Abstract
We developed a fusion toxin, DT388IL3, consisting of the catalytic and translocation domains of diphtheria toxin (DT388) linked to interleukin 3 (IL3) for the treatment of patients with acute myeloid leukemia (AML). Our goal in this study was to estimate a range for the maximum tolerated dose (MTD) and to evaluate the dose-limiting toxicity (DLT) of DT388IL3 in cynomolgus monkeys (Macaca fasicularis), which possess cross-reactive IL3 receptors. In our previous study, we administered up to six infusions of DT388IL3 at 40, 60, or 100 μg/kg every other day to three pairs (one male monkey and one female monkey) of young adult monkeys. In five of six monkeys, results showed a dose-dependent increase in malaise and anorexia but no consistent abnormalities in serum chemistries or blood counts. There was no evidence of organ damage by blood tests or histopathology. However, the female treated at 100 μg/kg, died of moderate to severe vasculitis of multiple tissues. Based on these findings, this study repeated the 100 μg/kg group and added a group that received 150 μg/kg in an effort to confirm a dose response. Two female monkeys were treated with up to six infusions of DT388IL3 at 100 μg/kg or 150 μg/kg every other day. One additional female monkey was treated as a negative control. Monkeys in the 100 μg/kg group showed moderate malaise and anorexia, but no consistent abnormalities in blood counts or serum chemistries. Moderate elevations of liver enzymes were noted in the 150 μg/kg group in addition to severe malaise and anorexia. No significant findings were revealed at gross necropsy. The histopathological findings revealed regenerative myeloid hyperplasia and hepatic degeneration and regeneration in the 150 μg/kg group. Similar lesions of less severity were detected in the 100 μg/kg group. DT388IL3 plasma half-life was approximately 20 min with a peak concentration of approximately 2 μg/ml (30,000 pM). The IC50 for AML blasts in vitro was 6 pM. Collectively, our results suggest that DT388IL3 can be tolerated at doses up to 100 μg/kg in a nonhuman primate, which is higher than previously reported for other AML directed diphtheria toxin fusion proteins, and should in principle allow for dose escalation with reduced toxic side effects. Based on these findings a phase I clinical trial has recently been initiated with DT388IL3 for the treatment of AML.
Keywords: Diphtheria toxin, Cynomolgus monkey, Fusion protein, DTIL3, AML
Introduction
Acute myeloid leukemia (AML) is the most common form of leukemia in adults and accounts for approximately 15–20% of childhood leukemia. The average incidence of AML typically ranges between two and six cases per 100,000 individuals in the USA [22]. Despite aggressive treatment, the prognosis remains poor as most patients that attain complete remission from induction combination chemotherapy relapse and die from chemoresistant disease and complications of therapy [23]. Development of selective cytotoxic agents that can circumvent the mechanisms of chemoresistance could greatly improve AML therapy.
One novel class of therapeutics is fusion proteins composed of catalytic toxins covalently linked to AML-selective peptide ligands. The ligand directs the molecule to the surface of the immature myeloid cell and triggers receptor-mediated endocytosis. The toxin then translocates to the cytosol and catalytically inactivates protein synthesis leading to cell death.
Diphtheria toxin (DT) is a 535 amino acid residue protein with three domains [7]. The C-terminal domain (amino acid residues 390–535) has a β-sheet-rich tertiary structure and functions to bind the protein to the heparin-binding epidermal growth factor (EGF)-like cell surface receptor [6]. The middle domain (amino acid residues 201–389) is rich in amphipathic α-helices and facilitates translocation of the catalytic domain to the cytosol [24]. The N-terminal domain (amino acid residues 1–200) is an ADP-ribosylase and catalytically adds ADP to the diphthamide residue of elongation factor 2 (EF2) leading to inactivation of protein synthesis [5]. The C-terminal receptor-binding domain has been replaced with alternative ligands to generate fusion proteins [30].
Our initial attempt to target AML with fusion proteins was made using DT388GMCSF composed of the catalytic and translocation domains of DT (DT388) fused to human granulocyte-macrophage colony-stimulating factor (GM-CSF). In Phase I clinical trials, complete and partial remissions were seen; however, hepatic toxicity was dose limiting [13]. We recently demonstrated that DT388IL3 targeting IL3 receptors was better tolerated in rodent models and lacked hepatic injury [32]. We engineered DT388IL3 composed of DT (DT388) fused to human interleukin-3 (IL3) [12, 14, 18]. IL3 is a cytokine which supports the proliferation and terminal differentiation of multipotential and committed myeloid and lymphoid progenitors, but does not act on the most primitive hematopoietic stem cells [26]. Many human AML blasts express the IL-3 receptor and proliferate in response to IL3 [1]. In vitro studies of DT388IL3 showed potent blast cell kill (greater than 1 log) from 36% of patients under conditions which produced minimal damage to normal hematopoietic stem cells [2, 3, 10, 12, 14]. In vivo studies using NOD/SCID mice inoculated intravenously with human IL-3 receptor positive AML blasts treated with DT388IL3 daily for 5 days yielded a significantly improved median disease-free survival to >120 days (P<0.001) [4, 17]. The maximum tolerated dose (MTD) of DT388IL3 was 0.045 μg/g/day given as intra-peritoneal injections for 5 days [10]. In vivo studies using DT388IL3 did not produce Kupffer cell or liver damage, which had been previously observed with DT388GMCSF [32].
