Abstract
MAGE-3, a member of melanoma antigen (MAGE) gene family, is recognized as an ideal candidate for tumor vaccine because it is expressed in a significant proportion of tumors of various histological types and can induce antigen-specific immune response in vivo. There is now substantial evidence that heat shock proteins (HSPs) isolated from cancer cells and virus-infected cells can be used as vaccines to produce cancer-specific or virus-specific immunity. In this research, we investigated whether M. tuberculosis HSP70 can be used as vehicle to elicit immune response to its accompanying MAGE-3 protein. A recombinant protein expression vector was constructed that permitted the production of fusion protein linking amino acids 195–314 of MAGE-3 to the C terminus of HSP70. We found that HSP70-MAGE-3 fusion protein can elicit stronger cellular and humoral immune responses against MAGE-3 expressing murine tumor than those elicited by MAGE-3 protein in vivo, which resulted in potent antitumor immunity against MAGE-3-expressing tumors. Covalent linkage of HSP70 to MAGE-3 was necessary to elicit immune response to MAGE-3. These results indicate that linkage of HSP70 to MAGE-3 enhanced immune responses to MAGE-3 in vivo and HSP70 can be exploited to enhance the cellular and humoral immune responses against any attached tumor-specific antigens.
Keywords: MAGE-3, Heat shock protein 70, Vaccine, Cytotoxic T lymphocytes, ELISpot
Introduction
The identification of human tumor antigens opens up a possibility to develop cancer vaccines. The melanoma antigen(MAGE) gene family, which encodes antigens recognized by cytotoxic T lymphocytes (CTLs), was identified from a melanoma cell line (MZ2E) in 1991 [1]. MAGE-1 and MAGE-3 antigens have been termed as tumor-rejection antigens because tumors expressing these antigens on appropriate human leukocyte antigen (HLA) class I molecules are rejected by host CTLs [2]. MAGE-3, a closely related gene to MAGE-1, is more abundantly and frequently expressed than MAGE-1 [3]. MAGE-3 is expressed in a significant proportion of primary and metastatic tumors of various histological types, such as in 74% of metastatic melanoma and in 50% of carcinomas of esophagus, head and neck, bladder, and lung [4]. MAGE-3 is recognized as ideal candidate for tumor vaccine and has been applied in a variety of vaccines [5, 6].The MAGE-3 gene encodes a melanoma antigenic epitope recognized by specific CTLs [7]. Indeed, tumor regression responses were observed in melanoma patients treated with a peptide encoded by MAGE-3 gene [8]. However, tumor regressions have been observed in only 15% or 28% of patients vaccinated with MAGE-3 peptide or MAGE-3 recombinant protein, respectively [5, 8]. So, there is a need to increase the potency of tumor vaccines based on MAGE-3.
Heat shock proteins (HSPs), a family of highly conserved molecules, are found in all prokaryotes and in most compartments of eukaryotic cells and play essential roles in protein metabolism under both stress and non-stress conditions, including functions in protein folding and membrane translocation and the degradation of misfolded proteins [9]. There is now substantial evidence that HSPs isolated from cancer cells and virus-infected cells can elicit cancer-specific or virus-specific immunity [10, 11]. These observations suggest that HSPs chaperone antigenic peptides into antigen-presenting cells (APCs), potentially allowing peptides to enter the MHC class I pathway for loading onto MHC class I molecules, where they can be presented to cytotoxic CD8+ T cells. Previous studies demonstrated that soluble, adjuvant-free HSP70 fusion proteins could elicit substantial immune responses in mice [12].
Based on these observations, we constructed and purified a recombinant M. tuberculosis HSP70-MAGE-3 fusion protein and found that fusing HSP70 to MAGE-3 enhanced cellular and humoral immune responses to MAGE-3 in vivo, which resulted in potent antitumor immunity against MAGE-3-expressing tumors.
Material and methods
Expression vector constructs
pET28a (+) expression vector was the basic plasmid used for the preparation of expression vector constructs. The complete coding sequence containing M. tuberculosis HSP70 gene was amplified by PCR using the pcDNA3-HSP70 [13] as a template. The upstream primer (5′-GGCGCTA GCATGGCTCGTGCGGTCGGGATC-3′) contained an Nhe I site including the AUG translation initiation codon. The downstream primer (5′-GCGGAGCTCCTTGGCCTCCCGGCCGTC-3′or 5′-GCGGAGCTC TCACTTGGCCTCCCGGCCGTC-3′) contained a Sac I site either immediately after the last coding sequence or immediately after the translation stop codon. The portion of MAGE-3 (aa195-314) coding sequence was amplified by RT-PCR using total RNA of human melanoma LiBr cells as a template. The upstream primer (5′-TTTGAGCTCATCATGCCCAAG GCAGGC-3′) contained a SacI site immediately before amino acid 195 of MAGE-3. The downstream primer (5′-TTTCTCGAGTCACTCTTCCCC CTCTCTC-3′) contained an XhoI site, immediately after the translation stop codon. For the generation of pET-HSP70, the full-length HSP70 was subcloned into Nhe I and SacI sites of pET28a (+). MAGE-3 was subcloned into SacI and Xho I sites of pET28a (+) to generate pET-MAGE-3. For the generation of pET-HSP70-MAGE-3, MAGE-3 was subcloned into Sac I and Xho I sites of pET-HSP70. All vectors were propagated in E. coli DH5α. Recombinant protein expression was conducted in E. coli BL21(DE3)pLysS.
