Abstract
In this study, we demonstrated that chemoimmunotherapy using S-1, a novel oral fluoropyrimidine anticancer drug, combined with lentinan (LNT), a β (1→3) glucan, was effective in vivo, and we clarified the augmentation of the function of dendritic cells (DCs) in vivo and in vitro. The survival period of Colon-26–bearing mice treated with S-1+LNT was significantly more prolonged than that of mice treated with S-1 alone (P<0.05). On the other hand, LNT did not prolong the survival period when combined with S-1 in Colon-26–bearing athymic mice. The frequency of CD86+ DCs infiltrated into Colon-26 was increased in mice treated with S-1+LNT, and splenic DCs harvested from mice treated with S-1+LNT showed more potent T-cell proliferation activity than that of DCs from mice treated with S-1 alone (P<0.05). Furthermore, the activity of cytotoxic T lymphocytes (CTLs) in splenocytes of S-1+LNT–treated mice was specific and more potent than that of CTLs from mice treated with S-1 alone (P<0.05). These results suggest that modulation of specific immunity with LNT has a significant role in enhanced antitumor effects through the modification of DC function. We demonstrated that DCs might play an important role in chemotherapy, and the combination therapy of S-1 and LNT presents a promising chemoimmunotherapy, which might lead to better survival for cancer patients.
Keywords: Chemoimmunotherapy, Colon-26, CTL, DCs, Lentinan, S-1, Specific immunity
Introduction
Except in the early stages and in certain types of cancer, we have not been satisfied with the outcome of anticancer therapy. To overcome this situation, new strategies for cancer treatment are required. One of them, chemoimmunotherapy, has been advocated as a modality of combination chemotherapy and immunotherapy since the 1970s [1, 2]. Recent attention has been paid to their combinatorial effect on the host immunological response. Actually, some cytokines (IL-2, GM-CSF, IFN-α, and IFN-β, etc.) or biochemical response modifiers (BRM, BCG [2], LNT [3], etc.) have been used clinically, and these have advantages in eliciting an antitumor immunological response. Especially, the elucidation of immunological molecular mechanisms has provided us with strategies to utilize them as a weapon to treat cancer patients [4].
When addressing the priming of immune response, we can not disregard the role of dendritic cells (DCs) as professional antigen-presenting cells (APCs). In immune response to tumors, immature DCs acquire tumor antigens in tumor tissues, process the antigens, and then migrate into regional lymph nodes. They differentiate into mature DCs and present the tumor antigens on their surface MHC class I and class II molecules to prime naïve T cells [5]. The activated T cells show specific cytotoxic activity to the target cells and mediate tumor destruction. Utilizing these immunological features, DCs have been used in cancer immunotherapy in several ways. For example, DCs have been pulsed in vivo and ex vivo with peptides [6] or tumor lysate [6, 7] and have been genetically modified with cytokine and cancer antigen–expressing constructs [8, 9]. As we learn more and advance, promising treatments are expected. Recently, a new strategy using the combination of intratumoral injections of DCs and systemic chemotherapy has been reported with intriguing results [10]. However, almost all of these strategies need multistep manipulations ex vivo. Besides, it is controversial which is the best method for isolating, activating, and loading antigens to DCs [4]. Furthermore, the generation of an effective immune response in tumor-bearing hosts has been proven to be more difficult than the induction of protective immunity in normal animals [11]. These defects have been attributed to suppression of T-cell function and defective function of APCs [11–13]. Elucidation of the function of the professional APCs and DCs may help in improving the manipulation of antitumor immune response, and prolong the survival period of tumor-bearing hosts.
Lentinan (LNT), β-(1→3)-D-glucan, an extract from the edible mushroom, Lentinus edodes, has been reported to show direct antitumor effects [14, 15] and various immunomodulatory effects. For instance, LNT is able to activate CTLs [16, 17], LAK cells, NK cells [18], and macrophages [18]. It is also able to improve Th1/Th2 cytokine balance [19]. LNT has been used to treat cancer patients as a biological response modifier (BRM) with cancer chemotherapy, and has been reported to improve the quality of life of the patients and prolong their survival periods [3]. Recently, the β-glucan receptor was identified and characterized [20, 21], and β-glucan has been reported to affect phagocytosis of monocytes and macrophages.
Cancer chemotherapy may bring some antitumor effects, but it is hard to prolong survival. S-1 is a novel oral form of 5-FU consisting of tegafur (prodrug of 5-FU), gimeracil (inhibits degradation of 5-FU and maintains 5-FU level in effective range for long time), and oteracil potassium (regulates the phosphorylation of 5-FU in the gastrointestinal tract and reduces gastrointestinal toxicities) in the molar ratio of 1:0.4:1. S-1 was developed to enhance antitumor activity of 5-FU, not accompanied by gastrointestinal toxicity in vivo [22–25]. S-1 has shown potent antitumor effects and fewer gastrointestinal adverse effects due to the longer persistency of 5-FU from the actions of these two modulators as shown in recent clinical trials [26, 27].
Chemoimmunotherapy has shown promise as being advantageous to our conventional chemotherapy. To understand the mechanism of chemoimmunotherapy in the treatment of cancer, we evaluated the role of cellular immunity in cancer-bearing mice and rats treated with the combination of S-1+LNT. We especially focused on the function of the DCs in vivo and in vitro.
Material and methods
Cells and reagents
Colon-26, a BALB/c-derived colorectal adenocarcinoma cell line, was maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) [28]. Meth A, a methylcholanthrene-induced BALB/c-derived fibrosarcoma cell line that was obtained from the RIKEN cell bank, and YAC-1, an A/Sn-derived murine lymphoma cell line, were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. Rat lung cancer adenocarcinoma, Sato lung carcinoma (SLC) [29], was maintained intraperitoneally (i.p.) in 5-week-old male Donryu rats and was collected for further experiments 4–7 days after i.p. implantation.