In our previous study [9], we evaluated the toxicology and pharmacokinetics of DT388IL3 in cynomolgus monkeys because they possess cross-reactive receptors to human IL3. We administered up to six infusions of DT388IL3 at 40, 60, or 100 μg/kg every other day to three pairs of young adult cynomolgus macaques (one female and one male per pair). We reported the MTD of DT388IL3 to be 60 μg/kg for six doses. Monkeys treated with 100 μg/kg DT388IL3 exhibited severe malaise and anorexia with the female monkey dying of severe vasculitis of multiple organs. In this study, we attempt to estimate a more accurate range for the MTD and to add data regarding the dose-limiting toxicities.
Methods
Animals
Three young adult female cynomolgus monkeys weighing 2.5–3.5 kg were obtained from Charles River Company, Sierra Biomedical Division, Sparks, NV, USA and two were obtained from Worldwide Primates, Miami, FL, USA and quarantined for 60 days at the Comparative Medicine Clinical Research Center of Wake Forest Health Sciences. All procedures involving nonhuman primates were conducted in compliance with state and federal laws of the US Department of Health and Human Services and guidelines established by the Wake Forest University Institutional Animal Care and Use Committee. Monkeys were housed in single cages. Individual physical exams and baseline laboratory data to include complete blood count, biochemical panel, urinalysis, and clotting profile were recorded for each monkey.
Catherization and tethering system
Three weeks prior to catheter implantation, each monkey was acclimated to a nylon jacket (Alice King Chatham, Inc., Los Angeles, CA, USA) and tether for 3 weeks. After becoming adapted to the jacket tether system, each monkey had a silastic vascular catheter implanted into the femoral vein using sterile techniques. Surgery was performed under anesthesia using ketamine hydrochloride (Fort Dodge, Ft. Dodge, IA, USA), diazepam (Abbott Laboratories, North Chicago, IL, USA), and buprenorphine (Reckitt & Colman, Richmond, VA, USA) (15.0, 1.0, and 0.01 mg/kg, respectively). The catheter was inserted into the femoral vein and advanced into the caudal vena cava. The free end of the catheter was tunneled, subcutaneously, to exit the back between the scapulae and threaded through a flexible metal tether and attached to the swivel apparatus (Alice King Chatham) on the back of the cage. Normal saline with 1 U heparin/ml was infused at a constant rate of 3 ml/h through a three-way stopcock to maintain catheter patency.
Toxicology study
Two pairs of female monkeys were administered intravenous injections of 100 μg/kg or 150 μg/kg of DT388IL3 every other day for a total of up to six doses. A fifth female monkey served as a negative control. The every other day regimen allows for observations of toxicities between doses and matches the planned therapeutic regimen in human patients. DT388IL3 was diluted in 250 mM NaCl and 10 mM Tris HCL to a final volume of 1 ml for injection. All monkeys received prophylactic administration of vancomycin hydrochloride, 10 mg/kg, (Abbott Labs) and ceftazidime, 30 mg/kg, (GlaxoSmithKline, England) intravenously throughout the length of the study. Infusion rate of heparinized saline was increased up to 6 ml/h as needed to maintain hydration. Animals were monitored daily for signs of clinical toxicity to include depression, lethargy, anorexia, diarrhea, vomiting, and pain. A toxicity grading system was adapted for monkeys as previously described [27]. Animals were treated with 3 mg/kg of ketoprofen, (Fort Dodge) or 0.1 mg/kg of buprenorphine, as needed for analgesia. Complete blood chemistries were performed daily and serum chemistries performed at least every 3 days. Chemistries included total protein, albumin, alanine transferase (ALT), alkaline phosphatase (ALKP), aspartate aminotransferase (AST), bilirubin, lactate dehydrogenase, blood urea nitrogen (BUN), creatinine, electrolytes, cholesterol, and triglycerides.
Pathology
Postmortem examinations were performed on all monkeys. Monkeys in the 100 μg/kg group (#7228, #7229) were euthanized on day 14. Monkeys in the 150 μg/kg were euthanized on day 6 (#7225) and day 16 (#7226). Samples from the adrenal glands, bone marrow, brain, catheter implantation site, cecum, cervix, colon, duodenum, eyes, heart, ileum, jejunum, kidney, liver, lungs, lymph nodes, mammary glands, nerve (sciatic), ovary, pituitary gland, prostate, skeletal muscle, skin, spleen, stomach, testis, thymus, thyroid gland, vagina, urinary bladder, and uterus were removed. The tissues were fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined by a board certified veterinary pathologist (JMC).