To construct the pcDNA- MAGE-3, MAGE-3 was amplified by PCR. The upstream primer (5′-TTTGCTAGCATGCCTCTTGAGCAGAGGAG-3′) contained an Nhe I site including the AUG translation initiation codon. The downstream primer (5′-CGGCTCGAGTTAGACTCCCTCTTCCTCCT-3′) contained an Xho I site immediately after the translation stop codon. The fragment was subcloned into the Nhe I and Xho I sites of pcDNA3.1(+) vector.
The presence of the inserted fragment was confirmed using restriction enzyme digestion and gel electrophoresis. All constructs was confirmed by DNA sequencing.
Protein purification
Overnight cultures of BL21 (DE3) pLysS were diluted 1/100 in SOB medium containing kanamycin (30 μg/ml) and chloramphenicol (50 μg/ml). The cultures were grown to an OD600 of 0.5 and recombinant protein production was induced at 25°C by addition of isopropyl thiogalactoside (IPTG) (0.5 mM) for 4 h. The cells were harvested and cell pellets were frozen at −20°C. Recombinant protein was purified as soluble protein using Ni-NTA Agarose (Qiagen Inc., USA) according to the manufacturer’s protocol. Protein purity was verified by SDS-PAGE and protein fractions were pooled and dialyzed against 0.1 mM PBS at 4°C for 48 h. Protein concentration was determined by Bradford assay.
Cell lines
The mouse melanoma cells line B16 that had no homologous form of MAGE-3 was transfected with the plasmid pcDNA-MAGE-3 using Lipofectamine 2000 (Invitrogen, USA) and then selected in the presence of 600 μg/ml G418 (Sigma Chem. Co.). The G418-resistant clones were subcloned and then screened for MAGE-3 expression by Western blot using the anti-MAGE-3 antibody (NeoMarkers, USA). The positive B16-MAGE-3 cells were maintained at 37°C in 5% CO2 in DMEM containing 10% fetal bovine serum and 200 μg/ml G418. On the day of tumor challenge, B16 or B16-MAGE-3 cells were harvested and finally resuspended in 1×PBS for injection.
Mice
Female C57BL/6 mice (6–8 weeks old) were obtained from the Laboratory Animal Center of the Fourth Military Medical University (Xi’an, China). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Protein vaccinations
Groups of six mice were intraperitoneally (i.p.) injected with 3.2 μg (200 pmol) MAGE-3, 15.8 μg (200 pmol) HSP70, 18.6 μg (200 pmol) HSP70-MAGE-3, or 1.6 μg (100 pmol) MAGE-3 mixed with 7.9 μg (100 pmol) HSP70 and mice i.p. injected with 100 μl PBS were used as control. A second vaccination was performed 2 weeks later. The splenocytes were harvested and pooled 2 weeks after the boost.
IFN-γ enzyme-linked immunosorbent spot (ELISpot) assays
Mouse IFN-γ ELISpot assay was performed in PVDF-bottomed 96-well plates (Millipore, Bedford, MA, USA) by using a murine IFN-γ ELISpot kit (Diaclone, Besancone, France) according to the manufacturer’s instructions with minor modifications. Briefly, plates were coated overnight at 4°C with anti-IFN-γ capture antibody and washed three times with PBST (PBS+0.05% Tween20). Plates were blocked for 2 h with 2% skimmed dry milk. 1×106 cells/well splenocytes were then added together with the indicated number of lethally irradiated (10,000 cGy) B16 or B16-MAGE-3 cells (5×104/well respectively) and incubated for 24 h at 37°C. Cells were then removed and a biotinylated IFN-γ detection antibody was added for 2 h. Free antibody was washed out, and the plates were incubated with streptavidin-alkaline phosphatase for 1 h at 37°C, followed by extensive washing with PBST, and with PBS. Spots were visualized by the addition of the alkaline phosphatase substrate BCIP/NBT. The number of dots in each well was counted using a dissection microscope. The number of MAGE-3-specific T-cell precursors in splenocytes was calculated by subtracting the IFN-γ+ spots of splenocytes on B16 stimulating cells from that on B16-MAGE-3 cells.
Cytotoxicity assays
The CytoTox 96 non-radioacive cytotoxicity assay (Promega Inc.) was performed to determine the cytotoxic activity of the splenocytes in mice vaccinated with various proteins against B16 and B16-MAGE-3 tumor cells, according to the manufacturer’s protocol with minor modification. Briefly, splenocytes of vaccinated mice were cultured in the presence of human IL-2 (40 U/ml) and irradiated B16-MAGE-3 cells. After 3 days, B16 and B16-MAGE-3 target cells were plated at 1×104 cells/well on 96-well U-bottomed plates (Costar), then the splenocytes (effector cells) were added in a final volume of 100 μl at 1:2.5, 1:5, and 1:10 ratio, respectively. The plates were incubated for 45 min in a humidified chamber at 37°C, 5% CO2, and centrifuged at 500×g for 5 min. 50 μl aliquots were transferred from all wells to a fresh 96-well flat-bottom plates, and an equal volume of reconstituted substrate mix was added to each well. The plates were incubated at room temperature for 30 min and protected from light. Then 50 μl stop solution was added, and the absorbance values were measured at 492 nm. The percentage of cytotoxicity for each effector: target cell ratio was calculated from the equation: [A (Experimental) − A (Effector Spontaneous) − A (Target Spontaneous)]×100/[A (Target maximum) − A (Target spontaneous)]. Percentage of MAGE-3-specific lysis was calculated by subtracting the lysis percentage of splenocytes on B16 from that on B16-MAGE-3 target cells.
