Abstract
Purpose
We investigated and compared the biodistribution of Albuleukin, a human serum albumin (HSA)-interleukin-2 (IL-2) fusion protein, with those of IL-2 and HSA. The objective was to determine whether Albuleukin distributes differently to normal organs and lymphoid tissues than IL-2 by virtue of its genetic fusion with HSA.
Methods
The chelating agent 2-(p-isothiocyanato-benzyl)-cyclohexyl-diethylenetriaminepentaacetic acid (CHX-AII was selected for radiolabeling with 111In, and conjugation with CHX-AII did not alter bioactivities of IL-2 and Albuleukin on proliferation of CTLL-2 cells. The radiolabeled proteins were injected intravenously into mice, uptake in organs was measured, and whole-body autoradiography was performed.
Results
Striking differences in the biodistribution of IL-2 and Albuleukin were noted. 111In-IL-2 cleared from blood rapidly, with less than 1% ID/g (percentage of injected dose per gram of tissue) at 20 min after injection. At this time, the kidneys showed more than 120% ID/g uptake, and these high levels persisted through 6 h. 111In-Albuleukin, by contrast, showed significantly longer circulation (14% ID/g at 6 h), lower kidney uptake (<6% ID/g), and higher localization in liver, spleen, and lymph nodes (maximal uptake ~22% ID/g for all three organs). Uptake in liver, spleen, and lymph nodes appears to be mediated in part by the IL-2 component of Albuleukin because 111In-HSA showed significantly lower accumulation in those tissues despite more prolonged circulation in blood.
Conclusion
These data support the hypothesis that Albuleukin targets tissues where lymphocytes reside to a much greater extent than does IL-2, and suggest that Albuleukin may exhibit improved efficacy and reduced toxicity in the treatment of solid tumors. Clinical trials underway will determine whether the improved targeting in the mice translates into a better therapeutic index in humans.
Keywords: Albuleukin, Interleukin-2, Biodistribution, Whole-body autoradiography, 111In-radiolabeling, Mice
Introduction
Interleukin-2 (IL-2) plays an important role in a variety of immune functions, and it regulates T-cell growth as well as the activities of natural killer cells and B-cells [23]. The immunomodulatory activities of IL-2 are believed to stimulate a host response against antigens associated with neoplastic cells [17]. Recombinant IL-2 has become an accepted therapy for melanoma and renal cell carcinoma [5]; however, the drug is associated with toxicity and a narrow therapeutic index [25], and it is only modestly successful in the treatment of cancer.
Due to its small molecular size, IL-2 is rapidly cleared through the kidneys, where it is metabolized completely [2, 3, 14, 18]. IL-2 has a very short plasma half-life [2, 8], and virtually no IL-2 was traceable in the serum 30 min after injection [14]. Increasing the molecular size of IL-2 may lead to reduced renal clearance, thereby achieving prolonged circulation [6], although it is unclear whether this creates a more favorable biodistribution. We have explored an alternate strategy of using human serum albumin (HSA) as a genetic fusion partner with the smaller therapeutic protein to create a new drug with the combined biological properties of IL-2 and HSA, including improved pharmacokinetic properties. Albuleukin, a fusion protein of IL-2 and HSA produced as a single polypeptide molecule, has a significantly extended half-life, has proved to be an effective agent for suppressing tumor growth in mice [11], and is currently being evaluated in a phase-I study in patients with solid tumors.
In addition to having a reasonably long circulation half-life, a biologically active molecule must also reach its target. Biodistribution studies with 125I or 14C labeled IL-2 have been performed at multiple institutions [4, 14, 18, 19], and these have shown that IL-2 is not highly targeted to tissues where T-cells reside. The use of albumin as a fusion partner offers advantages in addition to enhancing its circulating half-life. Albumin distributes to most fluids and tissues of the body and a large fraction returns to the vascular pool via the lymphatics; therefore, we hypothesized that Albuleukin may distribute to lymphatic tissues to a greater extent than does IL-2. Unfortunately, as radio-iodine is released from a cell upon internalization and intracellular degradation of a directly radio-iodinated protein, sites of the catabolism are not accurately traced and detected clearly [4, 14, 18, 19]; therefore, a radionuclide, such as 111In, which is retained in a cell after internalization, is more appropriate for conducting a biodistribution study like IL-2.
