Abstract
We previously characterized the expression of CD80 in different murine head and neck squamous cell carcinoma (HNSCC) clones derived following tumor progression in the absence of T cell–mediated immunity in severe combined immunodeficient (SCID) mice. We found that HNSCCs that did not express CD80 grew as progressors, while those that expressed CD80 were regressors when grown in immune-competent animals. In the present study, we characterized expression of a repertoire of immunoregulatory cytokines in these HNSCC lines, and found that HNSCCs that express cytokines IL-1α, IL-6, and GM-CSF do not express CD80, suggesting the hypothesis that these cytokines may down-modulate expression of CD80. Cytokine-conditioned medium from progressor HNSCC and recombinant IL-1α, IL-6, and GM-CSF caused a reduction of CD80 expression in regressor HNSCCs without affecting proliferation. Conversely, the decrease in CD80 expression in progressor HNSCCs could be restored by IFN-γ, a known inducer of CD80 expression. These data strongly suggest that high levels of cytokines IL-1α, IL-6, and GM-CSF expressed by tumor cells can down-regulate CD80 expression in HNSCC, and that IFN-γ can independently stimulate expression. These data provide evidence for a novel mechanism of cytokine-mediated down-modulation of CD80 during malignant progression of HNSCC that can be restored by IFN-γ.
Keywords: Cytokines, IFN-γ, CD80, Squamous cell carcinoma, Regulation
Introduction
T lymphocytes must receive two signals in order to become activated. One signal is mediated by the interaction between T cell and peptide bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The second signal is provided by costimulatory molecules on the APC interacting with specific counter-receptors on the surface of T cells. The B7 family of costimulatory molecules, which are members of the immunoglobulin superfamily, include CD80 (B7-1) and CD86 (B7-2) [1, 2]. Although other costimulatory molecules have been recently described, CD80 and CD86 provide the most potent costimulatory signals found to date. Engagement of CD80 by the CD28 receptor on T cells leads to IL-2 production, which is critical for T cell activation and growth. Lack of CD80 T-cell costimulation may lead to T cell anergy [3, 4]. Squamous epithelia can express CD80, but CD80 is not often detected in squamous cell carcinomas of the head and neck (HNSCCs), which are usually poorly immunogenic.
T lymphocytes from patients with HNSCC have shown impaired proliferative responses and depressed cytotoxicity [5, 6, 7]. One possible mechanism could involve decreased or absent costimulation, since normal squamous epithelia can express CD80 costimulatory molecules on their surface, while HNSCCs usually do not [8, 9]. Consistent with this, we previously derived clonal murine oral squamous cell carcinoma lines following tumor progression of transformed keratinocytes in immune-deficient SCID mice, and found a subset of these HNSCCs to be CD80-positive [10]. When implanted in immune-competent syngeneic BALB/c mice, the HNSCC that expressed CD80 became regressors, while those that did not express CD80 grew as progressors [10].
Little is known concerning the mechanism of decreased expression of CD80 during tumor progression of HNSCCs or other cancers. However, concomitant with decreased expression of CD80, others and we have detected constitutive expression of a repertoire of cytokines by squamous cell carcinoma that have immunomodulatory functions. Cytokines IL-1α, IL-6, GM-CSF, and IL-8 are detectable in tumor homogenates and cell lines from patients with HNSCC [11, 12, 13]. Murine squamous cell carcinomas have been shown to express homologues of these cytokines, including mIL-1α, mIL-6, and mGM-CSF. The expression of these cytokines by neoplasms has been previously associated with various cancer-promoting effects, including increased proliferation [14, 15], inflammation [16], and decreased immune responsiveness [17]. Therefore, we hypothesized that expression of cytokines such as IL-1α, IL-6, and GM-CSF could modulate CD80 expression in HNSCC. The results from studies presented here support our hypothesis and describe a means of restoring expression of an immune molecule important for tumor recognition and destruction using IFN-γ.
Materials and methods
Cell lines and cell culture
The derivation and characterization of CD80 expression and malignant potential of murine HNSCC cell lines B6C3-4scid, B6D8-4scid, B7E3-4scid, B7E11-4scid, and B4B8-4scid has been previously described [10]. HNSCCs were cultured at 37°C with 5% CO2 in MEM containing 10% FCS (Invitrogen Life Technologies, Carlsbad, CA) and antibiotics.
