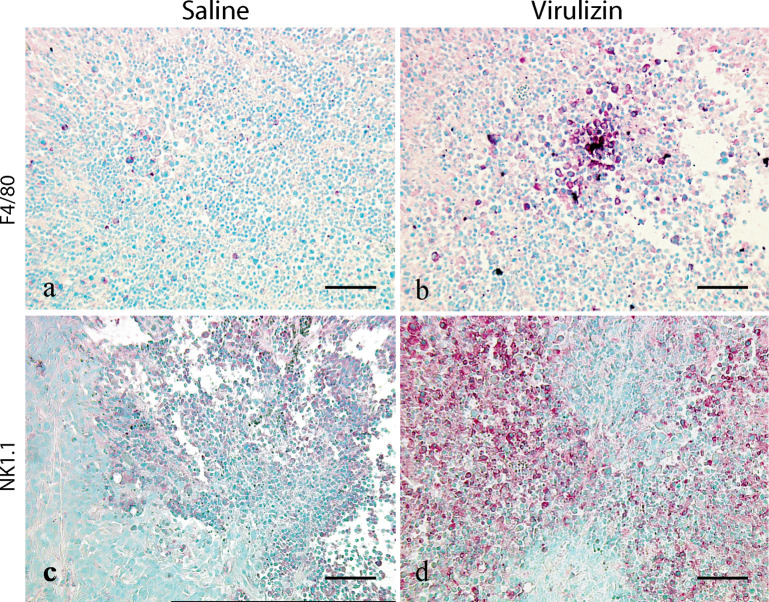
Fig. 2.
Virulizin-induced infiltration of macrophages and NK cells into tumors. Tumors were collected from mice treated with saline or Virulizin at the end of the experiments described in Fig. 1. Immunohistochemical staining for F4/80 (a–b) and NK1.1 (c–d) was performed on the paraffin-embedded tumor sections as described in “Materials and methods.” The number of both F4/80 and NK1.1 immunopositive cells in tumors was increased markedly in the Virulizin-treated group compared with those in the saline-treated group. A scale bar in each panel represents 100 μm.
In addition to tumor cytotoxicity as a result of direct cell-to-cell contact and release of tumor-killing agents, macrophages can elicit antitumor activity via a mechanism in which secretion of inflammatory cytokines and chemokines leads to recruitment to, and activation of, cytotoxic lymphocytes, such as NK cells, at the tumor site. To assess whether this mechanism of action is functional during Virulizin treatment, the extent of NK cell infiltration in human tumor xenografts was also evaluated by immunohistochemistry. As shown in Fig. 2, an increase in NK cells was found in tumors from mice treated with Virulizin (Fig. 2d) compared with mice treated with saline (Fig. 2c). Consistent with the pattern observed with macrophage infiltration, few NK1.1-positive cells were detected in the subcapsular or focal regions of tumors in the control group, while the number of NK1.1-positive cells was significantly increased in both the subcapsule of tumors and the region of inflammatory cell infiltration following the treatment with Virulizin. In addition, there was colocalization of macrophages and NK cells within the same areas of tumors isolated from Virulizin-treated mice. These results suggest that there may be crosstalk between macrophages and NK cells in the Virulizin-treated mice. To corroborate these results, flow cytometric analysis of tumor samples was performed and consistent with these results demonstrated increased NK cell infiltration into tumor xenografts (Fig. 8).
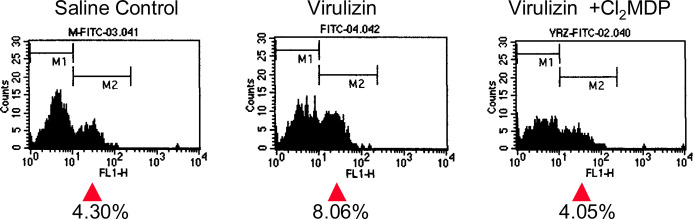
Fig. 8.
Diminished Virulizin-mediated tumor infiltration of NK cells in macrophage-depleted mice. CD-1 nude mice harboring C8161 human melanoma xenografts were depleted of macrophages using liposome-Cl2MDP, and then treated with saline or Virulizin (0.2 ml). Tumors were isolated from the mice at the end of the experiment. A cell suspension was prepared from tumors and stained with anti-DX5 (NK cell–specific) antibody conjugated with FITC. The number of NK cells in tumors were assessed by flow cytometry.