Pharmacokinetics
On day 1 of DT388IL3 infusion, 0.3 ml blood samples were collected via intravenous catheter at 0, 5, 30, 60, 90, 120, and 240 min. Samples were collected from the same catheter through which the experimental drug was administered. Serum samples were stored at −80°C until assayed. The concentration of DT388IL3 in the blood was measured using a sensitive biological assay as reported previously [16]. Briefly, proliferation of TF1HRas leukemic cells in response to serial dilutions of serum was measured by tritiated thymidine incorporation. The serum concentration of the drug was derived from a standard curve using known concentrations of DT388IL3. This assay yields reproducible values with a range of 30% with a limit of detection at 0.1 ng/ml. All assays were performed in triplicate. A nonlinear regression algorithim with GraphPad Prism was used to determine the t1/2.
Detection of monkey IgG response to DT388IL3
Pre-DT388IL3-treatment and post-DT388IL3-treatment blood samples (1.0 ml) were collected from the 150 μg/kg group and the serum separated and stored at −80°C until assayed. Serum anti-DT levels were detected by ELISA as previously described [16]. All samples were run in duplicate, and the mean values were calculated for analysis.
Results
Monkeys in the 100 μg/kg (#7228 and #7229) group received DT388IL3 via intravenous injection every other day for six doses. In the 150 μg/kg dose group, monkey #7225 received DT388IL3 via intravenous injection every other day for three doses (days 1, 3, and 5) and #7226 received five doses (days 1, 3, 5, 8 and 11).
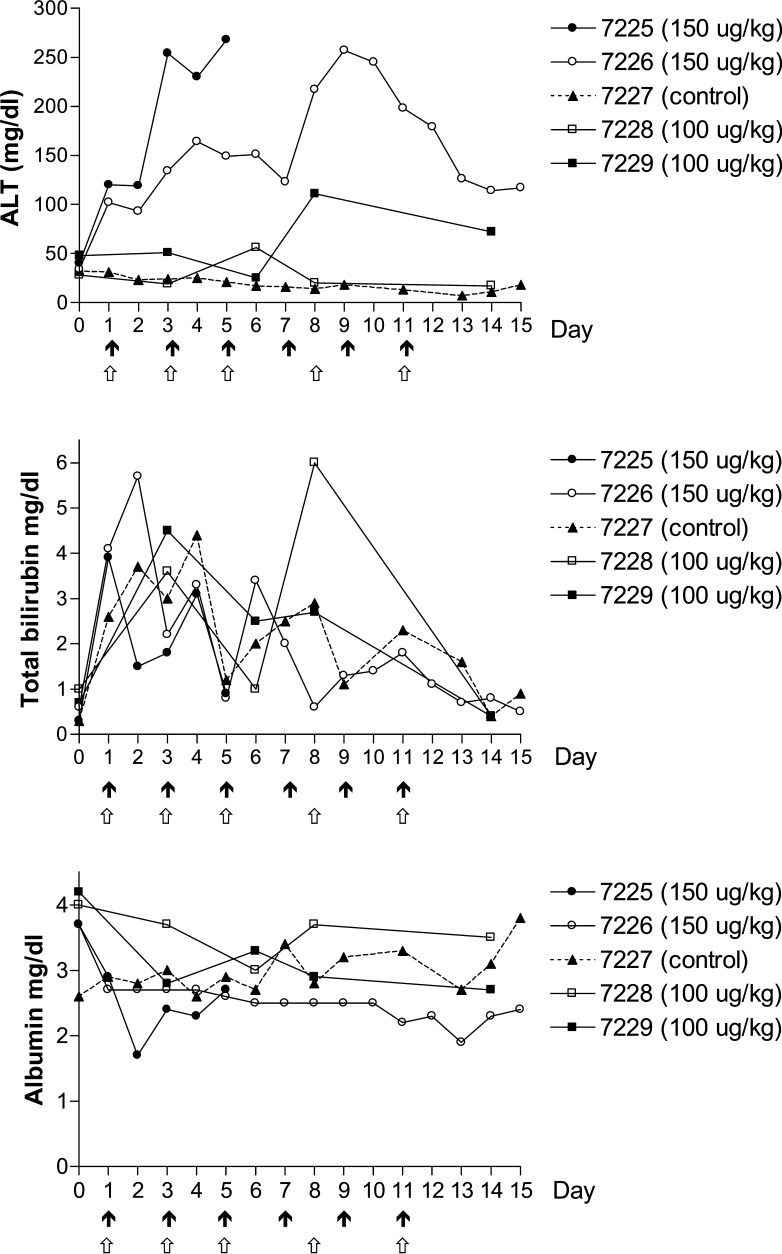
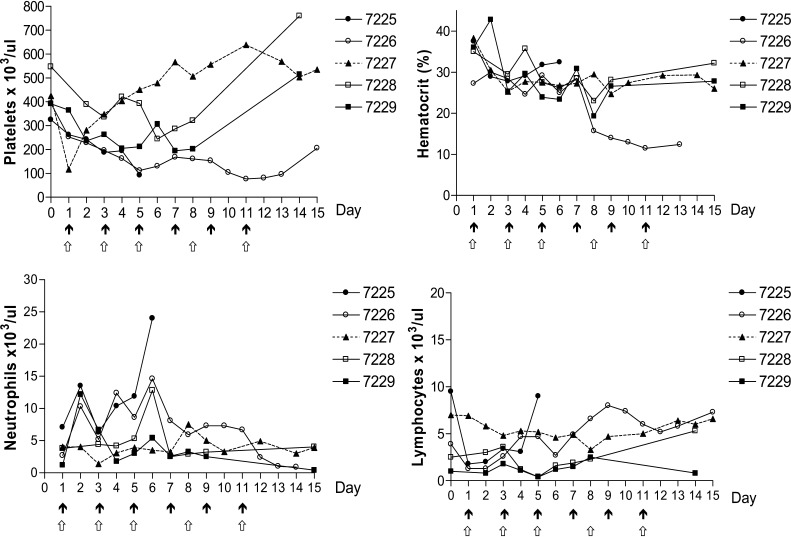
Clinical observations and toxic changes for each monkey are summarized in Table 1. Monkeys in the 100 μg/kg group experienced little to no malaise or anorexia. Serum chemistry results revealed a transient elevation in bilirubin values in both monkeys and a mild elevation in ALT in monkey #7229 (Fig. 2). There was no serum biochemical or clinical evidence of renal disease. Hematological results revealed no significant change in hematocrit, neutrophil or lymphocyte counts from baseline (Fig. 1).
Table 1.
Side effects in cynomolgus monkeys treated with DT388IL3 intravenously for up to six every other day doses
| Dose (μg/kg every other day IV×6) | |||||
|---|---|---|---|---|---|
| 100 | 100 | 150 | 150 | Control | |
| Toxicity | 7228 | 7229 | 7225a | 7226b | 7227 |