Herein we describe studies using 111In labeled proteins that clearly demonstrate the different biodistribution profiles of IL-2, Albuleukin, and HSA.
Materials and methods
Proteins
Albuleukin, produced at Human Genome Sciences, Inc., is recombinant human native IL-2 genetically fused to recombinant HSA. Albuleukin has a molecular mass of approximately 81.8 kDa. The HSA used in this study was also produced at Human Genome Sciences, Inc. Both proteins were produced in yeast Saccharomyces cerevisiae. IL-2 (Proleukin, Chiron Corporation, Emeryville, Calif.) was purchased commercially.
CHX-AII conjugation and 111In radiolabeling
All of the proteins were conjugated with 2-(p-isothiocyanato-benzyl)-cyclohexyl-diethylenetriaminepentaacetic acid (CHX-AII) [7, 12]. Protein to be conjugated was diluted in conjugation buffer (50-mM carbonate buffer, with 150 mM NaCl and 50 mM EDTA), a 20–50 mol ratio excess of CHX-AII was added, and the pH was adjusted to 8.5. The reaction mixture was allowed to stand at room temperature for 3 h to overnight and applied to a PD-10 column for purification.
One molecule of CHX-AII is capable of chelating one molecule of metal. CHX-AII conjugated proteins were radiolabeled with 111In (PerkinElmer Life Sciences, Boston, Mass.) at a specific activity of 18.5–37 kBq/μg for in vitro analysis and in vivo biodistribution study. Protein solution and 111InCl3 were mixed in 0.4 M citrate buffer and allowed to stand at room temperature for 30 min and purified with a PD-10 column. Labeling efficiencies, the percent incorporated into the protein of the total radioactivity added, of 45–60% were achieved.
High-performance liquid chromatography analysis and purification
IL-2 is susceptible to formation of aggregates during conjugation; therefore, radiolabeled IL-2 was further purified with size exclusion high-performance liquid chromatography (HPLC; Shodex GFC column, KW 802.5) to obtain the monomeric species. The HPLC analysis of Albuleukin and HSA with the same column showed that these proteins did not form aggregates during conjugation, and they were used in the biodistribution studies without purification.
CTLL-2 cell binding
111In labeled IL-2 or Albuleukin, 5 or 7 ng, was incubated with 3 million CTLL-2 cells in 0.175 ml for 1 h at room temperature. After centrifugation, the supernatant was aspirated and the cell pellet was counted with a gamma counter. Non-specific binding was determined using 25 μg of unlabeled protein.
CTLL-2 bioassay
Details of the CTLL-2 bioassay are described elsewhere [10]. Briefly, CTLL-2 cell lines, grown in RPMI containing 10% FCS, 50 μM β-mercaptoethanol (Sigma) and 5 ng/ml IL-2 (R&D System), were used and the cells were washed twice in PBS prior to the assay. An amount of 1×104 cells were seeded per well in RPMI containing the indicated amount of Albuleukin or IL-2 in a total volume of 200 μl per well. After 72 h, the cells were treated with 20 μl/well of Alamar Blue for 8 h. Absorbance at 530/590 was used as a measure of cell proliferation and the effective concentration 50% (EC50) was then determined by nonlinear regression analysis (GraphPad Prism, San Diego, Calif.).
Animals
Female BALB/c mice were supplied by ACE (Boyertown, Pa.). They were 10–19 weeks old and 22.6±2.2 g of body weight, at the time of the biodistribution studies. Animal experimentation was conducted in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and under the supervision and approval of the Institutional Animal Care and Use Committee. Principles of laboratory animal care (NIH publication no. 85–23, revised 1985) were followed.