Murine oral squamous cell carcinoma
We previously established a murine model of HNSCC from oral keratinocytes of male BALB/c mice that consist of five clonal cell lines transformed by the carcinogen 4-1-nitroquinolone-1-oxide in vitro and five progeny cell lines derived from tumors that formed from these clones in immune-deficient SCID mice [10].
Reagents
Murine recombinant cytokines IL-1α, IL-6, and GM-CSF were purchased from Biosource International (Camarillo, CA). Neutralizing antibodies against IL-1α (hamster antimouse monoclonal antibody; BD-PharMingen, San Jose, CA), IL-6 (rat antimouse monoclonal antibody; Biosource International) and GM-CSF (rat antimouse monoclonal antibody; BD-PharMingen) were aliquoted and prepared in PBS and stored at 20°C until use. Isotype controls, hamster antimouse IgG for IL-1α and rat antimouse IgG2a for IL-6 and GM-CSF (BD-PharMingen) were used in MTT assays for cell proliferation. For quantification of CD80 expression by flow cytofluorometry, FITC-conjugated hamster antimouse CD80 mAb (BD-PharMingen) was used with FITC-conjugated hamster as isotype control (BD-PharMingen). IL-1α and GM-CSF probes were obtained from American Type Culture Collection (ATCC; #63106, #39924).
Flow cytometric analysis
The relative levels of surface CD80 expression on the cell lines were compared by immunofluorescence staining and flow cytometry as previously described [10]. The stained cells were analyzed in a flow cytometer (FACScan, Becton Dickinson, San Jose, CA), which had been calibrated with standard beads at an excitation wavelength of 488 nm. The log fluorescence intensity of 104 cells was plotted and analyzed.
Enzyme-linked immunosorbent assay (ELISA)
Quantitation of cytokine secretion was determined by ELISA. HNSCC reisolate cell lines were grown in culture for several passages. Cells (3×106) were transferred into 75-cm2 tissue culture flasks with 12 ml of appropriate fresh medium. The supernatant was collected after 72 h, filtered through a 0.22-μm sterile filter unit (Millipore, Bedford, MA), aliquoted, and stored frozen at −20°C until used in ELISA. The quantity of cytokine secreted was standardized to reflect amount secreted in pg/ml/106 cells. ELISA was performed according to manufacturer’s instructions (Endogen, Cambridge, MA). The optical density at 450 nm was quantitated using an 8-channel spectrophotometer (Biotek Systems, Winooski, VT). A computer software package (Delta-Soft 3, BioMetallics, Princeton, NJ) was used to generate a standard curve by plotting the average absorbance obtained for each standard concentration on the vertical axis (Y) and the corresponding concentration on the horizontal axis (X). The concentration of the samples was calculated by interpolating from the standard curve.
Cell proliferation assay
Cell proliferation assays (MTT based, Boehringer Mannheim, Indianapolis, IN) were performed as follows. Regressor cell lines B6C3-4scid and B6D8-4scid and progressors B4B8-4scid, B7E3-4scid, and B7E11-4scid cells were dispensed into 96-well flat-bottom microtiter plates in 100 μl at a concentration of 5×103 cells/well. Various dilutions of IL-1α (0–5,000 pg/ml), IL-6 (0–5,000 pg/ml), and GM-CSF (0–5,000 pg/ml) in serum-free medium were used against regressor and progressor HNSCC lines (progressor lines were used as controls for specificity). Anti-IL-1α, anti-IL-6, or anti-GM-CSF against progressor HNSCC lines were added to triplicate wells for each cytokine concentration or for each blockade condition in a volume of 100 μl/well (regressor lines were used as controls for specificity). Control cells for the antibody assays were treated with 100 μl of isotype antibodies: hamster IgG for anti–IL-1α and rat IgG2a for anti–IL-6 and anti–GM-CSF (BD-PharMingen). Microtiter plates were prepared in triplicate for analysis on days 1, 3, and 5, and incubated at 37°C with 5% CO2. On the day of analysis, MTT labeling reagent and solubilization solution were added to each well according to manufacturer’s instructions. The plates were incubated overnight at 37°C and the absorbance at wavelengths between 550 and 600 nm measured using an 8-channel spectrophotometer (Biotek Systems, Winooski, VT) and analyzed by a computer software package (Delta-Soft 3, BioMetallics, Princeton, NJ). Cell proliferation was plotted and the graphs statistically analyzed for significant differences between groups.
