Abstract
Multiple myeloma (MM) cells produce monoclonal immunoglobulin (Ig) which serves as a truly tumor-specific antigen. The tumor-specific antigenic determinants are localized in the variable (V)-regions of the monoclonal Ig and are called idiotopes (Id). We review here the evidence obtained in a T-cell receptor (TCR) transgenic mouse model that Id-specific, MHC class II–restricted CD4+ T cells play a pivotal role in immunosurveillance and eradication of MHC class II-negative MM cells. In brief, monoclonal Ig secreted by MM cells is endocytosed and processed by antigen-presenting cells (APCs) in the tumor. Such tumor-resident dendritic cell APCs in turn present Id peptide on their class II molecules to Id-specific CD4+ T cells which become activated and indirectly kill the MHC class II-negative myeloma cells. However, if the Id-specific CD4+ cells fail to eliminate the MM cells during their initial encounter, the increasing number of tumor cells secretes so much monoclonal Ig that T-cell tolerance to Id is induced. Extending these findings to MM patients, Id-specific immunotherapy should be applied at a time of minimal residual disease and when new Id-specific T cells have been educated in the thymus, like after high-dose chemotherapy and autologous stem cell transplantation.
Keywords: Cancer, Idiotype, Immunosurveillance, T cells, Tolerance
Introduction
Targets for immune attack on tumor cells
Immunotherapy of cancer rests on the premise that tumors express antigens that serve as targets for immune-mediated attack. Such targets may be differentiation antigens, cancer-testis antigens, or truly tumor-specific antigens caused by somatic mutations or DNA rearrangements unique to the tumor cell [69]. The differentiation and cancer-testis antigens have an advantage in that they may be expressed by most tumors of a certain type, such as, e.g., malignant melanoma; therefore, a single universal vaccine may be applied to most patients with the same cancer disease. There are major drawbacks, however. Because differentiation antigens and cancer-testis antigens are also expressed to some extent in normal tissues, the immune system is often tolerant and only low-avidity responses may be induced, even by powerful vaccination. Moreover, if an immune response is elicited, autoimmunity might follow, like vitiligo induced by antimelanoma vaccines [49]. Truly tumor-specific antigens do not suffer from these shortcomings. However, their use is limited by the fact that it is usually difficult to find unique tumor-specific antigens for a given cancer.
Ig V-region idiotypes are truly tumor-specific antigens on B-cell tumors
B-cell malignancies are distinct from other types of cancer in that a highly tumor-specific antigen can be defined with ease, namely the variable (V)-regions of the monoclonal immunoglobulin (Ig) that each B-cell tumor clone produces. This is so because the vast diversity among Ig V-regions, contributed mainly by clonal rearrangements of V(D)J gene segments as well as somatic hypermutation [66], generates unique antigenic determinants in the V-regions of each monoclonal Ig. These V-region antigenic determinants are called idiotopes, and the sum of the idiotopes represent the idiotype (Id) of the monoclonal Ig. Id has distinct advantages as a tumor-specific antigen. First, because V-regions of Ig H and L chains may be amplified and sequenced for each individual B-cell tumor, tailor-made DNA-based Id vaccines can be relatively easily made for each patient. Second, because monoclonal Ig can readily be purified from patient serum or transfected cells, protein-based Id vaccines may be prepared without too much effort.
Basic immunology: Id vaccination elicits B- and T-cell responses in mice
Early studies demonstrated that Id is immunogenic in mice of the same inbred strain in which the B-cell tumor originally arose [58]. This observation is important because a premise for Id vaccination is that patients with B-cell malignancies must be able to respond to the Id of their own M-component. Id vaccination of syngeneic mice induced both anti-Id antibodies [58] and Id-specific T cells [25, 26].
Later studies demonstrated that Id-specific CD4+ T cells could be cloned and that they recognize short V-region sequences, called Id peptides, presented on major histocompatibility complex (MHC) class II molecules [3, 6, 7]. For this to occur, Ig has to be partially degraded (processed) by antigen-presenting cells (APCs) in order to generate short Id peptides that bind MHC class II molecules [70]. Such antigen processing of Ig for MHC class II presentation occurs in two ways. First, as in the conventional MHC class II pathway, Ig in extracellular fluid can be endocytosed by professional APCs like dendritic cells (DCs) that process the Ig and then present Id-peptides on their class II molecules to CD4+ T cells [6, 18, 19, 70]. Second, B-lymphoma cells [15, 56, 70, 71] as well as transgenic and normal B cells [46, 60] constitutively process their endogenously produced Ig and present Id peptides on their class II molecules to Id-specific T cells. In addition to Id-specific CD4+ T cells, Id-specific CD8+ cells have also been described. In the latter case, myeloma cells process endogenous Ig and present V-region peptides on MHC class I molecules to CD8+ T cells [14, 75].
Immunotherapy of MM in mice and humans: relevance of Id-specific antibodies and Id-specific CD4+ and CD8+ T cells
Given that both Id-specific antibodies and T cells are elicited by immunization with Id, what role does each of them play in elimination of MM? Id-specific antibodies are unlikely to play a major role, the reason being that myeloma cells secrete large quantities of myeloma protein resulting in a high concentration of Id+ Ig in the extracellular fluid. Such large amounts of ubiquitous myeloma protein are expected to block Id-specific antibodies long before they reach the surface of myeloma cells. And even if Id-specific antibodies were not completely blocked, myeloma cells express little or no surface Ig so that Id-specific antibodies should most often be of little consequence (Fig. 1a). As concerns Id-specific CD8+ T cells, they might well have a role in immunosurveillance because myeloma cells usually express MHC class I molecules. Indeed, a mouse myeloma cell line MOPC21 was shown to process its endogenous Ig and present an Id peptide (representing amino acids 49–58) on the MHC class I molecule H-2Kd to cloned cross-reactive influenza hemagglutinin–specific CD8+ T cells [14] (Fig. 1b). Similar evidence has also been obtained with human cells in vitro [38, 74]. However, despite these studies, there is not yet much information on the role of Id-specific CD8+ T cells in vivo in MM. As concerns Id-specific CD4+ T cells, their role in elimination of myeloma cells in vivo, and their mechanism of action, has been firmly established particularly in the MOPC315 mouse model. Myeloma protein secreted by the tumor is endocytosed and processed by APCs within the tumor. Resulting Id peptides are presented on MHC class II molecules to Id-specific CD4+ T cells that, once activated, indirectly kill the tumor cells (Fig. 1c) [9, 18, 19, 35, 36]. Id-specific CD4+ T cells have also been described in humans (see below), but their relevance to eradication of MM cells in patients is largely unknown.