Formulation of injections
Injection solutions were prepared by mixing radiolabeled proteins with respective unlabeled proteins. Total protein dose of 0.1, 0.5 or 0.4 mg/kg of IL-2, Albuleukin, or HSA, respectively, was injected into the tail veins at a dose volume of 10 ml/kg. These mass doses are approximately equivalent on a molar basis.
Biodistribution experiment
Following various intervals after administration of the radiolabeled protein, animals were sacrificed by CO2 inhalation. Blood was then sampled, and the following tissues were removed: mesenteric lymph nodes, spleen, one kidney, section of liver, thymus, lung, heart, stomach, duodenum, femur, and muscle. All tissues were weighed and counted with gamma counter (LKB Wallac Model 1480), using a window of 120–540 keV. The counter corrects for background and decay. Radioactivity measurements (cpm) were divided by tissue or fluid weight and the injected cpm (normalized to a 20-g mouse) to obtain the percentage of injected dose per gram of tissue (%ID/g).
Whole-body autoradiography
Animals for autoradiography were sacrificed by CO2 inhalation, then spread on a metal plate and submersed into a slurry of dry ice and isopentane for 1 min. Extremities were severed from the frozen carcass and the mice were embedded in OCT. The blocks of tissue were stored at –80°C. Sectioning was performed on a Hacker-Bright 8250 Cryrostat (Fairfield, N.J.), maintained at –20°C. Using a tape transfer system, 20-μm sections were sliced. The tapes with attached tissue were placed in between sheets of acetate for transport and placement into autoradiography cassettes. The same slices were then stained with hematoxylin and eosin.
The tissue tapes were placed in an autoradiography cassette along with 14C standards and Fuji phosphor imaging plate (IP). The exposure time was 16 h to 7 days according to estimated radioactivity. The IPs were digitized with a Fujifilm BAS-2500 image reader, set to: 16-bit gradation, 50-μm pixel size, latitude four orders of magnitude (L4) and sensitivity at S/10,000. The digital image was analyzed with Image Gauge V3.0 software (Fuji, Tokyo, Japan).
Results
In vitro characterization
HPLC analysis
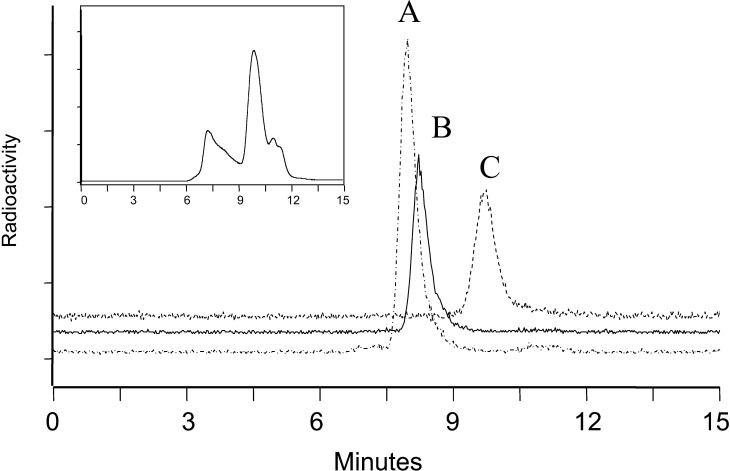
IL-2 has a tendency to form large aggregates, and size exclusion HPLC analysis of the CHX-A” conjugated IL-2 showed a broad distribution of large molecular weight entities as large as several hundred kilodaltons (Fig. 1, inset). Since large aggregates localize to the liver and spleen in vivo after administration, it is necessary to remove them from an injection solution in order to properly characterize the biodistribution of the native protein. Figure 1 shows the size exclusion chromatograms of 111In labeled IL-2 after HPLC purification and 111In labeled Albuleukin and HSA without purification. These demonstrate that each of the injected proteins showed a single peak whose retention times corresponded to the expected molecular weights of the monomeric based on the retention times of molecular weight standards (data not shown).
