Abstract
Purpose: A human monoclonal antibody (L612 HuMAb) that binds to ganglioside GM3 has been developed in our laboratory. L612 HuMAb is a 100% human IgM protein. L612 HuMAb binds to cell surface of melanoma and can kill the cells in the presence of complement. The primary objective of this study was to test the toxicity and pharmacokinetics associated with administration of L612 HuMAb to melanoma patients whose tumor cells expressed GM3. Experimental design: Nine patients with measurable metastatic melanoma (American Joint Committee on Cancer stage IV) were entered in the study. Eight had failed previous treatments that included chemotherapy, radiation therapy, melanoma cell vaccine, and/or biological therapy. All patients received a 48-h continuous infusion of L612 HuMAb at a dose of 960 mg, 1,440 mg, or 1,920 mg. Five of these patients received a second infusion and one patient received a third infusion, all with the previous dose. Results: Toxicity was limited to transient and mild pruritus and skin rash. One patient complained of pain at the site of subcutaneous metastases. Serum antibody levels peaked 24 to 48 h after starting the infusion. Two patients, one receiving a single course of 960 mg (612 mg/m2) and the second receiving two courses of 1,440 mg (911 mg/m2) followed by surgical therapy, are without evidence of disease >5 years after antibody infusion. Conclusions: The human IgM monoclonal antibody, L612 HuMAb, was well tolerated. Infusion of L612 HuMAb appears to produce significant antitumor activity in melanoma patients.
Keywords: Clinical trial, Cancer, Treatment, Monoclonal antibody, Ganglioside
Introduction
Because the toxicity of mouse monoclonal antibodies (MAbs) prevents optimal therapeutic doses, investigators are looking at human, humanized, or chimeric MAbs. Chimeric MAbs are created by grafting variable (V) domains of mouse MAb to the human IgG constant (C) domain; humanized MAbs are created by grafting V-complementary determining regions of mouse MAbs to human IgG C domains plus human V-framework regions. These humanized or chimeric MAbs have undergone several phases of clinical trials, most of which have tested larger antibody doses [1, 2, 3, 12, 13, 17, 19, 22]. These MAbs have demonstrated less toxicity and greater clinical efficacy than mouse MAb. Two of these MAbs, the chimeric IgG anti-CD20 MAb (rituximab) and the humanized IgG anti-HER2/neu MAb (Herceptin), have been approved and used worldwide for the treatment of CD20-positive non-Hodgkin’s lymphoma and breast cancer, respectively. More recently, the United States Food and Drug Administration (FDA) Oncologic Drugs Advisory Committee has recommended marketing the third antibody, I-131 labeled anti-CD20 antibody (Bexxar) [10], for the treatment of non-Hodgkin’s B cell lymphoma. Bexxar awaits full FDA approval. Humanized and chimeric MAbs that are under clinical trials or approved for marketing are of the IgG subclasses.
As one of the first groups to investigate human monoclonal antibodies, we focused on the IgM isotype [5, 7]. We hypothesize that IgM MAb might be more effective for killing cancer cells in vivo. This hypothesis was derived from (1) the correlation between prolonged survival and the high serum levels of IgM but not IgG antitumor antibodies in patients with metastatic melanoma [9, 21], and (2) the complete or partial regression of skin metastases of melanoma following intratumoral injection of human IgM antibodies [6, 8]. The L612 HuMAb tested in the present study is a 100% human IgM protein developed by transformation of human B cells using the Epstein-Barr virus (EBV) [26]. The antigen epitope recognized by L612 HuMAb is a terminal sialic acid-galactose residue of gangliosides such as GM3 and GM4 [4]. L612 HuMAb binds to the surface of various human cancer cells [4, 18] and can kill antigen-positive cancer cells in the presence of human complement in vitro [16, 21]. IgM anti-GM3 autoantibodies are found in melanoma patients without immunization, or induced by immunization with irradiated melanoma cells with no evidence of bystander autoimmune disease [21].
This report presents the results of intravenous administration of L612 HuMAb in patients whose distant metastatic melanoma (AJCC stage IV disease) did not respond to conventional and/or experimental treatment modalities. This study is the first clinical trial investigating systemic administration of an IgM HuMAb against cancer.