Anti-MAGE-3 ELISA
The anti-MAGE-3 antibody in the sera of vaccinated mice was determined by ELISA. Briefly, a 96-well flat-bottom ELISA plate was coated overnight at room temperature with 50 μl of 2.5 μg/ml MAGE-3 protein (aa 195–314). The plate was rinsed with PBS, incubated with blocking buffer (5% nonfat dry milk powder and 0.2% Tween 20 in PBS) for 2 h at 37°C. Mouse serum was 1:50 diluted in blocking buffer, added to the plate, and incubated for 2 h at 37°C. After rinsing with PBS, the plate was incubated with horseradish peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotech Inc. Santa Crus, CA, USA) for 1 h at 37°C. Tetramethyl-benzidine substrate was added, followed by incubation for 20 min at room temperature. The reaction was stopped with 2 M H2SO4. The ELISA plate was read at 450 nm.
In vivo tumor treatment experiments
Mice (six per group) were s.c. challenged with B16-MAGE-3 or B16 tumor cells (2×106 cells/mouse, respectively) in the right legs. 3 days later, mice were i.p. vaccinated with 200 pmol/mouse various proteins. One week later, these mice were boosted with the same regime as the first vaccination. Tumor volumes (length × width2 × π/6) were measured for each individual mouse and were plotted as the mean tumor volume of the group (± standard error, SE) versus days post tumor challenge. Once tumors became palpable, measurements were taken twice a week. The survival time of mice was recorded and Kaplan–Meier curves were generated.
Results
Production and purification of recombinant proteins
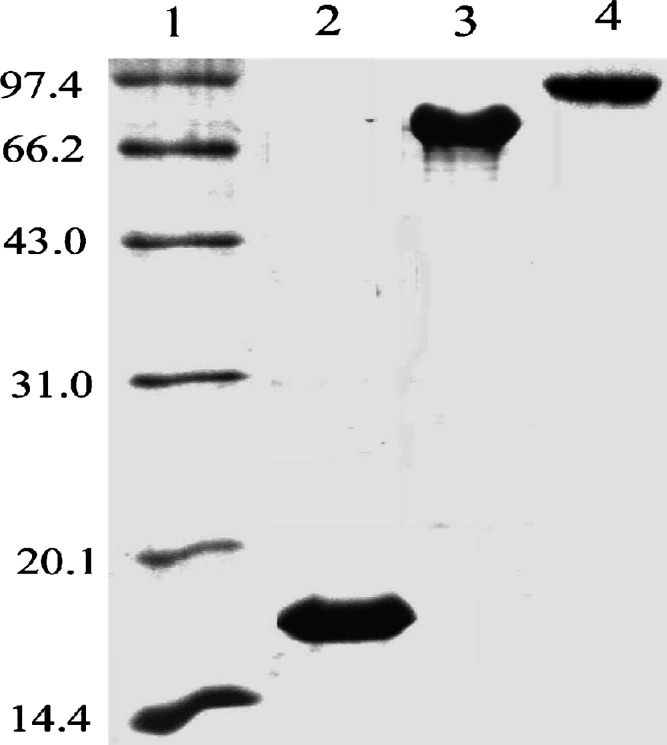
A recombinant system permitting production of M. tuberculosis HSP70 fusion protein in E. coli was developed to link aa 195–314 of MAGE-3 to the C terminus of HSP70. A comparable recombinant MAGE-3 protein (aa 195–314) and recombinant HSP70 protein were also produced. The selected portion of MAGE-3 contains many immunodominant epitope recognized by CTLs. The HSP70-MAGE-3 fusion protein, the MAGE-3 (aa 195–314) protein and HSP70 protein were expressed at high levels in E. coli (Fig. 1.). These proteins were purified as soluble proteins.
Fig. 1.
Production and purification of recombinant proteins. Purified proteins were examined by SDS-PAGE and visualized by Coomassie staining. Lane 1 low molecular weight markers; Lane 2 MAGE-3 protein (aa 195–314); Lane 3 HSP70 protein; Lane 4 HSP70-MAGE-3 protein
Escherichia coli-derived recombinant proteins could be contaminated with endotoxins, which have nonspecific immunostimulatory activities. The level of endotoxins in three purified recombinant proteins was determined using Limulus amebocyte lysate assay. All of the proteins had less than 0.05% endotoxin by weight. So, endotoxin contamination of the recombinant proteins was negligible.