Fig. 1.
Size exclusion high-performance liquid chromatography (HPLC) profiles of 111In labeled A Albuleukin, B human serum albumin (HSA), and C interleukin-2 (IL-2) proteins used in the biodistribution studies. Inset 111In labeled IL-2 before HPLC purification
CTLL-2 cell binding
Both 111In labeled Albuleukin (7 ng) and IL-2 (5 ng) showed specific binding to CTLL-2 cells, 12.5 and 19.4%, respectively, after subtraction of non-specific binding with 25 μg of unlabeled proteins.
CTLL-2 bioassay
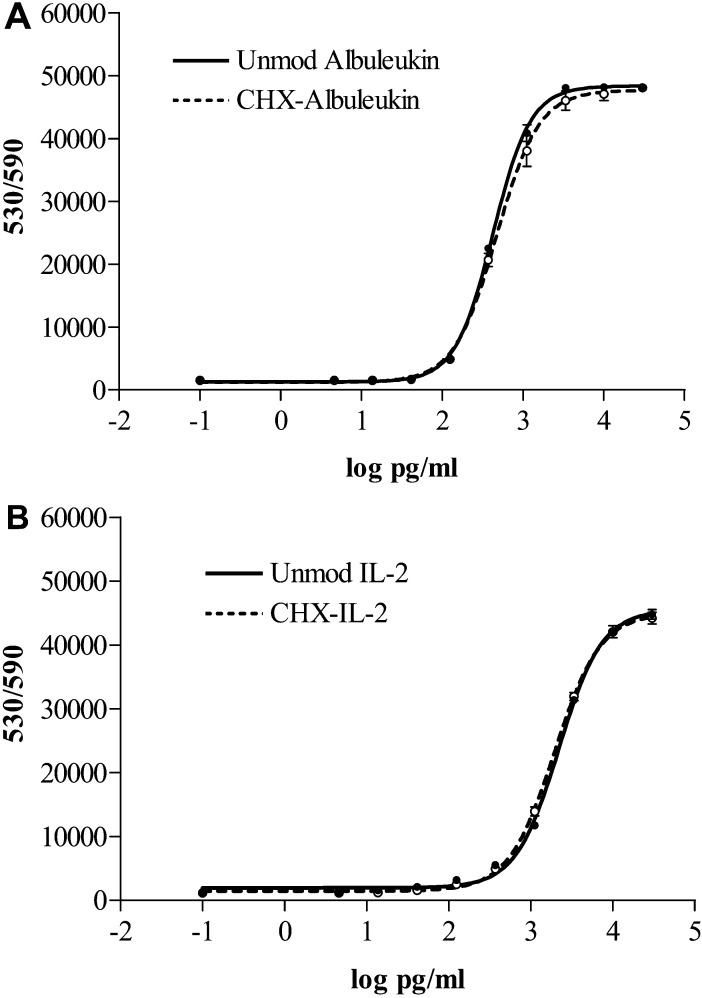
Both Albuleukin and IL-2 showed similar bioactivity curves on CTLL-2 cell proliferation before and after conjugation with CHX-A” (Fig. 2). The EC50s were calculated as 422.8 and 469.7 pg/ml for unmodified and CHX-A” conjugated Albuleukin, respectively, and 2194 and 1948 pg/ml for unmodified and CHX-A” conjugated IL-2, respectively.
Fig. 2A, B.
Comparison of CTLL-2 assay of CHX-A” conjugated proteins with unmodified proteins. A Albuleukin. B IL-2
In vivo biodistribution and autoradiography
IL-2
As shown in Figs. 3A and 4A, 111In labeled IL-2 is rapidly localized to kidney. At 20 min after i.v. injection, kidney uptake reached 123% ID/g while the blood concentration had dropped to 0.94% ID/g. High radioactivity was retained in the kidney through 6 h after administration, when blood concentration had dropped to 0.09% ID/g. Uptake in liver was less than 12% ID/g at all times and spleen and lymph nodes were less than 5% ID/g, all dramatically lower than in kidney. All other tissues showed even lower localization.
