Abstract
Immunosuppression is often identified in cancer patients. The aim of this study was to evaluate several immune parameters for patients with breast and lung cancer. Immunophenotyping analysis showed that the cancer patients investigated had significantly lower absolute numbers of peripheral blood lymphocytes than controls. The immunosuppression was more evident for the breast cancer subgroup. The most severe immune defect noticed was the marked impairment of IFN-γ secretion. A shift toward the Th2 phenotype as revealed by assessment of intracellular level of IFN-γ and IL-4 was also noticed. The secretion of proinflammatory cytokines IL-1β and TNF-α in whole blood cultures was not impaired. Although the proportion of activated cells was slightly lower than in the control group, our results showed that both peripheral T lymphocytes and NK cells of cancer patients could be induced to express early activation marker CD69 after ex vivo mitogen stimulation. In conclusion, our study revealed several immune defects in cancer patients. This suggests that an appropriate immunotherapeutical approach might be used to restore compromised immune functions with beneficial effects on both antitumor and general immunity.
Keywords: Breast cancer, CD69, Cytokine, Flow cytometry, Immunosuppression, Lung cancer
Introduction
A large body of evidence indicates that immunosuppression is associated with cancer, but the mechanisms underlying the immune defects noticed in cancer patients have not been fully elucidated [7, 5]. It has been shown that individuals with severe immunity deficits have a higher probability of developing cancers, and for a number of cancer patients, the signs of immunosuppression are evident even at the time of presentation to the physician [5, 12]. On the other hand, progressive tumor growth could lead to development of cellular immune deficiency involving both T lymphocytopenia and dysfunction [7, 9]. Specific antineoplastic therapy such as multidrug chemotherapy or radiotherapy has also been implicated in the impairment of several immune parameters [7, 9, 17]. Considerable effort has been made to find modalities to overcome the immune deficiency of cancer patients to improve both antitumor immunity and general immune functions. Therefore, it could be extremely useful to evaluate the immune status in cancer patients at different time points to identify the origin of immunosuppression and optimize the treatment for a maximum benefit with the fewest undesirable side effects.
This study was undertaken to evaluate several immune parameters of patients with breast and lung cancer, to examine the possibility of immune intervention aimed at improving their immune status.
Materials and methods
Patients
Eleven patients with breast cancer and 15 patients with lung cancer were included in this study after informed consent. Eleven healthy volunteers were included in the study as the control group. The main characteristics of the investigated subjects are summarized in Table 1. The age distribution between patients and controls was statistically similar (Student’s t-test).
Table 1.
Characteristics of cancer patients and healthy controls
| Characteristics | Number of subjects | ||
|---|---|---|---|
| Breast (n=11) | Lung (n=15) | Controls (n=11) | |
| Age (years) | |||
| Mean ± SD | 56±7.6 | 56.6±13.3 | 52.1±9.3 |
| Range | 51.5–60.5 | 49.9–63.3 | 42.8–61.4 |
| Gender | |||
| Male | – | 10 | 6 |
| Female | 11 | 5 | 5 |
| Clinical stage | |||
| I–II | 11 | – | Not applicable |
| III–IV | – | 15 | |
| Metastasis | – | 5 | Not applicable |
| Prior antineoplastic treatment | |||
| None | 6 | 7 | Not applicable |
| Chemotherapy | 5 | 8 | |
Whole blood cultures and cytokine measurement
Peripheral blood samples were mixed with equal volumes of RPMI 1640 (Sigma, St Louis, MO, USA) culture medium containing 5 U of heparin per ml, dispensed on 96-well tissue culture plates (250 μl/well), and incubated at 37°C in 5% CO2 for 24 h. Cultures were stimulated with lipopolysaccharide (10 μg/ml; LPS Escherichia coli; Sigma, St Louis, MO, USA) for TNF-α and IL-1β and with phytohemagglutinin (20 μg/ml; PHA; Sigma, St Louis, MO, USA) for the IFN-γ production. At the end of incubation, samples collected from five wells were centrifuged (quick run; 10 s) and the supernatants were stored at −70°C until tested for cytokine secretion. The level of cytokines in the samples was determined by enzyme-linked immunosorbent assays, using DuoSet ELISA Development Systems (R&D Systems).