Rapid induction of NK cell infiltration to tumors in Virulizin-treated mice
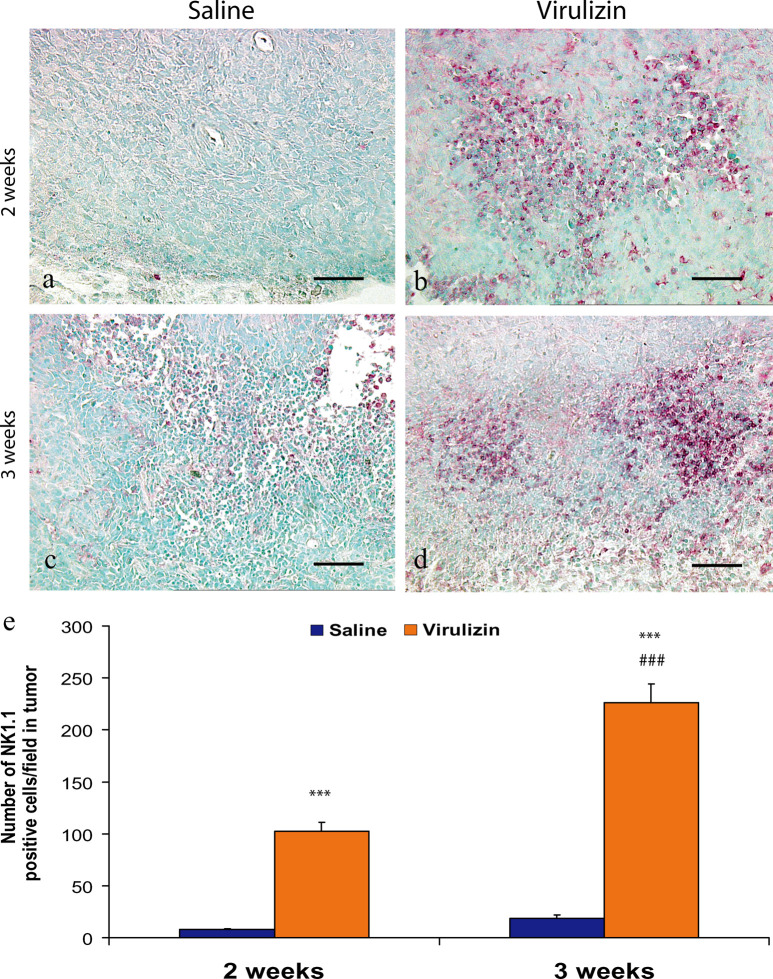
To determine the kinetics of NK cell infiltration to the tumor site and to assess whether NK recruitment is a direct consequence of Virulizin treatment, an in vivo time course experiment was performed. CD-1 nude mice bearing human C8161 melanoma xenografts received Virulizin or saline treatment. As illustrated in Fig. 3, increased infiltration of NK1.1-positive cells was observed for Virulizin treatment at weeks 2 and 3. Quantitative image analysis of data obtained from six tumor samples per group demonstrated that the average number of NK 1.1-positive cells per field at week 2 was 7.7 in the control group and 102.2 in the Virulizin-treated group. This number increased to 18.7 in the control group and 226.0 in Virulizin-treated group at week 3, indicating that NK cell infiltration was significantly elevated in tumors at 3 weeks as compared to 2 weeks following Virulizin treatment (p<0.001). No significant difference of NK cell infiltration was found between 2- and 3-week samples from mice treated with saline.
Fig. 3.
Analysis of NK cell infiltration into tumors over the course of treatments, by quantitative immunohistochemistry. CD-1 nude mice implanted with human C8161 melanoma were treated with saline or Virulizin as described in Fig. 1. At 2 or 3 weeks following the first treatment, the mice were sacrificed and tumors were collected. Immunohistochemical staining for NK1.1 was performed on the paraffin-embedded tumor sections as described in “Materials and methods.” A scale bar in each panel represents 100 μm. a–d NK1.1 immunopositive cells per field were determined by computer-assisted image analysis as described in “Materials and methods” and were presented as the average ± SE calculated from determinations of six tumor samples of each group. ***p<0.001 relative to saline control; ###p<0.001 relative to 2-week treatment
Increased NK cells in the mouse spleen and peripheral blood following Virulizin treatment
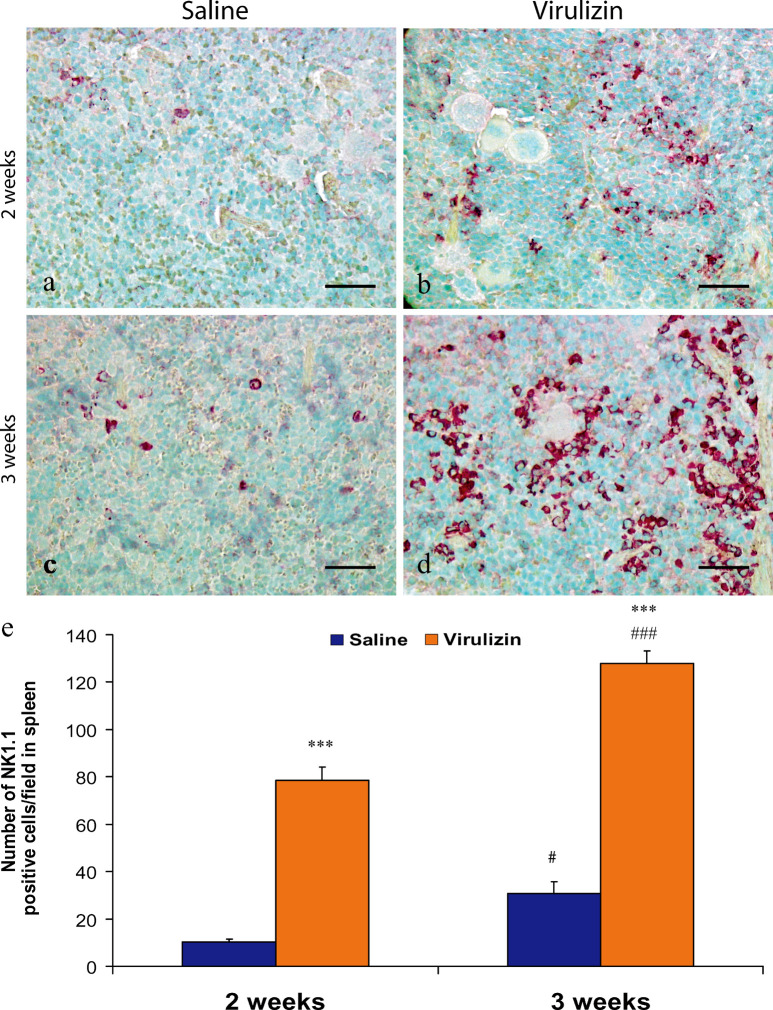
In view of the fact that the spleen is one of the main immune organs in the body, the next experiment examined whether Virulizin could expand the NK cell population in this lymphoid tissue. Immunohistochemical analysis demonstrated that the number of NK1.1+ cells increased in the spleen of Virulizin-treated mice (Fig. 4). An increase was evident as early as 2 weeks (compare Fig. 4a with b), and was more prominent at 3 weeks following Virulizin treatment (compare Fig. 4c with d). Computer-assisted image analysis showed that the number of NK cells was significantly higher in the Virulizin-treated group (78.5 and 127.8 cells/field at 2 and 3 weeks) as compared to that in the control group (10.3 and 30.7 cells/field at 2 and 3 weeks) with p<0.001 in both cases (Fig. 4e). In addition, the number of NK cells at 3 weeks was significantly higher (1.6-fold) than that at 2 weeks (p<0.001), while the number of NK cells in the spleen from the control group was also higher at 3 weeks than at 2 weeks (p<0.05), an increase likely due to tumor loading in mice.
