Abstract
Previous studies in small groups of patients suggested that immunization of melanoma patients with peptide epitopes recognized by T cells could induce regression of melanoma. This approach was tested in 36 patients with stage IV melanoma. The (MHC class I–restricted) peptides were from gp100, MART-1, tyrosinase, and MAGE-3. The gp100 and MART-1 peptides had been modified to increase their immunogenicity. In half the patients (groups 3 and 4) the peptides were given in the adjuvant Montanide-ISA-720, and half the patients in both groups were given GM-CSF s.c. for 4 days following each injection. Treatment was well tolerated except for two severe erythematous responses to Montanide-ISA-720 and marked inflammatory responses at sites of GM-CSF administration in three patients. There were no objective clinical responses but stabilization of disease for periods from 3 to 12 months were seen in seven patients. Five of these were patients given the peptides in Montanide-ISA-720. Delayed-type hypersensitivity (DTH) skin test responses were also seen mainly in the patients given the peptides in Montanide-ISA-720. GM-CSF did not increase DTH responses in patients in the latter group but may have increased DTH responses in those not given peptides in Montanide-ISA-720. Inflammatory responses around s.c. metastases or regional lymph nodes were observed in two patients. These results suggest that the peptides are more effective when given in the adjuvant Montanide-ISA-720. Nevertheless, results from this study, together with those from a number of comparable studies, indicate that peptide vaccines are currently of minimal benefit to patients and support the need for ongoing development of new strategies in treatment of this disease.
Keywords: Adjuvants, Clinical responses, Melanoma, Peptide vaccines, T-cell responses
Introduction
A large number of different antigen sources have been used to produce vaccines against melanoma, including whole cells [23], cell lysates [9, 22], purified proteins [21], peptide epitopes [12, 20], RNA [1] and DNA [31] coding for melanoma antigens. We previously conducted a large randomized trial to test whether immunotherapy with vaccinia viral lysates of an allogeneic melanoma cell line would be of therapeutic benefit as adjuvant to surgical treatment of AJCC stage IIb/III melanoma. After a median follow-up of 8 years the treated group had an approximate 20% improvement in survival compared with that in the control group but significance tests did not exclude the possibility that the results may have been due to chance [9].
During the conduct of this trial, a number of antigens recognized by T cells were discovered and their epitopes identified. Studies in animal models had suggested that peptide epitope vaccines were effective in inducing immune responses against tumors and regression of tumor growth [6, 45]. Studies on patients with melanoma also showed that peptides from the melanoma antigen gene (MAGE-1) [8, 11, 44], tyrosinase [4], MART-1/Melan-A proteins [14], and gp100 [33] could induce cytotoxic T-lymphocyte (CTL) responses against melanoma in vitro. Immunotherapy with peptide epitopes from well-defined tumor antigens potentially offers several advantages: e.g., the ability to monitor immune responses can be used to optimize dose and frequency of injections. Peptides can be selected on the basis of the HLA phenotype of the patient and the antigenic content of the patient’s melanoma. It should also be possible to identify whether particular peptides are more effective in inducing regression of melanoma.
Jaeger et al. [12] immunized six patients with peptides from MART-1, tyrosinase, and glycoprotein 100 (gp100). They reported induction of skin test responses in five of six of the patients and stabilization of disease in two patients. Subcutaneous injections of granulocyte-macrophage colony-stimulating factor (GM-CSF) increased skin test responses to the peptides, and clinical responses were seen in three of three patients treated with both GM-CSF and the peptides [13]. Marchand et al. [19] reported partial responses in three of six patients injected with MAGE-3 peptide restricted by HLA-A1. After additional accrual, responses were seen in 7 of 25 patients (3 CR, 4 PR) [20]. Rosenberg et al. [29] immunized patients with a modified gp100 peptide (gp100 209-2 M) and reported responses in 13 of 31 patients receiving concurrent IL-2 therapy.
In view of these promising results, we commenced a phase I/II study to treat patients with stage IV measurable melanoma by injection of five melanoma peptides to determine whether this approach was associated with clinical and immunological responses, and whether adjuvants may increase the responses. Purified protein derivative (PPD) was included as a helper protein, and in half the patients peptides were given in a water-in-oil emulsion (Montanide-ISA-720), which was shown in animal studies to be more effective in inducing T-cell responses against CMV peptides than alum, incomplete Freund’s (IFA), immune-stimulating complexes (ISCOMS), and monophosphoryl lipid A (MPLA) adjuvants [34]. Approximately half the patients in both groups also received GM-CSF given subcutaneously. Our experience with this approach is described below.
Material and methods
Patients
Patients with stage IV measurable disease were entered into the study from the melanoma units in Sydney, Newcastle, and Adelaide from January 1997 to December 2001. Their performance status was ECOG 0 or 1. Ages ranged from 27 to 81 years. Inclusion criteria included low-volume disease, no previous chemotherapy or immunotherapy in past 4 weeks, and no other concomitant malignancy. All patients were HLA-A2-positive, as assessed by the Tissue Typing Unit of the NSW Red Cross Transfusion Service using sequence-specific oligonucleotide probes, as described elsewhere [15]. The studies were approved by the Hunter Area Research Ethics Committee, the Central Sydney Area Health Ethics Committee, and the Royal Adelaide Hospital Human Ethics Committee. Patients were randomly allocated to groups 1 and 2, and then to groups 3 and 4 (see below). Disease status was assessed by spiral computerized axial tomography (CAT) scans and in the case of subcutaneous metastases, by physical measurement with calipers. Low volume disease was not rigidly defined but generally included patients with metastases less than 3 cm in diameter and less than six in number. Tumor responses were assessed by RECIST criteria [40]. Tumor measurements were made before and 2 weeks after the 6th vaccine administration. If the patient had stable disease (SD) or its progression was not marked (<30% increase), the vaccinations would be continued at the discretion of the investigator.