For determination of CD69 expression, whole blood samples were incubated for 18–24 h in the absence or in the presence of PHA (20 μg/ml) or LPS (10 μg/ml).
Flow cytometry analysis
The percentages of different lymphocyte subsets were evaluated by two-color flow cytometric analysis using a Simultest IMK Plus kit (BD Biosciences, San Jose, CA, USA) according to the instructions of the manufacturer. Briefly, 100 μl of blood was added to 20-μl of different fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated monoclonal antibody (mAb) combinations and incubated for 30 min at room temperature in the dark. After incubation, 1 ml of provided Lysing Solution diluted 1:10 was added to samples to lyse erythrocytes. After centrifugation and washing, the samples were fixed using 0.5 ml of 1% paraformaldehyde. The following mAb combinations were used: CD3 FITC/CD19 PE, CD4 FITC/CD8 PE, CD3 FITC/anti-HLA-DR PE, and CD3 FITC/CD16+CD56 PE. Isotype controls using IgG1 FITC/IgG2a PE were included in each experiment. Samples were analyzed within 24 h using a BD FACS Calibur flow cytometer with Cell Quest software for data acquisition and analysis. A lymphocyte acquisition gate was set on a forward scatter / side scatter (FSC/SSC) light dot plot, and data for a minimum of 5,000 events were acquired in the lymphocyte gate and analyzed.
The expression of CD69 upon activation was determined on the surface of T lymphocytes after PHA stimulation and on the surface of NK cells after PHA or LPS stimulation. After 24 h of incubation, whole blood samples (100 μl) were mixed with 10 μl of CD3 FITC/CD16+CD56 PE reagent, and 10 μl of anti-CD69 antibody tagged with peridinin chlorophyll protein (PerCP). Incubation with conjugation antibodies, lysis, wash, and fix steps were performed as for phenotyping analysis. Acquisition gates were set on a FL1/SSC dot plot for T lymphocytes (CD3+) and a FL2/SSC dot plot for NK cells (CD16+CD56+). Data for a minimum of 5,000 events were acquired within these gates. The percentages of CD69+ cells were determined in histograms of CD69-PerCP fluorescence established for the gated populations of T lymphocytes and NK cells, respectively.
For detection of intracellular IFN-γ and IL-4, the FastImmune Cytokine System (BD Biosciences, San Jose, CA, USA) was used according to the manufacturer’s instructions. Briefly, heparinized peripheral blood samples were stimulated with 25 ng/ml phorbol 12-myristate 13-acetate (PMA), 1 μg/ml of ionomycin, and 10 μg/ml of brefeldin-A at 37°C in 5% CO2 for 4 h. For surface staining, whole blood samples were then incubated with anti-CD3-PerCP antibodies for 30 min at room temperature in the dark. After lysis of erythrocytes, 500 μl FACS permeabilizing solution was added, and samples were incubated for an additional 10 min at room temperature in the dark. For staining of intracellular cytokines, 20 μl of FITC-conjugated anti-IFN-γ/PE-conjugated anti-IL-4 antibodies was added, and samples were incubated for 30 min at room temperature in the dark. FITC-conjugated IgG2a/PE-conjugated IgG1 antibodies were used as negative controls. After washing, samples were suspended in 500 μl of 1% paraformaldehyde and analyzed using a flow cytometer. A minimum of 10,000 events was acquired in a T-lymphocyte gate set on a FL3/SSC.
Statistical analysis
Data were statistically analyzed using paired Student’s t-test for two-tailed values. Values for p less than 0.05 were considered significant. Correlation was evaluated using the Correl function of Microsoft Excel program. Results were presented as the mean ± SD.
