Abstract
MUC1 is a glycoprotein overexpressed in tumors as a hypoglycosylated form. A vaccine composed of a 100–amino acid peptide corresponding to five 20–amino acid long repeats, and SB-AS2 adjuvant, was tested in a phase I study for safety, toxicity, and ability to elicit or boost MUC1-specific immune responses. Patients with resected or locally advanced pancreatic cancer without prior chemotherapy or radiotherapy were eligible. Escalating doses of the peptide (100, 300, 1,000, and 3,000 μg) were admixed with SB-AS2 and administered intramuscularly every 3 weeks for three doses, in cohorts of four patients. Sixteen patients were enrolled. Common adverse effects were grade 1 flu-like symptoms, tenderness, and erythema at the injection site. Delayed-type hypersensitivity (DTH) sites showed few or no T cells prevaccination (Pre V), but increased T-cell infiltration postvaccination (Post V). There was an increase in the percentage of CD8+ T cells in the peripheral blood Post V. An increase in total MUC1-specific antibody was seen in some patients, and several patients developed IgG antibody. Two of 15 resected pancreatic cancer patients are alive and disease free at follow-up of 32 and 61 months. MUC1 100mer peptide with SB-AS2 adjuvant is a safe vaccine that induces low but detectable mucin-specific humoral and T-cell responses in some patients. No difference was seen between different peptide doses. Further evaluation is warranted to examine the effect on disease-free survival and overall survival, especially in early disease and in the absence of immunosuppressive standard therapy.
Keywords: Clinical trial, Immune response, Pancreatic cancer, Peptide vaccine
Introduction
Pancreatic cancer results in 28,400 deaths per year in the United States and is the fourth most common cause of cancer death [11]. Long-term survival is uncommon even following surgery and only seen in a few patients with early stage, node-negative disease [12]. The benefit of adjuvant therapy in patients with resected pancreatic cancer is a subject of controversy, with randomized trials showing contradictory results [13, 14, 17]. In this group of patients, targeted therapy in the form of vaccines that result in minimal toxicity presents an attractive alternative. In the last few years, vaccine development has shown remarkable progress, and technological advances have permitted identification of a growing number of antigens on cancer cells. One such antigen is MUC1 mucin [6]. MUC1 is a large transmembrane glycoprotein that consists of an extended polypeptide core that is highly glycosylated by O-linked carbohydrates. Much of the glycosylation is found within a region of tandemly repeated sequences of 20 amino acids per repeat. When cells undergo malignant transformation they produce hypoglycosylated MUC1 that has lost its luminal polarity [22]. These characteristics allow recognition by the immune system resulting in low-titer MUC1 antibodies and MUC1-specific cytotoxic T lymphocytes (CTLs) that can be found in most cancer patients with MUC1+ tumors [1, 5]. The lack of naturally occurring strong immune response to this tumor antigen is postulated to be due to the lack of efficient processing of tumor MUC1 protein by a patient’s antigen-presenting cells and therefore lack of induction of MUC1-specific T-helper (Th) cells [9]. A MUC1 vaccine that can boost effector pathways that induce Th-cell responses may be effective in controlling or curtailing tumor growth. We have chosen the 100mer synthetic peptide because in the in vitro studies with human T cells, we have been able to document its efficient processing by antigen-presenting cells and its presentation to, and elicitation of, CD4+ helper T cells [8]. Studies in vivo in chimpanzees have shown that when loaded onto dendritic cells, MUC1 peptide can elicit both helper and cytotoxic T-cell responses [2]. Furthermore, studies in MUC1-transgenic mice have shown that this synthetic peptide can induce immune responses when administered with various adjuvants [21]. In this study we used the adjuvant SB-AS2. It is composed of MPL (monophosporyl lipid A) and QS-21 in an oil-in-water emulsion. SB-AS2 has the ability to induce high titers of IgG antibodies, primarily Th1-dependent subclasses, and both helper and cytotoxic T-cell responses [3, 16].
In the first clinical study of a MUC1 vaccine, 100 μg of a similar synthetic peptide (105 aa) was administered with bacillus Calmette-Guérin (BCG) as adjuvant, for three doses every 3 weeks [7]. The main toxicity was skin ulceration at the vaccination sites, attributed to the BCG component of the vaccine. A twofold to fourfold increase in mucin-specific CTLs after vaccination was seen in 7 out of 22 patients tested. In skin biopsy specimens, a profound delayed-type hypersensitivity (DTH) reaction to the long peptide as well as its shorter (9 aa) fragments was demonstrated by the intensity of T-cell infiltration. Some increase in antibody responses was seen, but all antibodies remained IgM with no evidence that helper cell responses were generated that could promote isotype switching. This may have been due to the use of BCG as adjuvant known to induce primarily Th1-type T cells and CTL responses. It may also reflect the fact that the patients on that trial were all in advanced stages of their disease and severely immunosuppressed in their ability to process and present antigen and to prime new helper cell responses [18, 19]. For this reason in the current study we restricted eligibility to recently diagnosed patients with locally advanced pancreatic tumors or surgically resected disease. In this study, a change in the start site on the tandem repeat where the synthesis was initiated, gave us the same five tandemly repeated epitopes as in the 105mer peptide, but now contained within 100 amino acids.
Patients and methods
Objectives
The primary clinical objectives were to evaluate the toxicity and safety of the MUC1 vaccine. The immunologic endpoints were evaluation of DTH against MUC1 peptide, and evaluation of MUC1-specific antibody and cellular responses. The secondary objective was to evaluate the disease-free and overall survival of patients.