Fig. 4.
Immunohistochemical analysis of NK cells in the spleen. CD-1 nude mice implanted with human C8161 melanoma were treated with saline or Virulizin as described in Fig. 1. The mice were sacrificed and spleens were collected at 2 or 3 weeks following the first treatment. Immunohistochemical staining for NK1.1 was performed on the paraffin-embedded spleen sections as described in “Materials and methods.” A scale bar in each panel represents 100 μm. a–d NK1.1 immunopositive cells per field were determined by computer-assisted image analysis as described in “Materials and methods” and were presented as the average ± SE calculated from determinations of six spleen samples of each group. ***p<0.001 relative to saline control; #p<0.05 relative to saline control; ###p<0.001 relative to 2-week treatment.
Moreover, the number of NK cells in the mouse spleen and peripheral blood were analyzed by flow cytometry following Virulizin treatment. The data shown in Table 1 indicate that the percentage of NK cells was significantly increased in the spleen and peripheral blood with p values of 0.010 and 0.041, respectively.
Table 1.
Flow cytometric analysis of NK cells in the spleen and peripheral blood
| Group | Saline (% mean ± SE) | Virulizin (% mean ± SE) | p Value |
|---|---|---|---|
| Blood | 33.87±1.21 | 44.6±2.60 | 0.041 |
| Spleen | 21.06±1.83 | 29.23±0.35 | 0.010 |
Enhanced level of NK cell activity in Virulizin-treated mice
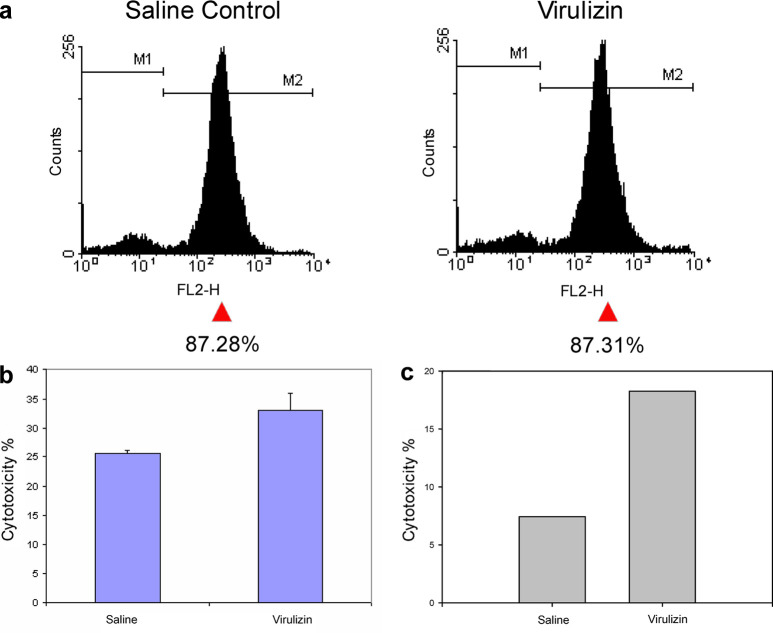
To determine whether Virulizin could enhance NK cell activity in vivo, NK cell–mediated cytotoxicity against NK-sensitive YAC-1 cells was evaluated using a standard LDH release assay. C57BL/6 mice were treated with Virulizin, and NK cells were isolated from the spleens and analyzed by flow cytometry. The purity of NK cells was determined to be around 87% in both saline- and Virulizin-treated groups (Fig. 5a). Increasing numbers of isolated NK cells were then cocultured with YAC-1 target tumor cells in vitro. As shown in Fig. 5b, a significant increase in NK cytotoxicity (29% in percentage cytotoxicity) was observed with NK cells isolated from Virulizin-treated mice at one of the effector to target ratios (5.2:1). In addition, a significant increase (p=0.023) was evident when LU20 was calculated for saline- and Virulizin-treated groups from a dose-response curve relating percentage specific lysis to effector to target ratio. LU20/107 NK cells was 428.5±7.7 in Virulizin-treated mice as compared to 370.8±5.3 in saline-treated mice, whereas the LU20/total NK cells per spleen was 158.5±2.6 in Virulizin-treated mice as compared to 137.2±2.0 in saline-treated mice. The NK cell–mediated cytotoxicity to human melanoma cells (C8161) also increased upon Virulizin treatment (Fig. 5c). Further studies are currently underway to fully evaluate NK cell cyctotoxicity to C8161 and to determine the LU20 using a range of effector to target cell ratios. These results suggest that NK cells in the spleens can be activated by Virulizin treatment in vivo, and activated NK cells possess enhanced cytolytic function that may contribute to the antitumor activity of Virulizin.
Fig. 5.