Peptides used in the study
The melanoma peptides were produced by the University of Pittsburgh Peptide Facility according to the instructions of current Good Manufacturing Practice, part 21 of the Code of Federal Regulations, US Food and Drug Administration (cGMP 21 CFR, FDA). They were supplied as freeze-dried preparations. Lyophilized peptide was reconstituted in sterile, apyrogen phosphate–buffered saline, pH 6.8, to a concentration of 1.5 mg/ml. (If peptides were insoluble in PBS they were dissolved in a small amount of DMSO prior to addition of PBS.)
The solution was passed through a 0.22-μm, low-protein-binding nonpyrogenic sterilization filter (cat. SLGV 025 BS; Millipore), and the peptide molarity of the solution checked by measuring the OD275 and the solution adjusted to 1 mg/ml and stored in 300-μl aliquots in sterile 1-ml conical-bottom cryotubes (cat. 366656; Nunc). Aliquots were stored at −80°C. The influenza matrix peptide used as a positive control was kindly supplied by Dr Andrew Scott, Ludwig Cancer Institute, Heidelberg, Victoria, Australia.
Patients were immunized with the HLA-A2-restricted peptides MAGE-3A.2 (FLWGPRALV), tyrosinase (YMDGTMSQV), gp100 209-2 M (IMDQVPFSV), gp100 280-9 V (YLEPGPVTVP), and MART-1 26–35 (ELAGIGILTV). The peptide GILGFVFTL from influenza matrix (58–66) was included as a control.
The peptides from MART-1 and gp100 used in the study were made more immunogenic by replacing the anchor residues with amino acids having higher binding affinities: i.e., V for A in position 9 in gp100 280-9 V, M for T at position 2 in gp100 209-2 M [25], and L for A in position 2 for MART-1 [41]. The peptide from tyrosinase was a naturally occurring variant resulting from deamination of asparagines (N) to aspartic acid (D) and was shown to be a target for CTL against melanoma [37]. The MAGE-3.A2 peptide was discovered by generating epitopes with binding motifs to HLA-A2. (See Van der Bruggen et al. [10, 44].)
Study design
Peptide administration
One hundred microliters of purified protein derivative (PPD) (10 IU; Commonwealth Serum Laboratories) was added to each vial of peptide (300 μg), and each peptide was given intradermally about 2 cm apart over deltoids or anterior part of the thigh each 2 weeks for six doses. In approximately half the patients (groups 3 and 4) the peptides and PPD were emulsified in Montanide-ISA-720 (Seppic, France, supplied by Tall Bennett Group [Waratah Street, Mona Vale, NSW, Australia]). Montanide-ISA-720 (0.7 ml) was added to 0.3 of peptide mixture and emulsified by aspiration into and out of the syringe. The emulsified peptides were given subcutaneously over the lower abdomen or anterior thighs each 2 weeks for six vaccinations.
GM-CSF administration
GM-CSF (Schering Plough, Baulkham Hills, NSW, Australia) was supplied in quantities of 400 μg (4.4×106 IU) per vial. Each vial was reconstituted with 16 ml of saline and given s.c. near the site of the peptide injections by constant infusion with a Graseby pump over 72 h commencing at the time of the peptide injections.
Skin tests
Nonspecific cell-mediated immunity (CMI) was assessed with the multitest CMI applicator (Institut Merieux, CSL Parkville, Victoria, Australia), as described elsewhere [2]. Skin tests with the peptides used for treatment and the control influenza peptide were carried out prior to and at 6 and 12 weeks after commencement of therapy, as described by Jaeger et al. [12, 13]. Peptides (100 μg) were given in 100 μl of PBS by intradermal injection on the volar aspect of the forearm. DTH reactions were evaluated at 48 h after injection. Reactions were considered positive when palpable skin induration was 2 mm or greater in diameter.
IFN-γ cytokine production assays
Blood samples from the patients were taken pretreatment and at 2, 4, 8, and 12 weeks during treatment, separated on Ficoll-Hypaque, resuspended in DMEM (Trace Bioscience, Castle Hill, NSW, Australia) at 5×106/ml and then added to an equal volume of FCS+20% DMSO. Vials of 1 ml were placed in a Handy freeze tray (Taylor Wharton) in the neck of a 35 l VHC (Taylor Wharton) liquid nitrogen container overnight and then stored in liquid nitrogen. After thawing they were cultured overnight in DMEM plus 10% heat-inactivated human AB serum at 37°C. Assay procedures are as described previously [10]. The peptide processing defective T2 cells [24] were pulsed with individual peptides (10 μg/ml) in AIM-V (serum free) media (Gibco BRL, Invitrogen, Melbourne, Victoria, Australia) overnight at 37°C. PBLs (5×105/ml) were then incubated with T2 peptide-pulsed cells in DMEM plus 10% human AB serum at a ratio of 1:1 for 2 days at 37°C, as described by Salgaller et al. [33] and Parkhurst et al. [25]. Positive controls were either 5×105 PBLs plated onto anti-CD3 (OKT3)-coated wells or 5×105 PBLs stimulated with PHA-P (cat. no. L8754; Sigma). Negative controls were non-peptide-pulsed T2 cells and HIV reverse transcriptase476-484-pulsed T2 cells. Cultures were in duplicate. Supernatants were harvested after centrifugation at 600 g to remove cells, then stored at −80°C. Assay of IFN-γ in the supernatants was carried out by ELISA using a commercially available kit (PharMingen, Becton Dickinson, North Ryde, NSW, Australia) and read in a plate reader (model 450, Bio-Rad) with a minimal detectable concentration of 2 pg/ml.