Methods
L612 HuMAB
L612 HuMAb is a human IgM-κ antibody that binds to human cancer cells expressing GM3 [4, 26]. L612 HuMAb is secreted from the cell line L612 grown in a serum-free medium (AIM, GIBCO Laboratories, Grand Island, NY) by a hollow-fiber culture system (AcuSyst 1000 Model, Endotronics, Minneapolis MN). This cell line is free from human viruses including HIV-1, HIV-2, HBA, CMV, HHV-6, retrovirus and adventitious virus, and it does not generate biologically active EBV. To purify IgM, the L612 spent medium containing the antibody was processed using four steps of column chromatography (Sephadex G-25 gel filtration, Q-Sepharose anion exchange, Superose 6 gel filtration, and Polymyxin B-Sepharose 4B affinity columns). The final product was adjusted to 1 mg/ml in phosphate-buffered saline (PBS) (pH 7.2) and stored at −80°C until use. The purity of the final product was greater than 99.9%. DNA contamination was less than 10 pg/mg antibody. Endotoxin contamination was 0.06 EU/ml or less, and the product was free from bacteria, fungi, mycoplasma, HIV, CMV, and hepatitis.
Patients
A clinical study of L612 HuMAb was approved by the Saint John’s Health Center Investigational Review Board for a study population of nine patients with metastatic melanoma. Eligibility criteria included the following: (1) diagnosis of AJCC stage IV melanoma with measurable disseminated disease; (2) tumor cells (obtained from incisional or excisional biopsy) that tested positive for antigen by the immune adherence (IA) assay; (3) no immediate hypersensitivity reaction to intradermal injection of 0.1-mg L612 HuMAb; (4) failure to respond to other therapies (surgery, chemotherapy, radiation therapy, biotherapy, and/or immunotherapy); (5) no other anticancer treatment 4 weeks prior to antibody therapy; and (6) no brain metastases.
Characteristics of the nine patients are outlined in Table 1. Except for patient 2, their life expectancy was less than 6 months.
Table 1.
Characteristics of the patients
| Patient (sex/age) | Prior treatmenta | Site of metastasesb | Antigen expressionc |
|---|---|---|---|
| 1 (M/49) | Surgery, chemotherapy, IFN-α, IL-2 | Skin (abdominal wall), liver, bilateral lung, small bowel | 4 |
| 2 (F/69) | Surgery | Skin (lower leg and back) | 2 |
| 3 (F/48) | Surgery, two types chemotherapy | Skin (multiple sites), spleen, pancreas, lung, intestine, lymph node | 4+ |
| 4 (F/65) | Chemotherapy, radiation therapy, intralesional BCG, PV, IFN-α, IL-2 | Skin (leg), lymph node | 4+; 0d |
| 5 (M/44) | Surgery, chemotherapy, PV | Skin (leg, back, buttock), lymph node, liver | 3 |
| 6 (M/63) | Surgery, PV, intralesional BCG | Skin (scalp, ear, neck), lung, lymph node | 3 |
| 7 (M/66) | Surgery, chemotherapy, radiation therapy, PV | Skin (leg, arm), abdomen, liver, lung, lymph node | 4+ |
| 8 (M/72) | Surgery | Liver, lymph node | 4+ |
| 9 (F/68) | Surgery, intralesional BCG, PV, IFN-α, IL-2 | Skin (scalp), liver, lung | 3 |
aPrior treatments for AJCC stage IV melanoma; surgery intended for complete resection of disease
bMetastases at the time of antibody infusion
cGM3 expression on melanoma cells was assessed by immune adherence assay (see “Materials and methods”)
dInguinal lymph node metastases were removed 13 weeks after L612 HuMAb infusion and melanoma cells were tested for antigen expression
Antibody administration
Patients were admitted to Saint John’s Health Center for a continuous infusion of L612 HuMAb via an indwelling peripheral venous catheter. L612 HuMAb was prepared for infusion by thawing and formulating with saline, and was administered continuously over 48 h in two 24-h infusions. Patients were divided into three sequential groups of three patients each. The first group received 480 mg/1,000 ml each 24 h (960 mg in 48 hours). After documentation of any intrainfusional or postinfusional toxicity in the first group, the second group received 720 mg/1,000 ml each 24 h (1,440 mg in 48 h). After documentation of any toxicity in the second group, the third group received 960 mg/1,000 ml each 24 h (1,920 mg in 48 h). Patients who responded to the infusion (partial response, mixed response, or stabilization of disease) by 4 weeks could receive an additional infusion of the same dose of L612 HuMAb.
The initial dose (960 mg) was determined from our previous experience with compassionate use of L612 HuMAb. Four AJCC stage IV melanoma patients who had received 560 mg, 688 mg, 1,000 mg, and 1,000 mg L612 HuMAb, respectively, showed only minimal toxicity: mild and transient pruritus and rash (two patients) and pain at the tumor site (one patient).