Elevated NK cell cytotoxicity in Virulizin-treated mice. C57BL/6 mice were treated with Virulizin or saline daily for 5 days. NK cells were isolated from the spleens 1 h after the last injection and cocultured with YAC-1 or C8161 target tumor cells to assess NK cell cytotoxicity using LDH release assay. In a the purity of NK cells is shown as analyzed by flow cytometry. b A standard bar graph was used to demonstrate the differences in cytotoxicity level with each bar representing mean percentage cytotoxicity values calculated from three independent experiments. The results for one effector to target (YAC-1) ratio (5.2:1) are shown. c A standard bar graph was used to demonstrate the differences in cytotoxicity level when splenic NK cells were cocultured with C8161 cells in a ratio of 10:1.
Increased apoptosis in tumors from Virulizin-treated mice
Although Virulizin does not appear to show direct antiproliferative activity against tumor cells in vitro [26], it clearly increases the level of macrophage and NK cell infiltration to tumors. Since these immune effector cells can activate apoptotic pathways in tumor cells, the extent of apoptosis in tumors following Virulizin treatment was determined. The apoptotic index was determined from tumor sections by TUNEL staining [35] followed by quantitative image analysis as described in “Materials and methods.” An increase in the number of apoptotic cells was observed as early as 2 weeks in tumors from Virulizin-treated group (compare Fig. 6a with b), while more apoptotic cells were detected in tumors isolated from mice treated with Virulizin for 3 weeks (Fig. 6d). Quantitative image analysis showed that the difference in the apoptotic index between Virulizin and saline-treated groups was statistically significant at both 2 and 3 weeks after treatment (p<0.001), with the percentage of apoptotic tumor cells being 6.2% and 6.7% in the control group, respectively, and 28.1% and 64.1% in the Virulizin-treated groups, respectively. Furthermore the apoptotic index was significantly higher in the Virulizin-treated 3-week samples (64.1%) than in the Virulizin-treated 2-week samples (28.1%) (p<0.001).
Fig. 6.
Elevated tumor cell apoptosis in Virulizin-treated mice. CD-1 nude mice implanted with human C8161 melanoma were treated with saline or Virulizin as described in Fig. 1. At 2 or 3 weeks following the first treatment, the mice were sacrificed and tumors were collected. The extent of tumor cell apoptosis was assessed using TUNEL assay on the paraffin-embedded tumor sections as described in “Materials and methods.” A scale bar in each panel represents 25 μm. a–d The number of total and apoptotic tumor cells per field was determined by computer-assisted image analysis as described in “Materials and methods.” e The percentage of apoptotic tumor cells was calculated from determinations of six tumor samples of each group and presented as the average ± SE. ***p<0.001 relative to saline control; ###p<0.001 relative to 2-week treatment.
Significantly compromised antitumor activity of Virulizin in SCID/beige mice
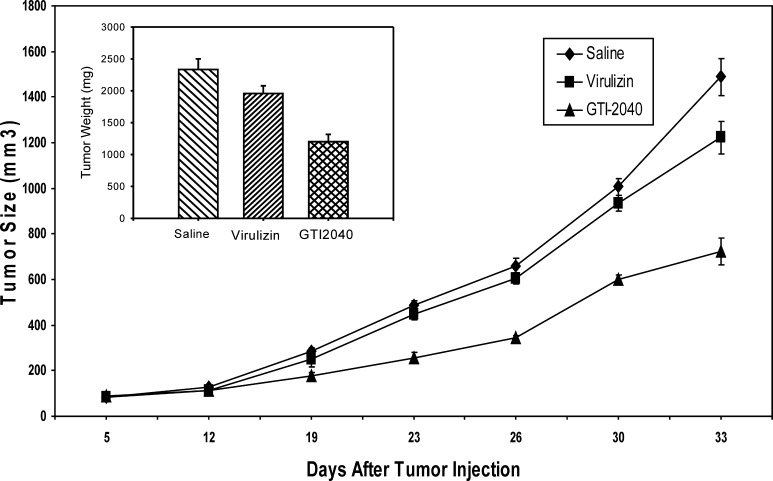
To further assess the contribution of NK cells to tumor growth suppression by Virulizin, the in vivo efficacy of Virulizin was evaluated in NK cell–deficient SCID/beige mice. Despite identical treatment schedule and drug dose, the antitumor activity consistently observed in CD-1 nude mice (Fig. 1) was markedly impaired in NK cell–deficient SCID/beige mice (Fig. 7). The average tumor weight difference between saline- and Virulizin-treated mice (2,335 and 1,958 mg, respectively) was insignificant with a p value of 0.082. In contrast, an antisense drug, GTI-2040, exhibited significant antitumor activity in CD-1 nude mice [29], the extent of which was not compromised in SCID/beige mice. GTI-2040 is a 20mer oligonucleotide that is complementary to a coding region in the mRNA of the R2 small subunit component of human ribonucleotide reductase, an only enzyme responsible for the synthesis of 2′-deoxyribonucleotides from the corresponding ribonucleoside 5′-diphosphates. GTI-2040 acts as a target-selective and sequence-specific anticancer agent whose antitumor activity was largely due to sequence-specific down-regulation of R2 expression and was not attributable to CpG-mediated immune stimulation [29]. The average tumor weight in GTI-2040–treated mice (1,202 mg) was significantly smaller than that in saline-treated mice (p<0.001). The clear lack of Virulizin-mediated antitumor effects in SCID/beige mice suggests an important role for NK cells in the mechanism of action of Virulizin.
Fig. 7.