Northern analysis
Total RNA was isolated from tumor cell lines when 80–90% confluent in 75-cm2 or 150-cm2 tissue culture flasks, using Trizol reagent according to the manufacturer’s instructions (Gibco BRL). Each purified DNA fragment (25–50 ng) to be used for hybridization was added to the random priming reaction with 50-μCi α-dCTP (Amersham Life Science, Arlington Height, IL) using the Prime-It RmT kit (Stratagene, La Jolla, CA) following manufacturer’s instructions. After hybridization with labeled probes, 2×106 cpm/2 ml, and washing under high stringency conditions, the membrane was exposed to a X-OMAT AR film with an intensifying screen at −80°C, and the film was developed by a M35 X-OMAT film processor (Kodak, Rochester, NY). Each blot was reprobed with labeled GAPDH cDNA to confirm loading and the integrity of the RNA run on the gels.
Cytokine stimulation
HNSCC cells were treated with exogenous cytokines or anticytokine antibodies. Briefly, regressors B6C3-4scid and B6D8-4scid or progressors B4B8-4scid and B7E3-4scid were plated in 25-cm2 tissue culture flasks until 75–80% confluency. Various dilutions of IL-1α (500, 5,000, and 10,000 pg/ml), IL-6 (250, 500, 5,000 pg/ml), or GM-CSF (500, 5,000, 10,000 pg/ml) in serum-free medium or anti–IL-1α, anti–IL-6 or anti–GM-CSF at 10, 100, or 200 ng/ml were added to each progressor or regressor cell line. Control cells were treated with equal volume of PBS/medium or isotype antibodies. The plates were incubated at 37°C for 48 h (cytokine treatment) or 24 h (anticytokine antibody treatment). Since cytokine stimulation for 48 h effected the greatest down-regulation in CD80 expression in preliminary experiments (data not shown), this incubation time was chosen for all subsequent cytokine stimulation experiments. Cells were then trypsinized and 1×106 cells were transferred over a nylon mesh into falcon tubes for staining for CD80 molecule and flow cytometry.
Stimulation with conditioned medium
HNSCC cells (3×104) were plated in 25-cm2 tissue culture flasks and allowed to incubate with 8 ml of MEM containing 10% FCS (Invitrogen Life Technologies, Carlsbad, CA) and antibiotics for 5 days. Using sterile techniques, the medium contained in each flask was then centrifuged to remove cellular debris. The resultant supernatants from the five HNSCC lines were placed over B6C3-4scid regressor cells that had been incubated in 6-well plates at a concentration of 2×104 cells/well for 5 days at approximately 90% confluency. Supernatants of the regressor cell lines B6C3-4scid and B6D8-4scid served as controls. After 24 or 48 h of incubation, the supernatants were removed and the cells stained with antibodies against CD80 costimulatory molecules for flow cytometric analysis, as previously described. Statistical analysis was performed using Student’s t-test. Differences were judged significant at p<0.05.
Reverse transcriptase (RT)-PCR
Messenger RNA isolation was obtained by use of a commercially available kit and following the manufacturer’s instructions (Micro-FastTrack, Invitrogen, Carlsbad, CA). The concentration of the elute mRNA was determined by measuring the absorbance at 260 nm on a spectrophotometer. RT-PCR was performed following the Invitrogen protocol (Carlsbad, CA). Samples were subjected to one cycle at 94°C for 2 min followed by 30 cycles of 45 s at 94°C, 45 s at 55°C, and 45 s at 72°C, with 5 min at 72°C on the last cycle and were run on a Stratagene robocycler (La Jolla, CA). Primers for the mouse IFN-γ and β-actin were synthesized by Sigma (St. Louis, MO). The primer sequences used were 5′-TAC TGC CAC GGC ACA GTC ATT GAA-3′ and 5′-GCA GCG ACT CCT TTT CCG CTT CCT-3′ for IFN-γ , and 5′-GTG GGC CGC TCT AGG CAC CA-3′ and 5′-CGG TTG GCC TTA GGG TTC AGG GGG G-3′ for β-actin (18). PCR products were separated on a 1% agarose gel in TAE buffer (0.04-M Tris-acetate and 0.001-M EDTA, pH 8). The gel was stained with ethidium bromide and bands were visualized by UV illumination. PCR of IFN-γ and β-actin produced bands of 405 and 245 base pairs, respectively.