Results
Clinical responses
In total, 36 patients were entered into the study. In 18 patients, peptides were given in Montanide-ISA-720. Seventeen patients received GM-CSF at the time of the peptide administration. Clinical details of the patients are summarized in Table 1. The treatment group and trial center are summarized in Table 2. There were three women in groups 1 and 2 and seven in groups 3 and 4. As shown in Table 2, there were no clinical responses (CR or PR) but seven patients (patients number 11, 17, 19, 22, 24, 32, and 33) had stable disease (SD) for periods in excess of 3 months. With the exception of patient 17, patients with SD had lung metastases and/or s.c. metastases. Five of the latter were in patients treated with peptides in Montanide-ISA-720, suggesting the latter may have increased clinical responses (χ2 test = 3.4, p=0.06). There may also have been a trend for more stabilization in patients receiving GM-CSF plus Montanide (patients 24, 32, and 33), but patient numbers were too small to place any significance on these results.
Table 1.
Patients receiving peptides with or without GM-CSF
| Patient no. | Age and sex | Time from diagnosis to metastases (months) | Site of metastases | Previous therapy | Time from first metastasis to treatment (months) | Adverse events during treatment | No. of vaccines | Best response | Duration to death/follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| From diagnosis (months) | First metastasis (months) | |||||||||
| 1 | 40 M | 38 | Multiple SC, lung, liver | 2 | Nausea, anorexia, Headache | 9 | PD | 52 | 14 | |
| 40 | Bone | Erythema at injection site | ||||||||
| Flu symptoms | ||||||||||
| 2 (GM-CSF) | 56 M | 78 | Lung | 19 | Tiredness, headaches | 13 | PD | 107 | 29 | |
| 87 | SC | Redness and swelling at GM-CSF site | ||||||||
| 99 | Brain | Flu symptoms | ||||||||
| 3 | 64 M | 64 | Lung | 2 | Headaches, blurred vision, dizziness, breathlessness | 12 | PD | 77 | 13 | |
| 66 | SC Liver | |||||||||
| 4 (GM-CSF) | 51 F | 96 | Abdo/pelvis LNs | 2 | Sore neck/throat | 5 | PD | 124 | 13 | |
| 96 | Lung, SC | Erythematous SC lesion | ||||||||
| Erythema at GM-CSF site | ||||||||||
| Tiredness | ||||||||||
| 5 (GM-CSF) | 49 M | 12 | SC | 1 | Dyspepsia, lethargy | 4 | PD | 20 | 8 | |
| Pain in neck and shoulder | ||||||||||
| Erythema at injection site | ||||||||||
| 6 | 81 M | 1 | SC abdomen | 6 | Insomnia, itchy SC metastases | 3 | PD | 9 | 9 | |
| Painful SC metastases | ||||||||||
| 7 (GM-CSF) | 35 M | 19 | Upper arm SC | VMCL | 72 | Tiredness, feadaches, nausea | 9 | PD | 99 | 80 |
| 23 | Pelvic LNs | Recurrent herpes | ||||||||
| 38 | Lung | Sore/swollen injection sites | ||||||||
| 42 | Chest SC | |||||||||
| 48 | Abdo/pelvis LN | |||||||||
| 58 | Para-aortic LN | |||||||||
| 72 | Adrenal, SC | |||||||||
| 8 (GM-CSF) | 60 M | 4 | Lower leg SC | 12 | Severe joint pain | 7 | PD | 23 | 19 | |
| Abdomen SC | Localized redness and soreness at injection sites | |||||||||
| Liver, bone | ||||||||||
| 9 | 70 M | 37 | Lung, SC | Surgery | 2 | None | 12 | PD | 47 | 10 |
| CNS | ||||||||||
| 10 (+GM-CSF) | 45 M | 196 | Lung | DTIC | 6 | Redness at injection site | 6 | PD | 208 | 12 |
| Liver | ||||||||||
| 11 | 54 M | 14 | SC, Lung | Surgery | 3 | Redness at injection site | 12 | SD | 45 | 31 |
| CNS | VMCL | |||||||||
| 12 (GM-CSF) | 83 M | 0 | L. great toe | Surgery | 0 | None | 0 | PD | 5 | 5 |
| 2 | Lung | |||||||||
| 13 (GM-CSF) | 72 M | ? | R. Thigh | Surgery | 13 | Cellulitis | 5 | PD | 25 | |
| Redness at injection site | ||||||||||
| 14 (GM-CSF) | 43 F | 0 | R shoulder, liver, lung, R lower rib | Surgery | 70 | Light-headedness, | 4 | PD | 78 | 78 |
| Palpitations, SOB | ||||||||||
| 15 (GM-CSF) | 67 F | 202 | L calf | Surgery | 2 | Nausea, fainting, vomiting/nausea, fever | 2 | PD | 205 | 4 |
| L groin | Surgery | |||||||||
| 16 | 40 M | 154 | Pulmonary | Surgery | 1 | None | 4 | PD | 158 | 4 |
| L parietal brain | ||||||||||
| R axilla | ||||||||||
| 17 | 44 M | 19 | Mediastinal LN | Surgery | 0 | None | 3 | SD | 21 | 4 |
| Skeletal, neck, subclavicular, brain, abdomen | ||||||||||
| 18 | 47 F | 0 | Liver, anal region | 7 | None | 5 | PD | 27 | 20 | |
| 6 | L inguinal | |||||||||
| Montanide-ISA-720 | ||||||||||
| 19 | 69 M | 20 | Lung | 0 | Nausea, dry wretching | 7 | SD | 47 | 27 | |
| Fatigue, chest pain | ||||||||||
| 20 | 63 M | 15 | Lym. neck | VMCL | 30 | Local discomfort and redness at injection sites | 6 | PD | 41 | 26 |
| 29 | Lung | Flu-type symptoms | ||||||||
| 32 | Bone, abdo LN | |||||||||
| 38 | Adrenal | |||||||||