Results
Lymphocyte phenotype
Flow cytometric analysis revealed that cancer patients had generally lower percentages of lymphocyte sets and subsets in peripheral blood as compared to controls, but the difference was not statistically significant (data not shown). One notable exception was the proportion of lymphocytes in the lung cancer group (21.7±8.2 vs 30.2±6.6 for the control, p<0.05). In addition, the proportion of activated T cells (i.e., CD3+HLA-DR+) in the group of lung cancer patients was significantly greater than that for control subjects (19.2±10.6 vs 8.2±3.9). When the absolute number of cells was calculated on the basis of total cell counts, more differences became evident (Table 2).
Table 2.
Immunophenotyping analysis for cancer patients and normal subjects. Values indicate average and range (95% confidence interval) for the total number of cells/μl
| Parameter | Total number | ||
|---|---|---|---|
| Breast cancer (n=11) | Lung cancer (n=15) | Controls (n=11) | |
| White blood cells | 4,954 (4,090–5,818)** | 8,060 (6,667–9,453) |
7,550 (6,149–8,951) 7,411 (6,404–8,418) |
| Granulocytes | 2,947 (2,285–3,609)** | 5,582 (4,397–6,767) | 4,513 (3,675–5,351) |
| Monocytes | 361 (268–454) | 417 (291–543) | 433 (390–476) |
| Lymphocytes | 1,427 (1,112–1,742)** | 1,630 (1,393–1,867)** | 2,229 (1,831–2,627) |
| B lymphocytes (CD19+) | 147 (90–204) | 87 (60–114)** | 181(107–255) |
| T lymphocytes (CD3+) | 953 (715–1,191)** | 1,141 (933–1,349)* | 1,594 (1,267–1,921) |
| T helper cells (CD4+) | 576 (398–754)* | 614 (469–759)* | 886 (692–1,080) |
| T cytotoxic/suppressor cells (CD8+) | 403 (305–501)** | 597 (476–718) | 735 (592–878) |
| CD4/CD8 | 1.52 (1.07–1.97) | 1.21 (0.84–1.58) | 1.22 (1.02–1.44) |
| NK cells (CD16+CD56+) | 213 (106–320) | 330 (251–409) | 328 (207–449) |
| Activated T lymphocytes (CD3+HLA-DR+) | 121 (89–153) | 208 (137–279) | 131 (81–181) |
*p<0.05; **p<0.01 (Student’s t-test)
As can be seen, cancer patients had significantly lower absolute numbers of lymphocytes than healthy subjects, the difference being more prominent for the breast cancer group. For these latter patients, statistically significant lower values for both subsets of T lymphocytes (CD4+ and CD8+) were obtained. In the lung cancer group, a significant decrease in the total number of both B and T lymphocytes (CD4+ subset) was noticed. Although not significant, a slight increase in the total number of CD3+HLA-DR+ cells was also obtained for lung cancer patients versus healthy subjects. The number of granulocytes was significantly lower than controls in the group of breast cancer patients, while lung cancer patients presented comparable or even higher values than healthy subjects. These differences between the two groups of patients seem to reflect subtle differences in the immune mechanisms of the two types of cancer as well as in the way these are affected by the tumor.
When the patients were considered according to the antineoplastic treatment they received previously (i.e., chemotherapy vs none), several differences came out. Thus, for the breast cancer patients treated with systemic chemotherapy (5 out of 11), the total number of lymphocytes was significantly lower than controls (1,378±429 vs 2,229±609, p<0.05). The differences were significant for both subsets of T lymphocytes, CD4+ (425±176 in breast cancer patients vs 886±297 in healthy control group, p<0.05) and CD8+ (420±153 in breast cancer patients vs 735±219 in healthy control group, p<0.05). In the case of patients investigated before starting chemotherapy, the decrease was only noticed for the CD8+ subset (388±191 vs 735±219, p<0.05). In the lung cancer group, the difference was more remarkable between patients who received chemotherapy and those who did not. For the patients who did not receive chemotherapy (7 out of 15), we obtained a significantly lower number of B lymphocytes (85±36 vs 181±113 in controls, p<0.05). On the contrary, the treated patients had significantly lower numbers of both B lymphocytes (90±68 vs 181±113 in controls, p<0.05), T lymphocytes (1,028±430 vs 1,594±501 in controls, p<0.05) and the CD4+ subset (496±285 vs 886±297 in controls, p<0.05). Although the differences were not statistically significant, the number of T lymphocytes expressing the activation marker HLA-DR was slightly increased in both subgroups of lung cancer patients (174±72 in treated patients and 242±178 in untreated patients) when compared with the control subjects (131±77).