Vaccination of mice with the HSP70-MAGE-3 enhances MAGE-3-specific T-cell-mediated immune responses
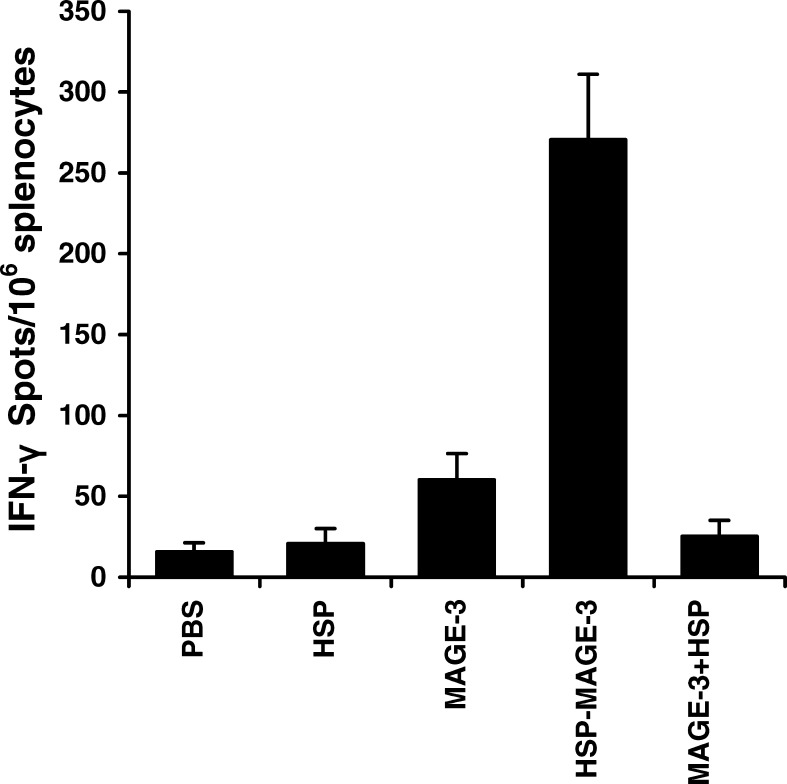
CD8+ CTLs are one of the most crucial components among antitumor effectors [14]. To determine the MAGE-3-specific CD8+ T-cell precursor frequencies generated by HSP-70-MAGE-3, ELISpot was performed. ELISpot assay is a sensitive functional assays used to measure IFN-γ production at the single-cell level, which can thus be applied to quantify antigen-specific CD8+ T cells. As shown in Fig. 2, the number of spot-forming T-cell precursor specific for MAGE-3 in the splenocytes from mice vaccinated with HSP-70-MAGE-3 was about four to five times greater than that from mice with MAGE-3 alone. The MAGE-3-specific IFN-γ producing T cells/106 splenocytes derived from mice vaccinated with MAGE-3 or MAGE-3 mixed with HSP70 were slightly higher than those from mice vaccinated with HSP70 or PBS. The results suggest that the HSP70-MAGE-3 can enhance the generation of IFN-γ-producing MAGE-3-specific T-cell precursors.
Fig. 2.
ELISpot assays of MAGE-3-specific T-cell precursors from the splenocytes of vaccinated mice. C57BL/6 mice were intraperitoneally vaccinated with MAGE-3, HSP70, HSP70-MAGE-3, or a mixture of MAGE-3 with HSP70. The control group received PBS. A second vaccination was performed 2 weeks later. The number of INF-γ producing MAGE-3-specific T-cell precursors was determined by using the ELISpot assay. The spot-forming numbers were the mean of ±SE in each group. Statistical analysis by a paired Student’s t-test revealed that mice vaccinated with HSP70-MAGE-3 generated the higher IFN-γ spot number than MAGE-3 or a mixture of MAGE-3 and HSP70 (p<0.01, n=6)
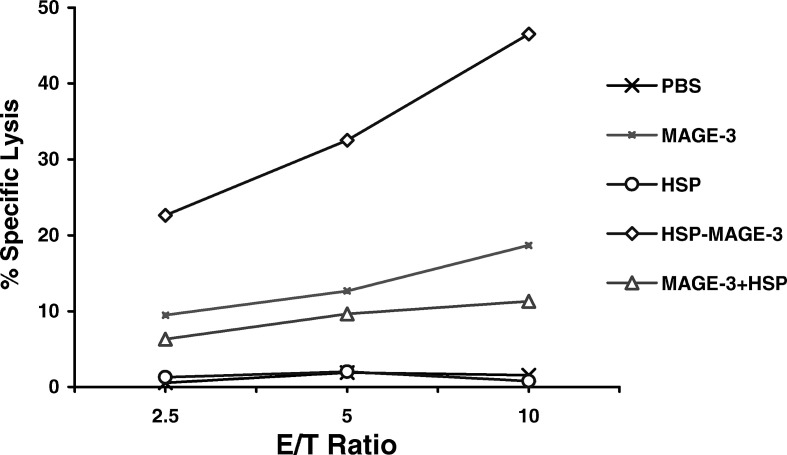
We also performed cytotoxicity assays to determine the MAGE-3- specific lysis of MAGE-3-expressing cells by CTLs induced by vaccination with HSP70-MAGE-3. As shown in Fig. 3, the MAGE-3-specific lysis of CTLs from mice vaccinated with HSP70-MAGE-3 was greater than that from mice vaccinated with MAGE-3, whereas mice vaccinated with MAGE-3 or MAGE-3 mixed with HSP70 showed slightly higher than the HSP70-vaccinated mice or controls (PBS). Using these two methods, we have demonstrated that HSP70-MAGE-3 vaccination led to the enhancement of the MAGE-3-specific T-cell-mediated immune response.