Fig. 3A–C.
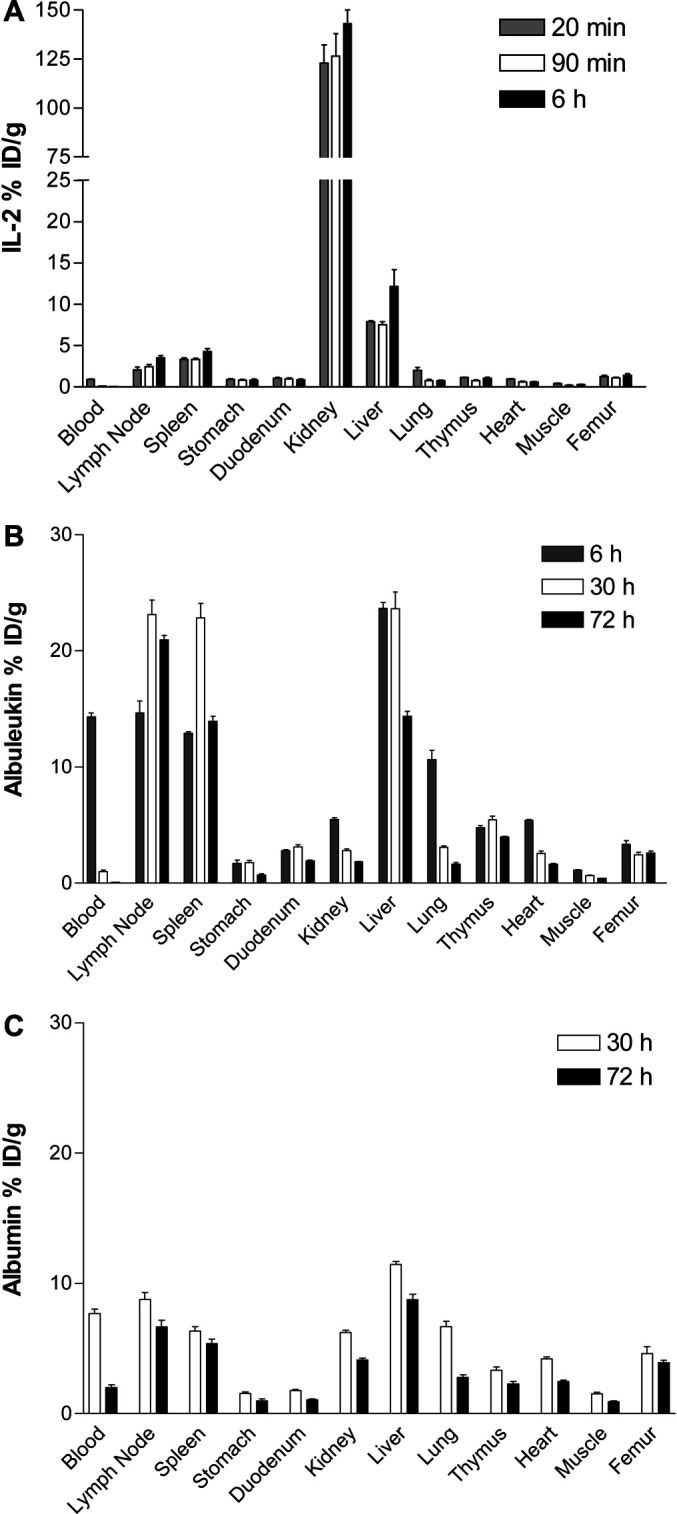
Biodistribution of 111In labeled A IL-2, B Albuleukin, and C HSA in BALB/c mice after i.v. injection. Data are expressed as mean±SEM of percent of injected dose per gram of tissue (% ID/g) with 5 mice per group
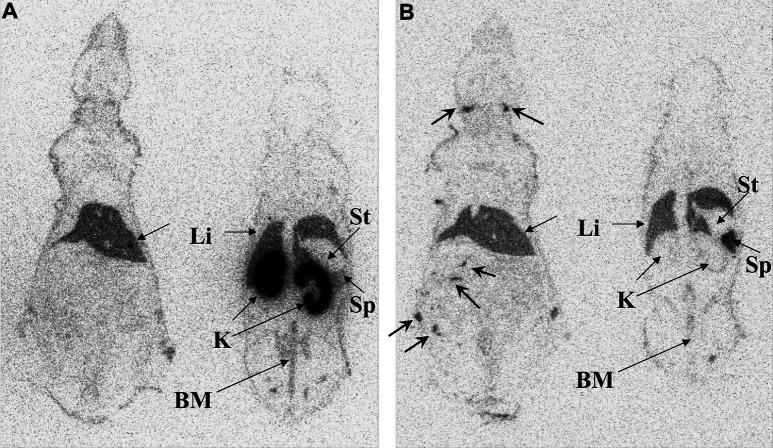
Fig. 4A, B.
Autoradiography of mice injected with 111In labeled IL-2 at A 90 min and B Albuleukin at 30 h, respectively. Images on the left of each panel show mainly liver (Li) and lymph nodes (arrows), whereas images on the right of each panel show kidneys (K), liver, spleen (Sp), and bone marrow (BM) of pelvis and spine. Stomach (St) and vena cava had low activity
Whole-body autoradiography images at 90 min clearly show markedly higher radioactivity levels in kidney as compared with liver and spleen, some radioactivity in bone marrow, and the absence of radioactivity in other organs.
Albuleukin
111In labeled Albuleukin showed a strikingly different biodistribution pattern than IL-2 (Figs. 3B, 4B). Six hours after injection, blood concentration was high, 14.3% ID/g, and kidney uptake was low, only 5.49% ID/g. Furthermore, there was significantly higher accumulation of radioactivity in liver, spleen, and lymph nodes, 22, 12 and 15% ID/g, respectively, as compared with the corresponding values for IL-2 (p<0.001). At 30 h after injection, blood concentration dropped to 1.03% ID/g, but high uptake in lymph nodes, spleen, and liver remained or even increased (~22% ID/g in all three organs). At 72 h, blood concentration dropped to 0.06% ID/g, whereas the lymph node, spleen, and liver concentrations were all above 12% ID/g.
Whole-body autoradiography images at 30 h illustrated the absence of accumulation in the kidneys and localization in liver and spleen. Several lymph nodes were also clearly visualized.