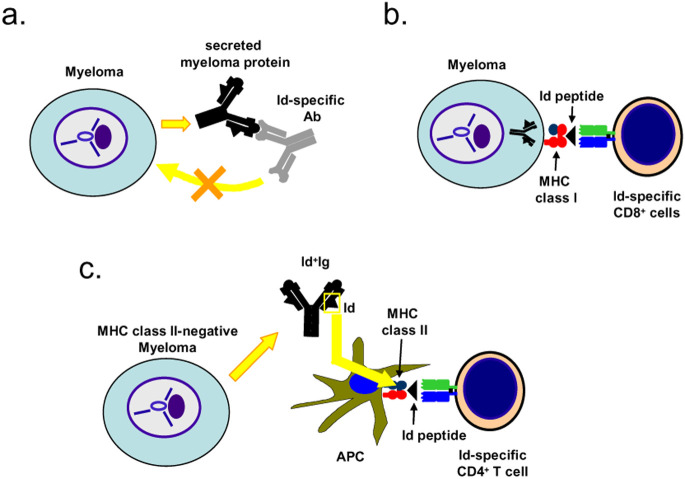
Fig. 1a–c.
Role of Id-specific antibodies, CD8+ and CD4+ T cells in eradication of MM cells. a Id-specific antibody is likely to be blocked by the high concentration of myeloma protein and will thus not reach the MM cells. Moreover, MM cells usually express little or no surface Ig. Consistent with this, Id-specific antibodies on their own seem to be of little therapeutic value [11, 12]. b MM cell line MOPC21 has been shown in vitro to process its endogenous Ig and present Id peptide on its MHC class I molecules. Thus, an influenza-specific CD8+ T cell, which cross-reacted to the Id peptide, killed the MM cells in vitro [14]. c MM cells are usually MHC class II-negative, like MOPC315. However, secreted myeloma protein is endocytosed and processed by tumor APCs which present Id peptides on their class II molecules to Id-specific CD4+ T cells that become activated and kill the MM cells by a yet-undefined mechanism [9, 18, 19, 35, 36]
An experimental mouse model for MM
The MOPC315model: early studies
In certain strains of inbred mice like BALB/c, intraperitoneal injection of mineral oil induces oil granulomas in the peritoneal cavity that over months develop into plasmacytomas (called MM herein) [51]. Among the different MM tumors generated is the mineral oil–induced plasmacytoma 315 (MOPC315), which produces an IgA myeloma protein [20]. These MM cell lines, including MOPC315, are typically maintained by s.c. or i.p. passages, but in vitro lines have been adapted for some tumors including MOPC315.
In 1972, Lynch, Eisen, and coworkers [43] reported a seminal observation: mice immunized with myeloma protein M315 in complete Freund’s adjuvant (CFA) were resistant to a subsequent challenge with MOPC315 cells. Immunization with a distinct IgA myeloma protein, M460, did not induce any resistance to a MOPC315 challenge. Thus, Id vaccination endowed the mice with the ability to reject myeloma cells in an Id-specific fashion. This basic observation has been repeated in a number of labs in other MM (and B-cell lymphoma) models.
Apparently, anti-Id antibodies induced by Id vaccination with M315 [58] had only minor cytotoxic activity on their own against MOPC315 cells in vivo [11, 12]. Thus, other tumoricidal mechanisms had to be sought. Jorgensen and Hannestad described immunization with M315 in CFA that induced T cells that recognized M315 in hapten-based adoptive transfer experiments [26]. It was subsequently shown that such polyclonal Id-specific T cells could be elicited by immunization with a fragment (representing amino acids 88–117) of the lambda L chain of M315 (called λ2315) [30] and that responses were under MHC-linked immune response (Ir) gene control [27, 28, 31]. Further work demonstrated that residues in positions 94, 95, and 96 were crucial for expression of the Id determinant [5, 23], these residues are different in λ2315 (Phe94Arg95Asn96) compared with germline λ2 (Tyr94Ser95Thr96) due to somatic mutations in the Vλ2 gene segment in MOPC315 cells [10]. Immunization of BALB/c mice with λ2315 in CFA induced a partial resistance to a challenge with MOPC315, indirectly suggesting that Id-specific T cells could play a role in protection against myeloma [29].
Later studies in the MOPC315 model: Id-specific CD4+ T cells recognize Id peptides presented on MHC class II molecules
From BALB/c mice immunized with λ2315 in CFA, CD4+ clones were derived that had exactly the same specificity as predicted from the in vivo studies described above in that they recognized a synthetic peptide covering amino acids 91–101 of the λ2315 chain presented on the class II molecule I-Ed on APCs [3, 6, 7]. Prior to presentation, the λ2315 Ig needed to be partially degraded intracellularly in APCs (processed) by the conventional class II pathway [70]. The TCR repertoire for the Id (λ2315) peptide presented on I-Ed was severely limited [59], and the frequency of CD4+ T cells specific for Id (λ2315) was correspondingly low [7] (Fig. 2).
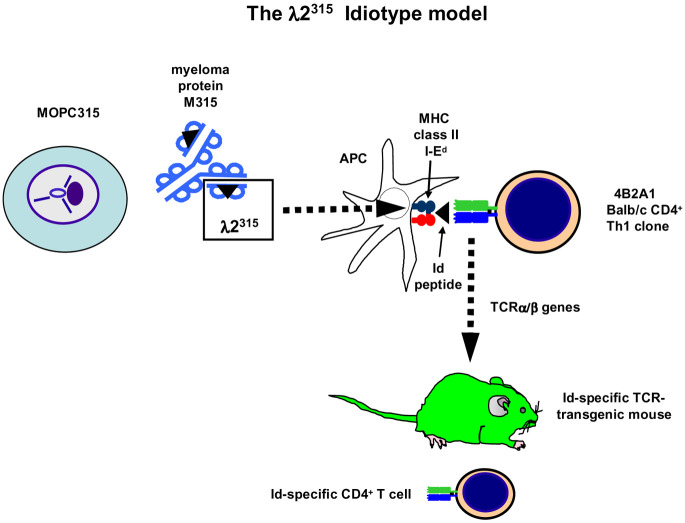
Fig. 2.