Eligibility criteria
Eligible patients had surgically resected or locally advanced pancreatic cancer. Performance status was Eastern Cooperative Oncology Group (ECOG) 0–2. Prior chemotherapy or radiotherapy was not permitted. Patients had adequate bone marrow, liver, and kidney function at study entry, and were required to have a white cell count (WBC) >3.5 mm3, platelets >100,000 mm3, serum creatinine ≤1.5 gm/dl, and total bilirubin ≤2.0 mg/dl. A minimum of 3 weeks was required after surgery. A life expectancy of more than 4 months was required. Patients being treated with glucocorticoids and nonsteroidal anti-inflammatory drugs were excluded. Patients were required to have at least one positive response to a panel of common recall antigens. When this panel became commercially unavailable, this was not required for the last four patients. Prior to study entry, all patients gave written informed consent according to the University of Pittsburgh Institutional Review Board guidelines, and the study was carried out under the Investigational New Drug (IND) No. 7632.
Vaccine preparation and administration
A 100–amino acid synthetic MUC1 peptide with the molecular structure of H2N-(GVTSAPDTRPAPGSTAPPAH)5-CONH2, was synthesized under GLP conditions at the Department of Molecular Genetics and Biochemistry Peptide Synthesis Facility, University of Pittsburgh School of Medicine. The required dose of MUC1 peptide was made up to a volume of 0.1 ml. The adjuvant SB-AS2 (a gift from SmithKline Beecham Pharmaceuticals) was formulated as a mixture of MPL and QS-21 in 250 μl of an oil-in-water emulsion. The preparation of SB-AS2 was delivered in vials of 0.5 ml to be mixed with 0.1 ml of the ordered dose of MUC1. The total volume of 0.6 ml was given intramuscularly into the upper arm by injection. Patients received three injections of the vaccine, once every 3 weeks. Following completion of the study, patients could be treated with chemotherapy or radiotherapy at the discretion of the primary oncologist. If there was no evidence of recurrent disease, patients were offered a booster vaccine dose of 300 μg of peptide plus adjuvant, every year starting 1 year after the last vaccination dose, for two doses.
Dose escalation and dose-limiting toxicity
Patients were treated at dose levels of 100, 300, 1,000, and 3,000 μg of peptide. Four patients were entered at each dose level. A patient was considered for evaluation if at least two doses of vaccine were given as scheduled. A delay of 4 weeks was permitted for any reason. Doses were to be escalated until dose-limiting toxicity (DLT) was observed in one of four patients entered at a given dose level. Dose escalation continued if zero out four or two or fewer of eight patients at that level had DLT. The maximum tolerated dose (MTD) was considered to have been reached if more than two patients in a cohort experienced DLT. The maximum dose was 3,000 μg of vaccine. All toxicity was graded using the NCI Common Toxicity Criteria version 1.0. DLT was defined as grade ≥3 nonhematologic toxicity, except skin toxicity; grade 4 vomiting, despite the use of antiemetic; grade 4 neutropenia lasting more than 7 days and/or accompanied by infection and/or fever; and grade 4 thrombocytopenia.
Pretreatment assessment and follow-up studies
All patients had a complete history and physical examination and routine laboratory tests within 2 weeks of starting therapy. All these tests were repeated prior to every injection of the vaccine. Radiologic tests including a chest X-ray and computerized tomographic scan of the abdomen were required within 2 weeks of study entry, and repeated 2 weeks after completion of the third vaccine dose. A complete blood count with differential, and a complete chemistry profile with serum amylase and lipase were done weekly during the period of vaccination.
Immunologic studies
Peripheral blood lymphocytes and plasma were collected immediately before the first vaccine (prevaccination [Pre V] sample) and immediately before each additional injection (postvaccine 1, postvaccine 2), and 3 weeks after the last injection (postvaccine 3). The cells were frozen at −80°C and plasma at −20°C, and thawed just before use. All time points were tested simultaneously.
ELISA assay for anti-MUC1 antibody testing
Microtiter plates (Immulon 4; Dynatech Labs) were coated with 0.5 μg of synthetic 100mer MUC1 peptide in 100 μl of phosphate-buffered saline (PBS). Following overnight incubation at 4°C, the plates were washed twice with PBS and blocked with 100 μl of 2.5% bovine serum albumin (Sigma Chemical) in PBS (PBS-BSA). A duplicate plate was also prepared as a control for nonspecific antibody binding. This plate was not coated with MUC1 peptide but was otherwise treated the same way, including blocking. The blocking reagent was removed, and 50 μl of various dilutions of patient’s plasma was added. Dilutions of the plasma were made in 2.5% BSA-PBS. After 1 h the plates were washed five times with PBS–0.01% Tween 20. Each well then received 50 μl of one of the secondary antibodies, alkaline phosphatase–conjugated goat antihuman Ig, polyvalent (Sigma No. A3313) diluted 1:1,000 in BSA-PBS, antihuman IgM, or antihuman IgG. Following a 1-h incubation the plate was washed five times with PBS–0.01% Tween 20, and 100 μl of Sigma 104 phosphatase substrate at 3 mg/ml in 0.5 mM MgCl2, 0.05 M Na2CO3. The reaction was terminated after 1 h by adding 50 μl of 0.05 M NaOH. The results are read at OD250 nm on a spectrophotometer. The OD values from the control wells without MUC1 peptide were subtracted from the OD values in test wells with MUC1 peptide. Each dilution is tested in triplicate wells and each plasma sample was tested multiple times. Samples from all time points from one patient were always tested simultaneously. Each patient served as his own control for background versus specific values and for prevaccination and postvaccination (Post V) values.
