Abstract
In this study, we developed two Her-2/neu-derived E75 altered peptide ligands (APLs) that demonstrate increased affinities for the HLA-A*0201 allele compared with wild-type E75 peptide. The APLs contain amino acids from E75(369–377), an immunodominant Her-2/neu-derived peptide, and preferred primary and auxiliary HLA-A*0201 molecule anchor residues previously identified from combinatorial peptide library screening with the recombinant molecule. CTL lines were generated against wild-type E75 peptide (KIFGSLAFL) and APLs by multiple rounds of peptide stimulation of peripheral blood mononuclear cells (PBMCs) from HLA-A2+ antigen normal individuals. CTL lines raised on wild-type E75 peptide cross-reacted with APLs and similarly, CTL lines raised on APLs cross-reacted with wild-type E75 peptide, as measured by IFN-γ ELISpot and target cell lysis assays. One of five individuals demonstrated specificity for APL 2 (FLFGSLAFL), whereas APL 5 (FLFESLAFL)-specific responses were observed from all five individuals tested. Molecular models of the E75, APL 2, and APL 5/HLA-A2 complexes indicated that the substitution of glycine with glutamic acid at position four of APL 5 resulted in the presentation of a large, negatively charged side chain that interacts with the outer edge of the HLA-A2 antigen alpha helix and is freely available to interact with cognate T-cell receptors. The results of this study further substantiate the concept that rational design of T-cell epitopes may lead to stronger peptide immunogens than natural, wild-type peptides.
Keywords: Her-2/neu, Altered peptide ligand (APL), Cytotoxic T lymphocyte (CTL), Human leukocyte antigen (HLA), T-cell receptor (TCR)
Introduction
Twenty to forty percent of adenocarcinomas, including breast, ovary, pancreas, colon, and lung, overexpress the Her-2/neu protein. Her-2/neu overexpression occurs in both primary tumors as well as metastatic sites and expression is stable [1]. Overexpression is often associated with a poor prognosis, a metastatic phenotype, and resistance to chemotherapy [2, 3]. Fisk et al. identified E75 (KIFGSLAFL, residues 369–377) as an immunodominant peptide derived from Her-2/neu [4]. Four of four ovarian-specific CTL lines recognized the E75 peptide [4], and furthermore, other groups have also substantiated E75 as an important HLA-A2 antigen-restricted CTL epitope [5, 6]. Several clinical trials have demonstrated both the efficacy and safety of E75 peptide immunization of HLA-A2+ antigen breast or ovarian cancer patients whose tumors overexpressed Her-2/neu [7, 8, 9, 10]. Collectively, these studies suggest that peptides derived from E75 may be potentially useful antigens in immunotherapies aimed at Her-2/neu-expressing tumors.
A current issue in the development of cancer vaccines is the induction of CTL responses against self-derived tumor antigens. Therefore, attempts have been made to overcome tolerance to self-antigens by increasing the immunogenicity of self-peptides. HLA-A*0201 allele-binding peptides are frequently nonamers, with positions two and nine considered primary anchor residues [11, 12]. These HLA anchor residues are essential, but alone are inadequate to bind to HLA class I alleles, indicating that other peptide residues are important for binding. Auxiliary anchor residues are considered critical for forming stable peptide-HLA class I complexes [13, 14, 15, 16]. Using a novel synthetic peptide bead library screening technique, HLA-A*0201 allele secondary anchor residues were elucidated [17].
For peptide-based therapies to be effective, the HLA-peptide must be immunogenic. Several studies have shown that two components are important in determining the immunogenicity of HLA-peptide complexes: (1) the affinity of a peptide for MHC class I molecules and (2) the stability of the complex itself [18, 19, 20]. A direct correlation exists between immunogenicity of peptides and MHC class I binding affinity [18]. Peptides with high affinities for MHC class I molecules are present on cell surfaces for a longer time than peptides with low affinities for MHC class I molecules [21]. Therefore, peptides with increased affinity for MHC class I molecules have an increased potential to induce T-cell-mediated immune responses.
Modification of self-derived peptides may activate T cells by two potential mechanisms: (1) activation of existing T cells that are tolerant of, or have weak affinities to, self-antigens, or (2) activation of a new population of T cells specific for the modified tumor peptide that cross-react with wild-type peptide. The studies described here contribute to the rational design of effective peptide-based immunotherapies that aim to activate tumor-specific T cells.