| Hematology | |||||
| Leukopenia | 0 | 0 | 0 | 0 | 0 |
| Leukocytosis | 1 | 0 | 3 | 2 | 1 |
| Thrombocytopenia | 0 | 0 | 1 | 2 | 0 |
| Anemia | 2 | 3 | 2 | 3 | 2 |
| Gastrointestinal/appetite | |||||
| Anorexia | 1 | 1 | 3 | 3 | 0 |
| Weight loss | 0 | 0 | 1 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 | 1 | 0 |
| Liver | |||||
| Alanine transferase elevation | 0 | 1 | 2 | 2 | 0 |
| Hypoalbuminemia | 2 | 2 | 3 | 2 | 0 |
| Renal/metabolic | |||||
| Elevated blood urea nitrogen | 0 | 0 | 0 | 0 | 0 |
| Elevated creatinine | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 1 | 1 | 0 |
| Overall health | |||||
| Activity level | 0 | 0 | 3 | 2 | 0 |
| Discomfort/malaise | 1 | 1 | 3 | 3 | 0 |
Uckun toxicity grading system with grades 0–3 (0 within normal limits, 1 mild, 2 moderate, 3 severe)
aThrough three doses
bThrough five doses
Fig. 2.
Serum biochemical results of monkeys treated with up to six intravenous infusions of DT388IL3. Black arrows denote dosing schedule for the 100 μg/kg group; white arrows denote schedule for the 150 μg/kg group
Fig. 1.
Hematological results of monkeys treated with up to six intravenous infusions of DT388IL3. Black arrows denote dosing schedule for the 100 μg/kg group; white arrows denote dosing schedule for the150 μg/kg group
In contrast, both monkeys in the 150 μg/kg group (#7225 and #7226) presented with moderate to severe malaise and anorexia. Moderate elevations in ALT were observed in addition to mild to moderate hypoalbuminemia and transient hyperbilirubinemia in both monkeys (Fig. 2). However, there was no serum biochemical or clinical evidence of renal disease. Moderate to marked leukocytosis was observed in both female monkeys (Fig. 1). On day 6 of treatment, after receiving three DT388IL3 infusions, Monkey #7225 presented moribund and was euthanized. A marked neutrophilia (40.7×103/ul) with a left shift (16% bands), an elevated ALT value of 439 u/l, and a hypoalbuminemia of 2.7 g/dl was present at the time she was euthanized. Monkey #7226 had a similar, but less severe response and treatment intervals were extended to allow for recovery. Platelet counts in both dose groups and the negative control showed variability with the 150 μg/kg dose group showing mild to moderate thrombocytopenia (Fig. 2). Inadvertent blood loss due to catheter complications on days 4, 9, and 10 (monkey #7226) and days 2 and 9 (control monkey #7227) resulted in low hematocrit values (Fig. 1).