Fig. 3.
MAGE-3-specific lysis against B16-MAGE-3 cells by cytotoxic T lymphocytes (CTLs) induced by vaccination with various proteins. Mice were vaccinated as described in the Fig. 2. The splenocytes of mice were harvested and restimulated with irradiated B16-MAGE-3 cell. The percentage of specific lysis of CTLs on B16-MAGE-3 target cells was determined by a cytotoxicity assays. Percentage of MAGE-3-specific lysis was calculated by subtracting the percentage lysis of CTLs on B16 from that on B16-MAGE-3 target cells. The MAGE-3-specific lysis of CTLs from mice vaccinated with HSP70-MAGE-3 was higher than those vaccinated with MAGE-3, or a mixture of MAGE-3 and HSP70 (p<0.01, n=6)
Vaccination of mice with the HSP70-MAGE-3 enhances MAGE-3-specific humoral immune responses
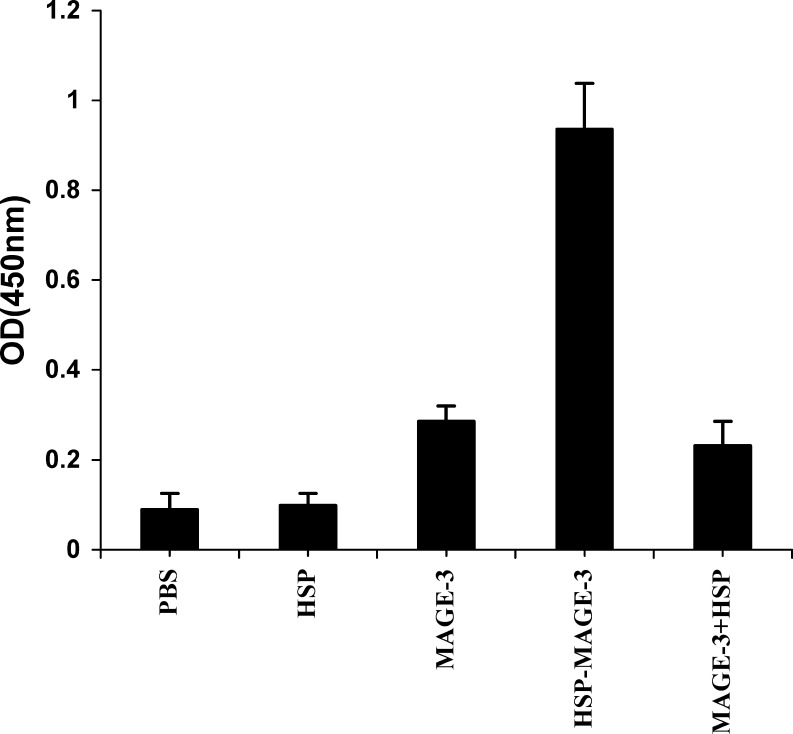
We investigated whether mice would elicit an anti-MAGE-3 antibody response after vaccination with the HSP70-MAGE-3. The quantity of anti-MAGE-3 antibody in the sera of the vaccinated mice was determined by ELISA 2 weeks after the last vaccination. Mice vaccinated with MAGE-3, MAGE-3 mixed with HSP70, HSP70 or PBS showed low or undetectable levels of anti-MAGE-3 antibody. In contrast, mice vaccinated with HSP70-MAGE-3, had significantly high levels of anti-MAGE-3 antibody. The results show that the HSP70-MAGE-3 can elicit the MAGE-3-specific humoral immune responses (Fig. 4).
Fig. 4.