Id-specific TCR transgenic mice in the MOPC315 model. MOPC315 secretes an IgA with λ2 L chains (λ2315). Myeloma protein is endocytosed and processed by APCs and a fragment encompassing residues 91–101 is presented on the MHC class II molecule I-Ed to cloned Id-specific 4B2A1 CD4+ T cells. Recognition by Id-specific CD4+ cells depends on residues 94, 95, and 96, which are different from germline-encoded residues due to somatic mutations in the Vλ2 gene segment of MOPC315 [3, 6, 7, 70]. The TCR α and β chains from the Id-specific CD4+ 4B2A1 cells were cloned [59] and a TCR-transgenic mouse was generated [8]
Id-specific CD4+ T cell in immunosurveillance of myeloma in TCR-transgenic mice
To study the role of Id-specific CD4+ T cells in immunosurveillance and immunotherapy of MM, it was necessary to amplify their frequency as well as to have a source of naïve Id-specific T cells. We therefore made mice transgenic for the αβ TCR (Vα19 Jα1, Vβ8.2 Jβ1.2) of a BALB/c Id-specific CD4+ T-cell clone 4B2A1 [8]. A clonotype-specific monoclonal antibody for this TCR was established so that myeloma-specific transgenic T cells could be tracked by flow cytometry and immunohistochemistry [4]. To avoid problems of alloreactivity during challenge with MOPC315, the Id-specific TCR-transgenic mice were extensively backcrossed (>x20) against the BALB/c background in which the MOPC315 myeloma arose.
TCR-transgenic mice were significantly protected against 1.6×105 MOPC315 cells injected s.c., while they succumbed to the control myeloma J558 which express an Id different from the one of MOPC315. Thus, the protection was Id specific. Moreover, the protection required TCR transgenes because nontransgenic littermates were not protected against MOPC315 [36]. Since most Id-specific TCR-transgenic CD4+ T cells express endogenous TCR genes, and thus dual TCR, other specificities could have contributed in these first experiments [36]. We therefore repeated the experiments with Id-specific TCR-transgenic mice which had been made recombination deficient by breeding them homozygous for the scid mutation. Such Id-specific TCR-transgenic SCID mice were even better protected against the MOPC315 myeloma, in addition, protection could be transferred to SCID recipients with purified Id-specific CD4+ T cells [9]. These experiments conclusively demonstrated that Id-specific CD4+ T cells could protect mice against s.c. myeloma challenge in the absence of CD8+ T cells and B cells (i.e., antibodies) (Fig. 3).
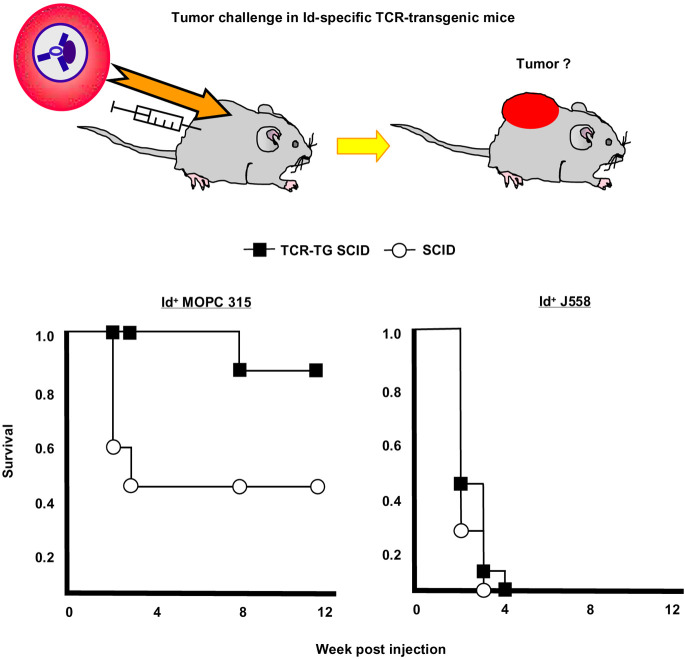
Fig. 3.
Id-specific TCR-transgenic SCID mice are protected against Id+ MOPC315. TCR-transgenic SCID and SCID littermates were injected s.c. with either Id+ MOPC315 or Id− J558 and observed for tumor development. An Id-specific tumor protection was observed since protection required Id-specific transgenes as well as Id expression by the tumor. Since Id-specific TCR-transgenic SCID mice lack B cells and CD8+ T cells, and since their CD4+ T cells only express the transgenic TCR and not endogenous TCR chains, Id-specific CD4+ T cells protect against tumor development. Protection could be transferred with purified CD4+ T cells to SCID recipients [9]. This Figure is redrawn from Fig. 3 in [9] with permission from the publishers
Id-specific CD4+ T cells protect against MHC class II negative MOPC315: importance of Id-primed APCs
Similar to most myeloma cells, MOPC315 cells do not express MHC class II molecules in vitro or in vivo [35], which precludes a direct recognition by Id-specific CD4+ T cells (Fig. 4a). However, when splenic APCs were added to the mixture of MOPC315 cells and Id-specific CD4+ T cells, growth inhibition of MOPC315 cells was obtained provided that the added APCs expressed the proper class II molecule (I-Ed) for Id presentation [35]. Similarly, tumor-derived APCs from H-2d SCID mice with MOPC315 tumors could stimulate Id-specific T cells ex vivo, while their counterpart APCs from MHC-incompatible H-2b Rag2-/- mice with MOPC315 tumors could not [18]. These results indicated that class II+ host APCs could endocytose and process M315 myeloma protein secreted by the MOPC315 tumor not only in vitro [35], but also in vivo [18]. Furthermore, this work demonstrated that host APCs could be isolated ex vivo from MOPC315 tumors, and that such isolated APCs were Id primed since they could stimulate Id-specific CD4+ T cells without adding exogenous Id+ protein into the assay [18, 19]. In tumors, APCs were localized beneath the endothelium of CD31+ blood microvessels (Fig. 4c). A large fraction had a phenotype consistent with a dendritic cell of myeloid origin (CD11b+, CD11c+), but APCs were heterogeneous, probably representing a mixture of DCs in various maturation stages and possibly some macrophages. Some Id-primed APCs were phagocytic (Fig. 4b) and could stimulate Id-specific T cells at a single-cell level in vitro [19].