Necropsies were performed on all monkeys in both dose groups including the negative control. Monkeys #7228 and #7229 (100 μg/kg group) were euthanized 3 days after the sixth drug infusion on day 14. Monkey #7225 (150 μg/kg group) was euthanized 1 day after the third drug infusion on day 6 and #7726 (150 μg/kg group) 5 days after the fifth infusion on day 16. The control monkey (#7227) was euthanized on day 16. Grossly, no significant findings were observed in either dose group. Histopathological results for each monkey are shown in Table 2. Myeloid hyperplasia of mature cells was observed in treated monkeys except for #7225 of the 150 μg/kg group. In this animal, there was bone marrow necrosis with fibrin deposition and myeloid hyperplasia of immature cells. Evidence of vascular wall necrosis of venules of the lymph node was also observed. Additionally, within the kidney, there were mild, multifocal intraglomerular fibrin thrombi and tubular necrosis with regeneration. Evidence of renal pathology was not found in any other monkeys. Mild, diffuse lymphoid depletion of the spleen was noted in both 150 μg/kg treated monkeys. Extramedullary hematopoiesis was observed in the liver of the 100 μg/kg monkeys and in the adrenal glands of the 150 μg/kg monkeys.
Table 2.
Histopathological findings of cynomolgus monkeys treated with DT388IL3 intravenously for up to six every other day doses
| Histopathological findings | 100 ug/kg dose group | 150 ug/kg dose group | |||
|---|---|---|---|---|---|
| #7228 | #7229 | #7225 | #7226 | #7227 | |
| Hepatocellular swelling | 1 | 1 | 1 | 1 | |
| Extramedullary hematopoeisis | 1 | 1 | 1 | 2 | |
| Myeloid hyperplasia | 1 | 1 | 2 | 3 | |
| Lymphoplasmacytic gastritis or colitis | 1 | 1 | 1 | ||
| Lymphoid depletion, spleen | 1 | 1 | |||
| Lymphoid follicular hyperplasia | 2 | 2 | |||
1 mild, 2 moderate, 3 marked, 4 severe
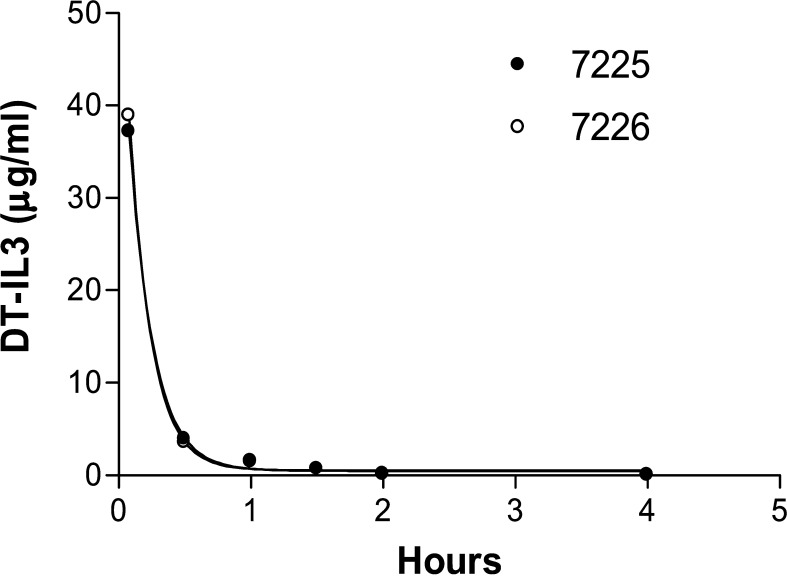
The calculated half-life for DT388IL3 was 20 min based on the results from the 150 μg/kg group (Fig. 3). DT388IL3 concentration at 30 min post-intravenous infusion was 3.9 μg/ml. Antibody responses were minimal in the 150 μg/kg group evaluated at 6 (#7225) or 16 (#7726) days post-treatment with anti-DT388IL3 levels <1 μg/ml, see Table 3.
Fig. 3.
Serum levels of DT388IL3 for monkeys in the 150 μg/kg group. The half-life was determined to be approximately 20 min
Table 3.