Material and methods
HLA-A*0201 allele-binding synthetic peptides
The following HLA-A*0201 allele-binding peptides used in this study were Her-2/neu peptides: E75 (residues 369–377, KIFGSLAFL), modified E75 peptide 2 (APL 2, FLFGSLAFL), and modified E75 peptide 5 (APL 5, FLFESLAFL); and hepatitis B core antigen (FLPSDYFPSV, residues 18–27). The hepatitis B virus core antigen (FLPSDYFPSV) was synthesized with a cysteine residue substituted for the tyrosine residue (FLPSDCFPSV). This cysteine residue was conjugated to fluorescein (Fl-peptide) for use in competitive inhibition studies to measure the affinity of modified Her-2/neu E75 peptides for the HLA-A*0201 allele. Peptides were manufactured by PeptidoGenic Research (Livermore, CA) and were greater than 95% pure as assessed by HPLC and mass-spectrometric analysis.
Cell lines, media, and antibodies
T2 cells (CRL-1992; American Type Culture Collection, Manassas, VA) were used as antigen-presentation cells for ELISpot and 51Cr release assays. T2 cells, K562 (NK-sensitive erythroblastoma cell line), and MCF-7 tumor cells (HLA-A2+, Her-2/neu +) were cultured in cRPMI 1640 medium (RPMI 1640 medium (Cellgro, Herdon, VA) supplemented with 10% heat-inactivated FBS (Omega Scientific, Tarzana, CA), 1,000 U/ml penicillin-streptomycin, and 2-mM l-glutamine). A Her-2/neu-overexpressing tumor cell line, MCF-7-2/18 (HLA-A2+), was kindly provided by the Cancer Research Institute, University of California at San Francisco, and cultured in DMEM-G-418 (Dulbecco’s Modified Eagle’s Medium (Invitrogen, Rockville, MD) supplemented with 10% heat-inactivated FBS, 1,000 U/ml penicillin-streptomycin, 2-mM l-glutamine, and 500 μg/ml G-418).
A mouse antihuman HLA-A2 antibody (US Biologicals, Swampscott, MA) was used to identify HLA-A2+ antigen normal individuals (1.0 μg/ml/1×106 cells) followed by staining of the HLA-A2-labeled PBMC with a FITC-conjugated goat antimouse antibody (1.0 μg/ml; BD Pharmingen, San Jose, CA). Acquisition and analysis of data was performed using a FACScan flow cytometer (BD Biosciences, Mountain View, CA) and CellQuest software, version 4.0 (Beckton Dickinson).
HLA-A*0201 allele-binding affinity of modified Her2/neu E75 peptides
To measure the relative affinity of the APLs for HLA-A*0201 molecules, a competitive peptide inhibition assay was performed as previously described [22] with slight modifications [23]. Mean fluorescence intensity (MFI) values were used to determine inhibition of a reference peptide (Fl-peptide), FLPSDCFPSV, fluoresceinated at the cysteine residue (C6) from binding to HLA-A*0201 molecules on T cells by modified E75 peptides. Percentage inhibition was calculated as (1 − [MFI T2 + Fl-peptide + modified peptide − MFI T2 only] / [MFI T2 + Fl-peptide − MFI T2 only]) x 100. The IC50 of the APL was determined by calculating the concentration of peptide required to inhibit binding of 50% of the Fl-peptide binding to T2 cells (IC50 in μM).
Generation of CTLs specific for Her-2/neu E75 and E75 APLs
PBMCs were obtained from normal donors either by leukapheresis (HSC-0096) or peripheral blood draws (HSC A01.88). Human blood was obtained from normal donors according to the guidelines set forth by the Human Subjects Committee at the University of Arizona. PBMCs were purified using standard white blood cell separation by density centrifugation with Ficoll-Hypaque Plus (Amersham Pharmacia Biotech, Piscataway, NJ). HLA-A2+ antigen peptide-specific CTLs were generated as follows: on day 0, HLA-A2+ PBMCs (2×106/ml) were plated in 2 ml of Iscove’s Modified Dulbecco’s Medium (IMDM; Life Technologies, Rockville, MD) with 25-mM HEPES, supplemented with 10% heat-inactivated human AB serum (Gemini Bioproducts, Calabasas, CA), 1,000 U/ml penicillin-streptomycin, 2-mM l-glutamine, and 0.5 mg/ml amphotericin B in 24-well plates. IL-7 (R&D Systems, Minneapolis, MN) was added at a concentration of 10.0 ng/ml to the cells. E75, APL 2, or APL 5 was added at 1.0 μg/ml. Starting on day 1, 300 IU/ml of IL-2 (Chiron, Emeryville, CA) was added every 2–3 days. Weekly peptide stimulations began 7 days after the first stimulation. Autologous, irradiated PBMCs were pulsed with 1.0 μg/ml peptide. Induction cultures (nonadherent lymphocytes) were transferred to wells containing peptide-pulsed irradiated cells in cIMDM (10:1 ratio of effector cells to stimulator cells). IL-2 was added every 2 to 3 days. At the end of the third stimulation, IFN-γ ELISpot assays were performed to determine peptide recognition.