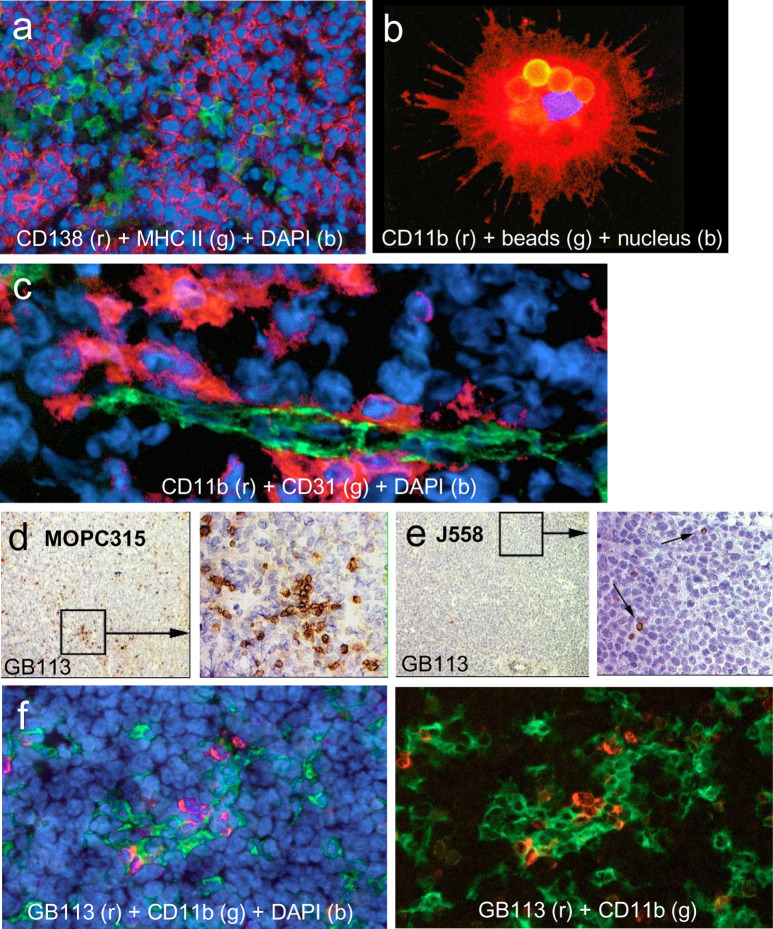
Fig. 4a–f.
Antigen-presenting cells and Id-specific CD4+ T cells in tumors. a MOPC315 tumors were stained for MHC class II expression (green) and MM marker CD138 (syndecan-1, red). MOPC315 cells are MHC class II-negative but aggregates of MHC class II APCs are seen in the tumor. b CD11b+ (red) APCs can be purified from MOPC315 tumors by their ability to phagocytose magnetic beads coated with IgG2a FITC. The phagocytosed beads are seen as yellow spheres. Such phagocytic tumor APCs were CD11b+CD11c+, I-Ed+, CD80+, CD86+, CCR5+, and CCR7+, indicating that they represent a mixture of immature and mature DCs of myeloid origin. Such ex vivo tumor APCs were spontaneously Id-primed and able to stimulate Id-specific CD4+ T cells at the single-cell level [19]. c CD11b+ (red) tumor APCs are located beneath CD31+ (green) endothelial cells in tumor vessels. d,e In TCR-transgenic mice, Id-specific CD4+ T cells stained with the clonotype-specific GB113 monoclonal antibody accumulate in small Id+ MOPC315 tumors (d) but not in J558 tumor (e). f Id-specific CD4+ T cells (GB113+, red) and CD11b+ (green) APCs colocalize in small MOPC315 tumors in TCR-transgenic mice. Figures are reproduced from [18] and [19] with permission from the publishers
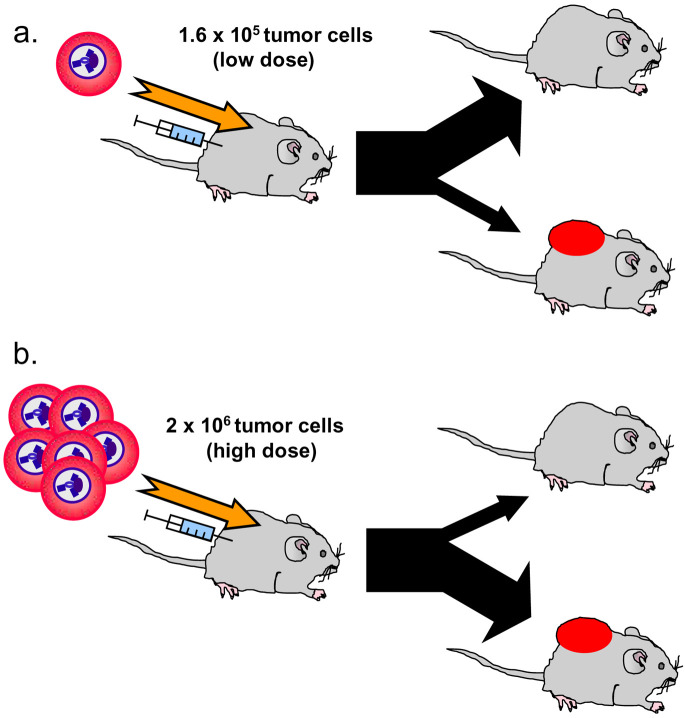
A crucial question was whether the Id-primed APCs could stimulate Id-specific CD4+ T cells at the site of MOPC315 injection and cause rejection. This was difficult to study because tumor cells apparently were rejected very early after injection, leaving no tumor specimen for investigation. Therefore, to be able to address this issue at all, we injected high amounts of MOPC315 cells (2×106) to partly overcome the resistance of Id-specific TCR-transgenic mice (Fig. 5) [18]. Small MOPC315 tumors established in such a way in TCR-transgenic mice were analyzed under the assumption that T-cell activation in small tumors would reflect the situation when MOPC315 actually were rejected. By this approach, we found that (1) Id-specific CD4+ T cells were activated and proliferated in small MOPC315 tumors, (2) CD4+ T cells were much more frequent in Id+ MOPC315 tumors than in Id− tumors, and (3) Id-specific CD4+ T cells colocalized with class II+ tumor APCs [18] (Fig. 4d–f). The mechanism by which activated CD4+ T cells kill myeloma cells is presently obscure, but FasLigand-Fas interaction and interferon γ might play a role.