Results
Progressor HNSCC cell lines secrete high levels of cytokines IL-1α, IL-6, and GM-CSF
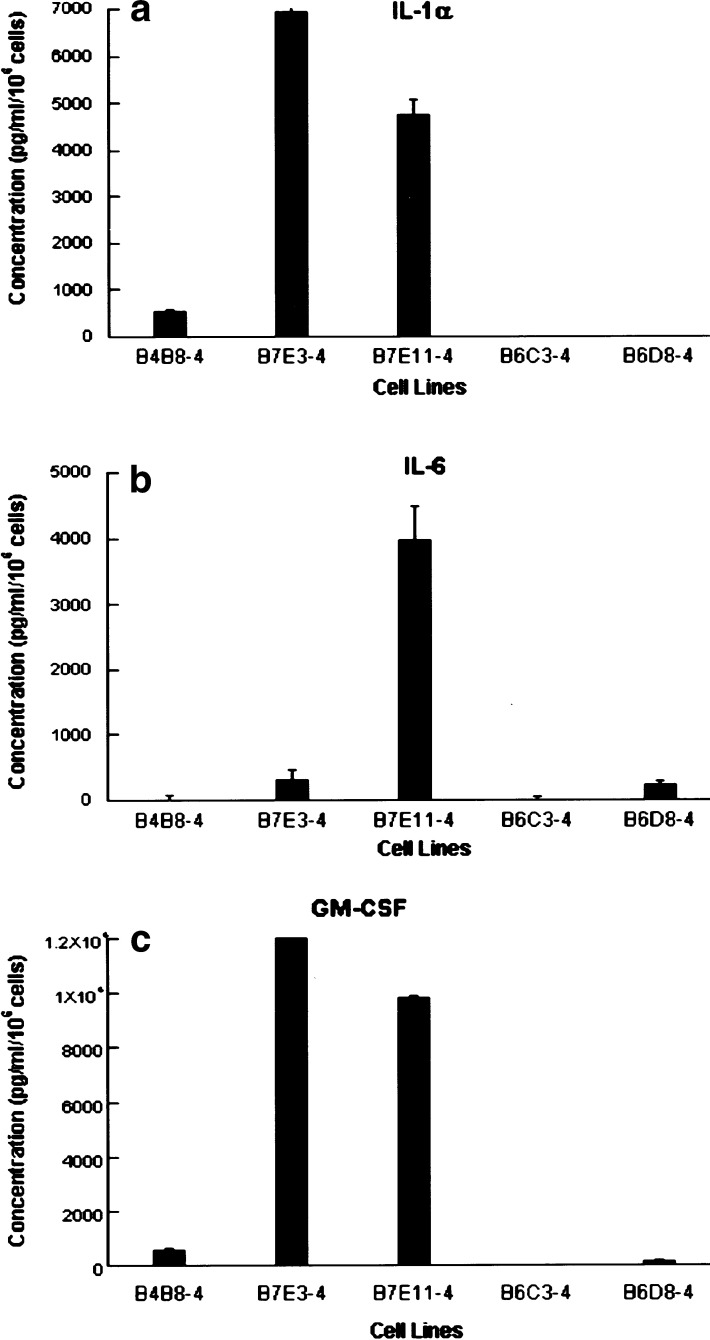
We measured the secretion of cytokines in culture supernatants of the HNSCC cell lines derived from SCID mice by ELISA. Consistent with our previous studies of cutaneous squamous cell carcinoma (PAM 212 reisolates), several of the oral squamous cell lines expressed detectable levels of IL-1α, IL-6, and GM-CSF [11] (Table 1). In the HNSCC cell lines, we noted a potential relationship between cytokine secretion (Table 1), tumorigenicity in immune-competent mice, and low CD80 expression reported previously [10]. Cytokines IL-1α and GM-CSF were detected at increased levels together in the supernatants of the reisolate lines B4B8-4scid, B7E3-4scid, and B7E11-4scid, and IL-6 was detected in B7E3-4scid and B7E11-4scid lines that grew progressively in immune-competent mice (Fig. 1). The B6 sublines produced all three cytokines at lower levels. Thus, we found that HNSCCs tumorigenic in SCID mice exhibit an association between expressions of cytokines IL-1α, IL-6, and GM-CSF, and progressor or regressor phenotype in immune-competent mice.
Table 1.
Cytokine profile of HNSCC lines
| Cell lines | Cytokines (pg/ml/106 cells)a | |||
|---|---|---|---|---|
| IL-1α | IL-6 | GM-CSF | ||
| Progressor | B4B8-4scid | 556±32 | 0±76 | 564±52 |
| Progressor | B7E3-4scid | 6,920±440 | 320±160 | 88,656±132 |
| Progressor | B7E11-4scid | 4,760±320 | 3,968±520 | 9,800±116 |
| Regressor | B6C3-4scid | 0±12 | 6±44 | 0±4 |
| Regressor | B6D8-4scid | 0±8 | 230±44 | 146±36 |
aConcentrations are reported as the average of each sample in triplicate with the respective standard deviation in pg/ml/106 cells
Fig. 1.