S-1 was prepared by mixing tegafur, gimeracil, and oteracil in the molar ratio of 1:0.4:1 and dissolved in 0.5% hydroxypropylmethylcellulose (HPMC) [22–24]. LNT [14, 15] was kindly provided by Ajinomoto (Kanagawa, Japan) and was dissolved in sterile saline solution at the final concentration of 0.5 mg/ml. Both S-1 and LNT were clinical grade reagents.
Animal experiments
Five-week-old BALB/c mice and BALB/c nu/nu mice were purchased from CLEA Japan (Tokyo, Japan). Five-week-old male C57BL/6 mice and 4-week-old male Donryu rats were purchased from Charles River Japan (Kanagawa, Japan). Animals were maintained in the specific pathogen-free Animal Facility of the Institute of Medical Science, The University of Tokyo, and Taiho Pharmaceutical and used for experiments after a 1-week quarantine.
Colon-26 cells (1×106) were subcutaneously (s.c.) inoculated into 6-week-old male BALB/c mice or athymic mice on day 0. Mice were randomized according to body weight (n=8) prior to start of the treatment on day 7. S-1 was orally administered 6.9 mg/kg as effective dose [23] on day 7, when cachectic symptoms were first observed, then for 14 consecutive days or for the rest of the animal’s life. LNT was administered i.p. at a dose of 0.1 mg/body [30] twice a week from day 7 to 21 or lifelong, based on clinical application.
SLC cells (2×105) were s.c. inoculated into the right axilla of 5-week-old male Donryu rats weighing about 150 g, and then the rats were randomized into treatment and control groups (n=7) based on body weight just after inoculation (day 0). Fifteen milligram per kilogram of S-1 as a minimum toxic dose [24] was orally administered from day 1–14, and 0.5 mg/body of LNT was administered i.p. [31] on days 1, 5, 8, 12, and 15, based on the clinical schedule.
For evaluation of the antitumor and anticachectic activity of S-1+LNT, body weight, tumor weight, and epididymal adipose tissue weight were measured.
Mixed lymphocyte reaction (MLR)
Splenic DCs were purified using a magnetic-activated cell sorter (MACS) as described previously [32]. Briefly, harvested spleens were treated with collagenase D (Rosche, Basel, Swiss) for 20 min at 37°C followed by a procedure to obtain single WBC suspensions. After incubating with antimouse CD11c mAbs (Miltenyl Biotec, CA, USA) for 15 min at 8–10°C, CD11c+ DCs were positively selected by being passed through a MACS VS+ column (Miltenyl Biotec, CA, USA) twice. CD11c+ DCs constituted 80–92% of the purified population. T cells were enriched using a nylon wool column from either C57BL/6 (H-2b) or BALB/c (H-2d) mice. CD3+ T cells represented >85% of their purified population. T cells (5×105) were cultured with different concentrations of X-ray irradiated (20 Gy) DCs in U-bottomed 96-well plates. After 72 h of culture, the cells were pulsed with 1 μCi of [3H]-thymidine for 18 h. [3H]-Thymidine incorporation was measured using a liquid scintillation counter.
Serum calcium and cytokine quantitation
Serum calcium level was determined using a calcium assay kit (Calcium E test; Wako). Serum levels of IL-6, IL-10, and TGF-β1 were quantified in murine serum using ELISA kits (OptEIA; BD Pharmingen) according to the manufacturer’s instructions.
Immunohistochemical analysis
Tumors were harvested 14 and 28 days after tumor inoculation, then immediately frozen, and embedded in OCT compound. Serial 4-μm sections were made from these tumors using a cryostat and underwent immunohistochemical staining using antibodies to CD11c, CD86 (Pharmingen, San Diego, CA, USA), and B220. The stained cells were counted in 20 fields at a magnification of ×200 for each sample. Evaluation of the results was performed in a blind fashion.
In vitro stimulation of spleen cells
Spleen cells were harvested from the mice that had received S-1 alone, and S-1+LNT on day 28. Spleen cells were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin G, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 1 mM nonessential amino acids, and 50 mM 2-mercaptoethanol (complete medium), and were cultured in 25-cm2 culture flasks in an upright position at a 10:1 ratio with Colon-26 cells irradiated with 100 Gy X-ray in the presence of rhIL-2 (10 IU/ml) for 5 days.
Cytotoxic assay
Cytotoxic activity was measured by a 4-h chromium 51 (51Cr)-releasing assay using the labeled syngeneic tumor cells described below. Target cells were labeled with 100 μCi of 51Cr for 1 h at 37°C. The labeled target cells were washed and resuspended to a mixture of 1×105 cells/ml in complete medium; 100 μl of the target cells was mixed with an equal volume of the effector cells at various ratios in duplicate in 96-well U-bottomed plates. After incubation for 4 h at 37°C in 5% CO2, supernatants were harvested, and the released radioactivity was determined with a gamma counter. The percentage of specific 51Cr release was calculated as: 100×[(experimental release) − (spontaneous release)] / [(maximal release) − (spontaneous release)] (%). Spontaneous release was determined using target cells without effectors, while total release was determined with target cells exposed to 1 N HCl. Spontaneous release and variation between replicates was less than 20%.
Statistical analysis
All results are expressed as mean ± SD. Comparisons between different means were performed by repeated analysis of variance (ANOVA) test. Mann–Whitney’s U test was used for comparison between two groups. P<0.05 was considered significant.