Detection of IFN-γ-producing cells by ELISpot
Nitrocellulose plates (Millititer, Millipore, Bedford, MA) were coated with a mouse antihuman IFN-γ monoclonal antibody (10 μg/ml/well; Pharmingen, San Diego, CA), diluted in PBS overnight at 4°C. T2 cells (2.5×104 cells/well) were loaded with the indicated peptide (10.0 μg/ml) and incubated with effector cells (5×104 cells/well) in a total of 200.0 μl of X-Vivo 15 (BioWhittaker, Walkersville, MD). After incubation for 36 h at 37°C in 5% CO2, cells were removed by washing five times with PBS / 0.05% Tween 20 (PBS-Tw). Captured cytokine was detected by incubation for 5 h at room temperature with biotinylated mouse antihuman IFN-γ monoclonal antibody (2.5 μg/ml/well, Pharmingen) diluted in PBS / 0.05% Tween 20 / 0.1% FCS (PBS-Tw-FCS). Streptavidin-HRP, diluted 1:1000 in PBS-Tw-FCS, was added for 1 h at room temperature. Staining was performed with 3-amino-9-ethylcarbazole (20.0 mg/ml, Sigma, St. Louis, MO) for 15–20 min at room temperature. Spots were counted by two individuals without well-identifiers, using a dissecting microscope.
Cytolytic activity of PBMCs cultured with Her-2/neu APLs
Chromium51 release cytotoxicity assays were performed to evaluate the ability of PBMCs to lyse the following target cells: T2 cells pulsed with 10.0 μg/ml of either wild-type E75 or HBV peptide, MCF-7, MCF-7-2/18, and K562 cell lines. Targets (5×103 cells/well) were labeled with 100 μCi of 51Cr (Amersham Pharmacia Biotech, Piscataway, NJ) for 45 min followed by incubation with peptide (if indicated) in X-Vivo for 1 h prior to assay. Percentage specific lysis was calculated as ([experimental 51Cr release − spontaneous release] / [maximum release – spontaneous release]) x 100.
Molecular modeling of the HLA-A2 molecule and E75/APL2/APL 5 complex
The coordinates of the HLA-A2 molecule complexed with an HIV-derived nonamer peptide, Tax, were downloaded from the Protein Data Bank (ID number 1DUZ) [24]. This file was used for manipulations in the Swiss-Pdb Viewer (DeepView) Model program [25]. The Tax peptide, which closely resembles the wild-type E75 peptide, was mutated manually to generate the E75, APL 2, and APL 5 peptides bound to the quarternary HLA-A2 antigen structure. Each HLA/peptide complex was submitted for energy minimization with the GROMOS96 feature of the Swiss-Pdb Viewer. Complexes were visualized in stereo using the computer graphics program O on a Silicon Graphics Octane computer to view all possible rotamers.
Statistical analyses
Lysis of target cells was compared using two-tailed, paired Student t-tests. Data sets are shown as mean ± SEM. Probability values of p<0.05 were considered to indicate significant differences between data sets.
Results
Modified E75 peptides and HLA-A*0201 allele-binding affinity
Published HLA-A2 antigen-binding motifs suggest that the HLA-A*0201 allele-binding Her-2/neu-derived peptide, E75, does not contain optimal HLA-A2 antigen-binding residues and is unlikely to be presented as efficiently to CTLs as peptides with optimal or preferred amino acids for the HLA-A*0201 allele. Two E75 altered peptide ligands (APLs) containing optimal HLA-A*0201 allele-binding amino acids for the E75 peptide were designed for these studies (Table 1). Wild-type E75 contains a lysine (Lys) and isoleucine (Iso) at positions one and two, respectively. According to the HLA-A2 antigen motif previously reported by Smith et al. [17], phenylalanine (Phe) was found to be a preferred auxiliary residue at positions one and three, and leucine (Leu) was a preferred anchor residue (position 2) for nonamer peptides that bind to the HLA-A*0201 molecule. Therefore, these alterations were incorporated to the wild-type peptide for both Her-2/neu APLs (designated APL 2 and APL 5). The glycine (Gly4) residue present at position four of E75 was replaced with a glutamic acid (Glu4) in APL 5, again a preferred HLA-A*0201 allele-binding amino acid.
Table 1.