Progressor HNSCC cell lines secrete cytokines IL-1α, IL-6, and GM-CSF. Secretion of proinflammatory cytokines IL-1α, IL-6, and GM-CSF in culture supernatants by reisolated SCID cell lines was determined by ELISA. Cytokines IL-1α and GM-CSF were detected at increased levels together in the supernatants of the reisolate lines B4B8-4scid, B7E3-4scid, and B7E11-4scid that grew progressively in immune-competent mice. A similar increase was not observed by cell lines B6C3-4scid and B6D8-4scid that regressed in immune-competent mice
Cytokine mRNA expression parallels cytokine secretion in murine HNSCC cell lines
To determine if the differences in cytokine secretion among the cell lines were associated with differences in cytokine mRNA expression, we compared cytokine mRNA expression of the HNSCC lines for IL-1α and GM-CSF by Northern blot analysis (Fig. 2). Overall, cytokine mRNA was detected by Northern blot analysis only in the cell lines in which cytokine protein was detected by ELISA at higher levels. An increase in IL-1α and GM-CSF cytokine mRNA signal was observed among the three HNSCC lines B4B8-4scid, B7E3-4scid, and B7E11-4scid that demonstrated increased expression of cytokines by ELISA (Fig. 2, lanes 1, 2, 3). Elevated expression of these cytokines was not observed for the B6D8-4scid and B6C3-4scid cell lines (lanes 4, 5). Thus, constitutive expression of cytokine mRNA was detected in HNSCC lines that constitutively secreted the highest levels of these cytokines.
Fig. 2.
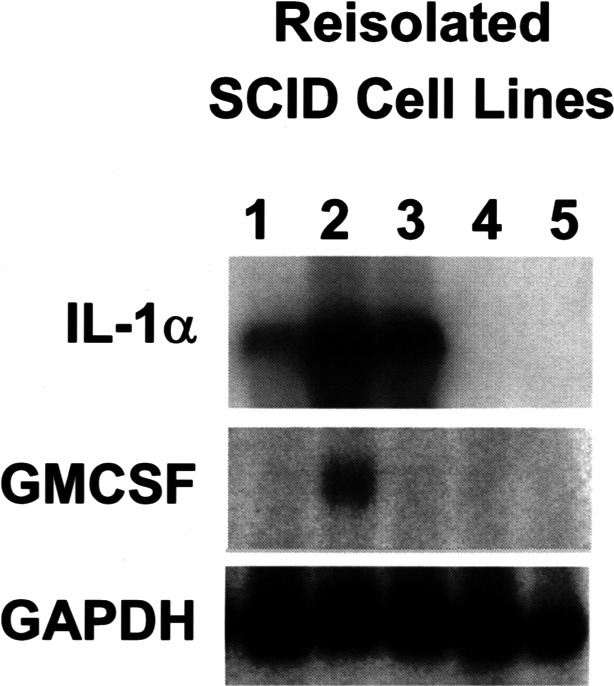
Cytokine mRNA expression parallels cytokine secretion in HNSCC cell lines. Cytokine total RNA expression of the HNSCC lines for IL-1α and GM-CSF was analyzed by Northern blot analysis. Increased expression of cytokine RNA was detected in progressor squamous cell lines that secreted the highest levels of these cytokines
Recombinant IL-1α, IL-6, GM-CSF decrease CD80 costimulatory molecule expression
The levels of cytokine expression in our model system suggested the hypothesis that constitutive expression of cytokines might down-regulate expression of CD80. In order to determine whether CD80 expression can be modulated by cytokines, regressor cell lines B6D8-4scid and B6C3-4scid that express high levels of CD80 costimulatory molecules were stimulated for 24, 48, and 72 h with exogenous cytokines IL-1α, IL-6, and GM-CSF. In preliminary experiments, we determined that stimulation with exogenous IL-6 (250–500 pg/ml) and IL-1α (2,500–5,000 pg/ml) at 48 h gave the greatest reduction in CD80 expression, as compared to 24 and 72 h (data not shown). Figure 3 shows that CD80 expression was significantly reduced by 48-h stimulation with cytokines in the B6C3-4scid cell lines, while in the B6D8-4scid cell line, CD80 expression was not reduced to significant levels. IL-6 stimulation had the most consistent effect, while stimulation with GM-CSF had the least effect on CD80 down-regulation. The pattern of modulation appears to be delayed (48 h) and not dose-dependent, since we were not able to achieve greater reduction of CD80 expression by increasing concentrations of cytokines. We have also noted that the reduction in CD80 expression was not complete, suggesting that other factor(s) may regulate CD80 expression.