| 21 | 73 F | 48 | Soft tissue | 85 | Abdominal cramping and vomiting | 6 | PD | 102 | 54 | |
| 83 | LN in pelvis, bowel | |||||||||
| 84 | Lung and SC | |||||||||
| 91 | Brain | |||||||||
| 22 | 57 F | 0 | Lung | Surgery | 4 | Tenderness at injection sites | 12 | SD | 55 | 55 |
| SC | ||||||||||
| 23 (GM-CSF) | 65 F | 53 | Leg soft tissue, SC | 116 | Flu-type symptoms | 6 | PD | 149 | 33 | |
| 108 | Chest soft tissue, | Severe joint aches | ||||||||
| 111 | Breast SC | Localized redness and swelling at injection sites | ||||||||
| SC | ||||||||||
| 24 (GM-CSF) | 49 M | 48 | Lung | Surgery | 1 | Flu symptoms | 4 | SD | 60 | 12 |
| Liver | Tenderness at injection sites | |||||||||
| Nausea, swelling and redness at tumor nodules | ||||||||||
| 25 (GM-CSF) | 67 F | 63 | Bone | Limb perfusion | 5 | Redness and tenderness at injection site, headaches | 5 | PD | 60 | 12 |
| Lymph node | Radiotherapy | Tenderness in lymph nodes | ||||||||
| Leg SC | ||||||||||
| 26 (GM-CSF) | 66 M | 73 | Lung, SC | Surgery | 1 | Redness at injection sites | 7 | PD | 81 | 8 |
| CNS | Radiotherapy | |||||||||
| 27 | 68 M | 4 | SC, Lung | 4 | Redness at SC sites | 6 | PD | 15 | 11 | |
| Adrenal, bone | ||||||||||
| 28 | 53 M | 4 | Bone, adrenals | Radiotherapy | 5 | Pain at injection site | 4 | PD | 15 | 11 |
| Lymph nodes, tonsils | DTIC | |||||||||
| Spleen, liver | 24 August 1999 | |||||||||
| 5 October 1999 | ||||||||||
| 29 (+GM-CSF) | 57 M | 6 | Lung | Radiotherapy | 7 | Flu symptoms | 12 | PD | 24 | 18 |
| Bone | Heartburn | |||||||||
| Hoarse voice | ||||||||||
| 30 | 51 F | 84 | Lung | 26 | Marked tiredness (grade 3) | 3 | PD | 121 | 37 | |
| Mediastinum LN | Painful injection sites | |||||||||
| 31 | 35 M | 174 | Brain | Surgery and radiotherapy | 24 | Tightness in chest | 8 | PD | 206 | 32 |
| 197 | Lung | |||||||||
| 32 (+GM-CSF) | M | 167 | Lung | Surgery | 0 | Painful injection sites | 16 | SD | 200 | 33 |
| 33 (GM-CSF) | 64 M | 13 | Lung | VMCL | 14 | Painful injection sites | 17 | SD | 48 | 35 |
| 34 | 55 M | 11 | Lung | DTIC | 3 | Slight lethargy | 5 | PD | 33 | 22 |
| NV06 | ||||||||||
| PNU | ||||||||||
| 35 | 27 F | 11 | Brain | Surgery 4/2/98 | 1 | None | 3 | PD | 16 | 5 |
| Lungs | R Axilla 13/7/98 | |||||||||
| Liver | DTIC 8/12/98 | |||||||||
| SC | IFN 13/10/98 | |||||||||
| Steroids | ||||||||||
| 36 | 64 F | 31 | SC leg | Surgery | 19 | None | 9 | PD | 62 | 31 |
| SC leg | ||||||||||
| LN groin | ||||||||||
| Lung | ||||||||||
Table 2.
Summary of patients entered and clinical outcome. SD stable disease for 3 months or longer
| Patient groups | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Treatment | ||||
| Montanide-ISA-720 | − | − | + | + |
| GM-CSF | − | + | − | + |
| Trial center | ||||
| NMU | 2 | 1 | 5 | 6 |
| SMU | 3 | 5 | 4 | 1 |
| RAH | 3 | 4 | 2 | 0 |
| 8 | 10 | 11 | 7 | |
| Best response | 1 SD (13%) | 2 SD (18%) | 3 SD (43%) | |
| Percentage with SD | 6% | 28% | ||
Toxicities
Practically all patients receiving Montanide-ISA-720 developed redness at the injection sites. Two patients developed extensive redness and induration over their lower abdomen, and one patient was admitted to hospital with a mistaken diagnosis of cellulitis. In both patients the swelling and redness subsided over a 2-week period. Both patients had a history of eczema and one of psoriasis, but the true nature of these severe reactions is unknown. Nevertheless, skin tests with small amounts of the reagent were carried out on subsequent patients to detect these idiosyncratic responses. GM-CSF administration was also associated with severe localized inflammatory responses in two patients, and most patients had systemic symptoms of inflammation to varying degrees.
CMI skin test responses
As shown in Table 3, measurement of cell-mediated immunity by the multitest CMI applicator revealed that 2 of 11 patients in groups 1 and 2, and 7 of 13 patients in groups 3 and 4, had a hypoergic score (<10 mm induration for men and 5 mm for women) [2] (difference not significant by χ2 tests). Five of the seven patients in groups 3 and 4 were completely anergic. There was no correlation between CMI results and development of DTH responses to peptides, in that four patients with negative CMI results had responses to the peptides (patients 19, 22, 30, and 32). Conversely, no DTH responses to the peptides were seen in eight patients (patients 3, 5, 8, 9, 11, 20, 24, and 33) with strong CMI scores. There was also no obvious correlation with development of SD, in that three patients with SD (patients 19, 24, and 32) had no responses in the multitest CMI tests.
Table 3.