Diminished antitumor activity of Virulizin in NK cell–deficient SCID/beige mice. C8161 cells (1×107 cells in 100 μl of PBS) were subcutaneously injected into the right flank of SCID/beige mice. The mice were then administered intraperitoneally with 0.2 ml of saline or Virulizin daily, or intravenously with 10 mg/kg of GTI-2040 every other day when tumors reached a volume of 50–100 mm3. Tumor dimension was periodically measured using calipers over a 4-week period. Each point represents average volume calculated from ten mice. Bars SE. Inset a day after the last treatment, tumors were excised from the animals, and tumor weight was measured. Antitumor activity was estimated by the reduction of tumor weight. The data are representative of two independent experiments.
Depletion of macrophages compromised Virulizin-induced NK cell infiltration into tumors
Activated macrophages can induce production of cytokines and chemokines that direct the innate immune responses by recruitment and activation of NK cells. An earlier study demonstrated that depletion of macrophages in vivo by liposome-Cl2MDP significantly compromised the antitumor activity of Virulizin using a human C8161 melanoma xenograft model. To examine whether the presence of macrophages is important for Virulizin-mediated NK cell infiltration into tumors, mice bearing C8161 human melanoma xenografts were depleted of macrophages and treated with Virulizin, and NK cell infiltration was assessed by flow cytometry. Analysis of CD11b-positive cells (monocytes/macrophages) in peripheral blood collected from mice indicated that injection of liposome-Cl2MDP successfully depleted macrophages systemically in these mice [24]. As shown in Fig. 8, macrophage depletion led to a significant decrease in the number of NK cells infiltrating into tumors. The number of NK cells increased from 4.30% to 8.04% in tumors following Virulizin treatment in nondepleted mice but was unaltered at 4.05% in tumors from the mice depleted of macrophages. Given that depletion of macrophages results in loss of Virulizin-induced increase in NK cell infiltration into tumors, these results support the hypothesis that Virulizin-activated macrophages promote tumor infiltration of NK cells and provide a clear basis for an antitumor mechanism of action that involves the interplay between cellular components of the innate immune system.
Discussion
Virulizin is a novel BRM that demonstrates strong antitumor efficacy in a variety of human tumor xenograft models including melanoma, pancreatic cancer, breast cancer, ovarian cancer, and prostate cancer [24, 26, 27]. Previous studies demonstrated that Virulizin could activate macrophages and induce release of inflammatory mediators and increase cytotoxicity, indicating that macrophages play a role in the antitumor activity of Virulizin. The present study extends these findings by demonstrating that Virulizin augments both macrophages and NK cell infiltration into the tumor site, in a time-dependent manner, where they could directly participate in tumor cell apoptosis. Infiltration of NK cells into the tumor site appears to depend on the presence of macrophages, as depletion of macrophages led to the impairment of NK cell infiltration into tumors. Furthermore, the antitumor activity of Virulizin was attenuated in NK cell–deficient SCID/beige mice. Taken together, these data suggest an antitumor mechanism of action that requires the interplay between the major components of the innate immune system.
Successful immunohistochemical study of NK cells in mice has rarely been reported using anti-NK1.1 and other available NK cell–specific antibodies. The current study, however, successfully demonstrated detection of mouse NK cells in the spleen and tumor tissue using anti-NK1.1 monoclonal antibody. This may be due to the application of PLP fixative in the critical fixation step. Cell surface antigens are very sensitive proteins, which can be easily denatured by routine fixation. The PLP fixative devised by McLean and Nakane [36] links carbohydrate moieties without excessive binding to protein antigenic sites. Mori et al. [37] also demonstrated excellent preservation of antigenicity for cell surface antigens on lymphocytes and macrophages when samples were fixed with PLP fixative. In addition, Miao and Scutt [38] showed an excellent preservation of alkaline phosphatase activity in paraffin-embedded decalcified tissues fixed with PLP but not in samples fixed with 10% neutral phosphate-buffered formalin.
Results from other investigators demonstrate that the number of tumor-infiltrating NK cells correlates with positive survival prognosis in colorectal, gastric, and lung cancers in humans [39–42]. An essential role for infiltrating NK cells in eradicating experimental tumors has also been reported [10, 13, 43, 44]. In addition, NK cells can recognize and attack cells in which class I MHC expression is reduced or abolished [45–49], a feature that renders some tumor cells resistant to recognition and lysis by class I–restricted CTLs (CD8+ T cells). The preferential recognition of MHC class I–deficient tumor cells by NK cells may be attributed to the combined function of the lack of interaction between NK cell inhibitory receptors including Ly49 (in mice), KIR (in humans), and CD94/NKG2A (in both mice and humans) with MHC class I molecules, and up-regulation of proteins that bind and activate a NK cell stimulatory receptor NKG2D [50–57]. These studies support the hypothesis that tumor-infiltrating NK cells can be effective in limiting initial tumor spread and metastasis even when tumor cells lose their sensitivity to CTLs or when the ability to elicit acquired antitumor immunity is significantly compromised. In the current study, NK cell infiltration into tumors was induced early in the tumor growth period and persisted over the entire course of Virulizin treatment in significantly immune-compromised mice. This positively correlated with increased tumor cell killing by NK cells isolated from Virulizin-treated mice. In previous studies, Virulizin demonstrated significantly enhanced antitumor efficacy in combination chemotherapy [27]. Taken together, these results suggest that Virulizin remains sufficiently effective in immune-compromised conditions, and altered NK cell functions are at least partly responsible for the observed antitumor activity.