Mean immunoglobulin levels were summarized across time points for IgG, IgM, and total Ig. Because of imbalances in available data, least squares mean estimates rather than raw means are presented, calibrated to a 20:1 dilution. A linear model was utilized for the logarithm (base 10) of the mean calibrated immunoglobulin levels, to evaluate the contrast between each time point and the baseline Pre V measurement, while controlling for patient and for the logarithm of the dilution. To control for multiple testing across time points, a randomization test was performed [4]. (Validity conditions were not met for Dunnett’s test for multiple comparisons with a common control. The Bonferroni correction can be inappropriately conservative when the endpoints are strongly correlated, as in this case.) A model including the logarithm of dose in place of a patient effect was similarly fitted and tested.
DTH to MUC1 and hepatitis B antigen
Delayed-type hypersensitivity testing to MUC1 peptide and hepatitis B antigen as control was done in all patients. Hepatitis B antigen at the dose of 1 μg/0.1 ml was placed on the right lower forearm, and 100 μg/0.1 ml MUC1 peptide was placed on the right upper forearm intradermally. Skin tests were read at 48–72 h, erythema and induration were recorded for each skin test. A 6-mm punch biopsy was obtained under local anesthesia of both DTH sites. Patients who completed the course of vaccination had repeated DTH testing and biopsies as described above, 2 weeks after the last vaccination. Skin biopsy samples were cut in half. One half was minced into small pieces and placed in tissue culture for infiltrating T cells to migrate out and expand to be used in phenotypic analysis by flow cytometry. The other half of the biopsy sample was frozen for immunohistology. Cells infiltrating the biopsy samples were expanded in culture for 2–3 weeks in RPMI 1640 (ICN, Costa Mesa, CA, USA) supplemented with 10% human AB serum (Mediatech, Herndon, VA, USA), 20 U/ml of IL-2 (DuPont), and 10 ng/ml IL-7. No antigen was given in vitro. They were analyzed for cell surface phenotype and intracellular cytokine production as described below.
Intracellular cytokine production and fluorescence-activated cell sorter (FACS) analysis
Cells were activated by incubation for 4 h at 37°C in 1 μM ionomycin (Sigma Chemical, St Louis, MO, USA) and 20 μg/ml phorbol myristate acetate (PMA; Sigma). A quantity of 2 μm of monensin (Sigma) was added to prevent secretion of cytokines into the extracellular space. The cells were washed once in FACS buffer (PBS with 5% fetal calf serum and 0.1% sodium azide) and then fixed for 20 min in 2% paraformaldehyde (Sigma). Following two washes in FACS buffer with 0.1% saponin (Sigma), the antibodies against CD3, CD4, CD8, and TCR ζ chain (Becton Dickenson, Franklin Lakes, NJ, USA), interferon γ (4S.B3; Pharmingen, San Diego, CA, USA), or IL-4 (8D4-8; Pharmingen) were added in saponin buffer for 30 min at 4°C. Flow cytometry was performed with a FACS Caliber instrument. Three-color analysis was used to detect the intracellular cytokines IFN-γ or IL-4 in the CD4+ and CD8+ populations.
Temporal changes in flow cytometry evaluations of IFN-γ and CD3ζ-positive PBLs were summarized to determine statistical significance. In principle, a measurement error model based on replication studies could be used to classify patients as responders, but such quality control data was not available due to a limited number of cells. Temporal changes were tested across the sample; it should be noted that the null hypothesis is symmetry between increases and decreases, not the absence of changes. Immunohistochemical evaluation of CD3, CD4, and CD8 cells in infiltrates at the hepatitis and mucin sites were handled similarly. Changes were tested with the Wilcoxon signed-rank test. Spearman correlations between pairs of changes were calculated and tested against a null hypothesis of zero correlation. These tests were not adjusted for multiple comparisons.
Results
Patient characteristics
Sixteen patients, 11 men and 5 women, were recruited at the University of Pittsburgh Cancer Institute from July 1998 to March 2001. Median age was 62 years (range 45–78 years). All but one patient had surgically resected pancreatic cancer. The patient with locally advanced tumor was staged as T3 N1. The resected tumors were T1 N1 (1), T2 N1 (1), T3 N1 (9), T3 N3 (3), and T4 N0 (1). In the group with resected cancer, the time from surgery to first vaccine dose ranged from 4 to 15 weeks (median = 7 weeks). All three doses of the vaccine were given as scheduled, except in three patients. One patient, no. 5 at the 300-μg dose with locally advanced disease, did not receive the third dose of the vaccine due to rapid progression of disease and declining performance status. Two patients, no. 12 and no. 13, at the 1,000-μg dose did not receive the third dose of the vaccine. In one patient there were complications related to surgery and in the other patient, vaccine was temporarily unavailable due to production issues.
Toxicity
Vaccine therapy was well tolerated. Most patients had mild symptoms. These were flu-like symptoms (grade 1, 25%), tenderness (grade 1, 38%), and erythema (grade 1, 31%; grade 2, 6%) at the injection site. One patient, no. 2 at the 100-μg dose with prior history of rheumatoid arthritis, had recurrent symptoms in the toe (grade 1 arthralgia) lasting for 2–5 days following every dose of the vaccine. There was no skin ulceration. No patient had grade 3 or 4 toxicity.
Clinical outcome
Of the 15 patients with resected pancreatic cancer, 13 patients have died, 2 patients are alive and disease free at follow-up of 32 and 61 months. The median survival is 12 months (Table 1). The protocol allowed patients to receive adjuvant therapy after completing three vaccinations at the discretion of the treating physician. Eight patients went on to receive chemotherapy or a combination of chemotherapy with radiation.
Table 1.