The clinical response was defined as follows: Complete response was complete disappearance of all measurable disease. Partial response was >50% reduction of tumor size. Mixed response was >50% reduction in some areas of metastasis without change or progression in other areas. Stabilization was <25% reduction or no change. Progression was an increase of tumor size.
Antigen expression on melanoma tissue
Each patient’s surgically excised melanoma metastases were minced and filtrated. Single-cell suspensions were tested for L612 antigen expression using IA [7] with 10 μg/ml L612 HuMAb. IA scores were defined as follows: 0, no positive cells; 1, 10–24% positive cells; 2, 25–50% positive cells; 3, 50–74% positive cells; 4, 75–89% positive cells; 4+, >90% positive cells.
Assay for L612 HuMAb in serum
Sera collected before, during (24, 36, and 48 h), and after (72 h and variably) antibody infusion were tested for L612 HuMAb levels by an enzyme-linked immunosorbent assay (ELISA). Purified GM3 (4 μg/well) was the antigen source. Phosphate-buffered saline (PBS) was used to dilute sera from 1/50 to 1/6,400 in 50 μl. Other procedures followed standard ELISA for ganglioside antigens [15]. The level of L612 HuMAb was calculated by subtracting the optical density (OD) of preinfusion serum from the OD of postinfusion serum. The resultant OD was converted to antibody titer (μg/ml) using reference OD values obtained by mixing known amounts of L612 HuMAb with preinfusion serum.
Assay for anti-L612 HuMAb antibodies in serum
Sera collected during (24, 36, and 48 h) and after (72 h and variably) antibody infusion were tested for antibodies to L612 HuMAb by ELISA. Purified L612 HuMAb was the antigen source and purified L55 HuMAb [15, 20] was the control antigen. Both antigens were adjusted to 20 μg/100 μl by carbonate buffer (pH 9.5). Sera (first antibody) were serially diluted from 1/50 to 1/800 in PBS containing 1% human serum albumin. A peroxidase-labeled goat antihuman IgG (γ-specific) was used as a second antibody. Other procedures followed standard ELISA procedures for protein antigens.
Results
Toxicity
The antibody infusion doses of 960 mg, 1,440 mg, and 1,920 mg were well tolerated (Table 2). When expressed in terms of each patient’s body surface area, these doses ranged from 473 to 1,223 mg/m2. The infusion rates were 20, 30, and 40 mg/h, respectively. The side effects were minor during and after infusion. Five patients (patients 3, 5, 6, 7, and 9) complained of mild and transient pruritus and rash during the infusion (ECOG toxicity grade 1). This was easily controlled by oral antihistamine and completely resolved during the infusion. Patient 2 complained of tumor pain at the sites of subcutaneous metastases on her back. Patients 3, 6, 7, and 9 received a second infusion with the same dose 4 to 7 weeks after the first infusion. Patient 4 received a second infusion 44 weeks after the first infusion. Patient 9 received a third infusion with the same dose 14 weeks after the second infusion; thus the total dose administered to patient 9 was 5,760 mg (3,669 mg/m2). No additional toxicity was observed with the second or third infusion.
Table 2.
Toxicity of L612 HuMAb administration
| Patient | Dose and number of courses | Infusion ratea (per hour) | Toxicityb | ||
|---|---|---|---|---|---|
| First infusion | Second infusion | Third infusion | |||
| 1 | 960 mg (473 mg/m2) | 20 mg | NS | – | – |
| 2 | 960 mg (612 mg/m2) | 20 mg | Tumor pain | – | – |
| 3 | 960 mg (593 mg/m2)×2c | 20 mg | Pruritus/rash | NS | – |
| 4 | 1,440 mg (911 mg/m2)×2c | 30 mg | NS | NS | – |
| 5 | 1,440 mg (727 mg/m2) | 30 mg | Pruritus/rash | – | – |
| 6 | 1,440 mg (692 mg/m2)×2c | 30 mg | Pruritus/rash | NS | – |
| 7 | 1,920 mg (753 mg/m2)×2c | 40 mg | Pruritus/rash | NS | – |
| 8 | 1,920 mg (1,060 mg/m2) | 40 mg | NS | – | – |
| 9 | 1,920 mg (1,223 mg/m2)×3c | 40 mg | Pruritus/rash | Pruritus/rash | Pruritus/rash |
aContinuous infusion for 48 h
b NS, not significant; itching/rash were mild and transient
cPatients 3, 4, 6, 7, and 9 received a second infusion with the same dose 5, 44, 7, 4 and 5 weeks, respectively, after the first infusion. Patient 9 received a third infusion with the same dose 14 weeks after the second infusion
Clinical response
Four weeks after the start of antibody infusion, one patient showed a partial response (patient 2), three patients showed a mixed response (patients 3, 6 and 7), and two patients showed stabilization of disease (patients 4 and 9). At weeks 12 to 16, patient 2 had a complete response, patient 7 showed further decreases in tumor size, patient 4 had a mixed response, and patient 9 maintained stable disease (Table 3).