Albumin
To investigate the contribution of the IL-2 portion on the biodistribution of Albuleukin, the biodistribution of HSA was also measured for comparison. As shown in Fig. 3C, 111In labeled HSA had a significantly higher concentration in blood, 7.68% and 2.01% ID/g at 30 and 72 h, respectively, as compared with 1.03 and 0.06% ID/g for Albuleukin (p<0.001), but significantly lower uptake in lymph nodes, spleen, and liver (Cmax <12% ID/g) than Albuleukin (Cmax ~22% ID/g, p<0.001).
Discussion
IL-2 is an immunomodulatory agent that does not kill tumor cells directly but rather activates T-cells, which have been shown to infiltrate solid tumors and unleash a chain of events that lead to tumor cell lysis [17]. Objective responses to IL-2 therapy have been achieved in melanoma and renal cell carcinoma [5, 17]. Unfortunately, toxicities of IL-2, such as hypotension, pulmonary edema, and sepsis, have limited its clinical application [5], and treatment of other types of solid tumors with IL-2 has not been as successful. The results of the experiments reported here using 111In-labeled IL-2 confirmed previous reports [1, 2, 9, 18] using IL-2 labeled with radio-iodine or other radionuclides that IL-2 is cleared very rapidly from circulation and that the kidney is the major site of catabolism. IL-2 showed low localization in the liver, spleen, and lymph nodes, and even lower uptake in other tissues. The low uptake in all other tissues may be a consequence of the rapid renal clearance of this molecule. The very small fraction of the IL-2 dose that reaches its intended target may be a factor contributing to poor clinical efficacy.