Fig. 5a,b.
Challenge of Id-specific TCR-transgenic mice with Id+ MOPC315 cells has two outcomes. a When a low dose (1.6×105) of tumor cells is injected, immunosurveillance is effective and most mice reject the tumor inoculum [9, 18, 36]. b When a high dose (2×106 cells) is injected, more mice develop tumors and fewer are protected. With progressive tumor growth, Id-specific T cells are deleted in the thymus [37], in peripheral lymphoid organs, and in the tumor itself [2]
It should be noted that, in the experiments reviewed above, tumor cells were injected s.c. and tumors developed locally. Thus, the findings need to be extended to a MOPC315 variant that homes to the bone marrow, thereby mimicking MM in human.
Tolerance development to progressive Id+ MOPC315 tumors
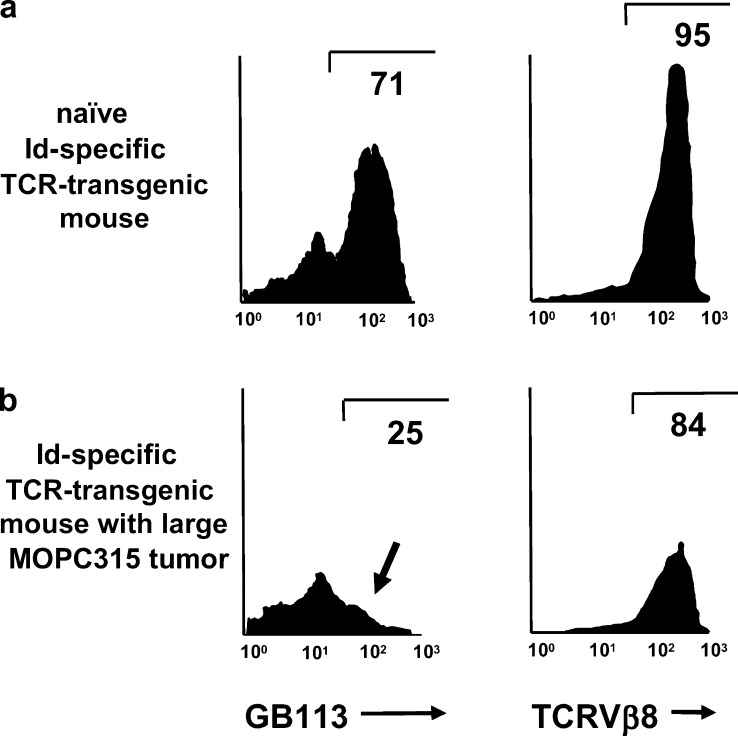
As stated above, if a higher dose of MOPC315 cells (2×106) was injected, the resistance of TCR-transgenic mice could be partly overcome. As tumors progressed in such mice, and myeloma protein concentration in serum exceeded 50 μg/ml, T-cell tolerance to Id progressively developed. The T-cell tolerance manifested itself as (1) deletion of Id-specific thymocytes [37], (2) deletion of Id-specific CD4+ T cells in the peripheral lymphoid organs (Fig. 6) and in the tumor [2], and (3) as functional unresponsiveness of ex vivo T cells to stimulation with Id peptide / APCs in vitro [2, 37]. Only a few residual low-avidity T cells with dual TCRs due to expression of endogenous TCRα chains persisted; whether these cells have any tumor protective role at all remains to be demonstrated. Possibly, tumor cells and the secreted myeloma protein could both have contributed to T-cell tolerance. However, since deletion of thymocytes could be induced by injection of purified myeloma protein, the latter was sufficient for induction of this important mechanism for T-cell tolerance development [37]. The experiments established that Id-specific CD4+ T cells have a role in immunosurveillance of Id+ MM; however, if T cells fail to eliminate the MM cells upon their initial encounter, T-cell tolerance ensues and MM tumors progress (Fig. 6).
Fig. 6a,b.
Deletion of Id-specific CD4+ T cells in TCR-transgenic mice with progressive growth of s.c. MOPC315 tumors. In order to partly overcome their tumor resistance, TCR-transgenic mice were injected with high loads of MOPC315 cells. Lymph node cells from naïve Id-specific TCR-transgenic mice (a), or from TCR-transgenic mice with a large MOPC315 tumor (b) were analyzed by flow cytometry. In uninjected mice, about 70% of gated CD4+ T cells express the transgenic TCR, detected by the clonotype-specific antibody GB113. In mice with large MOPC315 tumors, the GB113+ population disappears (arrow in b) while most cells maintain expression of the transgenic β chain detected by the TCR Vβ8-specific F23.1 monoclonal antibody. Thus, CD4+ T cells expressing the transgenic TCR are deleted while those expressing endogenous TCR α chains together with the transgenic TCR β chain are spared. The latter cells have low responsiveness to Id in vitro. This Figure is redrawn from Fig. 3 in [2] with permission from the publishers
Experimental T-cell therapy of Id+ tumors
The experiments described above show that Id-specific CD4+ T cells have a role in immunosurveillance. We have also tested if Id-specific T cells may have a role in immunotherapy. To this end, Id-specific T cells were transferred to mice that previously had been injected with Id+ tumor cells. This approach was successful in an Id+ (λ2315-transfected) B-lymphoma model where naïve Id-specific CD4+ T cells could be transferred as late as 17 days after tumor cell injection and still cure about 50% of the mice [42]. Whether T-cell immunotherapy could be successful also in the MOPC315 MM model remains to be tested.