The data presented in this study suggest that Virulizin likely exerts its antitumor activity via an indirect mechanism that involves enhancement of antitumor immunity. Virulizin augmented recruitment of macrophages and NK cells into the tumor site, and apoptosis was often detected in the area with increased number of F4/80- and NK1.1-positive cells, suggesting that these immune effecter cells may contribute to increased tumor cell apoptosis. This is consistent with a large amount of experimental evidence that macrophage- and NK cell-mediated cytotoxicity against solid tumors is mainly through induction of apoptosis [58–61]. Macrophages and NK cells can kill tumor cells upon direct cell–cell contact and/or indirectly via paracrine mechanisms involving cytokines and granules. For example, engagement of Fas ligand (FasL) or TNF-α on the NK cell with Fas (CD95) or TNFR on tumor cells, respectively, have been identified as important mediators of NK cell–mediated target cell apoptosis [61]. NK cells can also initiate the process of calcium-dependent formation of perforin pores and the release of cytolytic granule serine proteases that activate the caspase cascade in tumor cells to effect apoptosis [62, 63]. Furthermore, NK cells were identified as the chief effectors mediating tumor regression in vivo, caused by both apoptotic and necrotic tumor cell death [64]. Future studies on the mechanism of action of Virulizin will focus on the detailed molecular events that are involved in its antitumor activity including the above pathways.
The number of NK cells significantly increased in the spleen and peripheral blood of mice that received Virulizin treatment, suggesting that Virulizin can stimulate expansion of NK cells. Normally, NK cells differentiate from CD34+ primitive hemopoietic progenitor cells under the influence of various cytokines [2, 15, 65–68] and then distribute within a variety of lymphoid and nonlymphoid tissues [1, 65, 69, 70]. Although the exact mechanism involved in NK cell expansion in Virulizin-treated mice remains to be determined, preliminary data suggest an involvement of cytokines such as IL-12, whose production was induced in vivo upon Virulizin treatment (manuscript in preparation). IL-12 not only induces cytotoxicity of tumor cells but also acts as a growth factor for activated T cells and NK cells and promotes proliferation of these cells in synergy with other mitogenic or costimulatory signals [71–75]. IL-12 can also synergize with stem cell factors and the other colony-stimulating factors to induce the proliferation and differentiation of hematopoietic stem cells [75–78]. The observation that NK cell infiltration is compromised upon depletion of macrophages suggests that macrophages play a critical role in NK cell expansion. Current studies are assessing whether macrophage depletion alters NK cell expansion in the spleen. As previously suggested, Virulizin can activate monocytes/macrophages and stimulate the production of inflammatory cytokines such as IL-12, TNF-α ,and IL-1 [24, 25]. Taken together, it is interesting to speculate that Virulizin-activated monocytes/macrophages stimulate cytokine secretion to further induce proliferation of NK cells. Furthermore, Virulizin may also affect bone marrow stromal cells directly to promote NK cell differentiation and proliferation. Studies are currently underway to further explore these possibilities.
In conclusion, the present study demonstrated Virulizin’s ability to induce increased infiltration of NK cells and macrophages to tumors and to cause enhanced tumor cell apoptosis. The critical involvement of NK cells in the antitumor mechanism of Virulizin has been further confirmed in vivo. In addition, Virulizin was shown to be capable of not only modulating NK cell expansion but also enhancing NK cell–mediated cytotoxicity against tumor cells in mice. Drug-induced expansion and activation of NK cells without causing significant toxicity is a very attractive immunotherapeutic approach that can replenish the number and activity of NK cells frequently lost in cancer patients as well as in patients with viral infections [79, 80]. With a strong safety profile observed both in preclinical and clinical studies, Virulizin appears to be an excellent candidate to provide a safe and efficient immunotherapeutic approach in the treatment of human cancers.
Acknowledgements
We are grateful to Dr D.R. Welch for providing human melanoma cell line C8161. We also thank Dr T. Benatar for technical assistance, Dr A. Vassilakos and members of Lorus Therapeutics for helpful discussion and critical reading of the manuscript.
Abbreviations
- BRM
Biological response modifier
- MHC
Major histocompatibility complex
- NK cells
Natural killer cells
- TUNEL
Terminal deoxynucleotidyltransferase-mediated X-dUTP nick end-labeling
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654–2670. [PubMed] [Google Scholar]
- 3.Dima VF, Ionescu MD, Balotescu C, Dima SV, Vasiliu V, Lacky D. New approach to the adoptive immunotherapy of Walker-256 carcinosarcoma with activated macrophages combined with photodynamic therapy. Roum Arch Microbiol Immunol. 2001;60:237–256. [PubMed] [Google Scholar]
- 4.Chen GG, Lau WY, Lai PB, Chun YS, Chak EC, Leung BC, Lam IK, Lee JF, Chui AK. Activation of Kupffer cells inhibits tumor growth in a murine model system. Int J Cancer. 2002;99:713–720. doi: 10.1002/ijc.10412. [DOI] [PubMed] [Google Scholar]
- 5.Thiounn N, Pages F, Mejean A, Descotes JL, Fridman WH, Romet-Lemonne JL. Adoptive immunotherapy for superficial bladder cancer with autologous macrophage activated killer cells. J Urol. 2002;168:2373–2376. doi: 10.1097/00005392-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Shaw SG, Maung AA, Steptoe RJ, Thomson AW, Vujanovic NL. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161:2817–2824. [PubMed] [Google Scholar]