Cytokine production
Cytokines represent key soluble mediators of immune response. The ability of peripheral blood immune cells from cancer patients and controls to produce cytokines ex vivo was investigated in a whole blood assay. Samples were incubated for 24 h in the presence of LPS for induction of proinflammatory cytokines and of PHA for IFN-γ. The level of cytokines in whole blood culture supernatants is presented in Table 3.
Table 3.
Cytokines concentration in whole blood culture supernatants
| Cytokine | Stimulus | Mean value (pg/ml) | ||
|---|---|---|---|---|
| Breast (n=11) | Lung (n=15) | Controls (n=11) | ||
| TNF-α | LPS | 7,025±4,538 | 11,148±5,151 | 8,203±4,499 |
| IL-1β | LPS | 7,930±4,842 | 10,125±5,847 | 7,108±2,588 |
| IFN-γ | PHA | 644±826*** | 1,811±2,294* | 5,036±2,211 |
*p<0.05, compared to controls; ***p<0.001, compared to controls,
From data in Table 3, it can be seen that while in the group of breast cancer patients the level of inflammatory cytokines (TNF-α and IL-1β) was comparable to the controls, the values for lung cancer patients were higher. Although not statistically significant, this increase in the level of inflammatory cytokines for lung cancer patients probably reflects the inflammatory status associated with the disease. In terms of immune reactivity, our results suggest that the secretion of IL-1 and TNF-α is not impaired in peripheral blood cells of cancer patients. As expected, a positive correlation was found between the values for TNF-α and IL-1 for both groups of cancer patients (p=0.664).
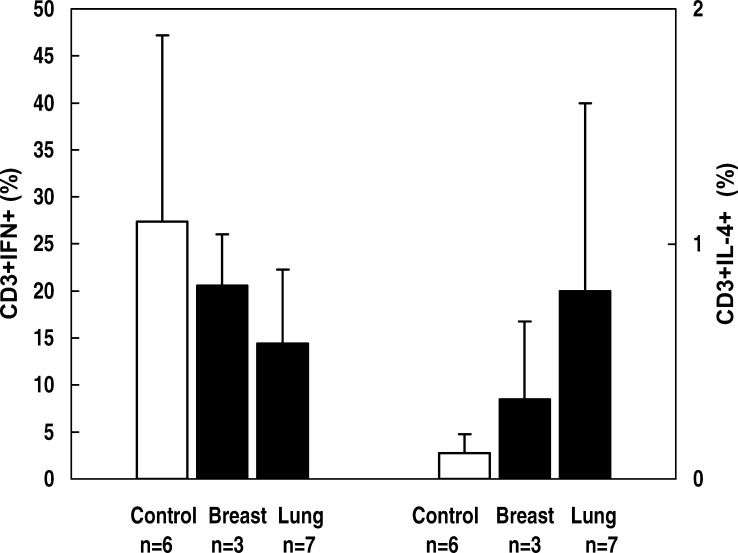
On the contrary, when comparing the level of IFN-γ, significantly lower values were noticed in both groups of cancer patients than in controls. Although most of the patients were T lymphocytopenic, no correlation was found between IFN-γ concentration and the number of T lymphocytes, which suggests a defect at the level of Th1-type cytokine secretion. The impairment in IFN-γ production could have dramatic consequences, as it is known that an effective antitumor immunity involves the participation of Th1 cells. The fact that nine patients (three with lung cancer and six with breast cancer) did not produce IFN-γ at all is noteworthy. Among them, seven patients (three in the group with lung cancer and four in the group with breast cancer) were analyzed before starting chemotherapy. It appears that the impairment of IFN-γ production was a characteristic of a compromised immune status in cancer patients rather than a direct consequence of chemotherapy. To test the possibility of a shift toward Th2 phenotype, we checked for IL-4 production in the same samples of PHA-stimulated whole blood culture supernatants used for IFN-γ measurement. The level of IL-4 in mitogen-stimulated cultures in both cancer patients and controls was below the detection limit of the ELISA test. For a limited number of patients (three breast cancer and seven lung cancer), we tested the capacity to produce Th1- versus Th2-type cytokines by measuring the proportion of T lymphocytes producing IFN-γ and IL-4, respectively, compared to six healthy subjects. As unstimulated cells produce few or no cytokines, we induced cytokine gene expression by stimulating peripheral whole blood samples with PMA and ionomycin. The proportions of CD3+ IFN-γ+ and CD3+ IL-4+ cells in stimulated samples are shown in Fig. 1.