Clinical summary
| Dose (μg) | Patient | Stage | Time to recurrence from surgery (months) | Survival from surgery (months) | Adjuvant therapya |
|
|---|---|---|---|---|---|---|
| Alive | Died | |||||
| 100 | 1 | T3 N1 | 11.0 | 28 | Yes | |
| 2 | T3 N1 | – | 61 | – | Yes | |
| 3 | T3 N1 | 11.0 | 12 | Yes | ||
| 4 | T3 N1 | 17.0 | 21 | Yes | ||
| 300 | 5b | T3 N1 | 3.0 | 4 | No | |
| 6 | T3 N1 | 7.0 | 9 | No | ||
| 7 | T3 N0 | 9.0 | 10 | Yes | ||
| 8 | T3 N0 | 15.0 | 20 | Yes | ||
| 1,000 | 9 | T3 N1 | – | 32 | – | Yes |
| 10 | T3 N1 | 7.0 | 8 | No | ||
| 11 | T2 N1 | – | 23 | No | ||
| 12 | T1 N1 | 7.0 | 8 | No | ||
| 3,000 | 13 | T3 N1 | 6.0 | 10 | No | |
| 14 | T3 N0 | 20.0 | 22 | Yes | ||
| 15 | T3 N1 | 10.0 | 13 | No | ||
| 16 | T4 N0 | 10.0 | 17 | No | ||
aAdjuvant therapy consisting of external beam radiation therapy for 40–54 Gy with radiosensitizing doses of 5 FU was given in seven patients. Patient no. 2 received one cycle (3 weeks) of gemcitabine hydrochloride as adjuvant therapy
bLocally advanced disease
Immunologic responses
Anti-MUC1 antibody response and isotype switching to IgG
Prevaccination antibody levels were measured against the immunizing 100mer peptide in plasma from 15 of the 16 patients (Table 2). ELISA assays of anti-MUC1 serum antibody levels Pre V showed that four patients—no. 3, no. 7, no. 8, and no. 11 (for patient treatment, see Table 1)—had very low levels (OD values at 1:20 dilution of 0.2 or lower), five patients—no. 4, no. 6, no. 9, no. 10, and no. 14—had intermediate levels (OD values at 1:20 dilution of 0.2–0.6), and five patients—no. 5, no. 12, no. 13, no. 15, and no. 16—had relatively high antibody levels (OD values at 1:20 ranging from 0.6 to 1.2). As previously reported, most of the antibody was of the IgM isotype [15]. One patient (no. 3) from the lowest antibody group, two patients (no. 4 and no. 6) from the intermediate group, and two patients (no. 12, and no. 15) from the high antibody group showed an increase in total antibody after vaccination. In all of them, except for no. 12, this was due to the increase specifically in IgG. The anti-MUC1 antibody detected in patient no. 12 who had neither IgM nor IgG, had IgA that did not change with vaccination. IgG OD values for patient no. 4 changed from 0.358 Pre V to 0.561 Post V; for patient no. 6 the values changed from 0.139 to 0.283; for patient no. 13, from 0.085 to 0.127; and for patient no. 15, from 0.112 to 0.368 (Table 2). In separate comparisons with baseline, the log of IgG level was significantly different only at the third Post V time point (P=0.042). After adjusting for multiple testing of the three Post V time points, the change from baseline to the third Post V time point was not statistically significant (P>0.05). Similar results held for total Ig. There were no significant changes for the log of the IgG/total Ig ratio. For both IgG and IgM, interpatient variation was highly significant. Even though the increase in IgG titers was seen in 3/4 patients vaccinated with the lowest dose and only in 1/4, 0/4, and 1/4 in the next three doses, the fact that it was seen at other doses as well suggests a lack of a significant relationship between the peptide dose and Post V IgG.
Table 2.
Anti-MUC1 serum antibodies Pre V and Post Va. The value in italics observed increases in total antibody and in IgG postvaccination. Values given are for 1:20 serum dilution. Results are identical for all dilutions tested, from 1:20–1:160. Pre V prevaccination serum sample, V1 serum sample at 3 weeks after the first vaccine, V2 serum sample at 3 weeks after the second vaccine, V3 serum sample at 3 weeks after the third vaccine
| Patient No. | Timea | Antibody Isotype | ||
|---|---|---|---|---|
| Total Ig | IgM | IgG | ||
| 1 | V2 | 0.719a | 0.544 | 0.086 |
| V3 | 0.536 | 0.481 | 0.071 | |
| 2 | Pre V | 0.848 | 0.325 | 0.043 |
| V1 | 0.621 | 0.287 | 0.118 | |