Table 3.
Tumor response to infusion of L612 HuMAb
| Patient | Total dose of HuMAb | Tumor responsea | Survival (months)b | |||
|---|---|---|---|---|---|---|
| 4 weeks | 12–16 weeks | Current | Post-Ab infusion | Post-stage IV diagnosis | ||
| 1 | 960 mg (473 mg/m2) | Progression | Unknown | Expired | 6.8 | 18.8 |
| 2 | 960 mg (612 mg/m2) | Partial | Complete | NEDa | >67 | >63 |
| 3 | 1,920 mg (1,186 mg/m2) | Mixed | Progression | Expired | 4.0 | 14.3 |
| 4 | 2,880 mg (1,822 mg/m2) | Stabilization | Mixedc | NEDa | >66 | >83 |
| 5 | 1,440 mg (727 mg/m2) | Progression | Progression | Expired | 5.1 | 11.1 |
| 6 | 2,880 mg (1,384 mg/m2) | Mixed | Progression | Expired | 14.7 | 15.0 |
| 7 | 3,840 mg (1,506 mg/m2) | Mixed | Partial | Expired | 6.3 | 8.5 |
| 8 | 1,920 mg (1,016 mg/m2) | Expiredd | 0.9 | 2.3 | ||
| 9 | 5,760 mg (3,669 mg/m2) | Stabilization | Stabilization | Expired | 20.2 | 21.2 |
a NED, no clinically evident disease
bMonths after start of HuMAb trial and after diagnosis of AJCC stage IV melanoma
cMultiple subcutaneous metastases were completely regressed and antigen-negative inguinal lymph node metastases were surgically resected at week 13
dThis patient’s death 27 days after the antibody trial was suspected to be the result of failure to thrive following hepatic resection
Patient 2 had two distant subcutaneous metastases (4 to 5 cm in diameter) and multiple local satellites. The antigenicity test of a satellite tumor that was biopsied in this patient showed only mild reactivity (IA=2). Nevertheless,4 weeks after administration of 960 mg (612 mg/m2) of L612 HuMAb, she showed a partial response; by week 14 the response was complete.
Patient 4 had previously failed to respond to intralesional bacille Calmette-Guérin (BCG), single-agent chemotherapy with vinblastine, combined-agent chemotherapy with the Dartmouth regimen (cisplatin, carmustine, dacarbazine, tamoxifen), radiation, a polyvalent melanoma cell vaccine (PV) [14], interleukin 2 (IL-2), and interferon α (IFN-α). This patient had extensive subcutaneous metastases on her right thigh (Fig. 1A, B), in addition to left inguinal lymph node metastases. She received 1,440 mg (911 mg/m2) of antibody. By week 4 her disease had stabilized, by week 7 the multiple subcutaneous metastases were flattened (Fig. 1C), and by week 13 all subcutaneous lesions had completely regressed. The lymph node metastases that were not responsive to L612 HuMAb were surgically removed at week 13. Nodal tumor proved to be antigen-negative.
Fig. 1A–D.