Fig. 1.
Proportion of IFN-γ+ and IL-4+ T lymphocytes in cancer patients and controls. Whole blood samples were incubated with PMA (25 ng/ml), ionomycin (1 μg/ml), and brefeldin-A (10 μg/ml) for 4 h. Stimulated samples were labeled with CD3-PerCP for surface staining and with IFN-γ–FITC/IL-4–PE for intracellular cytokines. Cells producing cytokines were identified by three-color flow cytometric analysis. Figure indicates percentages of IFN-γ+ and IL-4+ cells from gated CD3+ cells (T lymphocytes). Data are representative of samples from three patients with breast cancer, seven patients with lung cancer, and six healthy controls
From the data presented, it can be seen that the proportion of IFN-γ–producing T lymphocytes was lower in both groups of cancer patients as compared to healthy subjects, although the differences were not statistically significant—probably due to the high variability even in the control group. At the same time, the percentages of IL-4–secreting T lymphocytes were higher in cancer patient groups. Our results might suggest that a shift toward a Th2 phenotype could occur in cancer patients; however, the limited number of subjects investigated did not allow an accurate estimation of Th1/Th2 balance. We also noticed the presence of double-positive cells in both groups of cancer patients, i.e., cells secreting both cytokines (mean value = 0.6%, as compared to 0.2% for the control).
CD69 expression on activated T lymphocytes and NK cells
The functional capacity of T lymphocytes and NK cells was assessed by measuring the expression of CD69 on the cell surface upon activation. Whole blood samples from ten breast cancer patients, ten lung cancer patients, and eight healthy subjects were stimulated for 24 h with PHA (20 μg/ml) or LPS (10 μg/ml). Preliminary titration experiments showed that CD69 expression increased with the concentration of PHA and LPS, respectively. The concentrations that induced the largest proportion of cells expressing surface CD69 and did not affect cell viability were chosen as work concentrations. The proportions of activated CD69-positive cells in the population of T lymphocytes (CD3+) and NK cells (CD56+CD16+), respectively, were measured. As can be seen in Fig. 2, both T lymphocytes and NK cells of cancer patients could be activated by mitogen stimulation. Although breast cancer patients showed slightly lower values of CD69 expression on T lymphocytes and NK cells upon PHA, the level was, in general, comparable to healthy controls.
Fig. 2a, b.