Results
Chemoimmunotherapy using S-1 and lentinan prolongs the survival of Colon-26 tumor-bearing BALB/c mice
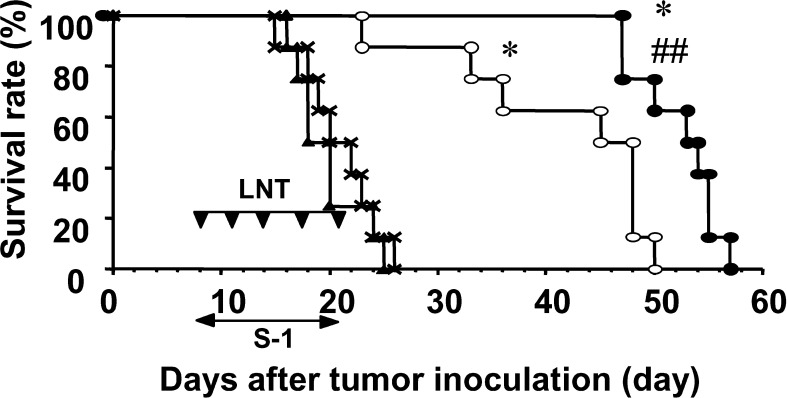
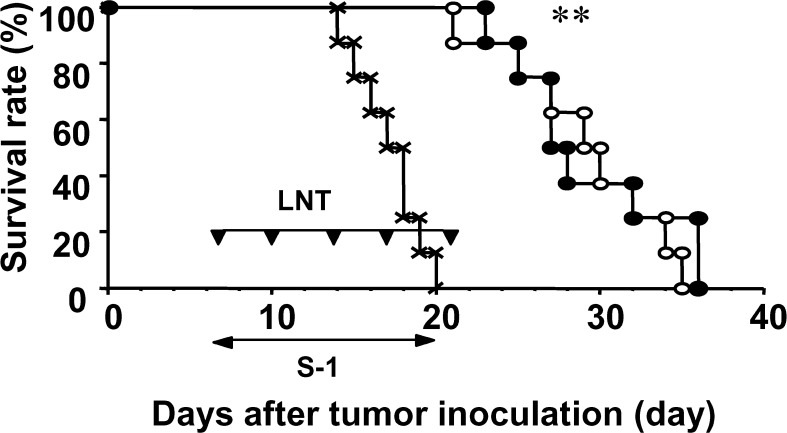
The mouse model used in this report has been reported to show cancer cachectic symptoms [28]. The survival periods of the no-treatment, the LNT alone, the S-1 alone, and the S-1+LNT groups were 20.9 ± 3.6, 19.8 ± 3.2, 41.4 ± 9.7, and 52.3 ± 3.8 days, respectively. The survival of Colon-26–bearing mice was not prolonged by the treatment with LNT alone. However, treatment with S-1 alone prolonged survival of the mice compared with the control group or the LNT-alone–treated group (P<0.05). Furthermore, the combination treatment of S-1 with LNT could make survival longer compared with that with S-1 alone with statistical significance (P<0.05) (Fig. 1). We performed lifelong chemoimmunotherapy using S-1+LNT. There was no statistically significant survival prolongation between treatment for 2 weeks and that for the life of the animal in each group (data not shown); however, no specific toxic effects due to continuous treatment were observed.
Fig. 1.
Chemoimmunotherapy using S-1 and LNT prolongs survival of Colon-26–bearing BALB/c mice. On day 0, mice were inoculated with 1×106 Colon-26 cells s.c. at their right axilla. Mice received HPMC p.o. as control (X), and either or both of LNT alone (0.1 mg/body) i.p. twice a week (filled triangles), and S-1 (6.9 mg/kg) p.o. every day from days 7 to 20, alone (open circles) or with LNT (filled circles). Data represent survival after tumor inoculation. Data are representative of independent two experiments. *P<0.01 vs control and LNT alone, P<0.01 vs S-1 alone.
Chemoimmunotherapy using S-1 and LNT prolongs the survival of Sato lung carcinoma–bearing Donryu rats
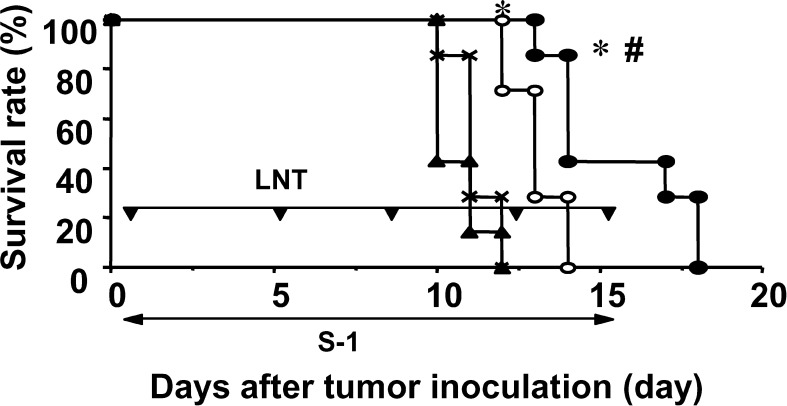
To examine whether survival prolongation could be observed in another tumor model, we used the rat tumor model. The survival periods of the no-treatment, the LNT alone, the S-1 alone, and the S-1+LNT group were 11.1 ± 0.7, 10.6 ± 0.8, 13.0 ± 0.8, and 15.4 ± 2.1 days, respectively. The survival of rats bearing SLC was not prolonged by treatment with LNT alone. Treatment with S-1 alone prolonged survival of the rats, compared with the control group or the group treated with LNT alone (P<0.05). Furthermore, combination treatment using S-1+LNT could prolong the survival of the rats compared with that of the group treated with S-1 alone (P<0.05) (Fig. 2).
Fig. 2.
Chemoimmunotherapy using S-1 and LNT prolongs survival of Donryu rats bearing SLC. On day 0, rats were inoculated with 2×105 SLC cells s.c. at their right axilla. Mice received HPMC p.o. as control (X), and either or both of LNT alone (0.5 mg/body) i.p. twice a week (filled triangles), and S-1 (15 mg/kg) p.o. every day from day 1 to 14, alone (open circles) or with LNT (filled circles). Data represent survival after tumor inoculation. Data are representative of two independent experiments. *P<0.01 vs control and LNT alone; #P<0.05 vs S-1 alone.