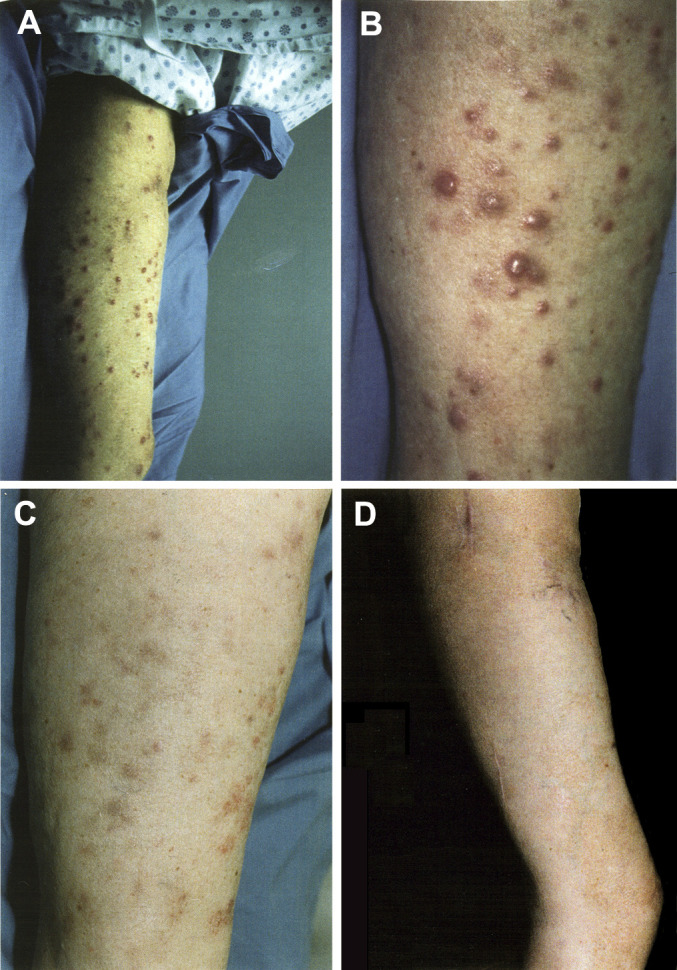
Subcutaneous metastasis on the right leg of patient 4 before infusion of L612 HuMAb (A and B), and 7 weeks (C) and 29 months (D) after the first infusion of L612 HuMAb (1,440 mg). This patient is currently without clinical evidence of disease at >48 months of follow-up
Patient 7, who had skin, liver, lung, and abdominal metastases, received 1,920 mg (753 mg/m2) of antibody. This patient had previously failed to respond to postoperative chemotherapy, radiation therapy, and PV. By week 4 of antibody infusion, all of the subcutaneous nodules on his right leg were flattened; by week 8 all had faded and pathological examination confirmed the complete regression of melanoma. Two remaining subcutaneous metastases on his arm were surgically removed. A computed tomography (CT) scan of the abdomen and pelvis at week 8 showed stable or decreased size of metastases and complete resolution of an iliac cystic lesion. A CT scan of the chest also showed stabilization or decrease in lesion size and complete resolution of chest pleural thickening. Disease remained stable between weeks 8 and 13.
Patient 3, who had extensive disseminated melanoma involving subcutaneous, intestinal, lung, spleen, lymph node, and pancreatic sites, received 960 mg (593 mg/m2) of antibody. At week 5, she showed partial regression of abdominal metastases, stabilization of subcutaneous metastases, and progression of lung metastases; she was given a second dose of 960 mg. She expired due to the overwhelming tumor burden 13 weeks after the second antibody infusion.
Patient 9 presented with rapidly growing metastases in the lung and liver and near the primary site on the scalp. She had failed previous treatments of IFN-α, IL-2, PV, and intralesional BCG. She received 1,920 mg (1,223 mg/m2) of antibody. The tumor on her scalp stabilized at week 4, and a CT scan of the chest showed stabilization of disease. At week 5 she received a second infusion of L612 HuMAb. The size of subcutaneous and lung metastases remained stable at weeks 4 to 16.
Follow-up
Patients 1, 5, and 6 showed progression of disease within 12 weeks and were transferred to chemotherapy and biological therapy protocols, but did not respond. They expired at 6.8, 5.1, and 14.7 months, respectively, after antibody infusion. Patient 7 showed improvement of his subcutaneous and hematogenous disease but was diagnosed with bilateral brain metastases of significant size at week 19. He died 2.1 months later from the brain metastases. Patient 2, who had shown a complete regression of her disease at week 14 and continued to be free of disease for >67 months, received PV treatment at week 22. Whether the PV treatment contributed to her long-term disease-free status has yet to be determined. Patient 4 was free of disease for 5 months until she developed several new nodules on her right leg. She received a second infusion with the same dose of L612 HuMAb. While some of the secondary subcutaneous metastases regressed or stabilized within 2 months of the infusion, other lesions began to grow slowly, at which time the patient began treatments with granulocyte macrophage colony-stimulating factor and PV. She did not respond to these treatments and the subcutaneous tumors were surgically removed. This patient is currently without clinical evidence of disease >66 months after the antibody trial (Fig. 1D) and >82 months after the diagnosis of stage IV melanoma. Patient 9 was diagnosed with breast cancer with nodal metastases 5.5 months after antibody infusion. She underwent segmental mastectomy and axillary lymphadenectomy followed by adjuvant chemotherapy. Six weeks after the breast operation, the remaining melanoma metastases on her scalp were surgically removed and treatment with PV was begun. Nevertheless her disease progressed and she expired 20.2 months after the first infusion of antibody. Whether the direct cause of death was breast cancer or melanoma is not known.