CD69 surface expression on T lymphocytes (a) and NK cells (b). Whole blood samples were cultured in the absence or presence of PHA (20 μg/ml) or LPS (10 μg/ml) for 24 h. Stimulated samples were labeled with CD3 FITC, CD16CD56 PE, and CD69 PerCP. Surface expression of CD69 was determined by three-color flow cytometric analysis. Analysis was performed by gating on CD3+ cells (T lymphocytes) or CD16+CD56+ cells (NK cells), and dead cells were excluded by forward/side scatter gating. Figures indicate percentages of CD69+ cells and are representative of samples from ten patients with breast cancer, ten patients with lung cancer, and eight healthy control subjects
Discussion
It is generally accepted that immunosuppression is associated with cancer and becomes more evident as the disease progresses. Besides increasing the risk of infections, immunodeficiency may also contribute to the progression of cancer [20]. Several immunosuppressive molecules are produced by the tumors (such as IL-10, TGFβ, or COX-2 metabolites), but specific therapies such as chemotherapy and radiotherapy contribute to alteration of immune system function to a great extent [3, 5]. Evaluation of overall immunocompetence of cancer patients could help to guide and monitor the treatment and to identify candidates for specific immune intervention (i.e., immunotherapy).
In the present investigation, we studied several immune parameters in two groups of cancer patients with the aim of identifying the degree of immunosuppression. The patients were either evaluated prior to chemotherapy (7 patients out of 15 in the lung cancer group and 6 out of 11 in the breast cancer group) or at least 6 months after the last series of chemotherapy.
Immunophenotyping analysis showed that cancer patients had significantly lower absolute numbers of peripheral blood lymphocytes than the controls (Table 2), which is in accordance with other published data [7, 15, 18]. In our study, lymphopenia was more evident in the group of breast cancer patients than in lung cancer patients, although the latter were all in more advanced stages of the disease. In both groups, patients who received chemotherapy presented a more dramatic decrease of the total number of lymphocytes, which affected more cellular types. This argues for a severe and long-term impact of chemotherapy on immune cells, as we tested the patients at least 6 months after the last series of cytostatic agents. A trend toward lower values of NK cells was evident again for the breast cancer group, but the difference was not significant. While the total number of T lymphocytes was lower in the lung cancer group, the proportion of CD3+ cells expressing the HLA-DR marker was increased as compared to healthy subjects. This observation was in accordance with other published data which describe the induction of HLA class II expression on peripheral blood T lymphocytes following thoracic radiochemotherapy [9]. However, we noticed this abnormality for all the investigated lung cancer patients irrespective of their prior treatment. Regarding the granulocytes, the absolute number was significantly lower in the breast cancer group but higher in the lung cancer patients compared with controls, probably due to inflammation associated with lung cancer. Our results showed that in terms of the total number of immunocompetent cells, immunosuppression was more pronounced in cancer patients who received chemotherapy. According to current knowledge, lymphopenia could be a consequence of antineoplastic cytostatic therapy, but it might be also an intrinsic characteristic of the advanced cancer stages. In our study, we noticed a decreased number of lymphocytes in all patients, irrespective of their clinical stage. Therefore, we hypothesized that it was probably due to the prior chemotherapy. The CD4 lymphopenia noticed in these patients was considered particularly relevant, as it might lead to impaired cell-mediated immunity. Our data are consistent with other studies which showed that whereas CD8+ T-cell numbers could recover relatively rapidly, CD4+ T cells could take much longer to recuperate after intensive chemotherapy [17]. The reduced number of competent cells in peripheral blood could seriously affect immune functions, including antitumor immunity.