Cancer cachexia markers and serum levels of immunosuppressive cytokines show no difference between S-1 alone and S-1+LNT treatment
Both the Colon-26 and SLC tumor model are well-known cancer cachexia models [28, 29]. We examined body weight, adipose tissue weight, serum calcium level, and the serum levels of immunosuppressive cytokines (IL-6, IL-10, and TGF-β1) as cancer cachexia markers. The group treated with LNT alone did not show any improvement of cancer cachexia markers. S-1 treatment almost completely improved body weight loss, adipose tissue weight, serum calcium level, and IL-6 when compared with the control group (P<0.05). Unexpectedly, cancer cachexia markers showed no significant difference between the groups treated with S-1 alone and the groups treated with S-1+LNT (Table 1). Thus, the improvement of cancer cachexia probably is not a major cause of the survival prolongation in group treated with S-1+LNT.
Table 1.
Cancer cachexia markers and serum levels of immunosuppressive cytokines showed no differences between S-1 alone and S-1+LNT treatment. Epididymal adipose tissue weight, which reflects systemic fat weight, and serum calcium level were measured on day 16. Body weights of mice were measured on day 21. Serum IL-6, IL-10, and TGF-β1 were evaluated by ELISA on day 14. Data represent the mean ± SD. LNT itself did not show any effects on body weight, adipose tissue weight, and serum calcium level as compared with those of no treatment group. S-1 treatment improved adipose tissue weight and serum calcium level (P<0.05), and serum levels of IL-6, IL-10, and TGF-β1. However, groups showed no differences between treatment with S-1 alone and treatment with S-1+LNT. NS Not significant
| Treatment (g) | Body weight (g) | Adipose tissue (mg/dl) | Serum Ca (pg/ml) | Serum IL-6 (pg/ml) | Serum IL-10 (pg/ml) | Serum TGF-β1 |
|---|---|---|---|---|---|---|
| Normal | 26.0±1.6 | 0.37±0.16 | 9.8±1.2 | 0 | 83.3±121.0 | 779.6±615.6 |
| Control | 23.0±2.2a | 0.09±0.09b | 19.7±3.4c | 109.6±64.0d | 23.4±44.6e | 440.0±718.7f |
| LNT | 21.8±1.9g | 0.11±0.08h | 19.5±4.1i | 350.2±417.7j | 35.4±60.5k | 213.1±247.0l |
| S-1 | 24.5±2.3m | 0.31±0.06n | 11.8±1.1o | 21.6±22.9p | 37.7±37.4q | 538.9±694.0r |
| S-1+LNT | 26.0±2.9s | 0.33±0.08t | 10.4±1.4u | 29.2±42.2v | 63.4±47.6w | 188.0±146.6x |
a,gNS; b,hNS; c,i,NS; d,jNS; e,kNS; f,lNS; g,mP<0.05; h,nP<0.05; i,oP<0.05; j,pP<0.05; k,qNS; l,rNS; m,sNS; n,tNS; o,uNS; p,vNS; q,wNS; r,xNS
Treatment with S-1+LNT is associated with the increased frequency of CD86+ cells in the tumor
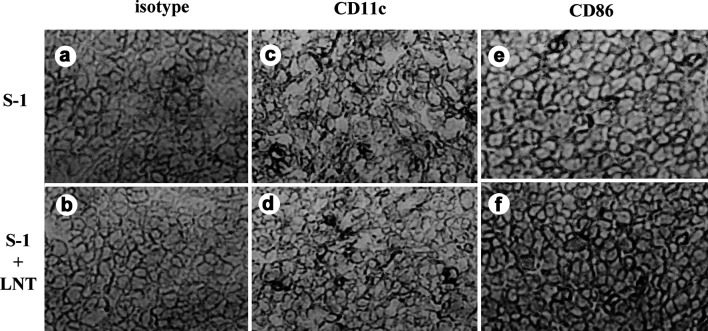
Several previous studies have shown a correlation between intratumoral DC infiltration and survival periods [11, 33, 34]. A greater frequency of DCs in the tumor is associated with a better prognosis. We examined tumor-infiltrating DCs with immunohistochemical staining using CD11c (a relatively specific marker of murine DCs) and CD86 (B7.2) on days 14, 28, and 42 to compare S-1 treatment alone, with S-1+LNT treatment. Treatment with S-1+LNT was associated with an increased number of CD86+ cells in the tumor at day 14 (P<0.05), whereas there was no difference in the number of CD11c+ cells in the tumors derived from groups treated with S-1 alone and those treated with S-1+LNT (Fig. 3, Table 2). The distribution of DCs through the tumor was not treatment specific. Typical morphological features suggest that the CD86+ cells could be DCs. Moreover, CD86+ cells did not stain with the B220 Abs in serial section, and we could not detect B220+ cells in the tumor (data not shown).
Fig. 3.
Immunohistochemical staining shows that treatment with S-1+LNT was associated with increased frequency of CD86+ cells in the tumor. a Isotype control of treatment with S-1 alone, b isotype control of treatment with S-1+LNT, c CD11c+ of treatment with S-1 alone, d CD11c+ with S-1+LNT, e CD86+ of treatment with S-1 alone, f CD86+ of treatment with S-1+LNT. The original magnification is ×200 for each sample.
Table 2.