Antibody titers in cynomolgus monkeys treated with 150 μg/kg DT388IL3 intravenously for up to five every other day doses
| Monkey | Sample days | Anti-DT388IL3 IgG (μg/ml) |
|---|---|---|
| 7225 | Day 0 | <0.02 |
| Day 6 | <0.02 | |
| 7226 | Day 0 | <0.02 |
| Day 16 | 0.5 | |
| 7227a | Day 0 | <0.02 |
| Day 16 | <0.02 |
aControl monkey, not treated
Discussion
In this study, we attempted to improve our knowledge regarding the safety and toxicity of the DT388IL3 in nonhuman primates. Combining this study with our previous work, we observed tolerance of 100 μg/kg of DT388IL3 in three of four animals. These data, while not statistically significant based on the small sample size, broaden the range of tolerated doses to include 100 μg/kg DT388IL3. Previously tested fusion proteins in nonhuman primates have shown MTD’s of 7.5 μg/kg/day for 5 days for DT388GMCSF, 20 μg/kg/day for ten days for DAB389EGF, 10 μg/kg/day for 14 days for DAB389IL2, and 100 μg/kg for two doses for FN18-CRM9 [9]. Thus, only the anti T cell fusion protein has a similar nonhuman primate tolerance. In comparison, the MTD in humans for these fusion proteins is 4 μg/kg/day for DT388GMCSF, 16 μg/kg/day for DAB389EGF, and 27 μg/kg/day for DAB389IL2 [20].
The dose-limiting toxicity (DLT) was vascular injury characterized by vascular leak syndrome (VLS) and fibrin deposition within blood vessels. This toxicity was dose related. In animals with mild to moderate vascular injury the removal of the drug resulted in reversal of the toxicity. Although recombinant human IL3 reacts with the macaque IL3 receptor and produces myeloid stimulation in vivo, the relative binding affinity of human IL3 for the monkey receptor had been reported to be 25-fold to 50-fold lower than for the homologous human receptor [28, 29]. Therefore, dose-limiting toxicities may not exactly parallel those seen in human patients.
Vascular leak syndrome is the dose-limiting side effect of many recombinant fusion toxin therapies including diphtheria, ricin and pseudomonas exotoxin fusion proteins [11, 25]. Vascular leak syndrome is characterized by an increase in vascular permeability accompanied by extravasation of fluids and proteins resulting in hypoalbuminemia, edema, weight gain, malaise, anorexia, fatigue, and dyspnea. Both female monkeys treated at 150 μg/kg DT388IL3 experienced clinical signs associated with VLS including moderate to severe hypoalbuminemia, malaise and anorexia similar to patients in phase II clinical trials of diphtheria fusion protein DAB389IL2 [11]. Symptoms of VLS were temporarily alleviated by withdrawal of the fusion protein in our study. Additionally, both monkeys were maintained on constant rate infusion of intravenous saline to maintain hydration, which may have minimized hypotension. There is no known method to prevent VLS, however, the side effects are generally self-limiting. Attempts to reduce VLS have included modification of the immunotoxin molecule to increase specificity and thereby minimizing damage to vascular endothelial cells [25]. Construction of a small molecule inhibitor, endothelial cell (EC) myosin light-chain kinase (MLCK), which has been shown to protect the endothelium from injury from disease related stress, may also prove valuable in preventing VLS [31].
Administration of DT388IL3 induced a mild to moderate rise in hepatic transaminases that subsided with cessation of the drug. These elevations correlate with the hepatocellular swelling and degeneration of hepatocytes observed histologically. Severity of the lesions was related to dose. This mild hepatotoxicity was transient and reversible as evidenced by decreasing ALT values and regenerative changes seen in hepatocytes histologically. Other fusion toxins have been shown to induce reversible liver damage including DAB389IL2, ricin toxin A chain, and pseudomonas exotoxin. This is in comparison to diphtheria fusion toxins DAB389EGF and DT388GMCSF, which cause irreversible damage to hepatocytes and Kupffer cells, respectively [8; Marlena Moors Westcott, unpublished observations].
Myeloid hyperplasia of mature cells within the bone marrow and extramedullary hematopoiesis were observed in monkeys of both dose groups. This hyperplastic response is likely due to stimulation of IL3 receptors on multipotential and committed myeloid progenitors [26]. In contrast, monkey #7225 of the 150 μg/kg group had a myeloid hyperplasia of immature cells and necrosis within the bone marrow. Additionally, a circulating neutrophilia was also present in this animal. One explanation for this difference may be that IL3 receptors were present in abundance and located on early and late progenitor cells [21, 26]. Alternatively, DT388IL3 administration at such a high dose may have induced cytokine release resulting in a severe inflammatory response. Although bacterial sepsis could also yield similar findings, no evidence of infection was found. The variability in platelet counts observed between dose groups and the negative control could be due to a variety of factors including inflammation, inadvertent blood loss in some animals, or the presence of an indwelling silastic catheter.