Pharmacokinetics of L612 HuMAb
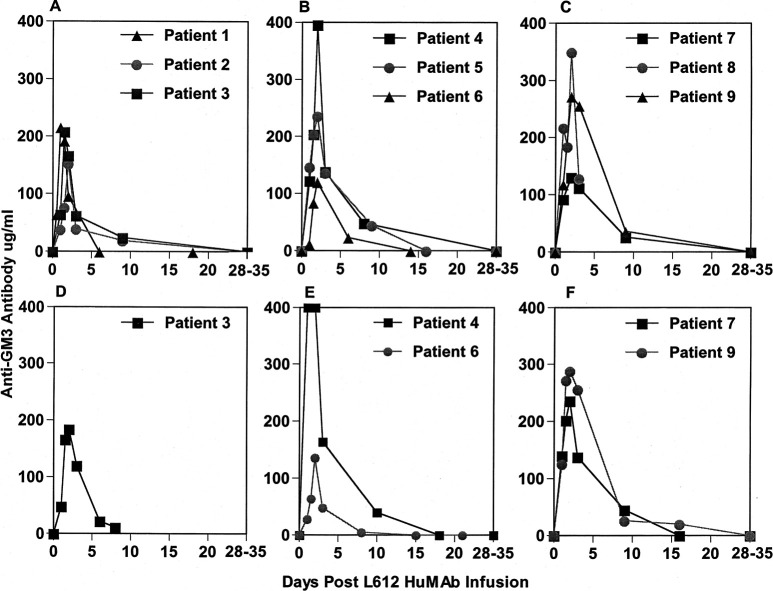
All patients had elevated antibody titers within 24 h after the onset of antibody infusion. Antibody titers peaked at 24 h in one patient, at 36 h in three patients, and at 48 h in five patients (Fig. 2A–C). Antibody pharmacokinetics appeared to be influenced by tumor volume, antigen density on melanoma cells, and site(s) of metastasis because the serum antibody levels did not always parallel the infusion dose or the dose per body surface area. Although the half-life of normal human IgM is 5 to 6 days, by day 3 the L612 HuMAb titer dropped to 26−37% of its peak in patients receiving 960 mg, to 31−57% of its peak in patients receiving 1,440 mg, and to 36−94% of its peak in patients receiving 1,920 mg. These results indicate that the binding of antibody to melanoma cells took place shortly after the onset of infusion. By week 4 to 5, antibody titers dropped to preinjection levels in all nine patients. Sera from five patients who received a second infusion with the same dose were tested by the same assay; in all cases, antibody levels matched or exceeded levels from the first infusion period (Fig. 2D–F).
Fig. 2A–F.
Serum IgM anti-GM3 antibody levels (μg/ml) in nine patients receiving L612 HuMAb. Antibody was tested by ELISA. Optical density (OD) values were converted to actual concentration using L612 HuMAb mixed with preinfusion serum as a reference antibody. A B, and C are from the first infusion period. D, E, and F are from the second infusion period
Pharmacokinetics of antibody responses to L612 HuMAb
The possible induction of anti-L612 HuMAb antibody responses was assessed in all of the sera up to 239 days after the first infusion. Patient 9, who received 3 courses of 1,920 mg antibody, was monitored for 138 days. None of the nine patients developed detectable anti-antibody responses (data not shown).
Discussion
The present study represents the first clinical trial of 100% human protein monoclonal antibody for cancer patients. The maximum total dose in this study, three courses of 1,920 mg (1,223 mg/m2), was higher than doses used in previous trials of MAb for patients with cancer. All nine patients tolerated antibody infusion well with minimal or no toxicity. The toxicity reported with a high dose of chimeric or humanized IgG MAb by other investigators has included fever, chills, nausea, and vomiting in most of the previous clinical trials, and less frequently cardiac dysfunction, hypertension, hypotension, rigor, bronchospasm, and myalgia, and a death. The low toxicity of L612 HuMAb may reflect a variety of factors: its 100% human origin, its specific binding to cancer cells, its purity for the protein, its adjustment to an optimal concentration and rate of infusion, and its low level of endotoxins. The mild pruritus and rash caused by L612 HuMAb has not been thoroughly investigated but might reflect antigen-positive nevi. Our previous studies using a very sensitive three-step immunohistochemical assay found that compound nevi and dysplastic nevi stained positive with L612 HuMAb [18]. The level of serum L612 antibody is unlikely to be a cause of side effects because patient 4 had maximum elevation of antibody titer but experienced no pruritus and rash (Table 2 and Fig. 2). Anti-antibodies were not detected in any patient; thus, pruritus and rash probably were not caused by an anaphylactic reaction due to a secondary response.