Treatment with S-1+LNT was associated with increased numbers of CD86+ cells in the tumor. Data show mean ± SD cells/field at a magnification of ×200. The stained cells were counted in 20 fields at a magnification of ×200 in each sample. Evaluation of the results was performed in a blind fashion. Tumor-infiltrating CD11c+ cells (DCs) showed no difference between groups. However, tumor-infiltrating CD86+ cells of S-1+LNT–treated mice accumulated two times more than those of S-1–treated mice (*P<0.05). Original magnification was ×200. NS Not significant
| Treatment | CD11c+ | CD86+ | |
|---|---|---|---|
| Day 14 | No treatment | 8.9±4.6 | 2.3±1.1 |
| LNT | 15.8±8.9 | 1.3±1.0 | |
| S-1 | 16.6±6.3 | 2.0±1.1 | |
| S-1+LNT | 10.4±8.4 (NS) | 4.9±2.3* |
*P<0.05
Mixed lymphocyte reaction is up-regulated with DCs harvested from cancer-bearing mice treated with S-1+LNT
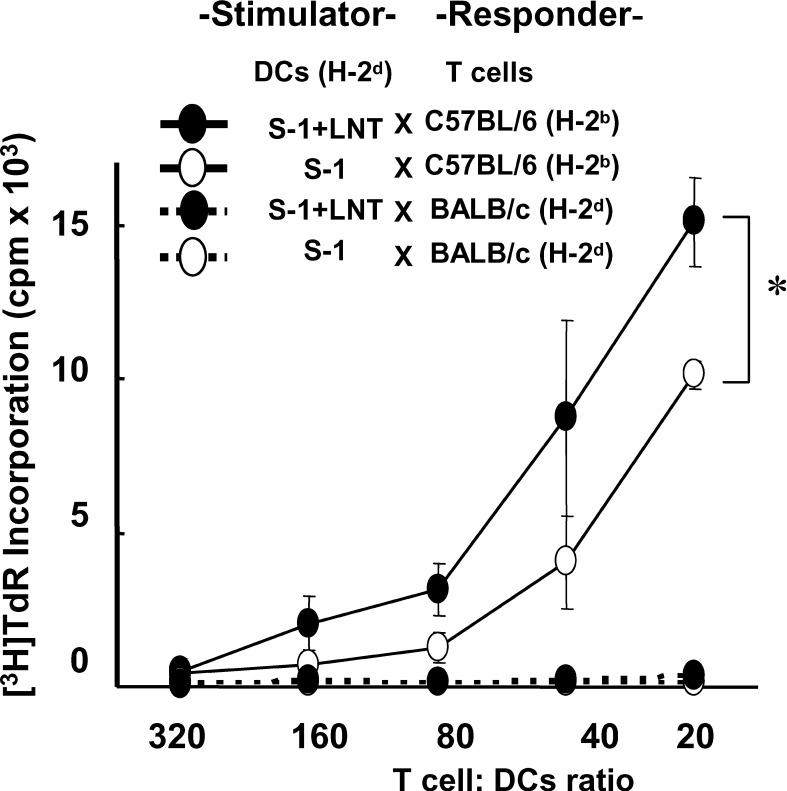
As mentioned above, the expression of a costimulatory molecule, CD86, was increased in tumor-infiltrating DCs in S-1+LNT–treated mice compared with those in mice treated with S-1 alone. To evaluate the APC function of DCs, we examined MLR using DCs obtained from cancer-bearing mice as a stimulator. DCs were sorted from the spleens of cancer-bearing mice using MACS. The purity of CD11c+ cells was greater than 80% (data not shown). As a responder, T cells were purified from the spleens of normal C57BL/6 (H-2b) and BALB/c (H-2d) mice using nylon wool columns. The purity of CD3+CD4+ or CD8+ cells was higher than 85% (data not shown). The DCs harvested from cancer-bearing mice treated with S-1+LNT showed approximately two times more potent stimulation of T-cell proliferation than those in mice treated with S-1 alone (P<0.05) (Fig. 4).
Fig. 4.
Mixed lymphocyte reaction was up-regulated with DCs harvested from cancer-bearing mice treated with S-1 and/or LNT. C57BL/6 (H-2b) and BALB/c (H-2d) splenic T cells enriched by nylon wool column were used as responders. X-ray irradiated (20 Gy), MACS-sorted CD11c+ splenic DCs from cancer-bearing mice (BALB/c, H-2d) were cultured at graded concentrations with 2×105/well responder T cells for 72 h. [3H]-Thymidine was added 18 h before harvesting. Data are expressed as mean ± SD of two separate experiments. There is a significant difference in [3H]-thymidine incorporation between CD11c+ splenic DCs originating from mice treated with S-1 alone and those originating from mice treated with S-1+LNT (*P<0.05). Data are representative of two independent experiments.
CTL activity is augmented in mice treated with S-1+LNT compared with S-1 alone
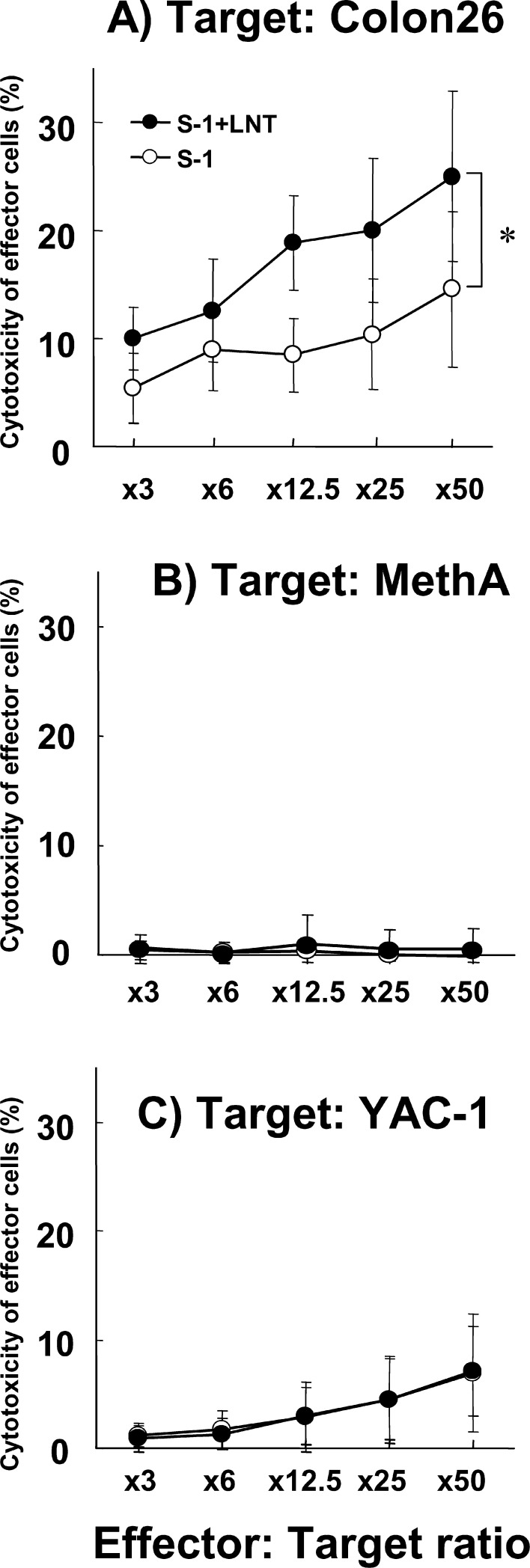
To examine the mechanism of survival prolongation, we examined the immune functions of effector cells from treated cancer-bearing mice on day 28 after the completion of treatment. The cytotoxic activity of the effector cells was more potent against Colon-26 cells when treated with S-1+LNT than with S-1 alone (P<0.05). The specificity of the effector cells was revealed using syngeneic fibrosarcoma cells, Meth A cells, and YAC-1 cells as a NK cell target. Cytotoxic effect was significantly enhanced only against the Colon-26 cells (P<0.05), but not against Meth A cells and YAC-1 cells in treatment with S-1+LNT (Fig. 5).