To evaluate the global immune reactivity of cancer patients, we investigated the capacity of peripheral blood cells isolated from these patients to produce cytokines. Our results showed that the level of inflammatory cytokines IL-1β and TNF-α in whole blood cultures of breast cancer patients was comparable to those obtained for normal subjects. In accordance with other studies, we detected increased levels of these cytokines for patients with lung cancer [13]. The elevated levels of proinflammatory cytokines such as IL-1β and TNF-α are considered as markers of macrophage activation, which could activate cellular immune response. However, for the same patients, as well as for the breast cancer group, the secretion of IFN-γ was strongly impaired. The deficient production of IFN-γ was noticed for both subgroups of patients (i.e., with or without chemotherapy), so it might be presumed that the reduced level of IFN-γ secretion was not merely an adverse effect of cytotoxic antineoplastic therapy. A reduced ability of blood lymphocytes from cancer patients to respond to mitogen stimulation in terms of IFN-γ secretion, despite a normal production of TNF-α, IL-1α, and IL-1β, was reported by other investigators as well [6]. The impaired production of IFN-γ upon mitogen stimulation was the most severe immune defect noticed in this study. In fact, a significant proportion of patients investigated before chemotherapy (7 out of 13) did not produce IFN-γ at all. Therefore, although we noticed that chemotherapy produced severe lymphopenia which persisted for several months, it might be possible that other factors, such as soluble factors secreted by tumor cells, have been involved in the diminished ability of lymphocytes in cancer patients to produce IFN-γ. Moreover, there was no correlation between the total number of T lymphocytes and NK cells and the ability to secrete IFN-γ ex vivo suggesting a dysregulation at the level of Th1-type cytokines secretion. The impaired production of IFN-γ together with a reduced expression of perforin noticed in cancer patients are considered as major alterations of cell-mediated immunity [21]. The antitumor activity of IFN-γ is complex and involves several mechanisms, such as up-regulation of MHC class I and class II molecules, shaping tumor immunogenicity, effects on cell proliferation, and antiangiogenesis [1, 16].
Current concepts in tumor immunology consider the production of Th1-promoting cytokines to be essential for an efficient antitumor immune response. Besides the enhancement of tumor-specific cytotoxic activity, Th1-type cytokines could also reduce tumor development by affecting several inflammatory and angiogenic mediators. As dysregulation of Th1/Th2 balance was noticed in cancer patients and animal models, agents such as cytokines and adjuvants able to promote a Th1-type immune response have been proposed to be used in several experimental studies and clinical settings [4, 14]. In our experiments, for a limited number of cancer patients, the mean value of the proportion of IFN-γ–secreting T lymphocytes was lower, while the mean value for IL-4 was greater, than controls. The differences were more evident for the lung cancer group than for the breast cancer group, and this might reflect the fact that lung cancer patients were in more advanced stages of the disease. However, the limited number of cases did not allow us to conclude that a shift toward a Th2 phenotype was a characteristic of cancer patients, and further studies are necessary to elucidate this issue. Another interesting observation was the presence of a subset of “double-positive” T lymphocytes, i.e. cells producing both IFN-γ and IL-4, in the samples from cancer patients.
Focusing on main cytotoxic effector cells of antitumor immune response, we looked for the activation potential of T lymphocytes and NK cells. Using a whole blood assay, we measured, by flow cytometry, the expression of early activation marker CD69 on the cell surface following stimulation with PHA for T lymphocytes, and PHA and LPS for NK cells. CD69 is not usually expressed on resting cells, but it is one of the earliest antigens expressed on T lymphocytes upon in vitro stimulation. For this reason, the expression of CD69 on the surface of T lymphocytes has been proposed as a method to assess cell activation and function [11]. Several studies have shown that stimulation with microbial compounds (such as LPS or muramyl dipeptide, MDP), mitogens (PHA), or even antigens could also induce the expression of CD69 on NK cells [2, 8, 10]. It was suggested that the high expression of CD69 is a consequence of NK activation by cytokines from stimulated T cells [19]. Our results showed that both peripheral T lymphocytes and NK cells of cancer patients could be induced to express CD69 after 24 h of stimulation. Moreover, all patients were responsive for both types of cells. With the exception of breast cancer patients who presented slightly lower values upon PHA stimulation, the proportion of cells expressing CD69 was comparable between patients and healthy subjects. This was an important finding because it suggested that effector cells in cancer patients could be activated.