Sequences, predicted HLA-A*0201 allele binding affinities, and IC50 values of wild-type and modified E75 peptides. Her-2/neu E75 was modified at HLA-A*0201 allele-binding residues. The affinity of these altered peptide ligands for the HLA-A*0201 allele was investigated using BIMAS (Bioinformatics and Molecular Analysis Section) that predicts half-time dissociation for HLA molecules based on coefficient tables
| Sequencea | BIMASb | IC50 μMc | |
|---|---|---|---|
| E75 | K I F G S L A F L | 481.19 | 27.0 |
| APL 2 | F L F G S L A F L | 4,599.39 | 19.0 |
| APL 5 | F L F E S L A F L | 18,857.49 | 15.0 |
aStandard single-letter amino acid code is used and the residues shown in bold represent deviations from Her-2/neu E75 (269–277)
bHLA half-time dissociation values calculated using BIMAS [14]
cIC50 values determined by the amount of peptide needed to inhibit an HLA-A2 allele-binding HBV reference peptide by 50%
To determine if the amino acid substitutions we introduced into the E75 peptide affected binding to the HLA-A2 molecules, we evaluated the APLs by computer predictions, and in vitro live cell binding assays (Table 1). The predicted half-time dissociation of the APLs for the HLA-A2 molecule was calculated using the BIMAS peptide-binding algorithm [26]. Based on coefficient tables, E75 APLs were assigned higher values compared with wild-type E75 (Table 1). To assess the relative HLA-binding affinity of E75 APLs, we employed a live cell binding assay to determine the amount of wild-type E75 and APL required to competitively inhibit the binding of an HLA-A2-binding fluoresceinated reference peptide (Fl-peptide) to the HLA-A*0201 allele, and calculated the concentration at which the Fl-peptide was inhibited by 50% (IC50). The substitution of preferred anchor and auxiliary HLA-A*0201 allele-binding residues in place of nonoptimal amino acids resulted in peptides with increased binding affinity for HLA-A2 molecules. Both APL 2 and APL 5 inhibited the binding of the Fl-peptide more strongly than wild-type E75 (Table 1). APL 5 demonstrated the highest relative affinity for the HLA-A*0201 allele compared with APL 2 and E75, and as predicted, APL 2 and APL 5 had lower IC50 values (19.0 μM and 15.0 μM, respectively) than wild-type E75 (27.0 μM).
Responses from CTLs raised on E75
We tested the immunogenicity of the APLs in normal HLA-A2+ individuals. To address the capacity to prime CTL responses in vitro, PBMCs were stimulated with wild-type E75, APL 2, or APL 5, as described in “Materials and methods.” PBMC cultures were monitored for peptide specificity by IFN-γ ELISpot assays after several rounds of peptide stimulation. Her-2/neu peptide-stimulated PBMCs with T2 cells pulsed with peptide (E75, APL 2, APL 5, or an irrelevant HLA-A*0201 allele-binding peptide) were cultured in IFN-γ ELISpot assays.
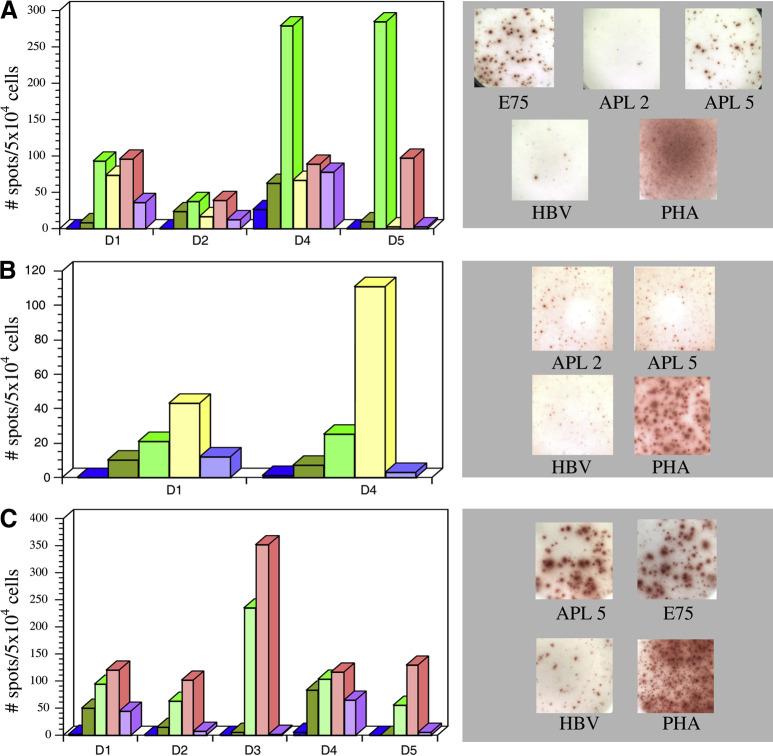
Wild-type E75 peptide elicited E75-specific responses from CTL lines derived from three of five individuals tested (D1, D4, and D5; Fig. 1A). These wild-type E75-specific CTLs cross-reacted with Her-2/neu APL 5 (Fig. 1A). Transient E75-specific activity was detected from one donor and APL 5 cross-reactivity was also observed (D2; Fig. 1A). In general, IFN-γ secretion was not observed when E75-specific CTLs were challenged with APL 2.