The half-life of 20 min is similar to those of other DT fusion toxins. There were no pretreatment serum antibodies to DT388IL3 and post-treatment antibody formation was low. Minimal exposure of up to 16 days to DT388IL3 may explain the low antibody response. This response is similar to that seen in cynomolgus monkeys treated with DT388GMCSF and may reflect the low-dose administration of DT monomer in both studies [16]. In contrast, human patients that have been immunized with diphtheria toxoid during childhood may have sufficient titers to minimize efficacy of administered DT388IL3 and should receive pretreatment screening [15].
In conclusion, this preclinical safety study suggests that an initial dose of DT388IL3 may be identified that can be safely administered. In the phase I clinical trial of DT388IL3, three patients received 4 μg/kg/day for six doses with minimal toxicity [19]. The results of this study should facilitate the design and testing of DT388IL3 in current phase I clinical trials for the treatment of refractory AML.
Acknowledgements
This work was supported by the National Institutes of Health (R01CS090263 (AF) R01CA76178, R21CA90550 (AF), and T32RR007009 (KC).
References
- 1.Ailles LE, Gerhard B, Hogge DE. Detection and characterization of primitive malignant and normal progenitors in patients with acute myelogenous leukemia using long-term coculture with supportive feeder layers and cytokines. Blood. 1997;90:2555–2564. [PubMed] [Google Scholar]
- 2.Alexander RL, Kucera GL, Klein B, Frankel AE. In vitro interleukin-3 binding to leukemia cells predicts cytotoxicity of a diphtheria toxin/IL-3 fusion protein. Bioconjug Chem. 2000;11:564–568. doi: 10.1021/bc000009q. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RL, Ramage J, Kucera GL, Caligiuri MA, Frankel AE. High affinity interleukin-3 receptor expression on blasts from patients with acute myelogenous leukemia correlates with cytotoxicity of a diphtheria toxin/IL-3 fusion protein. Leuk Res. 2001;25:875–881. doi: 10.1016/s0145-2126(01)00034-0. [DOI] [PubMed] [Google Scholar]
- 4.Black JH, McCubrey JA, Willingham MC, Ramage J, Hogge DE, Frankel AE. Diphtheria toxin-interleukin-3 fusion protein (DT(388)IL3) prolongs disease-free survival of leukemic immunocompromised mice. Leukemia. 2003;17:155–159. doi: 10.1038/sj.leu.2402744. [DOI] [PubMed] [Google Scholar]
- 5.Bodley JW, Dunlop PC, VanNess BG. Diphthamide in elongation factor 2: ADP-ribosylation, purification, and properties. Methods Enzymol. 1984;106:378–387. doi: 10.1016/0076-6879(84)06040-7. [DOI] [PubMed] [Google Scholar]
- 6.Brooke JS, Cha JH. Molecular characterization of key diphtheria toxin:receptor interactions. Biochem Biophys Res Commun. 2000;275:374–381. doi: 10.1006/bbrc.2000.3317. [DOI] [PubMed] [Google Scholar]
- 7.Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992;357:216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- 8.Cohen KA, Liu T, Bissonette R, Puri RK, Frankel AE. DAB389EGF fusion protein therapy of refractory glioblastoma multiforme. Curr Pharm Biotechnol. 2003;4:39–49. doi: 10.2174/1389201033378039. [DOI] [PubMed] [Google Scholar]
- 9.Cohen KA, Liu Cline JM, Wagner JD, Hall PD, Frankel AE. Toxicology and pharmacokinetics of DT388IL3, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human interleukin 3 (IL3), in cynomolgus monkeys. Leuk Lymphoma. 2004;45:1647–1656. doi: 10.1080/10428190410001663572. [DOI] [PubMed] [Google Scholar]
- 10.Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62:1730–1736. [PubMed] [Google Scholar]
- 11.Frankel AE, Fleming DR, Hall PD, Powell BL, Black JH, Leftwich C, Gartenhaus R. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin Cancer Res. 2003;9:3555–3561. [PubMed] [Google Scholar]
- 12.Frankel AE, McCubrey JA, Miller MS, Delatte S, Ramage J, Kiser M, Kucera GL, Alexander RL, Beran M, Tagge EP, Kreitman RJ, Hogge DE. Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia. 2000;14:576–585. doi: 10.1038/sj.leu.2401743. [DOI] [PubMed] [Google Scholar]
- 13.Frankel AE, Powell BL, Hall PD, Case LD, Kreitman RJ. Phase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemia. Clin Cancer Res. 2002;8:1004–1013. [PubMed] [Google Scholar]
- 14.Frankel AE, Ramage J, Kiser M, Alexander R, Kucera G, Miller MS. Characterization of diphtheria fusion proteins targeted to the human interleukin-3 receptor. Protein Eng. 2000;13:575–581. doi: 10.1093/protein/13.8.575. [DOI] [PubMed] [Google Scholar]