Anti-MAGE-3 antibody titers in C57BL/6 mice vaccinated with various recombinant proteins. C57BL/6 mice were vaccinated as described in Fig. 2. Serum samples were obtained from vaccinated mice 2 weeks after the booster vaccination. The anti-MAGE-3 antibody was examined by ELISA. The results of the 1:50 dilution are presented showing the mean absorbance (A450nm) ± SE. Mice vaccinated with MAGE-3, HSP70 or PBS had low or undetectable levels of anti-MAGE-3 antibody. However, mice vaccinated with HSP70-MAGE-3, had significantly higher titers of anti-MAGE-3 antibody than other groups (p<0.01, n=6)
Vaccination with HSP70-MAGE-3 delays tumor growth and prolongs survival time of mice challenged with B16-MAGE-3 tumor cells
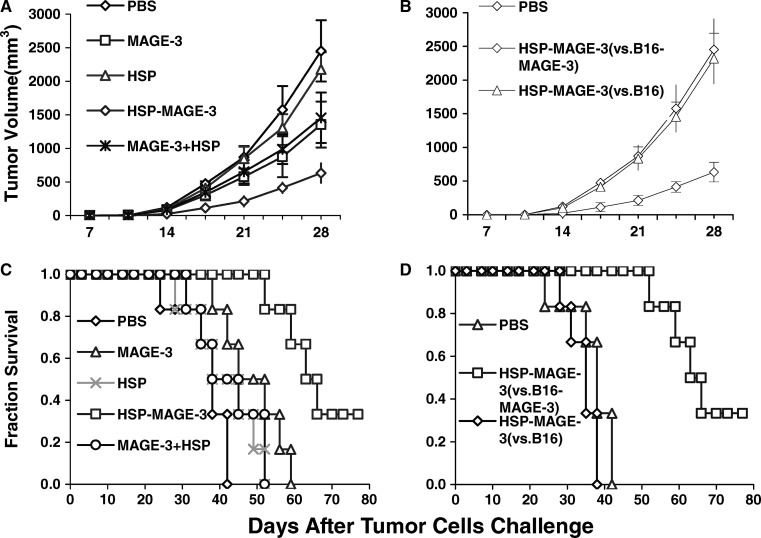
To test the efficacy of protein vaccines in eradicating established B16-MAGE-3 tumors, in vivo tumor treatment experiments were performed. B16-MAGE-3 cells were first s.c. inoculated into C57BL/6 mice at the dose of 1×106 cells/mouse. Three days later, each mouse was i.p. vaccinated with 200 pmol various protein, followed by a boost after 1 week. All mice challenged with tumor cells were monitored for tumor growth, and growth was recorded as the average tumor volume (Fig. 5a). 21 days after the B16-MAGE-3 tumor challenge, the average tumor volume in the control and in HSP70-immunized mice was greater than 800 mm3. The tumor growth was slightly delayed by MAGE-3 or MAGE-3 mixed with HSP70 vaccination. Vaccination with HSP-70-MAGE-3 significantly delayed tumor growth in B16-MAGE-3 tumor model compared to vaccination with PBS and other proteins. The survival of mice was recorded as the percentage of mice surviving after the tumor challenge (Fig. 5c). All the mice in control group died 42 days after tumor challenge. None of MAGE-3 or mixture of MAGE-3 and HSP70-immunized mice had survived 57 days after tumor challenge. In contrast, survival time of mice was significantly prolonged in the mice vaccinated with HSP70-MAGE-3 and 1 out 6 mice survived over 78 days.
Fig. 5.
The immunotherapy of pre-established B16-MAGE-3 melanoma (a, c) or B16 melanoma (b, d) with recombinant proteins. Groups of mice were s.c. inoculated with B16-MAGE-3 or B16 tumor cells (1×106 cells/mouse, respectively). Mice were intraperitoneally vaccinated with various proteins (or PBS) on day 3 and day 10. Tumor growth (a, b) was recorded as the mean tumor volume (in mm3). Error bars depict SE, n=6 mice/group. The survival of mice was recorded as the percentage of mice surviving after tumor challenge. For a, The tumor growth was slightly delayed by MAGE-3 or a mixture of MAGE-3 and HSP70 vaccination. Vaccination with HSP-70-MAGE-3 significantly delayed tumor growth in B16-MAGE-3 tumor model compared to vaccination with other proteins from day 21 (p<0.05, n=6). For b, HSP70-MAGE-3 vaccination delayed tumor growth in B16-MAGE-3 tumor model but not in B16 tumor model. Kaplan-Meier curves (c, d) were generated from survival data (n=6 mice/group). For c, Survival time of mice was significantly prolonged in the mice vaccinated with HSP70-MAGE-3 than other groups (p<0.01). For d, HSP70-MAGE-3 vaccination prolonged survival time of mice in B16-MAGE-3 tumor model but not in B16 tumor model
To test whether or not MAGE-3 is the main contributor to the anti-tumor response, we inoculated mice with either B16 or B16-MAGE-3 tumor cells, and vaccinated these mice with HSP70-MAGE-3. As shown in Fig. 5b, d, HSP70-MAGE-3 vaccination had no effect on delaying the tumor growth and prolonging the survival time in the B16 tumor model. The results demonstrate that the MAGE-3 substantially contributed to the anti-tumor immune response.
These experiments demonstrate that HSP70-MAGE-3 is potent therapeutic vaccines against the MAGE-3-expressing tumor.
Discussion
In this study, the HSP70 fusion vector described here enabled the expression of recombinant HSP70-MAGE-3 fusion protein. Both cellular and humoral immune responses against MAGE-3 could be elicited by vaccination with HSP70-MAGE-3, which resulted in potent therapeutic effects against MAGE-3-expressing tumors. Fusing HSP70 to MAGE-3 enhanced the immunogenicity of the MAGE-3 and obviated the need for an adjuvant.