Skin test responses in patients receiving peptides with or without GM-CSF (groups 1 and 2). SD stable disease for period of at least 3 months
| Patient no. | Sex | CMI score (mm/antigens postiive)b | Weeks | Tyrosinase | MAGE-3A2 | MART-1 | Gp100 | Gp100 209-2 M | Influenza |
|---|---|---|---|---|---|---|---|---|---|
| Groups 1 and 2 | |||||||||
| 1 (No)a | M | 8/3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | ||||
| 2 (Yes) | M | 11.8/4 | 0 | 0 | 0 | 0 | 0 | –d | – |
| 6 | 0 | 0 | 0 | 0 | – | – | |||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 3 (No) | M | 37/5 | 0 | 0 | 0 | 0 | 0 | – | – |
| 6 | 0 | 0 | 0 | 0 | – | – | |||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 4 (Yes) | F | 5.5/2 | 0 | 0 | 0 | 0 | 0 | – | – |
| 6 | 0 | 2 mm | 0 | 0 | – | – | |||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 5 (Yes) | M | 25/5 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 6 | 0 | 0 | 0 | 0 | 0 | – | |||
| 6 (No) | M | 282/6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 7 (Yes) | M | 9/3 | 0 | 0 | 0 | 0 | 0 | – | – |
| 6 | 0 | 0 | 4 mm | 0 | – | – | |||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 8 (Yes) | M | 22/5 | 0 | 0 | 0 | 0 | 0 | 0 | 10 mm |
| 6 | 0 | 0 | 0 | 0 | 0 | 10 mm | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 10 mm | |||
| 9 (No) | M | 16.5/4 | 0 | 0 | 0 | 0 | 0 | – | – |
| 6 | 0 | 0 | 0 | 0 | – | – | |||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 10 (Yes) | M | 12.5/3 | 0 | 0 | 0 | 0 | 0 | – | – |
| 6 | 0 | 3 mm | |||||||
| 12 | 0 | 0 | 0 | 0 | – | – | |||
| 11(No; SD)c | M | 18 mm/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Montanide-ISA-720 (groups 3 and 4) | |||||||||
| 19 (No; SD) | M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 6 | 0 | 10 mm | 0 | 0 | 0 | 0 | |||
| 12 | 0 | 0 | 4 mm | 0 | 0 | – | |||
| 20 (No) | M | 17.25/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 3 mm | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 21 (No) | F | 9.8/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 10 mm | 0 | 0 | |||
| 22 (No; SD) | F | Not done | 0 | 0 | 2 mm | 2.5 mm | 0 | 0 | 2 mm |
| 6 | 4 mm | 0 | 10 mm | 0 | 0 | 10 mm | |||
| 12 | 0 | 0 | 5 mm | 0 | 4 mm | 5 mm | |||
| 23 (Yes) | F | 7/10 | 0 | 0 | 0 | 0 | 0 | 0 | 4 mm |
| 6 | 0 | 0 | 3 mm | 0 | 0 | 0 | |||
| 24 (Yes; SD) | M | 25/6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 28 (No) | M | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 29 (Yes) | M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 30 (No) | F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 35 mm | 0 | 20 mm | 0 | 0 | 10 mm | |||
| 12 | 18 mm | 3 mm | 25 mm | 3 mm | 20 mm | 5 mm | |||
| 31 (No) | M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 32 (Yes; SD) | M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 6 mm | 0 | 0 | 0 | 0 | 0 | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 33 (Yes; SD) | M | 20/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 34 (No) | M | 2/1 | – | – | – | – | – | – | – |
aYes/no refers to whether patients received GM-CSF
bSum of diameters of induration/number of antigens positive
cSD patients with stable disease for period of at least 3 months
dNot done
DTH responses to peptides
Responses to influenza peptide were seen in one patient (patient 30) with negative CMI tests and three patients (patients 8, 20, and 23) with positive CMI tests. Responses to one or more of the melanoma peptides were seen in 3 of 11 patients in groups 1 and 2, and 6 of 12 patients in groups 3 and 4 (χ2 test = 1.02, p=0.31). The responses in groups 1 and 2 were in three of six patients receiving GM-CSF (group 2) but none in five patients in group 1. There were three of seven responses in group 3 and three of five in group 4 receiving GM-CSF (3/12 no GM-CSF vs 6/11 with GM-CSF; χ2 test = 1.49, p=0.22). The responses were generally weak (<5 mm) except in patients 19, 21, 22, and 30.
There were responses to MART-1 in 7 of 23 patients, MAGE-3.A2 in 4 of 23 patients, flu peptide in 4 of 23 patients, gp100 209-2 M in 2 of 17 patients, tyrosinase in 3 of 23 patients, and gp100 280-9 V in 2 of 23 patients. Skin tests were not done on nine patients from Adelaide and one patient from the Newcastle and Sydney Melanoma Units.