IgG MAbs have been favored over IgM MAbs because they are easier to prepare and purify and more stable. In general, they have stronger binding affinity and better penetration in tumor tissues, and they facilitate antibody-dependent cellular cytotoxicity. To our knowledge, L612 HuMAb is the first IgM MAb with a defined antigen specificity to be tested in cancer patients via systemic administration. The primary mechanism of action of L612 HuMAb appears to be a complement-mediated immune cytolysis that takes place on the antigen-positive cell membrane. L612HuMAb has been shown to kill cultured human melanoma cells expressing a higher level of GM3 on their surface in the presence of human complement [16, 21]. Due to their pentameric nature, antigen-bound IgM antibodies can activate complement more efficiently than do IgG antibodies. Previously we reported that when bound to the surface of melanoma cells, the IgM chimeric L612 HuMAb activates complement in vitro much more efficiently than does the IgG chimeric L612 HuMAb [4]. Retrospective analysis of patients with melanoma has also shown that high titers of serum IgM but not IgG antiganglioside antibodies correlate with good prognosis [9, 21]. Complement activation and cell destruction are usually protected by species-specific membrane inhibitors; thus human cells are not lysed by antibody and human complement [11].However, recent studies have shown that IgM antibodies to cell-surface glycolipid antigens, including gangliosides, can activate human complement to kill antigen-positive human cells [24, 25].These findings in conjunction with the high doses of L612 HuMAb may explain the clinical responses observed in our study.
We previously reported that GM3 was present in all of the human melanomas tested (52/52) by a biochemical assay [23]. The expression was widely heterogeneous. The average percentage of total lipid-bound sialic acids was 43.2%, ranging from 4.0 to 90.3%. In the present study we have chosen patients with moderately or highly antigen-positive melanoma when tested by the immune assay using L612 HuMAb. When 86 melanoma specimens obtained from patients undergoing resection of regional or distant metastatic melanoma were tested by IA assay with 10 μg/ml, 63 (73.3%) had an IA score >2 (Table 4).
Table 4.
Reactivity of L612 HuMAb to melanoma, carcinoma, and normal tissues. IH immunohistochemistry, IA immune adherence, MIF membrane immunofluorescence, PBL peripheral blood lymphocytes
| Biopsied, surgical, and autopsied tissues | Type of assay (L612 HuMAb dose) | No. positive/no. tested |
|---|---|---|
| Melanoma | IA (10 μg/ml) | 63/86 (73.3%)b |
| Melanoma | MIF (100 μg/ml) | 54/82 (65.9%)c |
| Melanoma | Direct IH (20 μg/ml) | 13/20 (65%) |
| Colon cancer | Direct IH (20 μg/ml) | 4/5 (80%) |
| Ovarian cancer | Direct IH (20 μg/ml) | 4/5 (80%) |
| Pancreas cancer | Direct IH (20 μg/ml) | 3/5 (60%) |
| Prostate cancer | Direct IH (20 μg/ml) | 2/5 (40%) |
| Breast cancer | Direct IH (20 μg/ml) | 2/5 (40%) |
| Kidney cancer | Direct IH (20 μg/ml) | 2/5 (40%) |
| Lung cancer | Direct IH (20 μg/ml) | 0/5 (0%) |
| Normal tissuesa | Direct IH (20 μg/ml) | 0/62 (0%) |
| Erythrocytes | IA absorption (100 μg/ml) | 0/44 (0%)d |
| PBL | IA absorption (100 μg/ml) | 0/32 (0%)d |
aSixty-two normal human tissues were obtained from 24 different organ sites: cerebellum (3), cerebrum (1), pons (2), spinal cord (2), eye (3), skin (2), muscle (3), breast (2), salivary glands (2), larynx (4), esophagus (4), stomach (2), duodenum (4), colon (2), liver (4), lung (2), heart (4), thyroid (4), spleen (2), lymph nodes (2), bladder (2), kidney (2), ovaries (2), and testis (2)
bPositive specimens were those with an IA score >2. Of the 86 specimens, 81 (94.2%) had an IA score >1 (data not shown)
cPositive specimens were those with an MIF score >25%. Of the 82 specimens, 76 (92.8%) had an MIF score >10% (data not shown)
dPreviously reported elsewhere [4]