Tumor vaccination strategies have been developed over the past years [15]. Recently, several genes or gene families encoding tumor-associated antigens have been verified [16]. Cancer/testis antigens, a subgroup of these tumor-associated antigens, are expressed in a variety of malignant neoplasms, and in the testis as the only normal tissue [17]. The MAGE gene family is the first cancer/testis antigen isolated, and 12 different MAGE genes have now been identified, of which MAGE-3 gene shows the typical cancer/testis antigen expression pattern [18]. For these reasons, MAGE-3 can be used as an attractive gene for tumor vaccines. The gene product of MAGE-3 has been verified as a 48-kDa cytoplasmic protein [19]. One clinical study reports that five valuable immune responses were observed among 33 melanoma patients which were MAGE-3-positive upon vaccination with recombinant MAGE-3 protein combined with adjuvant [5]. The need for more effective immunological prophylaxis and therapy for cancer has spurred intensive investigation of immunogens and immunization strategies aiming at eliciting effective CD8+ CTLs responses since the clinical effects will depend on the enhancement of T cell immunity.
Srivastava as well as others [20, 21] have demonstrated that HSP—peptide complexes isolated from tumor cells or mixing the peptides with HSPs can induce specific CTLs responses and tumor protection. However, there are limitations to immunization strategies when using HSPs and bound peptides isolated from cells or tissues of individuals. First, potentially immunogenic peptides may not bind to HSPs, yet still may bind to MHC class I molecules and stimulate T cells. Second, the isolation and purification of HSPs with bound peptides from human tissues and tumors are relatively more complicated. Third, the yield of purified HSP-peptide complexes can be low, and, therefore, substantial amounts or special handling of tissues and tumors may be required. Fourth, HSPs preparations must be made for each patient, and only patients with sufficient tissue or tumor are candidates for this treatment. Furthermore, the tissue or tumor must be accessible to surgical excision [22]. In contrast, recombinant HSP70-MAGE-3 fusion protein described here has several advantages. First, the HSP70 fusion proteins are easy to produce in large amounts, to purify, and to characterize. Secondly, the HSP70-MAGE-3 fusion protein provides a richer source of immunodominant epitopes available for binding to diverse MHC molecules. Third, the HSP70-MAGE-3 fusion protein can be used for all patients with MAGE-3 -positive tumor. Fourth, the strict tumor specificity of MAGE-3 should rule out damage of normal tissues following immunization.
The experiments described here demonstrate that linkage of HSP70 to MAGE-3 enhances MAGE-3-specific immune responses and antitumor effect is consistent with previous reports that linkage of HSPs to HIV-1 p24 [12], HPV16-E7 [23] or ova [24] proteins stimulate antigen-specific immune response. Despite the avid interest in this method of immunization, the underlying mechanisms of priming these responses are yet to be fully elucidated.
Immunization with the HSP70-MAGE-3 protein elicited a strong anti-MAGE-3 response. We suggest that the humoral response against MAGE-3 is enhanced by HSP70 linkage in the following manner. When mice are primed with HSP70-MAGE-3 protein, and then exposed to a second dose, a MAGE-3-specific B cell recognizes, internalizes and processes the fusion protein. The B cell then efficiently presents peptides derived from HSP70 on its surface in the context of an MHC molecule. This MHC/HSP70 peptide complex is bound by HSP70-reactive T cell, leading to the directed release of cytokines by the T cell to the B cell. These soluble factors stimulate the B cell to proliferate, differentiate, and secrete anti-MAGE-3 antibody.
Evidences suggest that HSPs may chaperone antigenic peptides into APCs, potentially allowing peptides to enter the MHC class I processing pathway in APCs and stimulating production of CD8+ CTLs. The mechanisms by which HSP70 enables covalently linked polypeptide fusion partners to enter into the MHC class I antigen-presenting pathway and to elicit CD8+ CTLs have been proposed to be (1) HSP70’s ability to assist protein folding and to facilitate the translocation of proteins into subcellular compartments [25, 26], (2) HSP70’s ability to facilitate the breakdown of intracellular proteins [12], and (3) the high frequency of T cells directed against HSP70. So we suggest that a major mechanism by which HSP70-MAGE-3 protein enhances MAGE-3-specific cellular immune responses may be that HSP70 improves direct MHC class I presentation of MAGE-3 to CTLs.
However, strong induction of CTLs by HSP70-MAGE-3 protein may require mechanisms other than simply increasing efficacy of antigen presentation through delivery of MAGE-3 to the MHC class I pathway. The effective priming of CD8+ CTLs requires activation and maturation of APCs. A recent study reported that HSPs fused to antigens might stimulate dendritic cells (DCs) to upregulate expression of MHC class I and costimulatory molecules [27]. So we suggest that HSP70 can activate APCs to prime CTL responses and augment cellular immune response to MAGE-3. γδ T cells may also contribute to the HSPs-associated antitumor immunity. Wei et al. [28] demonstrated that γδ T cells could kill the heat-treated autologous tumor cells through recognition of HSP70 on the target cells. Whether γδ T cells participate in the antitumor effect generated by HSP70-MAGE-3 vaccination has not been clarified. Further experiments must be performed to address the underlying mechanism.
MAGE-3-specific immune responses were enhanced by HSP70-MAGE-3 fusion protein but not by a mixture of MAGE-3 with HSP70, which indicated that covalent linkage of HSP70 to MAGE-3 was necessary to elicit immune responses. These results were consistent with previous report that immune responses was elicited in mice when HSP70 was crossed-linked but not simply mixed with the ova protein [12, 24]. These results imply that the linked-protein, which already contains numerous T cell epitopes, becomes more immunogenic due to the addition of HSP70 T cell epitopes.