| V2 | 0.974 | 0.121 | 0.132 | |
| 3 | Pre V | 0.202 | 0.073 | 0.141 |
| V1 | 0.543 | 0.181 | 0.290 | |
| V2 | 0.433 | 0.171 | 0.223 | |
| V3 | 0.401 | 0.146 | 0.223 | |
| 4 | Pre V | 0.452 | 0.137 | 0.358 |
| V1 | 0.591 | 0.135 | 0.373 | |
| V2 | 0.551 | 0.148 | 0.401 | |
| V3 | 0.631 | 0.203 | 0.561 | |
| 5 | Pre V | 0.815 | 1.425 | 0.145 |
| V1 | 0.740 | 1.317 | 0.145 | |
| V2 | 0.817 | 1.556 | 0.218 | |
| 6 | Pre V | 0.397 | 0.460 | 0.139 |
| V1 | 0.450 | 0.465 | 0.181 | |
| V2 | 0.432 | 0.435 | 0.197 | |
| V3 | 0.521 | 0.474 | 0.283 | |
| 7 | Pre V | 0.258 | 0.300 | 0.102 |
| V1 | 0.220 | 0.267 | 0.143 | |
| V2 | 0.218 | 0.182 | 0.198 | |
| V3 | 0.217 | 0.225 | 0.059 | |
| 8 | Pre V | 0.112 | 0.069 | 0.045 |
| V1 | 0.130 | 0.065 | 0.040 | |
| V2 | 0.111 | 0.060 | 0.040 | |
| V3 | 0.156 | 0.082 | 0.049 | |
| 9 | Pre V | 0.363 | 0.582 | 0.069 |
| V1 | 0.334 | 0.571 | 0.012 | |
| V2 | 0.345 | 0.629 | ND | |
| V3 | 0.375 | 0.719 | 0.062 | |
| 10 | Pre V | 0.313 | 0.565 | 0.104 |
| V1 | 0.268 | 0.547 | 0.111 | |
| V2 | 0.205 | 0.530 | 0.111 | |
| V3 | 0.325 | 0.642 | 0.141 | |
| 11 | Pre V | 0.210 | .048 | 0.025 |
| V1 | 0.140 | .062 | 0.008 | |
| 12 | Pre V | 0.852 | 0.167 | 0.235 |
| V1 | 0.872 | ND | ND | |
| V2 | 1.137 | 0.258 | 0.164 | |
| 13 | Pre V | 1.015 | 0.515 | 0.085 |
| V1 | 1.047 | 0.415 | 0.101 | |
| V2 | 1.156 | 0.364 | 0.127 | |
| 14 | Pre V | 0.551 | 0.253 | 0.062 |
| V1 | 0.646 | 0.265 | 0.053 | |
| V2 | 0.777 | 0.288 | 0.055 | |
| V3 | 0.614 | 0.283 | 0.046 | |
| 15 | Pre V | 0.625 | 0.103 | 0.112 |
| V1 | 0.635 | 0.050 | 0.168 | |
| V2 | 0.693 | 0.062 | 0.237 | |
| V3 | 0.964 | 0.177 | 0.368 | |
| 16 | Pre V | 1.199 | 0.971 | 0.162 |
| V1 | 0.866 | 0.575 | 0.129 | |
| V2 | 1.114 | 0.603 | 0.129 | |
| V3 | 0.829 | 0.522 | 0.147 | |
aNumbers correspond to OD values in ELISA
T-cell cytokine production
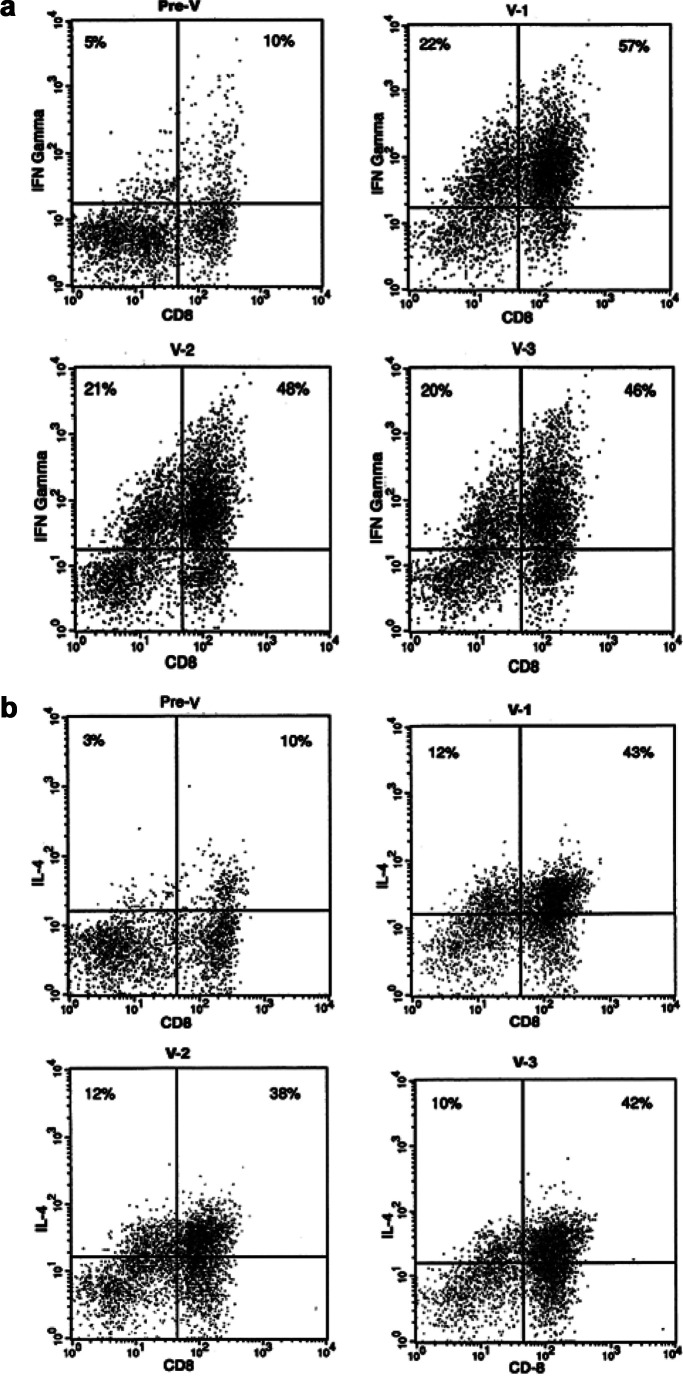
Prevaccination and postvaccination PBLs had been frozen. Samples from all time points were thawed at the same time and restimulated with the 100mer MUC1 peptide once in vitro prior to testing. The intent of the in vitro stimulation (IVS) was twofold: first, because the cells collected at different time points were stored at −80°C to be assayed simultaneously, one in vitro stimulation served to recover their viability; and, second, IVS was necessary to increase the number of antigen-specific T cells and facilitate their detection in ELISpot assays. However, numerous ELISpot assays performed on these cultures to detect MUC1-specific T cells were uninterpretable due to huge differences in background spots Pre V and Post V (data not shown). This prompted us to examine the ability of T cells Pre V and Post V to make cytokines against polyclonal activators. We saw in every patient an almost total suppression of their T-cell ability to make either IFN-γ or IL-4 Pre V (data not shown). In patients no. 3, no. 4, no. 6, no. 12, and no. 15 (see Table 1), however, the ability to make cytokines increased significantly Post V. An example of one patient (no. 4) that illustrates what was seen in all these patients is shown in Fig. 1. A short-term stimulation with PHA/PMA (as described in “Patients and methods”) of the Pre V PBLs resulted in a very low percentage of either IFN-γ (Fig. 1a) or IL-4 (Fig. 1b) producing cells. This number drastically increased post first vaccine and remained high through the next two injections. Recovery of responsiveness was independent of the dose of peptide they received, and, while it was most striking in patients no. 3, no. 4, no. 6, no. 12, and no. 15, it was seen to various degrees in 10/16 patients. Two patients had an intermediate level of cytokine production that was still below normal (50% of normal controls), which did not change Post V. Four of the sixteen patients never recovered their ability to respond, or recovered only marginally and transiently (data not shown).