- 15.Hall PD, Virella G, Willoughby T, Atchley DH, Kreitman RJ, Frankel AE. Antibody response to DT-GM, a novel fusion toxin consisting of a truncated diphtheria toxin (DT) linked to human granulocyte-macrophage colony stimulating factor (GM), during a phase I trial of patients with relapsed or refractory acute myeloid leukemia. Clin Immunol. 2001;100:191–197. doi: 10.1006/clim.2001.5066. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss CE, Hall PD, Cline JM, Willingham MC, Kreitman RJ, Gardin J, Latimer A, Ramage J, Feely T, Delatte S, Tagge EP, Frankel AE. Toxicology and pharmacokinetics of DTGM, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human granulocyte-macrophage colony-stimulating factor, in cynomolgus monkeys. Toxicol Appl Pharmacol. 1999;158:152–160. doi: 10.1006/taap.1999.8691. [DOI] [PubMed] [Google Scholar]
- 17.Kiser M, McCubrey JA, Steelman LS, Shelton JG, Ramage J, Alexander RL, Kucera GL, Pettenati M, Willingham MC, Miller MS, Frankel AE. Oncogene-dependent engraftment of human myeloid leukemia cells in immunosuppressed mice. Leukemia. 2001;15:814–818. doi: 10.1038/sj.leu.2402084. [DOI] [PubMed] [Google Scholar]
- 18.Liu TF, Urieto JO, Moore JE, Miller MS, Lowe AC, Thorburn A, Frankel AE. Diphtheria toxin fused to variant interleukin-3 provides enhanced binding to the interleukin-3 receptor and more potent leukemia cell cytotoxicity. Exp Hematol. 2004;32:277–281. doi: 10.1016/j.exphem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Misra D, Frankel AE, Hall PD, Liu TF, Black J, Moore JO (2004) The use of DT388IL3 fusion protein in patients with refractory acute myeloid leukemia (AML). Blood (in press)
- 20.Molnar I, Grover M, Frankel AE (2004) Fusion toxins in the treatment of cancer. Semin Oncol (in press
- 21.Nitsche A, Junghahn I, Thulke S. Interleukin-3 promotes proliferation and differentiation of human hematopoietic stem cells but reduces their repopulation in NOD/SCID mice. Stem Cells. 2003;21:236–244. doi: 10.1634/stemcells.21-2-236. [DOI] [PubMed] [Google Scholar]
- 22.Redaelli A, Botteman MF, Stephens JM, Brandt S, Pashos CL. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat Rev. 2004;30:237–247. doi: 10.1016/j.ctrv.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer CA. Acute myeloid leukemia in adults: where do we go from here. Cancer Chemother Pharmacol. 2001;48(Suppl 1):S45–S52. doi: 10.1007/s002800100304. [DOI] [PubMed] [Google Scholar]
- 24.Silverman JA, Mindell JA, Finkelstein A, Shen WH, Collier RJ. Mutational analysis of the helical hairpin region of diphtheria toxin transmembrane domain. J Biol Chem. 1994;269:22524–22532. [PubMed] [Google Scholar]
- 25.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, Vitetta ES. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 26.Suda T, Suda J, Ogawa M, Ihle JN. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985;124:182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- 27.Uckun FM, Yanishevski Y, Tumer N, Waurzyniak B, Messinger Y, Chelstrom LM, Lisowski EA, Ek O, Zeren T, Wendorf H, Langlie MC, Irvin JD, Myers DE, Fuller GB, Evans W, Gunther R. Pharmacokinetic features, immunogenicity, and toxicity of B43(anti-CD19)-pokeweed antiviral protein immunotoxin in cynomolgus monkeys. Clin Cancer Res. 1997;3:325–337. [PubMed] [Google Scholar]
- 28.van Gils FC, Budel LM, Burger H, van Leen RW, Lowenberg B, Wagemaker G. Interleukin-3 (IL-3) receptors on rhesus monkey bone marrow cells: species specificity of human IL-3, binding characteristics, and lack of competition with GM-CSF. Exp Hematol. 1994;22:248–255. [PubMed] [Google Scholar]
- 29.van Gils FC, Mulder AH, van den BC, Burger H, van Leen RW, Wagemaker G. Acute side effects of homologous interleukin-3 in rhesus monkeys. Am J Pathol. 1993;143:1621–1633. [PMC free article] [PubMed] [Google Scholar]
- 30.vanderSpek JC, Murphy JR. Fusion protein toxins based on diphtheria toxin: selective targeting of growth factor receptors of eukaryotic cells. Methods Enzymol. 2000;327:239–249. doi: 10.1016/s0076-6879(00)27280-7. [DOI] [PubMed] [Google Scholar]
- 31.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westcott MM, Abi-Habib RJ, Cohen KA, Willingham MC, Liu S, Bugge TH, Leppla SH, Frankel AE (2004) Diphtheria toxin-murine GMCSF-induced hepatoxicity is mediated by Kupffer cells. Mol Cancer Ther (in press) [PubMed]