In contrast, the biodistribution of Albuleukin showed significantly longer circulation, dramatically lower uptake in kidneys, and much higher uptake in lymph nodes, spleen, and liver. Elsewhere, we have reported that the plasma clearance of Albuleukin following i.v. injection in mice is 69-fold lower than that of IL-2 [11]. A slower rate of plasma clearance increases the likelihood that Albuleukin will encounter and bind to its intended receptor [24]. Interestingly, patients who underwent nephrectomy prior to IL-2 immunotherapy (and presumably had a lower rate of IL-2 clearance) had a higher response rate than patients with kidneys in place [25].
Compared with the biodistribution of HSA, Albuleukin showed significantly higher localization in lymph nodes, spleen, and liver, indicating that localization to these tissues is not simply due to albumin but is mediated in part by the IL-2 component of the Albuleukin molecule. As these tissues contain a large percentage of the target T-cells, the results suggest that greater biological activity might follow administration of Albuleukin compared with IL-2. Indeed, a more pronounced anti-tumor effect was obtained with Albuleukin compared with IL-2 when the two molecules were used to treat Renca tumors grown in mice [11]. Also, in melanoma tumor-bearing mice treated for 14 days at their respective maximum tolerated doses of IL-2 (1.0 mg/kg day−1) or Albuleukin (1.75 mg/kg QOD), the frequency of liver metastases was lower and the survival rate was greater in mice receiving Albuleukin compared with IL-2 [11].
It might be expected that the high percentage of T-cells in the thymus would result in high uptake of IL-2 and IL-2 analogs; however, these biodistribution studies demonstrated low uptake of radioactivity of around 5% ID/g for Albuleukin and around 1% ID/g for IL-2, respectively. The low uptake in thymus might reflect that the majority of thymic T-cells are naïve and do not express high levels of the high-affinity IL-2 receptor.
Previous studies have examined the biodistribution of IL-2 in animals using radionuclides such as 125I, 123I, 99mTc, 14C, and 35S, and useful information has been obtained or inferred [1, 4, 9, 13, 18, 19]. These studies demonstrated that the kidney is the site of catabolism of IL-2, and that cathepsin D, a major renal protease, is responsible for its hydrolysis [14]. Pepstatin A, an inhibitor of cathepsin D, increased the circulating half-life of IL-2 in a dose-dependent manner [15]. As IL-2 is rapidly internalized and metabolized, radio-iodine is not a suitable tracer because of its release as free iodine after catabolism of the radio-iodinated protein. In preliminary experiments using radio-iodinated Albuleukin, we concluded that this molecule is also internalized and catabolized based on the observation that the highest concentration of 125I radioactivity was found in the stomach (data not shown), a known site of iodine removal from blood [16], and low uptake of radioactivity was measured in other tissues. Distinct from iodine, 111In remains in a cell even after the radiolabeled protein is internalized and catabolized [20, 26]. 111In is a commonly used radionuclide with favorable physical properties for both pre-clinical and clinical studies. Use of 111In in biodistribution studies not only permits identification of the external sites where the protein is bound but also permits accounting for the internalized radioactivity. To label proteins with 111In, we selected the CHX-A” as the chelating agent because it allows conjugation to proteins under very mild conditions. The conjugation and radiolabeling were done at room temperature with high labeling efficiency, thereby preserving the bioactivity of the proteins. Both IL-2 and Albuleukin retained bioactivity, as shown by a cell-binding assay and a murine CTLL-2 proliferation bioassay.