IFN-γ ELISA assays
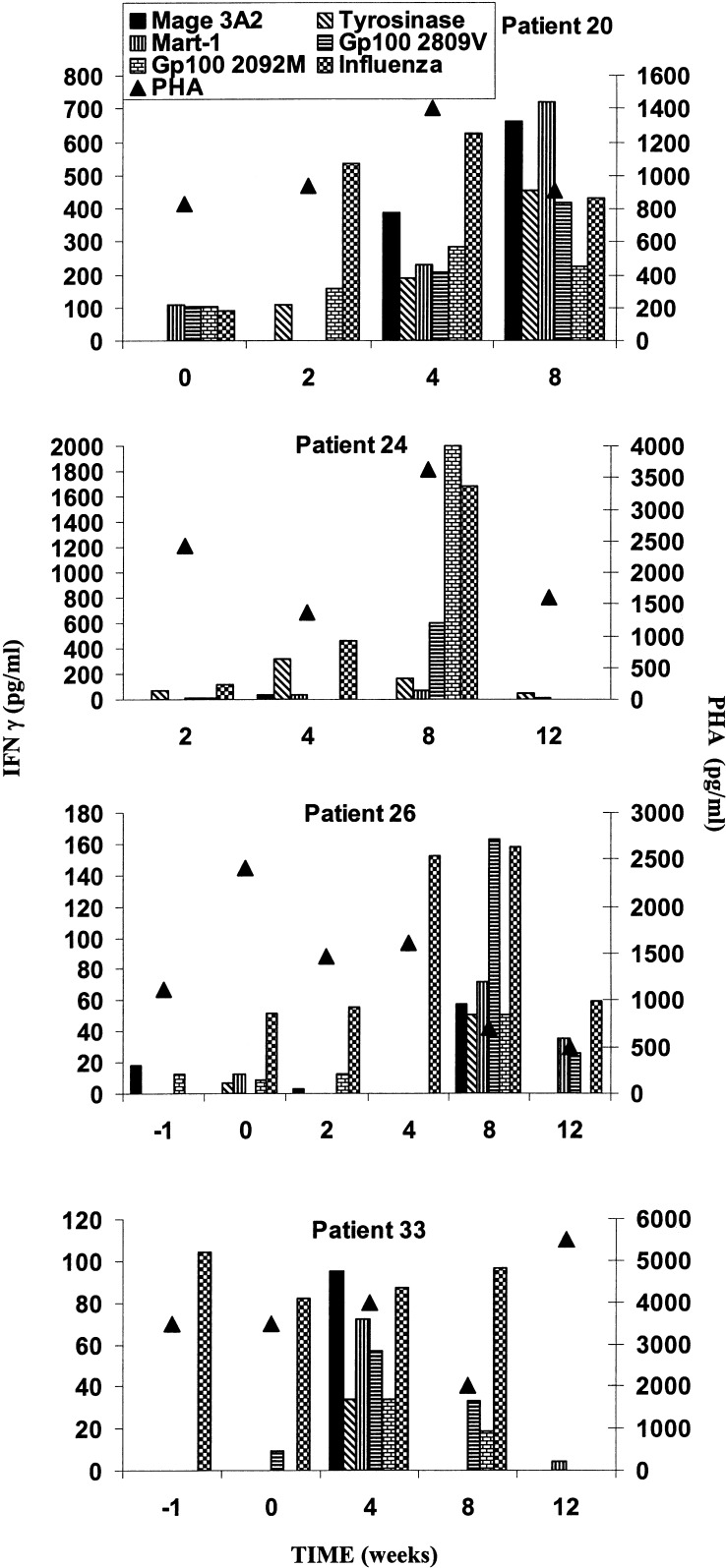
The results from these studies were compromised by loss of stored samples from groups 1 and 2, and loss of function in stored samples from 6 of 13 patients with sequential samples as defined by absence of response to PHA or anti-CD3. Figure 1 illustrates however that the peptides were able to induce IFN-γ production in one of two patients in group 3 (patient 20) and three of five in group 4 (patients 24, 26, and 33). Patient 20 had low responses to four of the peptides before treatment, but by week 4 and 8 had developed IFN-γ responses to all six peptides. In patient 24 there was development of strong responses by 8 weeks against the two melanoma gp100 peptides and influenza but responses were not detected at 12 weeks. Similarly, in patient 26, responses were seen against all the peptides at 8 weeks but only against influenza, MAGE, and gp100 209-2 M at 12 weeks. In patient 33 there was a response to influenza peptide at all time periods except week 12. Responses to all five melanoma peptides were seen at 4 weeks, but at 8 weeks responses were seen only against the two gp100 peptides. No responses to the peptides were seen in the remaining three patients with viable samples and good responses to PHA.
Fig. 1.
IFN-γ production from blood lymphocytes measured in ELISA in response to melanoma peptides at intervals during vaccination with melanoma peptides. All patients were in group 4, except patient 20.
Discussion
Many of the human melanoma antigens recognized by T cells have been characterized and shown to be nonmutated melanocyte differentiation antigens or members of the so-called cancer-testis group. Included in the latter are the series of MAGE antigens and the NY-ESO-1 antigen. At the time of commencing this trial, the main peptides under scrutiny were those from the differentiation antigens and the MAGE-3 antigen. The MAGE-3 epitope selected for this trial was subsequently shown not to be recognized on many melanoma cells [43] due to proteasome digestion [42] and in hindsight may not have been an effective target on melanoma cells for recognition by the immune system.
Results from the present study showed no evidence of clinical regression of melanoma but stabilization of disease was seen in 7of 36 patients, 5 of which were in patients given the peptides in Montanide-ISA-720. Melanoma in untreated patients may also undergo long periods of stable disease so it is not possible to say whether immunotherapy with the peptides was responsible for disease stabilization. A randomized trial would be needed to answer this question. We were therefore unable to confirm the promising results of small studies reported by others [12, 13, 20]. The reasons for this may be multiple. The peptides from differentiation antigens may have been poor immunogens due to previous recognition by the immune system [16] and development of anergy. This concern has been expressed in other studies [26], and much effort has been put into development of assays that measure high-affinity T-cell responses that may equate with clinical responses. These assays include measurement of median fluorescent intensity of tetramer binding to T cells. More recently, expression of CD107a from cytotoxic granules on the surface of cytotoxic T cells after exposure to tumor cells has been proposed as a simple method of measuring effective T-cell responses [32]. Such measures were not available in the present studies, and the main measures of immune responses were DTH skin test responses and IFN-γ production. DTH responses were seen in less than 50% of the patients and were mainly seen in patients given peptides in Montanide-ISA-720 (groups 3 and 4).
Patients receiving GM-CSF had a higher skin test response when peptides were given alone but not when given in Montanide-ISA-720, suggesting that GM-CSF may only be beneficial when peptides are not given in strong adjuvants like Montanide-ISA-720 [3]. Relatively low skin test responses were also reported in studies by Weber et al. [46] in patients with less advanced melanoma immunized with gp100 209-2 M and tyrosinase 370D. DTH responses were seen in 17 of 40 patients to the gp100 peptide, and 1 of 40 to the tyrosinase peptide, respectively. The patients in the present study had good ECOG performance scores but nevertheless, over half had low CMI scores in the CMI multitest skin tests and were perhaps incapable of responding to the peptides. In four patients (nos. 4, 6, 24, and 27) there was swelling and tenderness at the site of subcutaneous metastases, and in one (no. 25) tenderness of regional lymph nodes after each injection. These reactions had no relation to skin test responses to the peptides but provide evidence of biological responses induced by the vaccine.