Fig. 5.
CTL activity was augmented in mice treated with S-1+LNT compared with that in mice treated with S-1 alone. Cytolytic activity was assessed against Colon-26, Meth A, and YAC-1 cells at various E/T ratios from mice treated with S-1 alone and S-1 combined with LNT on day 28 after tumor inoculation. Data represent the mean ± SD of cytotoxicity of effector cells from splenocytes. There is a significant difference in cytolytic activity between effector cells from S-1 combined with LNT and S-1 alone (*P<0.05) against Colon-26 cells. Data are from two independent experiments.
Chemoimmunotherapy using S-1 and LNT shows no survival prolongation of Colon-26–bearing athymic mice
To confirm if the survival prolongation of the mice from S-1+LNT treatment was associated with the function of T cells, we examined athymic mice using the same procedure. The survival periods of the no-treatment, S-1 alone, and the S-1+LNT group were 17.1 ± 2.0, 29.1 ± 4.7, and 29.3 ± 4.9 days, respectively. Treatment with S-1 alone could prolong the survival of the mice with statistical significance (P<0.05). However, the survival prolongation from treatment with S-1+LNT was completely abrogated in athymic mice. These results strongly indicate that cytotoxic activity of the T cells has a major role in prolonging the survival period of the mice through the activation of DCs in the S-1+LNT treatment group (Fig. 6).
Fig. 6.
Chemoimmunotherapy using S-1 and LNT showed no survival prolongation of Colon-26–bearing athymic mice. On day 0, mice were inoculated with 1×106 Colon-26 cells s.c. at their right axilla. Mice received HPMC p.o. as control (X), S-1 alone (6.9 mg/kg) p.o. everyday (open circles), or with LNT (0.1 mg/body) i.p. twice a week (filled circles), from days 7 to 20. Data represent the survival after tumor inoculation. **P<0.01 vs control. There was no significant difference between S-1 and S-1+LNT.
Discussion
As reported previously, chemoimmunotherapy using combined 5-FU and LNT showed significant survival prolongation [35, 36]. However, little is known regarding the mechanism. We examined a novel anticancer drug, S-1, combined with the β-glucan, LNT, using a Colon-26 tumor and SLC tumor-bearing cachectic mouse model. We demonstrated here that the novel chemoimmunotherapy using the combination of S-1 and LNT showed survival benefits through increasing the frequency of tumor-infiltrating CD86+ cells, augmenting T-cell stimulating activity of the host’s own DCs, and inducing tumor-specific CTLs. This evidence suggests that chemoimmunotherapy using S-1+LNT may activate DCs in vivo, and the DCs may play an important role as a possible surrogate marker of chemoimmunotherapy.
There have been reports of immunosuppressive cytokines, such as IL-6 and TGF-β1, being detected in the serum of Colon-26 tumor–bearing mice [37, 38]. We confirmed that Colon-26 cells produced the immunosuppressive cytokines in vitro (data not shown). Generally, LNT has been reported to improve cancer cachexia through the modification of the Th1/Th2 cytokine balance [19]. In contrast to these previous reports, treatment with LNT alone showed neither any effect on survival prolongation, improvement of cancer cachectic markers, nor Th2 cytokines in our model (Fig. 1, Table 1). S-1 showed survival prolongation with improvement of cancer cachectic markers compared with the control group and the group treated with LNT alone (P<0.05). Surprisingly, the group treated with S-1+LNT showed more survival prolongation than the group treated with S-1 alone (P<0.05) without improvement of cancer cachectic markers (Fig. 1, Table 1). Moreover, survival benefit of chemoimmunotherapy with S-1+LNT was also observed in as SLC-bearing rat model (Fig. 2). These data suggest that LNT shows synergistic effects with S-1 treatment in survival prolongation. However, indicators of cancer cachexia and immunosuppressive cytokines showed no significant difference between groups treated with S-1 alone and those treated with S-1+LNT (Table 1).
To investigate the mechanism of survival prolongation of treatment with S-1+LNT, we hypothesized that certain cellular immune responses were involved in the survival benefit seen with S-1+LNT vs S-1 alone. Recently, a new receptor for β-glucan was identified and characterized. Furthermore, β-glucan was observed to mediate phagocytosis of macrophages and monocytes [20]. DCs are thought to be in the same differentiation lineage as macrophages and monocytes [39]. LNT has been reported to augment the cytotoxic activity of CTLs [16, 17], which need to prime the immune response by APCs. These results suggest that LNT could affect phagocytic activity and the prime immune response of DCs. However, LNT showed no effect on the maturation of murine bone marrow–derived DCs and human monocyte–derived DCs in vitro (data not shown). Besides, LNT does not affect the phagocytic activity of DCs in vitro (data not shown).