In summary, our results indicate that the HSP70-MAGE-3 fusion protein can elicit stronger MAGE-3-specific immune responses and antitumor effects against MAGE-3 expressing tumor than those elicited by MAGE-3 protein. The recombinant HSP70-MAGE-3 protein vaccine can be useful against a variety of human tumors which are MAGE-3-positive. Whatever be the underlying mechanism, HSP70 fusion proteins are attractive candidates for vaccines exploited to enhance the cellular and humoral immune responses in human.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Sudo T, Kuramoto T, Komiya S, Inoue A, Itoh K. Expression of MAGE genes in osteosarcoma. J Orthop Res. 1997;15:128. doi: 10.1002/jor.1100150119. [DOI] [PubMed] [Google Scholar]
- 3.Hoon DS, Yuzuki D, Hayashida M, Morton DL. Melanoma patients immunized with melanoma cell vaccine induce antibody responses to recombinant MAGE-1 antigen. J Immunol. 1995;1995(154):730. [PubMed] [Google Scholar]
- 4.Zhang Y, Chaux P, Stroobant V, Eggermont AM, Corthals J, Maillere B, Thielemans K, Marchand M, Boon T, van der Bruggen P. A MAGE-3 peptide presented by HLA-DR1 to CD4+ T cells that were isolated from a melanoma patient vaccinated with a MAGE-3 protein. J Immunol. 2003;171:219. doi: 10.4049/jimmunol.171.1.219. [DOI] [PubMed] [Google Scholar]
- 5.Marchand M, Punt CJ, Aamdal S, Escudier B, Kruit WH, Keilholz U, Hakansson L, van Baren N, Humblet Y, Mulders P, Avril MF, Eggermont AM, Scheibenbogen C, Uiters J, Wanders J, Delire M, Boon T, Stoter G. Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer. 2003;39:70. doi: 10.1016/S0959-8049(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 6.Bueler H, Mulligan RC. Induction of antigen specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte macrophagecolony stimulating factor and B71. Mol Med. 1996;2:545. [PMC free article] [PubMed] [Google Scholar]
- 7.Coulie PG, Karanikas V, Colau D, Lurquin C, Landry C, Marchand M, Dorval T, Brichard V, Boon T. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Lienard D, Beauduin M, Dietrich PY, Russo V, Kerger J, Masucci G, Jager E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219. doi: 10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.3.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Smith DF, Whitesell L, Katsanis E. Molecular chaperones: biology and prospects for pharmacological intervention. Pharmacol Rev. 1998;50:493. [PubMed] [Google Scholar]
- 10.Heikema A, Agsteribbe E, Wilschut J, Huckriede A. Generation of heat shock protein-based vaccines by intracellular loading of gp96 with antigenic peptides. Immunol Lett. 1997;57:69. doi: 10.1016/s0165-2478(97)00048-5. [DOI] [PubMed] [Google Scholar]
- 11.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 12.Suzue K, Young RA. Adjuvant-free Mycobacterium tuberculosis hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873. [PubMed] [Google Scholar]
- 13.Ye J, Chen GS, Song HP, Li ZS, Huang YY, Qu P, Sun YJ, Zhang XM, Sui YF. Heat shock protein 70/MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo. Cancer Immunol Immunother. 2004;53:825. doi: 10.1007/s00262-004-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Platsoucas CD, Fincke JE, Pappas J, Jung WJ, Heckel M, Schwarting R, Magira E, Monos D, Freedman RS. Immune responses to human tumors: development of tumor vaccines. Anticancer Res. 2003;23:1969. [PubMed] [Google Scholar]
- 16.Boon T, Old LJ. Cancer Tumor antigens. Curr Opin Immunol. 1997;9:681. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 17.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 18.De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora JP, De Smet C, Brasseur F, van der Bruggen P, Lethe B, Lurquin C. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics. 1994;40:360. doi: 10.1007/BF01246677. [DOI] [PubMed] [Google Scholar]
- 19.Kocher T, Schultz-Thater E, Gudat F, Schaefer C, Casorati G, Juretic A, Willimann T, Harder F, Heberer M, Spagnoli GC. Identification and intracellular location of MAGE-3 gene product. Cancer Res. 1995;55:2236. [PubMed] [Google Scholar]
- 20.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castelli C, Ciupitu Anne-Marie T, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61:222. [PubMed] [Google Scholar]
- 22.Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, Rothman JE, Alan N. Houghton Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA. 2000;97:3485. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papilloma virus (HPV) type 16 E7 expressing tumour by administration of fusion protein comprising Mycobacterium Bovisbacille Calmette Guerin(BCG) HSP65 and HPV16-E7. Clin Exp Immunol. 2000;121:216. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 27.Cho BK, Palliser D, Guillen E, Wisniewski J, Young RA, Chen J, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263. doi: 10.1016/s1074-7613(00)80179-x. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of autologous tumor killing by heat treatment of fresh human tumor cells: involvement of gamma delta T cells and heat shock protein 70. Cancer Res. 1996;56:1104. [PubMed] [Google Scholar]