Fig. 1A–C.
Her-2/neu E75 and APL-specific responses detected from HLA-A2+ antigen normal donors by IFN-γ ELISpot assays and representative wells of IFN-γ ELISpot. T2 cells (2.5×104 cells/well) pulsed with peptides (10.0 μg/ml) were used to stimulate bulk PBMC cultures (5×104 cells/well). T2 cells were pulsed with the following peptides: E75 (green), APL 2 (yellow), APL 5 (pink), HBV (purple), unpulsed T2 cells (dark green), or CTL alone (blue). A CTLs raised on E75 (results from donor 3 were not included, as no IFN-γ-secreting, peptide-specific CTLs were detected). Representative spots from donor 5 shown to the right. B CTLs raised on APL 2 (representative spots from donor 4 shown to the right). C CTLs raised on APL 5 (representative spots from donor 1 shown to the right)
Responses from CTLs raised on APL 2
Stimulation of PBMCs with APL 2 resulted in APL 2–specific responses from only one of five donors (D4; Fig. 1B). APL 2–specific CTLs demonstrated cross-reactivity to wild-type E75 (Fig. 1B). Transient IFN-γ production by PBMCs was observed from one donor when challenged with target cells pulsed with APL 2 (D1; Fig. 1B).
Responses from CTLs raised on APL 5
In contrast to APL 2, APL 5 consistently elicited CTL responses from all five individuals (Fig. 1C), suggesting that this peptide is more immunogenic than both wild-type E75 and APL 2. Of importance, CTL lines specific for APL 5 were stimulated by wild-type E75 (Fig. 1C).
Molecular modeling of the HLA-A2 molecule and E75 APL complexes
Both APL 2 and APL 5 bound with greater affinity to the HLA-A2 molecule compared with wild-type E75, but only the latter peptide proved to be immunogenic in five of five donors. Since our APLs only differed by one amino acid (APL 2 Gly4, APL 5 Glu4), we wanted to further understand the relationship of these peptides presented by the HLA-A2 molecule to the TCR to investigate if these mutations create different surfaces for TCR binding. Crystallographic information for the HLA-A2 molecule with bound peptide was available in the Protein Data Bank. The HIV-derived peptide, Tax, displays structural similarity to the Her-2/neu-derived E75 peptide. Therefore, we modeled the HLA-A2-E75 complex by replacing amino acids in Tax (LLFGYPVYV) with those of E75 (similar to that reported by [27]) using the Swiss Pdb Viewer Model program. CTL responses directed against APL 2 (FLFGSLAFL) were only detected from one of five individuals, whereas APL 5 (FLFESLAFL) stimulated specific responses from all donors tested. The glutamic acid residue introduced at position four in APL 5 resulted in a side chain that energetically points out of the binding pocket of the HLA-A2 molecule (Fig. 2, parts A, B, and C show E75, APL 2, and APL 5, respectively). The negatively charged Glu4 residue in APL 5 (black residue in Fig. 2C) sits near positively charged amino acids, on the top edge of the α1 domain of the HLA-A2 antigen alpha helix (Arg65 and Lys66), which are highly likely to play a role in peptide binding. Glutamic acid is a large residue compared with glycine and the carboxylate group may be better able to interact with the TCR, as the side chain points outward from the HLA molecule. Since the Glu4 residue is the sole difference between the APL 5 and APL 2, the position of the side chain suggests that this residue plays a key role in the immunogenicity of the peptide, resulting in CTL activation in five of five individuals.
Fig. 2A–C.