Fig. 1.
Increase in the percentage of CD8+ T cells and in the ability of all the patient’s T cells to make cytokines when activated following vaccination. Patient’s PBMCs were activated with PMA/ionomycin, and intracellular levels of INF-γ (a) or IL-4 (b) evaluated in CD3+ cells by flow cytometry, as described in “Patients and methods.” Samples are from Pre V (Pre-V), 3 weeks after the first injection (V-1), 3 weeks after the second injection (V-2), and 2 weeks after the third injection (V-3).
An additional and a very intriguing change between Pre V and Post V PBLs that was seen in all patients was the change in CD4+ to CD8+ T-cell ratio, also represented in Fig. 1. Compared with Pre V samples that have the expected 2:1 ratio of CD4+ to CD8+ T cells, Post V samples contain many more CD8+ T cells than CD4+ T cells.
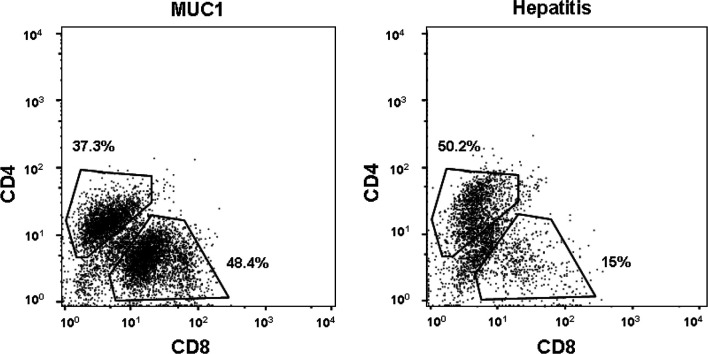
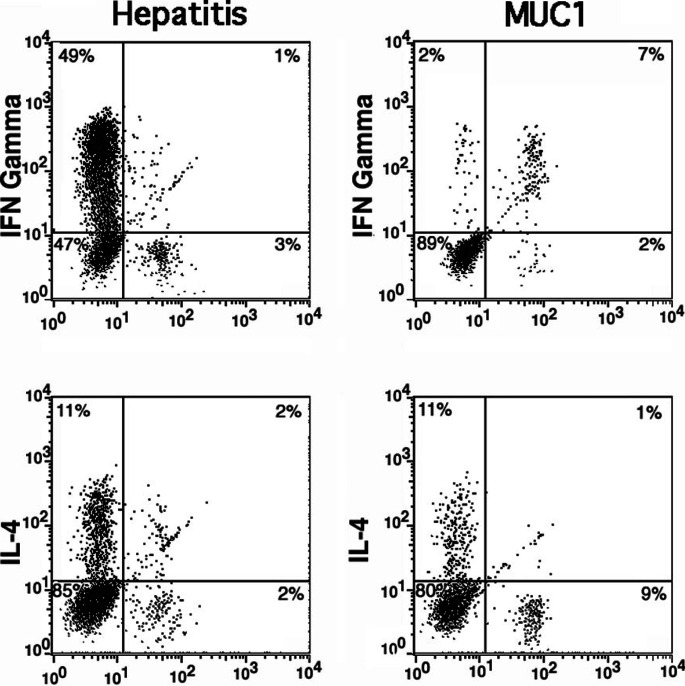
To determine whether this change was related to the adjuvant or the antigen, we examined T cells infiltrating DTH sites. As described in “Patients and methods,” all patients were skin-tested prior to the first vaccine and 3 weeks after the third injection. No classical DTH reaction, redness or indurration, was seen either before or after vaccination. We biopsied the DTH sites and looked by immunocytochemistry for the presence of infiltrating T cells . Small numbers of T cells were seen to infiltrate the hepatitis site in most patients, both Pre V and Post V (data not shown). The MUC1 site was devoid of T cells Pre V, and most patients showed small and varying numbers of both CD4 and CD8 T cells infiltrating the site Post V (data not shown). We expanded short term in vitro, in the absence of additional antigen stimulation, the T-cell population infiltrating the DTH site of the MUC1 peptide injection and compared it to the in vitro expanded T-cell population infiltrating the adjoining site of hepatitis B antigen injection. We found that in the hepatitis B site, CD4+ T cells were predominant and activated, with 49% producing IFN-γ and 11% producing IL-4 (Figs. 2 and 3). There were very few CD8+ cells. In the MUC1 site, the same proportion of CD4+ cells produced IL-4 as in the hepatitis B site, but almost none made IFN-γ. In contrast to those in the hepatitis B site, all CD8+ T cells in the MUC1 site were activated and produced IFN-γ.
Fig. 2.