Eighty-two of the 86 specimens were also tested by a membrane immune fluorescence (MIF) assay with 100 μg/ml L612 HuMAb: 54 specimens (65.9%) had >50% positive cells (Table 4). Twenty frozen melanoma specimen sections were tested by a direct immunohistochemistry (IH) assay with 20 μg/ml biotinylated L612 HuMAb. Thirteen specimens (65%) were positively stained. These findings indicate that about 70% of patients with regional or distant metastatic melanoma might benefit from infusion of L612 HuMAb. Table 4 shows the direct IH data from our recent studies of L612 HuMAb (20 μg/ml) reactivity to other cancer tissues and to 62 normal tissues derived from 24 different organ sites. Results indicate that the antibody may be of potential therapeutic value not only for melanoma but also for other cancers. IH-positive cancers included those of the colon (4/8 positive), ovary (4/5 positive), pancreas (3/5 positive), breast (2/5 positive), kidney (2/5 positive), and prostate (2/5 positive). None of the five lung cancer tissues tested in the current study were positive, but results of our previous studies using indirect IH assays indicate that L612 HuMAb could bind to lung cancer cells as well [4, 18]. None of the 62 normal tissues were positive. Overall, though the primary objective of the study was to determine the toxicity, the clinical responses in this small number of patients were remarkable. The two patients who have displayed no evidence of disease over 5 years had relatively smaller tumor burden as compared to the remaining 7 patients at the time of the antibody infusion. The result suggests that tumor volume is an important factor for metastatic melanoma patients to achieve a complete response by L612 HuMAb treatment.
Acknowledgements
We thank Dr Larry Nathanson and Dr Richard Essner for critical reading of the manuscript, and Ingrid Ting, Lan Sze, and Noriko Okamoto for technical assistance.
Footnotes
Dr. Ollila is currently affiliated with the University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA.
Supported by grant CA 30647 from the National Institutes of Health, National Cancer Institute.
References
- 1.Baselga J Clin Oncol. 1996;14:737. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh J Clin Oncol. 1999;17:2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 3.Goodman Cancer Immunol Immunother. 1993;36:267. doi: 10.1007/BF01740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoon Cancer Res. 1993;53:5244. [PubMed] [Google Scholar]
- 5.Irie Proc Natl Acad Sci U S A. 1982;79:5666. [Google Scholar]
- 6.Irie Proc Natl Acad Sci U S A. 1986;83:8694. [Google Scholar]
- 7.Irie RF, Saxton RE (1987) Human monoclonal antibodies: prospects for therapy of human melanoma. In: Kano K, Mori S, Sugisaki T et al (eds) Cellular, molecular, and genetic approaches to immunodiagnosis and immunotherapy. University of Tokyo Press, Tokyo, pp 73–86
- 8.Irie Lancet. 1989;8:786. doi: 10.1016/S0140-6736(89)92606-8. [DOI] [PubMed] [Google Scholar]
- 9.Jones J Natl Cancer Inst. 1981;66:249. [PubMed] [Google Scholar]
- 10.Kaminski J Clin Oncol. 2001;19:3918. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 11.Lachmann Immunol Today. 1991;12:312. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin J Clin Oncol. 1998;16:2825. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 13.McNeil J Natl Cancer Inst. 1995;87:658. [Google Scholar]
- 14.Morton Ann Surg. 1992;216:463. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishinaka Cancer Res. 1996;56:5666. [PubMed] [Google Scholar]
- 16.Nishinaka J Immunogenetics. 1998;48:73. doi: 10.1007/s002510050404. [DOI] [PubMed] [Google Scholar]
- 17.Ozkaynak J Clin Oncol. 2000;18:4077. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 18.Saito J Immunol Methods. 1990;134:121. doi: 10.1016/0022-1759(90)90120-K. [DOI] [PubMed] [Google Scholar]
- 19.Slamon N Engl J Med. 2001;344:783. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Tai Proc Natl Acad Sci U S A. 1983;80:5392. [Google Scholar]
- 21.Takahashi J Invest Dermatol. 1999;112:205. doi: 10.1046/j.1523-1747.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 22.Tolcher J Clin Oncol. 1999;17:478. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchida J Natl Cancer Inst. 1987;78:45. doi: 10.1093/jnci/78.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Wu Int Immunol. 1996;8:153. doi: 10.1093/intimm/8.1.153. [DOI] [PubMed] [Google Scholar]
- 25.Wu J Immunol. 1999;162:533. [PubMed] [Google Scholar]
- 26.Yamamoto J Natl Cancer Inst. 1990;82:1757. doi: 10.1093/jnci/82.22.1757. [DOI] [PubMed] [Google Scholar]