There have been reports that suggest a relationship between the frequency of tumor-infiltrating DCs (TIDCs) and the prognosis of cancer patients. The presence of more TIDCs is associated with a better prognosis [33, 34, 40]. To clarify the relationship between TIDCs and survival prolongation from treatment with S-1 alone and S-1+LNT in our models, we evaluated the TIDCs by immunohistochemical staining using anti-CD11c and anti-CD86 antibodies at the tumor site. CD11c has been reported as a relatively restricted marker of murine DCs [39], and CD86 (B7.2) has been reported to be a costimulatory molecule of DCs, B cells, and monocytes. CD11c+ DCs infiltrated into the tumor at a similar frequency in both of the treatment groups. In the group with tumors treated with S-1+LNT, interestingly, the number of CD86+ cells, which are thought to be activated APCs, infiltrated into the tumor twice as much as those treated with S-1 alone, with statistical significance (Fig. 3, Table 2). These CD86+ cells had typical DC morphological features; moreover, CD86+ cells do not show staining with B220 in serial section, and B220+ cells could not be detected in the tumors (data not shown). Taken together, these data suggest that TIDCs, especially activated DCs, might contribute to survival prolongation. These CD86+ TIDCs could acquire, process, and present to naïve T cells the antigens acquired from the tumor cells [4]. Actually, we found that S-1 could induce tumor apoptosis by terminal deoxynucleotidyltransferase-mediated nick end-labeling (TUNEL) staining, as reported previously (data not shown) [25]. On the other hand, Chaux et al. [41] reported that TIDCs have a poor capacity to prime tumor-specific immune response because of the lack of their expression of B7 and their poor capacity to stimulate T cells. To clarify the function of DCs, we examined the T-cell stimulating activity of splenic DCs obtained from cancer-bearing mice treated with S-1 alone or with S-1+LNT. In S-1+LNT treated mice, interestingly, [3H]-thymidine incorporation of responder T cells (H-2b) showed more augmentation than in mice treated with S-1, using DCs sorted from cancer-bearing BALB/c (H-2d) mice as a stimulator (Fig. 4). These data suggest that DC impact on T-cell stimulation is more potent in mice treated with S-1+LNT than mice treated with S-1 alone. In other words, LNT may improve DC function as an APC to stimulate T-cell proliferation in combination with S-1. No possibility that LNT could function as an antigen was thought likely, because DCs showed no difference in ability to stimulate syngeneic T-cell proliferation between these two groups (data not shown). This evidence suggests that LNT can activate the host’s own DC function, which plays a key role in the immune system, in this novel chemoimmunotherapy. This is the first report that a clinically applicable β-glucan, LNT, could affect DC function in vivo.
Next, to clarify the role of the immune response in the effector phase, we examined the cytotoxic activities of splenocytes from cancer-bearing mice after in vitro sensitization for 5 days. Splenocytes of S-1+LNT–treated mice showed more specific and potent cytotoxic activity than those of mice treated with S-1 alone (Fig. 5). Moreover, the survival prolongation of S-1+LNT–treated mice was abrogated in the athymic mice model (Fig. 6). These data strongly suggest that the T cells are involved in the observed survival prolongation.
Importantly, the combined chemoimmunotherapy could enhance the DC function in cancer-bearing mice, which could induce the generation of tumor-specific cytotoxic T cells, thus prolonging survival. Chemotherapy is a so-called double-edged sword, because it reduces host defense mechanisms [1]. Even if anticancer drug treatment shows tumor regression and sufficient death of tumor cells followed by providing antigens to DCs infiltrating in the tumor, it is difficult to achieve a survival benefit for clinical patients. Our data suggest that LNT may augment the function of DCs resulting in tumor-specific T-cell immunity. It is reported that to achieve long-tem survival, mild chemotherapy is recommended rather than intensive chemotherapy using maximum tolerable dose [28], and it follows that chemotherapy with low side effects is recommended to improve survival benefits. Therefore, combined use of S-1 with LNT has some possibility in prolonging the survival period of cachectic cancer patients. Moreover, DCs could play a possible role in cancer chemotherapy and chemoimmunotherapy in terms of TIDCs or T-cell–stimulating function.
More attention should be paid to chemoimmunotherapy focusing on host defenses to improve patient benefits in a clinical setting. The combined therapy using S-1 and LNT appears to be a promising chemoimmunotherapy, which could lead to a survival benefit for cancer patients.
Acknowledgements
We thank Dr James Vaughn Spencer for his critical review of the manuscript.
Abbreviations
- ANOVA
Analysis of variance
- CD
Cluster of differentiation
- CTL
Cytotoxic T lymphocyte
- ELISA
Enzyme-linked immunosorbent assay
- 5-FU
5-Fluorouracil
- i.p.
Intraperitoneally
- NK
Natural killer cells
- p.o.
Per oral
- s.c.