IL-2, labeled with 123I or 99mTc, has been used as a clinical imaging agent of lymphocyte infiltration [1, 21, 22]. Labeling with 111In could be more advantageous because imaging would reflect more truly the sites of binding, internalization, and catabolism. With a physical half-life of 2.8 days, 111In radiolabel may be ideally suited for monitoring the pharmacokinetics and biodistribution of Albuleukin and IL-2 in real-time with gamma scintigraphy.
Conclusion
In conclusion, Albuleukin, a genetically fused protein of IL-2 and HSA produced as a single polypeptide molecule, showed significantly prolonged circulation and reduced clearance from kidney than IL-2. The higher exposure of Albuleukin in liver, spleen, and lymph nodes where T-cells reside may lead to greater immune stimulation. These biodistribution results provide a rationale for investigating the biological activity of Albuleukin in treating in a broad range of solid tumors. Clinical trials underway will determine whether the improved targeting in the mice translates into a better therapeutic index in humans.
Acknowledgments
Acknowledgements
The authors thank T.S. Migone and C. Ward for assistance with the CTLL-2 cell bioassay, Y. Knight, H.L. Lin, C. Lincoln, J. Meadows, and R. Miceli for help with the animal experiments, and R. Melder, B. Osborn, and D. Russell for discussions.
References
- 1.Chianelli Nucl Med Biol. 1997;24:579. doi: 10.1016/s0969-8051(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 2.Donohue J Immunol. 1983;130:2203. [PubMed] [Google Scholar]
- 3.Donohue Cancer Res. 1984;44:1380. [PubMed] [Google Scholar]
- 4.Gennuso J Biol Response Mod. 1989;8:375. [PubMed] [Google Scholar]
- 5.Kammula Cancer. 1998;83:797. doi: 10.1002/(SICI)1097-0142(19980815)83:4<797::AID-CNCR25>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Katre Proc Natl Acad Sci USA. 1987;84:1487. [Google Scholar]
- 7.Kobayashi J Nucl Med. 1998;39:829. [PubMed] [Google Scholar]
- 8.Konrad Cancer Res. 1990;50:2009. [PubMed] [Google Scholar]
- 9.Koths Cell Molec Biol Lymphokines. 1985;3:779. [Google Scholar]
- 10.Kwack Mol Cell. 2000;10:575. doi: 10.1007/s100590000014. [DOI] [Google Scholar]
- 11.Melder 94th Annual Meeting of the American Association for Cancer Research. 2003;44:1298. doi: 10.1188/03.ONF.741-742. [DOI] [PubMed] [Google Scholar]
- 12.Milenic Nucl Med Biol. 2002;29:431. doi: 10.1016/S0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 13.Nadeau Drug Metab Dispos. 1995;23:904. [PubMed] [Google Scholar]
- 14.Ohnishi Tumour Biol. 1989;10:202. doi: 10.1159/000217617. [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi Cancer Res. 1990;50:1107. [PubMed] [Google Scholar]
- 16.Regoeczi E (1987) The body iodine pool. CRC Press, Boca Raton, Florida, p 48
- 17.Rosenberg Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 18.Sabo Lymphokine Cytokine Res. 1992;11:229. [PubMed] [Google Scholar]
- 19.Sands Int J Immunopharmacol. 1989;11:411. doi: 10.1016/0192-0561(89)90088-X. [DOI] [PubMed] [Google Scholar]
- 20.Sharkey Cancer Res. 1990;50:2330. [PubMed] [Google Scholar]
- 21.Signore Lancet. 1987;2:537. doi: 10.1016/S0140-6736(87)92925-4. [DOI] [PubMed] [Google Scholar]
- 22.Signore Nucl Med Commun. 1992;13:713. [PubMed] [Google Scholar]
- 23.Smith Science. 1988;240:1169. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 24.Sung Cancer Res. 1990;50:7382. [PubMed] [Google Scholar]
- 25.Wagner J Urol. 1999;162:43. [Google Scholar]
- 26.Yao J Nucl Med. 2001;42:1538. [PubMed] [Google Scholar]