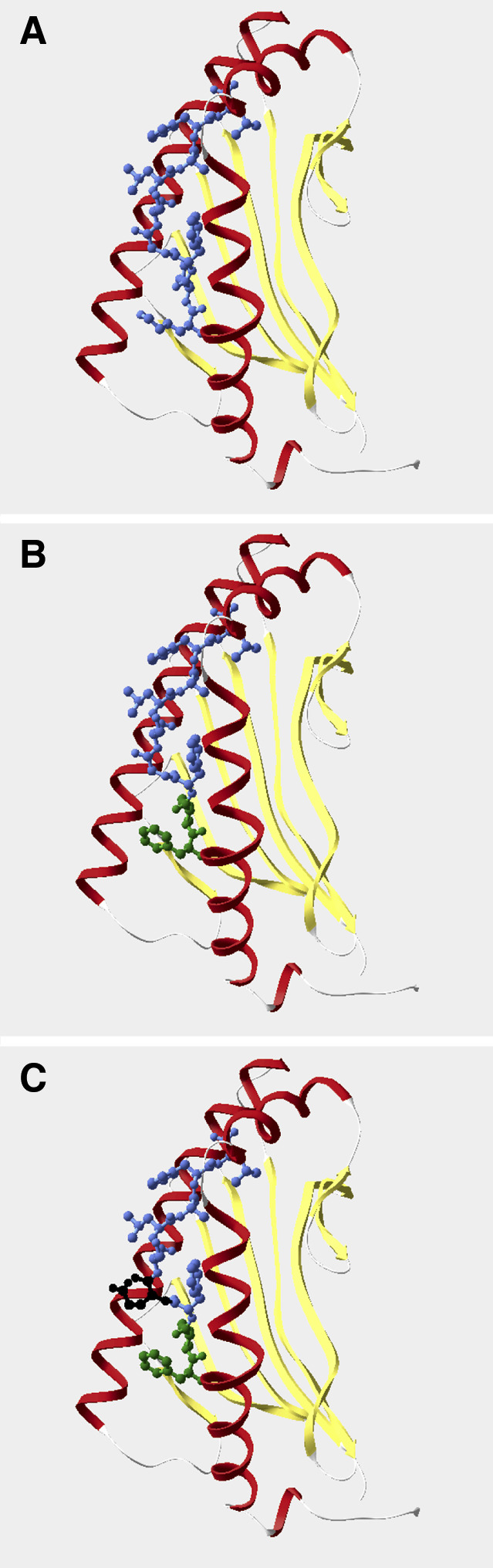
Molecular modeling of the HLA-A2 molecule complexed with E75 altered peptide ligands. The HLA-A2 antigen alpha helices are displayed in red (left: α1 domain, right: α2 domain) and the beta sheet in yellow. A HLA-A2-E75. B HLA-A2-APL 2; amino acids different from wild-type E75 are denoted by a change from blue to green. C HLA-A2-APL 5; Glu4 is represented by the black residue. The same orientation was used for all HLA-A2/peptide complexes
Cytolytic activity of APL-specific CTLs
To address whether IFN-γ-producing CTL lines could lyse targets cells pulsed with wild-type E75, cytotoxicity assays were performed. E75-specific CTLs lysed T2 cells pulsed with E75 peptide (Fig. 3A). Importantly, APL 2−specific and APL 5−specific cells lysed cells pulsed with wild-type E75 (Fig. 3, parts B and C, respectively). APL 5–specific CTLs showed increased lysis of wild-type E75-pulsed cells compared with APL 2-specific CTLs, correlating the results of our peptide affinity measurements and IFN-γ ELISpot assays. Collectively, CTLs from four of five individuals that demonstrated APL 5 specificity measured by ELISpot assays, lysed E75-displaying targets (D1, D3, D4, and D5; data not shown). APL 2 performed least well, inducing a specific response from one of five individuals as demonstrated by both IFN-γ ELISpot and cytotoxicity assays (D4). Although recognition of wild-type peptide by CTLs specific for both APL 2 and APL 5 occurred, lysis of MCF-7 (HLA-A2+ and Her-2/neu +) tumor cells was not observed. A tumor cell line transfected with a vector encoding the Her-2/neu antigen, MCF-7-2/18, was used to test CTLs specific for APL 5 (D1). Flow cytometric analysis demonstrated that these tumor cells overexpress Her-2/neu, compared with MCF-7 cells and express comparable levels of the HLA-A2 antigen (data not shown). Lysis of MCF-7-2/18 cells was observed, albeit low (7.39% ± 0.02%, the percentage lysis of the control, K562, was subtracted), at a 50:1 E:T ratio. When MCF-7-2/18 cells were pulsed with E75 peptide (10.0 μg/ml) prior to the cytotoxicity assay, a slight increase in lysis was observed (8.55% ± 0.001%).
Fig. 3A–C.