The relatively small number of the patients was one limitation of the current study. Despite this limitation, we were able to identify several aspects regarding the immune status of cancer patients. In agreement with literature data, reduced numbers of immunocompetent cells were obtained for cancer patients as compared to healthy subjects. The decrease in the total number of lymphocytes was more marked for those patients who underwent chemotherapy, although they were treated at least 6 months (usually 1 year) prior to this study. However, our results suggest that, besides chemotherapy, several other factors might contribute to the immunosuppression seen in cancer patients. The impairment in secretion of IFN-γ, which is considered the most severe immune defect observed in this study, was not a consequence of chemotherapy, as it was manifest to a great extent in untreated cancer patients.
Another interesting observation was that immunosuppression was more evident for breast cancer patients than for lung cancer patients, although we were not able to find a plausible explanation for this difference. It might be possible to get more information concerning this issue by selecting other immune parameters for investigation which might be more relevant to local immunity, especially in lung cancer. Interestingly, the immunosuppression was not a general phenomenon. We were able to identify several immune parameters, such as expression of early activation marker CD69 on T lymphocytes and NK cells upon ex vivo activation and secretion of proinflammatory cytokines TNF-α and IL-1, which were not impaired in cancer patients. This is considered an important finding because it suggests that immune cells of cancer patients could be responsive and an appropriate immunotherapeutical approach might be useful to restore compromised immune functions with beneficial effects on both antitumor and general immunity.
In conclusion, our data confirm the literature data regarding cancer-associated immunosuppression and suggest that evaluation of further immune parameters could be useful to get a complete view of the complexity of immune reactivity of cancer patients.
Acknowledgements
This work was supported by a grant from the Romanian Ministry of Education, Research and Youth (Program Biotech, grant 097). The authors gratefully acknowledge the skillful technical assistance of Camelia Tabarta, Rodica Tudorache, and Florica Chioseolu. The contribution of Iuliana Francisca Serbanescu to data analysis is also appreciated.
References
- 1.Beatty J Immunol. 2001;166:2276. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 2.Clausen Immunobiology. 2003;207:85. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 3.De Chest. 2000;117:365. doi: 10.1378/chest.117.2.365. [DOI] [PubMed] [Google Scholar]
- 4.Dredge Cancer Immunol Immunother. 2002;51:521. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 6.Fischer Ann Oncol. 1995;6:921. doi: 10.1093/oxfordjournals.annonc.a059360. [DOI] [PubMed] [Google Scholar]
- 7.Haden Int Immunopharmacol. 2003;3:1061. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 8.Haller Infect Immun. 2000;68:752. doi: 10.1128/IAI.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidecke Surgery. 2002;132:495. doi: 10.1067/msy.2002.127166. [DOI] [PubMed] [Google Scholar]
- 10.Heizelmann Immunopharmacol. 2000;48:117. doi: 10.1016/S0162-3109(00)00195-8. [DOI] [Google Scholar]
- 11.Lim Clin Diagn Lab Immunol. 1998;5:392. doi: 10.1128/cdli.5.3.392-398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackall Stem Cells. 2000;18:10. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 13.Matanic Scand J Immunol. 2003;57:173. doi: 10.1046/j.1365-3083.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto Int Immunopharmacol. 2001;1:1559. doi: 10.1016/S1567-5769(01)00071-6. [DOI] [PubMed] [Google Scholar]
- 15.Melichar Scand J Clin Lab Invest. 2001;61:363. doi: 10.1080/003655101316911404. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran Nature. 2001;410:1107. [Google Scholar]
- 17.Steele Leuk Res. 2002;26:411. doi: 10.1016/S0145-2126(01)00138-2. [DOI] [PubMed] [Google Scholar]
- 18.Tong Am J Clin Oncol. 2000;23:463. doi: 10.1097/00000421-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Werfel Eur J Allergy Clin Immunol. 1997;52:465. [Google Scholar]
- 20.Woo Cancer Res. 2001;61:4766. [PubMed] [Google Scholar]
- 21.Zanussi Cancer Immunol Immunother. 2003;52:28. doi: 10.1007/s00262-002-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]