The present study is similar to several others in reporting relatively low clinical response rates in patients immunized with melanoma peptides. Phan et al. [27] reported no responses in 22 patients immunized with modified gp100 209-2 M and MART-1 (26–35) 27L and one response in 19 patients treated with the same peptides plus class II DRB1*0401-restricted peptide from gp100 (44-5a). In previous studies, Rosenberg et al. [28] reported no responses in 23 patients treated with unmodified MART-1 peptide and no responses in 28 patients treated with three unmodified epitopes from gp100. Three mixed responses were seen in 11 patients treated with a modified gp100 (209-2 M) and 13 responses (1 CR) in 31 patients immunized with this peptide and given infusions of IL-2 [28]. A randomized trial is now in progress to assess the relative contributions of the peptide vaccine and the high dose IL-2 in this result. Similarly, Scheibenbogen et al. [36] reported one mixed response and two stable disease responses in 18 patients treated with four epitopes from tyrosinase. No clinical responses were seen in 28 patients treated with peptide epitopes from MART-1, gp100, and tyrosinase in MF59, or in 28 patients treated with the same peptides given with local injections of IL-12.
Several studies have supported the view [13] that GM-CSF may be an effective adjuvant to increase responses against melanoma peptides. Slingluff et al. [38] randomized 26 patients with stage IV melanoma to receive four peptides from gp100 and tyrosinase in Montanide-ISA-51 with GM-CSF or pulsed on dendritic cells. They reported higher T-cell responses in the group immunized with the peptides in GM-CSF and observed two objective responses in this group compared with one response in the dendritic cell vaccine treated group. There was also a trend for patients with AJCC stage II melanoma treated with gp100 and tyrosinase peptides in incomplete Freund’s adjuvant (IFA) and GM-CSF to have higher ELISpot responses than patients given the peptides in IFA alone [46]. A formal study on adjuvants to increase T-cell responses to tyrosinase and gp100 peptides found that GM-CSF was superior to IFA alone [35]. Administration of IL-2 to patients immunized with peptides resulted paradoxically in lower T-cell responses in blood, even though clinical responses were possibly higher [16, 28, 29].
It seems clear from these studies that clinical responses with regression of tumors following immunotherapy with melanoma peptides are infrequent, and it is not justified to offer this therapy to patients in general with metastatic melanoma. The question remains whether certain patients may be selected who will benefit from such therapy. We anticipated that nonspecific tests of CMI using the Institut Merieux Multitest Kit may help identify patients who may undergo responses to the peptides. Positive skin tests to peptides were seen, however, in four patients with negative CMI responses. Conversely, no skin test responses to peptides were seen in six patients with strong CMI scores. CMI scores could not therefore be used as an eligibility criteria. Tumor volume may also be important, but the majority of patients in the present study had early, low-volume disease. It was also noticeable that patients with SD, with one exception, had lung metastases with or without s.c. metastases. A number of other patients, however, with this disease distribution had PD so that site of disease was not the sole determinant of induction of SD.
The peptides from the differentiation antigens selected for study are known to be expressed in practically all melanomas so that selection on the basis of antigen expression is unlikely to be helpful. Immune responses in blood, as measured in ELISpot or cytokine release assays have not been predictive in other studies of clinical responses [16, 30]. Given the difficulty in identifying patients who may respond on clinical grounds, the present focus on development of more predictive tests and development of more novel treatment approaches appears well justified [26]. The latter includes new antigenic targets, such as MAGE-10 and NY-ESO-1, and inclusion of MHC class II antigens to induce helper responses [18]. Glycolipid antigens recognized in the context of CD1a on dendritic cells may also be important in immune responses against melanoma [5]. Approaches which target regulatory T cells in the host or allow expansion of CTLs appear promising, particularly the use of antibodies against CTLA-4 [27], or lymphocyte depletion approaches [7, 17, 39]. Identification of subgroups of patients that respond to these new treatment approaches remains an essential goal.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia and in part by the Sydney Melanoma Foundation and the Hunter Melanoma Foundation.