Lysis of target cells pulsed with wild-type E75 peptide. CTLs (at the indicated E:T ratios) were incubated with 51Cr-labeled target cells (5×103 cells/well), and 51Cr release from target cells was measured after 6–8 h. A E75-specific CTLs, B APL 2–specific CTLs, and C APL 5–specific CTLs lyse T2 cells pulsed with E75 (solid square). Lysis of the NK-sensitive cell line, K562 (open triangle), and lysis of T2 cells pulsed with HBV (open circle) served as background controls. Lysis of the MCF-7 breast cancer cell line (solid diamond) was also evaluated. Data are representative from donors 5, 4, and 1, respectively. Error bars represent the mean ± SEM of triplicate samples (*p<0.05)
Discussion
This study characterized two HLA-A*0201 allele-restricted, modified Her-2/neu E75 peptides for binding affinity to the HLA-A*0201 allele and for the ability to generate APL-specific CTLs that cross-react with wild-type E75 peptide. Our findings indicate that Her-2/neu APLs, designed for increased affinity for the HLA-A*0201 allele, can elicit specific peptide responses from normal donors. APL 5 had a significantly increased capacity to elicit IFN-γ from CTL lines compared with APL 2 (specific responses detected in 5/5 and 1/5 individuals, respectively). These APL-specific CTLs also cross-react with wild-type peptide, as demonstrated by IFN-γ ELISpot assays. APL-specific CTL lines lyse target cells pulsed with wild-type E75. E75-specific CTLs were also activated by APL 5, suggesting that cells activated in vivo by tumor-derived wild-type peptide, cross-react with, and are stimulated by, Her-2/neu APLs. In a therapeutic setting, CTLs that have a low affinity for wild-type peptide or that are partially anergic could be activated by Her-2/neu APLs.
APL 2 (FLFGSLALF) contains two amino acid modifications different from wild-type E75. Although these substitutions resulted in increased affinity for cell surface HLA-A*0201 molecules compared with wild-type E75, APL 2 proved to be inefficient at generating CTL lines from four of five individuals. However, APL 2–specific CTLs from the single responsive donor retained recognition of wild-type peptide as determined by IFN-γ production and lysis of target cells pulsed with wild-type E75. Since positions one and two of a nonamer peptide are buried in the HLA peptide-binding cleft, these residues are thought to be solely involved in HLA contact and may not affect TCR binding. Additionally, the isoleucine (Iso2) to leucine (Leu2) substitution was a subtle modification, as these amino acids are very similar in composition. Our results with APL 2 illustrate that peptides may possess increased affinity for HLA class I molecules compared with the wild-type peptide, but affinity alone is insufficient in rational design of a highly immunogenic peptide capable of T-cell activation.
The rational design of APL 5 (FLFESLAFL) also resulted in an increased affinity for HLA-A*0201 molecules compared with wild-type E75. In contrast to APL 2, APL 5 elicited peptide-specific responses from all five individuals tested. CTLs raised on APL 5 reacted most strongly to challenge with the same peptide and cross-reactivity with E75 was also observed when APL 5–specific CTLs were challenged with wild-type peptide. It has been suggested that this weak MHC binding is due to a lack of interaction with the center of the peptide [28]. The increase in the affinity of APL 5 for the HLA-A2 molecule may result from interaction of the center of the peptide (residue 4) with the HLA, resulting in the presentation of a peptide with more favorable TCR-binding characteristics compared with wild-type peptide.
Molecular modeling of the HLA-A2 molecule complexed with E75 APL provided further insight into the differences of peptide presentation by the HLA-A2 molecule to TCR. The side chain of the Glu4 residue in APL 5 appear to be extending out of the peptide-binding cleft, and may participate in the interaction with the TCR upon ligation of the TCR with the peptide-MHC complex. Therefore, increased peptide immunogenicity is likely due to the large side chain that interacts with the outer edge of the helix, allowing TCR contact. The wild-type E75 peptide and APL 2 contain a Gly4 residue, which is smaller in size compared with glutamic acid, and may not interact with the TCR. APL 2 differs from E75 by only the first two amino acids of the peptide, yet E75 was better able to stimulate CTLs than APL 2. Loss of CTL reactivity indicated that while the modifications introduced into APL 2 yielded a peptide with an increased affinity for HLA-A2 antigen, the conformation of the peptide/HLA complex was not favorable for TCR recognition. Studies have also demonstrated that substitutions introduced into anchor positions can alter residues involved in TCR binding [29]. While it is difficult to provide a concrete explanation for this finding, we might conclude that high affinity peptide/HLA complexes do not always translate to highly efficient cellular stimulators.
Although we observed minimal tumor cell lysis by our APL-specific CTL, one possible reason we could not demonstrate strong lysis of tumor cells was reported by Keogh et al. [30]. They found that CTL specific for APL, with more than one amino acid substitution compared with the wild-type peptide, were less frequently able to recognize the parental peptide. Several groups have also not been able to demonstrate lysis of tumor cells by peptide-specific CTLs [31, 32]. Additionally, low peptide density on the surface of the tumor cells may provide an explanation for the absence of tumor cell lysis. Zaks et al. [33] demonstrated that E75-specific CTLs isolated from patients immunized with E75 were unable to lyse tumor cells that expressed the Her-2/neu antigen. Similar to our findings, these CTLs lysed T2 cells pulsed with the E75 peptide. While the Her-2/neu antigen is over-expressed on tumor cells, the E75 peptide may not be present at high enough levels for TCR recognition. Studies have shown that increasing the peptide density on melanoma cells, via the addition of an exogenous melanoma-specific peptide (g209 2M), resulted in an enhanced sensitivity to peptide-specific CTLs [34]. We pulsed MCF-7 tumor cells that overexpressed the Her-2/neu antigen (MCF-7-2/18) with the E75 wild-type peptide and observed minimal lysis. It is possible that these cells secrete immunosuppressive factors that inhibit T-cell effector functions.