- 7.Smith DL, Cai J, Zhu S, Wei W, Fukumoto J, Sharma S, Masood R, Gill PS. Natural killer cell cytolytic activity is necessary for in vivo antitumor activity of the dipeptide L-glutamyl-L-tryptophan. Int J Cancer. 2003;106:528–533. doi: 10.1002/ijc.11253. [DOI] [PubMed] [Google Scholar]
- 8.Fan D, Liaw A, Denkins YM, Collins JH, Van Arsdall M, Chang JL, Chakrabarty S, Nguyen D, Kruzel E, Fidler IJ. Type-1 transforming growth factor-beta differentially modulates tumoricidal activity of murine peritoneal macrophages against metastatic variants of the B16 murine melanoma. J Exp Ther Oncol. 2002;2:286–297. doi: 10.1046/j.1359-4117.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen GG, Chak EC, Chun YS, Lam IK, Sin FL, Leung BC, Ng HK, Poon WS. Glioma apoptosis induced by macrophages involves both death receptor-dependent and independent pathways. J Lab Clin Med. 2003;141:190–199. doi: 10.1067/mlc.2003.22. [DOI] [PubMed] [Google Scholar]
- 10.Landstrom M, Funa K. Apoptosis in rat prostatic adenocarcinoma is associated with rapid infiltration of cytotoxic T-cells and activated macrophages. Int J Cancer. 1997;71:451–455. doi: 10.1002/(SICI)1097-0215(19970502)71:3<451::AID-IJC24>3.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Bonnotte B, Larmonier N, Favre N, Fromentin A, Moutet M, Martin M, Gurbuxani S, Solary E, Chauffert B, Martin F. Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J Immunol. 2001;167:5077–5083. doi: 10.4049/jimmunol.167.9.5077. [DOI] [PubMed] [Google Scholar]
- 12.Tsung K, Dolan JP, Tsung YL, Norton JA. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002;62:5069–5075. [PubMed] [Google Scholar]
- 13.Silobrcic V, Zietman AL, Ramsay JR, Suit HD, Sedlacek RS. Residual immunity of athymic NCr/Sed nude mice and the xenotransplantation of human tumors. Int J Cancer. 1990;45:325–333. doi: 10.1002/ijc.2910450220. [DOI] [PubMed] [Google Scholar]
- 14.Schattner A, Duggan DB. Natural killer cells—toward clinical application. Am J Hematol. 1985;18:435–443. doi: 10.1002/ajh.2830180415. [DOI] [PubMed] [Google Scholar]
- 15.Lian RH, Kumar V. Murine natural killer cell progenitors and their requirements for development. Semin Immunol. 2002;14:453–460. doi: 10.1016/S1044532302000805. [DOI] [PubMed] [Google Scholar]
- 16.Gilboa E. How tumors escape immune destruction and what we can do about it. Cancer Immunol Immunother. 1999;48:382–385. doi: 10.1007/s002620050590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengel H, Koszinowski UH. Interference with antigen processing by viruses. Curr Opin Immunol. 1997;9:470–476. doi: 10.1016/S0952-7915(97)80097-0. [DOI] [PubMed] [Google Scholar]
- 18.Glas R, Franksson L, Une C, Eloranta ML, Ohlen C, Orn A, Karre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanier LL. Turning on natural killer cells. J Exp Med. 2000;191:1259–1262. doi: 10.1084/jem.191.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham-Nguyen KB, Yang W, Saxena R, Thung SN, Woo SL, Chen SH. Role of NK and T cells in IL-12-induced anti-tumor response against hepatic colon carcinoma. Int J Cancer. 1999;81:813–819. doi: 10.1002/(SICI)1097-0215(19990531)81:5<813::AID-IJC24>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Kodama T, Takeda K, Shimozato O, Hayakawa Y, Atsuta M, Kobayashi K, Ito M, Yagita H, Okumura K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur J Immunol. 1999;29:1390–1396. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Schneeberger A, Koszik F, Schmidt W, Kutil R, Stingl G. The tumorigenicity of IL-2 gene-transfected murine M-3D melanoma cells is determined by the magnitude and quality of the host defense reaction: NK cells play a major role. J Immunol. 1999;162:6650–6657. [PubMed] [Google Scholar]
- 23.Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother. 1999;8:281–290. doi: 10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
- 24.Du C, Feng N, Jin H, Lee V, Wang M, Wright JA, Young AH. Macrophages play a critical role in the anti-tumor activity of Virulizin(R) Int J Oncol. 2003;23:1341–1346. [PubMed] [Google Scholar]
- 25.Ferdinandi ES, Braun DP, Liu C, Zee BC, Ely G. Virulizin(R)—a review of its antineoplastic activity. Expert Opin Investig Drugs. 1999;8:1721–1735. doi: 10.1517/13543784.8.10.1721. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Ferdinandi ES, Ely G, Joshi SS. Virulizin-2gamma, a novel immunotherapeutic agent, in treatment of human pancreatic cancer xenografts. Int J Oncol. 2000;16:1015–1020. doi: 10.3892/ijo.16.5.1015. [DOI] [PubMed] [Google Scholar]
- 27.Feng N, Jin H, Wang M, Du C, Wright JA, Young AH. Antitumor activity of Virulizin, a novel biological response modifier (BRM) in a panel of human pancreatic cancer and melanoma xenografts. Cancer Chemother Pharmacol. 2003;51:247–255. doi: 10.1007/s00280-002-0559-7. [DOI] [PubMed] [Google Scholar]
- 28.Fan H, Villegas C, Huang A, Wright JA. The mammalian ribonucleotide reductase R2 component cooperates with a variety of oncogenes in mechanisms of cellular transformation. Cancer Res. 1998;58:1650–1653. [PubMed] [Google Scholar]
- 29.Lee Y, Vassilakos A, Feng N, Lam V, Xie H, Wang M, Jin H, Xiong K, Liu C, Wright J, Young A. GTI-2040, an antisense agent targeting the small subunit component (R2) of human ribonucleotide reductase, shows potent antitumor activity against a variety of tumors. Cancer Res. 2003;63:2802–2811. [PubMed] [Google Scholar]
- 30.Du C, Feng N, Jin H, Wang M, Wright JA, Young AH. Preclinical efficacy of Virulizin in human breast, ovarian and prostate tumor models. Anticancer Drugs. 2003;14:289–294. doi: 10.1097/00001813-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–939. doi: 10.1210/en.142.2.926. [DOI] [PubMed] [Google Scholar]
- 32.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002;109:1173–1182. doi: 10.1172/JCI200214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryant J, Day R, Whiteside TL, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-U. [DOI] [PubMed] [Google Scholar]