Id-specific T-cell responses in humans
Naturally occurring Id-specific T-cell responses in patients
MM patients have for unknown reasons perturbations of their αβ TCR repertoire with clonal expansions particularly in the CD8 populations [22, 44, 62, 63]. Despite this, even in the absence of Id vaccination, low frequencies of Id-specific T cells have been detected by proliferation assays and cytokine secretion (ELISpot) assays in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma [48, 76]. These low-frequency Id-specific T cells are most likely not related to the clonally expanded T cells in these patients. The Id-specific T cells responded to synthetic peptides corresponding to the complementarity-determining regions (CDRs) of both H and L chains of the monoclonal Ig [21, 24, 73]. Id-specific CD4+ T cells appeared to be more frequent than CD8+ cells. While type I Th cells (with interferon γ production) dominated in the early stages of disease, type II Th cells (with IL-4 production) dominated with disease progression [77]. Id-specific cytotoxic T-cell lines could be generated that killed autologous primary myeloma cells in vitro [38, 74]. The cytotoxic T-cell lines consisted of both CD4+ [74] and CD8+ [38, 74] T cells, and killing was in one report solely MHC class I–restricted [38], while in the other report both class I– and class II–restriction was observed [74]. Collectively, these results could suggest that Id-specific T cells might be naturally occurring in MGUS and MM and that they might inhibit progression of disease. An alternative interpretation, which is in line with the results in the TCR-transgenic MOPC315 model reviewed above, is that in MM patients, most high-avidity Id-specific T cells have been deleted and that only low-avidity T cells remain. Although such low-avidity Id-specific T cells are detected by sensitive techniques in vitro, their significance in vivo is not known. It is indeed difficult to test if high-avidity Id-specific T cells actually are deleted in MM patients because the Id-specific repertoire prior to disease is unknown.
Id-specific vaccination in MM patients
A number of different strategies for Id vaccination have been employed. In some studies, untreated early stage MM patients were immunized with autologous alum-precipitated myeloma protein either with [47], or without [1], granulocyte-macrophage colony-stimulating factor (GM-CSF). In other studies, Id vaccination has been performed with conjugates of Id-keyhole limpet hemocyanin (Id-KLH) in conjunction with GM-CSF or IL-2 [45], or Id vaccination has been performed with Id-pulsed dendritic cells [17, 39, 55, 65, 72, 78]. Finally, Id vaccination may be performed as DNA vaccination [33, 61] or with Id-encoding recombinant adenoviruses [64]. Some of these studies were performed in untreated patients, while others were performed after high-dose chemotherapy and autologous stem cell support (ASCT). The frequency of patients exhibiting Id-specific T- (and B-) cell responses differed in these studies; but overall, a minority of patients developed detectable Id-specific T-cell responses. Only a few clinical responses have been observed, and it is too early to say whether Id vaccination and elicitation of Id-specific immune responses improve the prognosis of MM patients [16, 52].
Lessons from the MOPC315 model: possible therapeutic strategies for Id-specific immunotherapy in MM patients
The tolerance problem and high-dose chemotherapy / autologous stem cell transplantation
How do the results on T-cell tolerance development in the MOPC315 Id-specific TCR-transgenic mouse model apply to MM patients and the prospect of Id vaccination? It is likely that in MM patients, Id-specific T cells have already had their chance to kill off MM cells during the initial “immunosurveillance phase.” If immunosurveillance failed during the initial encounter, the T-cell repertoire for Id peptides derived from the myeloma protein most likely becomes deleted with tumor progression, at least to a certain extent. Thus, the Id-specific T-cell responses that have been observed in the MGUS and MM patients [48, 76] might well reflect residual low-avidity T cells that have not yet been completely tolerized. Such remaining low-avidity Id-specific T cells may possibly be stimulated by powerful Id vaccination, although the effect of vaccination on prognosis is uncertain. In this context, it is interesting to note that in the MOPC315 model, low-avidity Id-specific T cells appear to retard tumor growth ([2] and Munthe, Corthay, and Bogen, unpublished observations).
If this assessment of the immunological situation in MM patients is correct, what could be done to increase the efficiency of Id vaccination? It should be of overriding importance to reverse T-cell tolerance prior to Id vaccination. As judged from the Id-specific TCR-transgenic model, reversal might be obtained once the serum myeloma protein concentration has dropped to <50 μg/ml. However, note that this estimate is based on results with a single transgenic Id-specific TCR. In patients with a polyclonal Id-specific TCR repertoire, the serum concentration required for relief of tolerance might vary for individual Id-specific T cells. Moreover, myeloma protein concentration needed for T-cell tolerance induction could differ in mice and humans. Finally, although a high concentration of secreted myeloma protein seems to induce T-cell tolerance by itself [37], tumor burden could contribute. Thus T-cell tolerance induction could be influenced by the secretory status of the tumor and clearance of myeloma protein. Such a level of remission, with myeloma protein <50 μg/ml in serum, is likely to be obtained in only a fraction of MM patients undergoing high-dose chemotherapy and ASCT [54, 55]. In addition to this strong reduction in myeloma protein concentration, new high-avidity Id-specific T cells are needed; these may be educated in the thymus from committed thymocyte precursors resulting from the ASCT. Taking these considerations into account, it will be a challenge to find the best time point after ASCT to vaccinate: myeloma protein concentration should be at its nadir while new APCs (e.g., DCs) and a new T-cell repertoire should have emerged. The former requirement could be a problem since MM patients have been reported to have quantitatively and qualitatively deficient DCs [13, 53]. However, after ASCT, sufficient DCs have been obtained from MM patients to perform Id vaccination [17, 39, 55, 65, 72, 78]. The latter requirement, development of a new T-cell repertoire, might be difficult to fulfill as the T-cell receptor repertoire has been reported to be severely and long lastingly altered in MM patients both before [22, 44, 62, 63] and after ASCT [44]. Development of a new T-cell repertoire after ASCT could be a particular problem in MM patients with advanced age and thymic involution.
Even if ASCT does not result in a strong remission with myeloma protein <50 μg/ml, it might still be advisable to vaccinate at a time point when myeloma protein concentration is low. This suggestion is based on recent experimental data which suggest that the higher the antigen concentration, the more profound is the T-cell tolerance [57]. Thus, once myeloma protein concentration is reduced, low-avidity Id-specific T cells could regain some of their Id responsiveness.