T cells infiltrating postvaccination DTH sites. MUC1 peptide and hepatitis B antigen were injected intradermally 14 days after the last injection and injection sites biopsied 48 h later. One third of the biopsy sample was minced and cultured for 2 weeks to allow infiltrating T cells to exit the skin fragments. The cells were analyzed by flow cytometry. The example shown is from the 300-μg dose peptide group.
Fig. 3.
CD8+ T cells infiltrating the MUC1 DTH site are IFN-γ producers. MUC1 peptide and hepatitis B antigen were injected intradermally 14 days after the last injection, and injection sites biopsied 48 h later. One third of the biopsy sample was minced and cultured for 48 h to allow infiltrating T cells to exit the skin fragments. The cells were analyzed by flow cytometry. The example shown is from the 100-μg dose peptide group.
Analysis of CD8+ T cells prevaccination and postvaccination
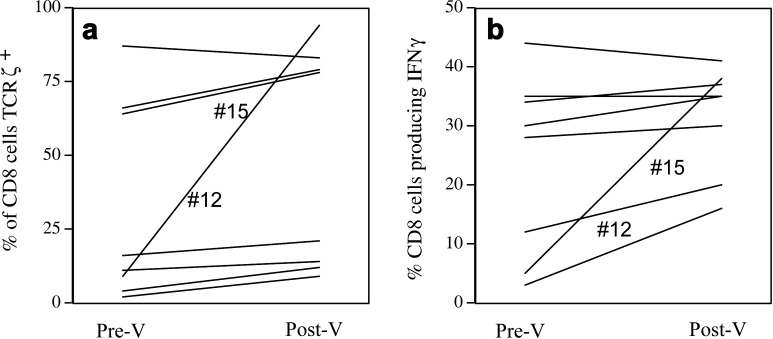
From eight patients we obtained sufficient number of cells to allow evaluation Pre and Post V of the CD8+ T cells and their ability to produce IFN-γ upon stimulation as well as their expression of the TCR ζ chain. We and others have previously published that cancer patients exhibit changes in their T-cell phenotype and function, reflected in the low-level expression or absence of the ζ chain and decreased ability to produce cytokines upon stimulation [18, 19]. Results are shown in Fig. 4. The proportion of CD8+ cells with high levels of ζ chain increased significantly (P=0.03) Post V (Fig. 4a). One correlation of interest was significantly different from zero: CD8+ cells making IFN-γ and CD8+ cells expressing higher levels of ζ chain (Spearman ρ=0.90, P=0.019, unadjusted). One patient (no. 15) had a very large increase in high ζ chain expressors (from 15% to 85%). The same patient also showed an increase in the percentage of CD8+ T cells producing IFN-γ (Fig. 4b).
Fig. 4.
Increase following vaccination in the percentage of CD8+ T cells that express normal levels of TCR ζ chain (a) and in the percentage of CD8+ T cells that produce IFN-γ upon stimulation (b). CD8+ T cells from patients’ PBMCs were evaluated for TCR ζ expression without activation. They were activated with PMA/ionomycin to measure intracellular levels of IFN-γ, as described in “Patients and methods.” Samples are from Pre V and the latest Post V time from which cells were available.
Discussion
In phase I trials of cancer vaccines administered to cancer patients, evaluation of the potential efficacy has been difficult, and the best methods for measuring antigen-specific immune responses have been a matter of debate [20]. Similarly, the adequate peptide dose for effective immunization has not been determined and will likely vary depending on the specific antigen. Our goal in this trial was to elicit MUC1-specific helper T cells, based on our in vitro experiments showing that the 100mer MUC1 peptide is processed and presented in HLA-DR and can stimulate class II–restricted CD4+ T cells to produce IFN-γ [8]. We planned to evaluate indirectly the efficacy of our vaccine to elicit helper T cells, by monitoring switching of Pre V anti-MUC1 IgM, a helper T-cell–independent isotype, to a helper-dependent isotype IgG Post V. We also anticipated that elicitation of helper T cells would stimulate expansion of preexisting MUC1-specific CD8+ T cells, thereby increasing their frequency that could be measured by ELISpot analysis. We selected the SB-AS2 adjuvant to further enhance both responses. SB-AS2 had been reported to be a good adjuvant for elicitation of both helper and cytotoxic T-cell responses [3, 16]. In a small number of patients, 5/16, belonging to all four groups, we saw for the first time evidence of MUC1-specific IgG antibody that may indicate peptide-specific helper T-cell activation. We were unable to directly measure MUC1-specific helper T cells due to the very high increase in nonspecific T-cell activation seen Post V.
Peptide dose escalation 30-fold, from 100 to 3,000 μg, did not result in toxicity. We did not escalate beyond 3,000 μg due to technical difficulties and cost of producing vaccine doses of this magnitude. We tested four different doses of peptide primarily to show that there is no toxicity involved in administering large doses of peptide, but also assuming that the larger peptide dose would yield a higher number of peptide/MHC class II complexes on the antigen-presenting cells. From our studies in MUC1-transgenic mice [21], we know that there is a degree of peripheral tolerance at the helper T-cell level and that primarily low-affinity T cells are available to respond. In that case, we reasoned, they were more likely to be stimulated with higher doses of antigen. Our data, however, show that high doses of peptide did not improve the outcome.
We have published previously that T cells from patients with pancreatic cancer have a reduced ability to be activated to produce cytokines [18, 19]. The patients on our trial were no exception in that their T cells had a severely reduced capacity to make either IFN-γ or IL-4. In some patients this changed drastically following the first injection of the vaccine. The ability of T cells to respond recovered to at least 50% that of healthy controls. Similarly, we saw an increase in the expression of ζ chain in CD8+ T cells Post V. We do not yet understand the mechanism of this recovery. In our study we are not able to correlate antibody or intensity of T-cell response following vaccination with time to recurrence or to the overall survival of patients.