- 34.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 35.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, Sawai T, Teshima T, Kyogoku M. A new decalcifying technique for immunohistochemical studies of calcified tissue, especially applicable to cell surface marker demonstration. J Histochem Cytochem. 1988;36:111–114. doi: 10.1177/36.1.3275709. [DOI] [PubMed] [Google Scholar]
- 38.Miao D, Scutt A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem. 2002;50:333–340. doi: 10.1177/002215540205000305. [DOI] [PubMed] [Google Scholar]
- 39.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 41.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103–108. doi: 10.1016/S0304-3835(00)00542-5. [DOI] [PubMed] [Google Scholar]
- 43.Ada GL. The immunological principles of vaccination. Lancet. 1990;335:523–526. doi: 10.1016/0140-6736(90)90748-T. [DOI] [PubMed] [Google Scholar]
- 44.Chapoval AI, Fuller JA, Kremlev SG, Kamdar SJ, Evans R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J Immunol. 1998;161:6977–6984. [PubMed] [Google Scholar]
- 45.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piontek GE, Taniguchi K, Ljunggren HG, Gronberg A, Kiessling R, Klein G, Karre K. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol. 1985;135:4281–4288. [PubMed] [Google Scholar]
- 47.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 48.Lanier LL. Natural killer cell receptors and MHC class I interactions. Curr Opin Immunol. 1997;9:126–131. doi: 10.1016/S0952-7915(97)80169-0. [DOI] [PubMed] [Google Scholar]
- 49.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–314. doi: 10.1016/S0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 50.Braud VM, Allan DS, O‘Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 51.Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. J Exp Med. 1999;190:1801–1812. doi: 10.1084/jem.190.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J Exp Med. 2000;192:1059–1068. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 55.Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev. 2001;181:158–169. doi: 10.1034/j.1600-065X.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 56.Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001;181:170–184. doi: 10.1034/j.1600-065X.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 57.Diefenbach A, Raulet DH. Innate immune recognition by stimulatory immunoreceptors. Curr Opin Immunol. 2003;15:37–44. doi: 10.1016/S0952-7915(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 58.Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358–5366. [PubMed] [Google Scholar]
- 59.Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 2001;167:2068–2073. doi: 10.4049/jimmunol.167.4.2068. [DOI] [PubMed] [Google Scholar]
- 60.Vujanovic NL. Role of TNF family ligands in antitumor activity of natural killer cells. Int Rev Immunol. 2001;20:415–437. doi: 10.3109/08830180109054415. [DOI] [PubMed] [Google Scholar]
- 61.Warren HS, Smyth MJ. NK cells and apoptosis. Immunol Cell Biol. 1999;77:64–75. doi: 10.1046/j.1440-1711.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 62.Kausalya S, Hegde SP, Bright JJ, Khar A. Mechanism of antibody-dependent natural killer cell-mediated AK-5 tumor cell death. Exp Cell Res. 1994;212:285–290. doi: 10.1006/excr.1994.1145. [DOI] [PubMed] [Google Scholar]
- 63.Bright JJ, Kausalya S, Khar A. Natural killer cell activation-associated induction of target cell DNA fragmentation in a spontaneously regressing rat histiocytoma. Immunology. 1995;85:638–644. [PMC free article] [PubMed] [Google Scholar]
- 64.Khar A, Anjum R. Host-tumor interactions during the regression of a rat histiocytoma, AK-5. Immunol Rev. 2001;184:244–257. doi: 10.1034/j.1600-065x.2001.1840122.x. [DOI] [PubMed] [Google Scholar]
- 65.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 66.Leclercq G, Debacker V, de Smedt M, Plum J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J Exp Med. 1996;184:325–336. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 68.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 69.van den Brink MR, Palomba ML, Basse PH, Hiserodt JC. In situ localization of 3.2.3+ natural killer cells in tissues from normal and tumor-bearing rats. Cancer Res. 1991;51:4931–4936. [PubMed] [Google Scholar]
- 70.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 71.Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–882. [PubMed] [Google Scholar]
- 72.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 73.Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, Herrmann SH, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perussia B, Chan SH, D’Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf SF, Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992;149:3495–3502. [PubMed] [Google Scholar]
- 75.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 76.Jacobsen SE, Veiby OP, Smeland EB. Cytotoxic lymphocyte maturation factor (interleukin 12) is a synergistic growth factor for hematopoietic stem cells. J Exp Med. 1993;178:413–418. doi: 10.1084/jem.178.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bellone G, Trinchieri G. Dual stimulatory and inhibitory effect of NK cell stimulatory factor/IL-12 on human hematopoiesis. J Immunol. 1994;153:930–937. [PubMed] [Google Scholar]
- 78.Hirayama F, Katayama N, Neben S, Donaldson D, Nickbarg EB, Clark SC, Ogawa M. Synergistic interaction between interleukin-12 and steel factor in support of proliferation of murine lymphohematopoietic progenitors in culture. Blood. 1994;83:92–98. [PubMed] [Google Scholar]
- 79.Vujanovic NL, Basse P, Herberman RB, Whiteside TL. Antitumor functions of natural killer cells and control of metastases. Methods. 1996;9:394–408. doi: 10.1006/meth.1996.0044. [DOI] [PubMed] [Google Scholar]
- 80.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/S0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]