Strategies for Id vaccination
The next question is how Id vaccination should best be performed. There are a multitude of different approaches to Id vaccination. Since the tumor-specific antigen, myeloma protein, can be readily purified from serum obtained prior to cytoreductive therapy, protein-based vaccines in conjunction with coupling to carrier proteins like KLH, various adjuvants like GM-CSF, and dendritic cells, may be used. Alternatively, it is relatively easy to amplify rearranged V(D)J genes from myeloma cells and produce Id vaccines in a DNA format.
Id-specific T-cell therapy?
Since Id-specific T cells probably are of low-avidity in MM patients, an alternative strategy may be warranted, namely transfer of high-avidity Id-specific T cells. This suggestion is based on the observation that donor lymphocyte infusion (DLI) has an effect in MM [41]. However, since the effect seems to be mediated by alloreactive T cells, it has been difficult to separate graft-versus-myeloma and graft-versus-host effects. If Id-specific T cells which are highly tumor specific could be transferred, the problem of graft-versus-host disease may be reduced while the graft-versus-myeloma effect is maintained. Indeed, Id-specific T cells for transfer could be obtained by immunizing related donors, followed by expansion and enrichment in vitro [32, 34, 38]. Whether infusion of such cells has any clinical effect is not known. The finding that transfer of Id-specific CD4+ T cells subsequent to Id+ B-lymphoma challenge could cure mice [42] suggest that this could be a valuable strategy.
Other tumor-specific antigens in MM that may be useful for vaccination
This review is focused on Id-specific immunotherapy. However, a number of other targets, like cancer-testis antigens MAGE-3 and NY-ESO-1 [50, 68], Muc-1 [67], and sperm protein 17 [40] may be useful, perhaps in conjunction with Id-specific immunotherapy.
Concluding remarks
Id is a truly tumor-specific antigen in MM and may be used as a target for immune attack. Experimental studies in mice suggest that Id-specific CD4+ T cells may be of particular importance but that these cells are tolerized in animals with advanced disease. Therefore, in MM patients, it is probably important to reduce tumor burden by conventional therapy prior to Id vaccination.
Acknowledgements
A number of investigators in the Bogen-lab have over the years contributed to the MOPC315 Id-specific TCR-transgenic model as indicated in the references. A.C. and K.U.L. are fellows of the Norwegian Cancer Society. This work was supported by grants from the University of Oslo, the Norwegian Research Council, the Norwegian Cancer Society, and the Multiple Myeloma Research Foundation. Suzanne Garman-Vik expertly prepared the manuscript.
Abbreviations
- APC
antigen-presenting cell
- ASCT
autologous stem cell transplantation
- CDR
complementarity-determining region
- CFA
complete Freund’s adjuvant
- DC
dendritic cell
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- H
heavy
- Id
idiotope or idiotype
- Ig
immunoglobulin
- IL
interleukin
- L
light
- M-component
monoclonal component
- MGUS
monoclonal gammopathy of undetermined significance
- MHC
major histocompatibility complex
- MM
multiple myeloma
- MOPC
mineral oil–induced plasmacytoma
- TCR
T-cell antigen receptor
- V
variable
Footnotes
A. Corthay and B. Bogen are joint corresponding authors for this article.
Contributor Information
Alexandre Corthay, Phone: +47-23073508, FAX: +47-23073510, Email: Alexandre.Corthay@labmed.uio.no.
Bjarne Bogen, Phone: +47 23073015, FAX: +47 23073510, Email: bjarne.bogen@labmed.uio.no.
References
- 1.Bergenbrant Br J Haematol. 1996;92:840. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogen Eur J Immunol. 1996;26:2671. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 3.Bogen EMBO J. 1989;8:1947. doi: 10.1002/j.1460-2075.1989.tb03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogen Eur J Immunol. 1991;21:2391. doi: 10.1002/eji.1830211015. [DOI] [PubMed] [Google Scholar]
- 5.Bogen Eur J Immunol. 1985;15:278. doi: 10.1002/eji.1830150313. [DOI] [PubMed] [Google Scholar]
- 6.Bogen Eur J Immunol. 1986;16:1373. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- 7.Bogen Eur J Immunol. 1986;16:1379. doi: 10.1002/eji.1830161111. [DOI] [PubMed] [Google Scholar]
- 8.Bogen Eur J Immunol. 1992;22:703. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- 9.Bogen Eur J Immunol. 1995;25:3079. doi: 10.1002/eji.1830251114. [DOI] [PubMed] [Google Scholar]
- 10.Bothwell Nature. 1982;298:380. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- 11.Bridges J Immunol. 1978;120:613. [PubMed] [Google Scholar]
- 12.Bridges J Immunol. 1978;121:479. [PubMed] [Google Scholar]
- 13.Brown Blood. 2001;98:2992. doi: 10.1182/blood.V98.10.2992. [DOI] [PubMed] [Google Scholar]
- 14.Cao J Exp Med. 1994;179:195. doi: 10.1084/jem.179.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chicz J Exp Med. 1993;178:27. [Google Scholar]
- 16.Coscia M, Mariani S, Battaglio S, Di Bello C, Fiore F, Foglietta M, Pileri A, Boccadoro M, Massaia M (2003) Long-term follow-up of idiotype vaccination in human myeloma as a maintenance therapy after high-dose chemotherapy. Leukemia [DOI] [PubMed]
- 17.Cull Br J Haematol. 1999;107:648. doi: 10.1046/j.1365-2141.1999.01735.x. [DOI] [PubMed] [Google Scholar]
- 18.Dembic Proc Natl Acad Sci U S A. 2000;97:2697. doi: 10.1073/pnas.050579897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dembic Blood. 2001;97:2808. doi: 10.1182/blood.V97.9.2808. [DOI] [PubMed] [Google Scholar]