Subcutaneously
- TGF-β1
Transforming growth factor-β1
References
- 1.Harris J, Sengar D, Stewart T, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976;37( suppl 2):1058. doi: 10.1002/1097-0142(197602)37:2+<1058::aid-cncr2820370813>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Gutterman JU, Mavligit G, Gottlieb JA, Burgess MA, McBride CE, Einhorn L, Freireich EJ, Hersh EM. Chemoimmunotherapy of disseminated malignant melanoma with dimethyl triazeno imidazole carboxamide and bacillus calmette-guerin. N Engl J Med. 1974;291:592. doi: 10.1056/NEJM197409192911202. [DOI] [PubMed] [Google Scholar]
- 3.Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Hepato-gastroenterology. 1999;46:2662. [PubMed] [Google Scholar]
- 4.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 5.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Geiger JD, Hutchinson RJ, Hohenkirk LF, Mckenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513. [PubMed] [Google Scholar]
- 8.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafli S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035. [PubMed] [Google Scholar]
- 10.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530. [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 12.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755. [PubMed] [Google Scholar]
- 13.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogl M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 14.Chihara G, Maeda Y, Hamuro J, Sasaki T, Fukuoka F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) Sing. Nature. 1969;222:687. doi: 10.1038/222687a0. [DOI] [PubMed] [Google Scholar]
- 15.Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom) Cancer Res. 1970;30:2776. [PubMed] [Google Scholar]
- 16.Hamuro J, Rollinghoff M, Wagner H. β (1→3) Glucan-mediated augmentation of alloreactive murine cytotoxic T-lymphocytes in vivo. Cancer Res. 1978;38:3080. [PubMed] [Google Scholar]
- 17.Suzuki M, Kikuchi T, Takatsuki F, Hamuro J. Curative effects of combination therapy with lentinan and interleukin-2 against established murine tumors, and the role of CD8-positive T cells. Cancer Immunol Immunother. 1994;38:1. doi: 10.1007/s002620050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tani M, Tanimura H, Yamaue H, Iwahashi M, Tsunoda T, Tamai M, Noguchi K, Arii K. In vitro generation of activated natural killer cells and cytotoxic macrophages with lentinan. Eur J Clin Pharmacol. 1992;42:623. doi: 10.1007/BF00265926. [DOI] [PubMed] [Google Scholar]
- 19.Yoshino S, Tabata T, Hazama S, Iizuka N, Yamamoto K, Hirayama M, Tangoku A, Oka M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000;20:4707. [PubMed] [Google Scholar]
- 20.Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 21.Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature. 2001;413:36. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 22.Takechi T, Nakano K, Uchida J, Mita A, Toko K, Takeda S, Unemi N, Shirasaka T. Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol. 1997;39:205. doi: 10.1007/s002800050561. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima M, Satake H, Uchida J, Shimamoto Y, Kato T, Takechi T, Okabe H, Fujioka A, Nakano K, Ohshimo H, Takeda S, Shirasaka T. Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol. 1998;13:693. doi: 10.3892/ijo.13.4.693. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima M, Shimamoto Y, Kato T, Uchida J, Yonekura R, Ohshimo H, Shirasaka T. Anticancer activity and toxicity of S-1, an oral combination of tegafur and two biochemical modulators, compared with continuous iv infusion of 5-fluorouracil. Anti-Cancer Drugs. 1998;9:817. doi: 10.1097/00001813-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Cao S, Lu K, Toth K, Slocum HK, Shirasaka T, Rustum YM. Persistent induction of apoptosis and suppression of mitosis as the basis for curative therapy with S-1, an oral 5-fluorouracil prodrug in a colorectal tumor model. Clin Cancer Res. 1999;5:267. [PubMed] [Google Scholar]
- 26.Ohtsu A, Baba H, Sakata Y, Mtachi Y, Horikoshi N, Sugimachi K, Taguchi T. Phase II study of S-1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma S-1 cooperative colorectal carcinoma study group. Br J Cancer. 2000;83:141. doi: 10.1054/bjoc.2000.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715. doi: 10.1016/S0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 28.Nukatsuka M, Fujioka A, Saito H, Uchida J, Nakano K, Takeda S, Unemi N. Prolongation of survival period and improvement of cancer cachexia by long term administration of UFT. Cancer Lett. 1996;104:197. doi: 10.1016/0304-3835(96)04247-4. [DOI] [PubMed] [Google Scholar]
- 29.Shimosato T, Watanabe K. Enzymorphological observation on irradiated tumor, with a particular reference to acid hydrolase activity. I. Light microscopic study. Gann. 1967;58:541. [PubMed] [Google Scholar]
- 30.Yamasaki K, Sone S, Yamashita T, Ogura T. Synergistic induction of lymphokine (IL-2)-activated killer activity by IL-2 and the polysaccharide lentinan, and therapy of spontaneous pulmonary metastases. Cancer Immunol Immunother. 1989;29:87. doi: 10.1007/BF00199282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeannin JF, Lagadec P, Pelletier H, Reisser D, Olsson NO, Chihara G, Martin F. Regression induced by lentinan, of peritoneal carcinomatoses in a model of colon cancer in rat. Int J Immunopharmacol. 1988;10:855. doi: 10.1016/0192-0561(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 32.Nonacs R, Humborg C, Tam JP, Steinman RM. Mechanism of mouse spleen dendritic cell function in the generation of influenza-specific, cytotoxic T lymphocytes. J Exp Med. 1992;176:512. doi: 10.1084/jem.176.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotze MT. Getting to the source: dendritic cell as therapeutic reagents for the treatment of patients with cancer. Ann Surg. 1997;226:1. doi: 10.1097/00000658-199707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwaab T, Weiss JE, Schned AR, Barth RJ., Jr Dendritic cell infiltration in colon cancer. J Immunother. 2001;24:130. doi: 10.1097/00002371-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Aoyagi K, Koufuji K, Yano S, Murakami N, Miyagi M, Takeda J, Shirouzu K. Long-term survival after gastric cancer with liver metastasis: a report of two cases. Kurume Med J. 2001;48:335. doi: 10.2739/kurumemedj.48.335. [DOI] [PubMed] [Google Scholar]
- 36.Kawarada Y, Imai T, Iwata M, Yokoi H, Noguchi T, Mizumoto R. Significance of multidisciplinary therapy for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31(supp l):S13. doi: 10.1007/BF00687098. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290. [PubMed] [Google Scholar]
- 38.Tanaka M, Miyazaki H, Takeda Y, Takeo S. Detection of serum cytokine levels in experimental cancer cachexia of colon 26 adenocarcinoma-bearing mice. Cancer Lett. 1993;72:65. doi: 10.1016/0304-3835(93)90012-X. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 40.Nestle FO, Burg G, Fah J, Wrone-Smith T, Nickoloff BJ. Human sunlight-induced basal-cell-carcinoma-associated dendritic cells are deficient in T cell co-stimulatory molecules and impaired as antigen-presenting cells. Am J Pathol. 1997;150:641. [PMC free article] [PubMed] [Google Scholar]
- 41.Chaux P, Favre N, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int J Cancer. 1997;72:619. doi: 10.1002/(SICI)1097-0215(19970807)72:4<619::AID-IJC12>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]