PBMCs stimulated for several rounds with wild-type E75 (KIFGSLAFL) demonstrated E75 specificity when challenged with T2 cells pulsed with E75 as determined by IFN-γ ELISpot assays. These E75-specific PBMCs were also activated by APL 5, suggesting that in vivo, existing anti-wild-type CTLs possess cross-reactive recognition of Her-2/neu APLs.
These findings indicate that the introduction of preferred amino acid residues into the Her-2/neu E75 peptide results in an increased affinity for the HLA-A*0201 allele compared with the wild-type peptide and may (1) activate existing CTLs specific for Her-2/neu and (2) elicit CTLs de novo that cross-react with wild-type peptide. Continuing studies are investigating CTL responses to APLs by wild-type anti-Her-2/neu CTLs from patients with Her-2/neu-positive tumors. These results will reveal if Her-2/neu APLs are capable of rescuing weakly responding or anergic CTLs in cancer patients.
Acknowledgements
We are grateful to Dr Sue Roberts for her contributions to the molecular modeling of HLA-A2/peptide complexes. This study was supported by a National Cancer Institute grant, RO1 CA94852.
References
- 1.Niehans J Natl Cancer Inst. 1993;85:1230. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- 2.Slamon Science. 1987;235:177. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Slamon Science. 1989;244:707. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Fisk J Exp Med. 1995;181:2109. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peoples Proc Natl Acad Sci U S A. 1995;92:432. [Google Scholar]
- 6.Brossart Cancer Res. 1998;58:732. [PubMed] [Google Scholar]
- 7.Brossart Blood. 2000;96:3102. [PubMed] [Google Scholar]
- 8.Knutson J Clin Invest. 2001;107:477. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray Clin Cancer Res. 2002;8:3407. [Google Scholar]
- 10.Knutson Clin Cancer Res. 2002;8:1014. [PubMed] [Google Scholar]
- 11.Falk Nature. 1991;351:290. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 12.Hunt Science. 1992;255:1261. [Google Scholar]
- 13.Parker J Immunol. 1992;149:3580. [PubMed] [Google Scholar]
- 14.Ruppert Cell. 1993;74:929. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 15.Kast J Immunol. 1994;152:3904. [PubMed] [Google Scholar]
- 16.Pogue Proc Natl Acad Sci U S A. 1995;92:8166. doi: 10.1073/pnas.92.18.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith Mol Immunol. 1998;35:1033. doi: 10.1016/S0161-5890(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 18.Sette J Immunol. 1994;153:5586. [PubMed] [Google Scholar]
- 19.Feltkamp Mol Immunol. 1994;31:1391. doi: 10.1016/0161-5890(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 20.Ressing J Immunol. 1995;154:5934. [PubMed] [Google Scholar]
- 21.Sigal Mol Immunol. 1995;32:623. doi: 10.1016/0161-5890(95)00031-9. [DOI] [PubMed] [Google Scholar]
- 22.van Hum Immunol. 1995;44:189. doi: 10.1016/0198-8859(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 23.Dionne Cancer Immunol Immunother. 2003;52:199. doi: 10.1007/s00262-002-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman Nucleic Acids Res. 2000;28:235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guex Electrophoresis. 1997;18:2714. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 26.Parker J Immunol. 1994;152:163. [Google Scholar]
- 27.Castilleja J Immunol. 2002;169:3545. doi: 10.4049/jimmunol.169.7.3545. [DOI] [PubMed] [Google Scholar]
- 28.Kuhns J Biol Chem. 1999;274:36422. doi: 10.1074/jbc.274.51.36422. [DOI] [PubMed] [Google Scholar]
- 29.Sharma J Biol Chem. 2001;276:21443. doi: 10.1074/jbc.M010791200. [DOI] [PubMed] [Google Scholar]
- 30.Keogh J Immunol. 2001;167:787. doi: 10.4049/jimmunol.167.2.787. [DOI] [PubMed] [Google Scholar]
- 31.Rivoltini J Immunol. 1995;154:2257. [PubMed] [Google Scholar]
- 32.Kawashima Hum Immunol. 1998;59:1. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 33.Zaks Cancer Res. 1998;58:4902. [PubMed] [Google Scholar]
- 34.Yang J Immunol. 2002;169:531. doi: 10.4049/jimmunol.169.1.531. [DOI] [PubMed] [Google Scholar]