- 20.Eisen Biochemistry. 1968;7:4126. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- 21.Fagerberg Int J Cancer. 1999;80:671. doi: 10.1002/(SICI)1097-0215(19990301)80:5<671::AID-IJC7>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Halapi Eur J Immunol. 1997;27:2245. doi: 10.1002/eji.1830270919. [DOI] [PubMed] [Google Scholar]
- 23.Hannestad Eur J Immunol. 1986;16:889. doi: 10.1002/eji.1830160803. [DOI] [PubMed] [Google Scholar]
- 24.Hansson Blood. 2003;101:4930. doi: 10.1182/blood-2002-04-1250. [DOI] [PubMed] [Google Scholar]
- 25.Janeway Proc Natl Acad Sci U S A. 1975;72:2357. [Google Scholar]
- 26.Jorgensen Eur J Immunol. 1977;7:426. doi: 10.1002/eji.1830070705. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen Nature. 1980;288:396. [Google Scholar]
- 28.Jorgensen J Exp Med. 1982;155:1587. [Google Scholar]
- 29.Jorgensen Scand J Immunol. 1980;11:29. [Google Scholar]
- 30.Jorgensen J Exp Med. 1983;158:2183. doi: 10.1084/jem.158.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen Scand J Immunol. 1985;21:183. [Google Scholar]
- 32.Kim Leuk Lymphoma. 2003;44:1201. doi: 10.1080/1042819031000076954. [DOI] [PubMed] [Google Scholar]
- 33.King Nat Med. 1998;4:1281. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- 34.Kwak Lancet. 1995;345:1016. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 35.Lauritzsen Cell Immunol. 1993;148:177. doi: 10.1006/cimm.1993.1100. [DOI] [PubMed] [Google Scholar]
- 36.Lauritzsen Proc Natl Acad Sci U S A. 1994;91:5700. doi: 10.1073/pnas.91.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauritzsen Int J Cancer. 1998;78:216. doi: 10.1002/(SICI)1097-0215(19981005)78:2<216::AID-IJC16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Li Blood. 2000;96:2828. [PubMed] [Google Scholar]
- 39.Lim Int J Cancer. 1999;83:215. doi: 10.1002/(SICI)1097-0215(19991008)83:2<215::AID-IJC12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Lim Blood. 2001;97:1508. doi: 10.1182/blood.V97.5.1508. [DOI] [PubMed] [Google Scholar]
- 41.Lokhorst Blood. 1997;90:4206. [PubMed] [Google Scholar]
- 42.Lundin Blood. 2003;102:605. doi: 10.1182/blood-2002-11-3381. [DOI] [PubMed] [Google Scholar]
- 43.Lynch Proc Natl Acad Sci U S A. 1972;69:1540. [Google Scholar]
- 44.Mariani Br J Haematol. 2001;113:1051. doi: 10.1046/j.1365-2141.2001.02871.x. [DOI] [PubMed] [Google Scholar]
- 45.Massaia Blood. 1999;94:673. [PubMed] [Google Scholar]
- 46.Munthe Eur J Immunol. 1999;29:4043. doi: 10.1002/(SICI)1521-4141(199912)29:12<4043::AID-IMMU4043>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Osterborg Blood. 1998;91:2459. [PubMed] [Google Scholar]
- 48.Osterborg Br J Haematol. 1995;89:110. doi: 10.1111/j.1365-2141.1995.tb08902.x. [DOI] [PubMed] [Google Scholar]
- 49.Overwijk Proc Natl Acad Sci U S A. 1999;96:2982. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pellat-Deceunynck Eur J Immunol. 2000;30:803. doi: 10.1002/1521-4141(200003)30:3<803::AID-IMMU803>3.3.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 51.Potter Physiol Rev. 1972;52:631. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- 52.Rasmussen Blood. 2003;101:4607. doi: 10.1182/blood-2002-06-1925. [DOI] [PubMed] [Google Scholar]
- 53.Ratta Blood. 2002;100:230. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 54.Reichardt Biol Blood Marrow Transplant. 1997;3:157. [PubMed] [Google Scholar]
- 55.Reichardt Blood. 1999;93:2411. [Google Scholar]
- 56.Rudensky Nature. 1992;359:429. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- 57.Singh J Exp Med. 2003;198:1107. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sirisinha Proc Natl Acad Sci U S A. 1971;68:3130. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snodgrass Eur J Immunol. 1992;22:2169. doi: 10.1002/eji.1830220832. [DOI] [PubMed] [Google Scholar]
- 60.Snyder J Immunol. 2002;168:3865. doi: 10.4049/jimmunol.168.8.3865. [DOI] [PubMed] [Google Scholar]
- 61.Syrengelas Nat Med. 1996;2:1038. doi: 10.1038/nm0996-1038. [DOI] [PubMed] [Google Scholar]
- 62.Sze Blood. 2001;98:2817. doi: 10.1182/blood.V98.9.2817. [DOI] [PubMed] [Google Scholar]
- 63.Sze Leuk Lymphoma. 2003;44:1557. doi: 10.1080/1042819031000097429. [DOI] [PubMed] [Google Scholar]
- 64.Timmerman Blood. 2001;97:1370. doi: 10.1182/blood.V97.5.1370. [DOI] [PubMed] [Google Scholar]
- 65.Titzer Br J Haematol. 2000;108:805. doi: 10.1046/j.1365-2141.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 66.Tonegawa Nature. 1983;302:575. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 67.Treon Blood. 1999;93:1287. [PubMed] [Google Scholar]
- 68.van Blood. 1999;94:1156. [PubMed] [Google Scholar]
- 69.Van Curr Opin Immunol. 1997;9:684. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 70.Weiss Proc Natl Acad Sci U S A. 1989;86:282. [Google Scholar]
- 71.Weiss Cell. 1991;64:767. [Google Scholar]
- 72.Wen Clin Cancer Res. 1998;4:957. [PubMed] [Google Scholar]
- 73.Wen Br J Haematol. 1998;100:464. doi: 10.1046/j.1365-2141.1998.00592.x. [DOI] [PubMed] [Google Scholar]
- 74.Wen Blood. 2001;97:1750. doi: 10.1182/blood.V97.6.1750. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto Eur J Immunol. 1987;17:719. doi: 10.1002/eji.1830170522. [DOI] [PubMed] [Google Scholar]
- 76.Yi Scand J Immunol. 1993;38:529. doi: 10.1111/j.1365-3083.1993.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 77.Yi Blood. 1995;86:3043. [PubMed] [Google Scholar]
- 78.Yi Br J Haematol. 2002;117:297. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]