The interesting observation that Post V patients have increased proportions of CD8+ T cells in circulation and that CD8+ T cells are uniquely infiltrating the MUC1 DTH sites, we ascribe to the specific action of the antigen. We have made a similar observation in preclinical studies of MUC1 vaccines in MUC1-transgenic mice [21]. Considering that CD8+ T cells are desirable antitumor effector cells, it is encouraging that MUC1 appears to exert its strongest effect on that cell population.
While this phase I study had a small number of patients, and their disease had rendered them severely immunosuppressed, we were still able to see marginal positive effects from the vaccine. In 10/16 patients we saw improvement in the ability of T cells to be activated. While the Post V increase in MUC1-specific antibody was not statistically significant, it is likely not due to random chance that we saw an this increase in antibody and evidence of isotype switching from IgM to IgG in the five patients who showed the greatest recovery in T-cell responsiveness. Since many of the patients opted for standard therapy following the last vaccine, we are unable to determine if the positive changes in overall and MUC1-specific immunity could have influenced progression of their disease. The median survival of 12 months in our study is comparable to historical controls. T3 disease or higher and nodal involvements are adverse prognostic factors and these patients have a particularly poor outcome. It is noteworthy that in our study, all patients were at high risk of recurrence with either T3–T4 disease or nodal involvement.
One other study evaluating a vaccine in pancreatic cancer has been reported. Jaffee et al. [10] conducted a phase I trial of a GM-CSF–secreting autologous pancreatic tumor vaccine in 14 patients with resected pancreatic cancer. Eight weeks after pancreaticoduodenectomy, patients received escalating doses of vaccine cells. No dose-limiting toxicities were encountered, and DTH responses to autologous tumor cells at the higher doses were seen in three patients Post V [10].
The MUC1 antigen appears to be a safe target for immune manipulation. In animal models it also appears to be a promising cancer vaccine. In MUCI-transgenic mice, a MUC1–dendritic cell vaccine showed evidence of tumor rejection [21], and we are now conducting a phase I study in patients with resected pancreatic or bile duct cancers. The difference between our studies in mice and the phase I studies we are conducting in patients is in the use of the MUC1 vaccine as prophylaxis in the former and therapy in the later. It remains to be seen in future trials if improvements in the vaccine design and immune monitoring will yield equally promising results in cancer patients.
Acknowledgements
This work was funded in part by Corixa Corporation, Nathan Arenson Fund for Pancreatic Cancer Research, and NIH/NCRR/GCRC/Grant No. 5MO1 RR 00056 to the University of Pittsburgh Medical Center. The authors wish to thank Dr Clauidine Bruck and Dr Martin Cheever for their comments on the manuscript and Alicia Depastino and Dolores Davis for secretarial assistance.
Footnotes
Work presented in part at the 36th Annual American Society of Clinical Oncology Meeting, New Orleans, LA, May 2000
References
- 1.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barratt-Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T-cell responses. Clin Cancer Res. 1999;5:1918–1924. [PubMed] [Google Scholar]
- 3.Doherty JF, Pinder M, Tornieporth N, Carton C, Vigneron L, Milligan P, Ballou WR, Holland CA, Kester KE, Voss G, Momin P, Greenwood BM, McAdam KP, Cohen J. A phase I safety and immunogenicity trial with the candidate malaria vaccine RTS, S/SBAS2 in semi-immune adults in The Gambia. Am J Trop Med Hyg. 1999;61:865–878. doi: 10.4269/ajtmh.1999.61.865. [DOI] [PubMed] [Google Scholar]
- 4.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- 5.Finn OJ, Jerome KR, Henderson RA, Pecher G, Domenech N, Magarian-Blander J, Barratt-Boyes SM. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev. 1995;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 6.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–353. doi: 10.1023/A:1011379725811. [DOI] [PubMed] [Google Scholar]
- 7.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 8.Hiltbold EM, Ciborowski P, Finn OJ. Naturally processed class II epitope from the tumor antigen MUC primes human CD4+ T cells. Cancer Res. 1998;58:5066–5070. [PubMed] [Google Scholar]
- 9.Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol. 2000;165:3730–3741. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 10.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Johnson C. Prognosis in pancreatic cancer. Lancet. 1997;349:1027–1028. doi: 10.1016/S0140-6736(96)10032-5. [DOI] [PubMed] [Google Scholar]
- 13.Kalser MH, Ellenberg SS. Pancreatic cancer Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 14.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat eitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. [PubMed] [Google Scholar]
- 16.Lalvani A, Moris P, Voss G, Pathan AA, Kester KE, Brookes R, Lee E, Koutsoukos M, Plebanski M, Delchambre M, Flanagan KL, Carton C, Slaoui M, Van Hoecke C, Ballou WR, Hill AV, Cohen J. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180:1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Buchler MW. European study group for pancreatic cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/S0140-6736(01)06651-X. [DOI] [PubMed] [Google Scholar]
- 18.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 19.Schmielau J, Nalesnik MA, Finn OJ. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res. 2001;7:933s–939s. [PubMed] [Google Scholar]
- 20.Simon RM, Steinberg SM, Hamilton M, Hildesheim A, Khleif S, Kwak LW, Mackall CL, Schlom J, Topalian SL, Berzofsky JA. Clinical trial designs for the early clinical development of therapeutic cancer vaccines. J Clin Oncol. 2001;19:1848–1854. doi: 10.1200/JCO.2001.19.6.1848. [DOI] [PubMed] [Google Scholar]
- 21.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Papadimitriou J, Finn OJ. Biology, biochemistry and immunology of carcinoma-associated mucins. Immunol Today. 1997;18:105–107. doi: 10.1016/S0167-5699(97)01028-1. [DOI] [PubMed] [Google Scholar]