References
- 1.Abdel-Wahab Z, Kalady MF, Emani S, Onaitis MW, Abdel-Wahab OI, Cisco R, Wheless L, Cheng TY, Tyler DS, Pruitt SK. Induction of anti-melanoma CTL response using DC transfected with mutated mRNA encoding full-length Melan-A/MART-1 antigen with an A27L amino acid substitution. Cell Immunol. 2003;224:86. doi: 10.1016/j.cellimm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CT, Roumiantzeff M, Kniker WT. The multitest system for assay of delayed cutaneous hypersensitivity (DCH) to ubiquitous antigens. J Allergy Clin Immunol. 1978;61:167. [Google Scholar]
- 3.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Brichard V, van Pel A, Wolfel T, Wolfel C, De-Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coventry CD1a in human. 2004;cancers:a. doi: 10.1016/j.it.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, an oncogenic self-protein. J Immunol. 1996;156:3151. [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber D, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Dura P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethe B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. Immunogenetics. 1994;39:121. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hersey P, Coates AS, McCarthy WH, Thompson JF, Sillar RW, McLeod R, Gill G, Coventry BJ, Dillon H, Simes RJ. Adjuvant immunotherapy of patients with high risk melanoma with vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol. 2002;20:4181. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 10.Hersey P, Menzies SW, Halliday GM, Nguyen T, Farrelly ML, DeSilva C, Lett M. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother. 2003;53:125. doi: 10.1007/s00262-003-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X, Chakraborty NG, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Enhancement of cytolytic T lymphocyte precursor frequency in melanoma patients following immunization with the MAGE-1 peptide loaded antigen presenting cell-based vaccine. Cancer Res. 1996;56:2479. [PubMed] [Google Scholar]
- 12.Jaeger E, Bernhard H, Romero P, Ringhoffer M, Arand M, Karback J, Ilsemann C, Hagedorn M, Knuth A. Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer. 1996;66:162. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.3.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger E, Ringhoffer M, Dienes HP, Arand M, Karbach J, Jager D. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer. 1996;67:54. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Apella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a melanoma antigen recognised by tumour infiltrating lymphocytes associated with in vivo tumour rejection. Proc Natl Acad Sci U S A. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy LJ, Poulton KV, Thomson W, Williams F, Middleton D, Howell WM, Tarassi K, Papasteriades C, Albert E, Fleischauer K, Chandanayingyong D, Tiercy JM, Juji T, Tokunago K, Ollier WER (1997) HLA class I DNA typing using sequence specific oligonucleotide probes (SSOP). In: Charron D (ed) Genetic diversity of HLA: functional and medical implication. Proceedings of the 12th International Histocompatibility Workshop, p 216
- 16.Lee KH, Wang E, Nielsen MB, Wunderlich J, Migueles S, Connors M, Steinberg SM, Rosenberg SA, Marincola FM. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292. [PubMed] [Google Scholar]
- 17.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 18.Mandic M, Almunia C, Vicel S, Gillet D, Janjic B, Coval K, Maillere B, Kirkwood JM, Zarour HM. The alternative open reading frame of LAGE-1 gives rise to multiple promiscuous HLA-DR-restricted epitopes recognized by T-helper 1-type tumor-reactive CD4+ T cells. Cancer Res. 2003;63:6506. [PubMed] [Google Scholar]
- 19.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwijck R, Humblet Y. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 20.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Lienard D, Beauduin M, Dietrich PY, Russo V, Kerger J, Masucci G, Jager E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219. doi: 10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.3.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Marchand M, Punt CJ, Aamdal S, Escudier B, Kruit WH, Keilholz U, Hakansson L, van Baren N, Humblet Y, Mulders P, Avril MF, Eggermont AM, Scheibenbogen C, Uiters J, Wanders J, Delire M, Boon T, Stoter G. Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer. 2003;39:70. doi: 10.1016/S0959-8049(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell MS, Darrah D, Stevenson L. Therapy of melanoma with allogeneic melanoma lysates alone or with interferon-alfa. Cancer Invest. 2002;20:759. doi: 10.1081/CNV-120002493. [DOI] [PubMed] [Google Scholar]
- 23.Morton DL, Hsueh EC, Essner R, Foshag LJ, O’Day SJ, Bilchik A, Gupta RK, Hoon DS, Ravindranath M, Nizze JA, Gammon G, Wanek LA, Wang HJ, Elashoff RM. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg. 2002;236:438. doi: 10.1097/00000658-200210000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nijman HW, Houbiers JGA, Vierboom MPM, Van Der Burg SH, Drijfhout JW, D’Amaro J, Kenemans P, Melief CJM, Kast WM. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1215. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 25.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539. [PubMed] [Google Scholar]
- 26.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 27.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Seipp CA, Einhorn JH, Roberts B, White DE. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Sznol M, Schwarz SL, Spiess PJ, Wunderlich JR, Seipp CA, Einhorn JH, Rogers-Freezer L, White DE. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690. [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE. Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum Gene Ther. 2003;14:709. doi: 10.1089/104303403765255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 33.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749. [PubMed] [Google Scholar]
- 34.Scalzo AA, Elliott SL, Cox J, Gardner J, Moss DJ, Suhrbier A. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatible adjuvant (Montanide ISA 720) J Virol. 1995;69:1306. doi: 10.1128/jvi.69.2.1306-1309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaed SG, Klimek VM, Panageas KS, Musselli CM, Butterworth L, Hwu W-J, Livingston PO, Williams L, Lewis JJ, Houghton AN, Chapman PB. T-cell responses against tyrosinase 368-376 (370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund’s adjuvant, granulcotye macrophage colony-stimulaitng factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8:967. [PubMed] [Google Scholar]
- 36.Scheibenbogen C, Schmittel A, Keilholz U, Allgauer T, Hofmann U, Thiel E, Schadendorf D (1999) Vaccination with tyrosinase peptides and GM-CSF in metastatic melanoma: a phase II trial. In: Proceedings of ASCO 18, p 436a [DOI] [PubMed]
- 37.Skipper JCA, Hendrickson RC, Gulden PH, Brichard V, van Pel A, Chen Y, Shabanowitz J, Wolfel T, Slingluff CL, Jr, Boon T, Hunt DF, Engelhard VH. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Terando A, Mule JJ. On combining antineoplastic drugs with tumor vaccines. Cancer Immunol Immunother. 2003;52:680. doi: 10.1007/s00262-003-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Nat Canc Inst. 2000;92:205. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 41.Valmori D, Fonteneau J-F, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini J-C, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750. [PubMed] [Google Scholar]
- 42.Valmori D, Gileadi U, Servis C, Dunbar PR, Cerottini JC, Romero P, Cerundolo V, Levy F. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J Exp Med. 1999;189:895. doi: 10.1084/jem.189.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valmori D, Lienard D, Waanders G, Rimoldi D, Cerottini J-C, Romero P. Analysis of MAGE-3-specific cytolytic T lymphocytes in human leukocyte antigen-A2 melanoma patients. Cancer Res. 1997;57:735. [PubMed] [Google Scholar]
- 44.van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, de Smet C, Traversari C, Townsend A, Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 45.Vierboom MPM, Nijman HW, Offringa R, Van Der Voort Eih, van Hall T, van den Broek L, Fleuren GJ, Kenemans P, Kast WM, Melief CJM. Tumor eradication by Wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186:695. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V, Lee PP, Groshen SG, Gee C, Jeffery GG, Sian S, Lee P. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected stage II melanoma. Cancer. 2003;97:186. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]