Fig. 3.
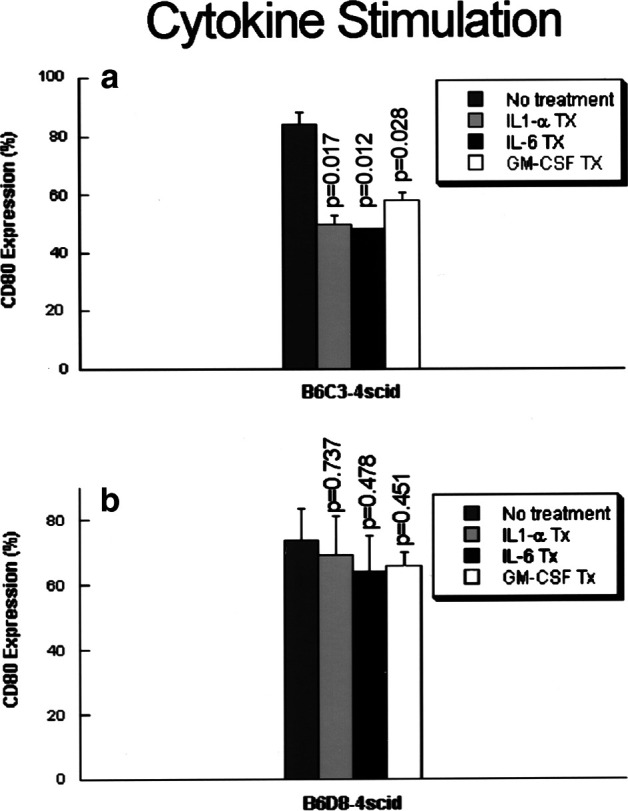
Recombinant IL-1α, IL-6, and GM-CSF decrease CD80 costimulatory molecule expression. Regressor cell lines B6D8-4scid and B6C3-4scid were treated for 48 h with exogenous cytokines IL-1α (5,000 pg/ml), IL-6 (250 and 500 pg/ml), and GM-CSF (5,000 or 10,000 pg/ml). Stimulation of B6C3-4scid cell line resulted in significant decreases in CD80 expression; while stimulation of the cell line B6D8-4scid did not. Results are from four independent experiments
Cytokine-conditioned medium from progressor HNSCC lines decreases CD80 costimulatory molecule expression
Since recombinant proinflammatory cytokines decreased CD80 expression, we would expect that supernatants from progressor HNSCC lines, which produce high levels of these cytokines, should cause comparable or greater reduction in CD80 expression. Therefore, we stimulated the regressor line B6C3-4scid with conditioned medium from progressor lines. Forty-eight hour stimulation of the regressor line B6C3-4scid with conditioned medium from all three progressor lines resulted in significant decreases in CD80 expression (Fig. 4), and showed that medium derived from the B7-clones (B7E3-4scid and B7E11-4scid) that show higher levels of cytokines had a greater effect on reduction of CD80 expression (p=0.026 and p=0.019, respectively) as compared to medium from the B4B8-4scid line (p=0.198) or from the regressor line B6D8-4scid that express insignificant amounts of cytokines (p=0.4624).These results confirm that the progressor cells secrete soluble factors that are functionally active and suppress CD80 expression. Moreover, they also demonstrate that the combination of cytokine(s) in conditioned medium derived from the B7-clone of HNSCC lines may be more effective in decreasing CD80 expression.
Fig. 4.
Cytokine-conditioned medium from progressor HNSCC lines decreases CD80 costimulatory molecule expression. Regressor cell line B6C3-4scid was stimulated for 48 h with conditioned medium from regressor and progressor cell lines. Medium derived from the B7-clones, B7E3-4scid, and B7E11-4scid, which contained high levels of proinflammatory cytokines IL-1α, IL-6, and GM-CSF had a greater effect on reduction of CD80 expression (p=0.026 and p=0.019, respectively) as compared to medium from the regressor line B6D8-4scid (p=0.4624) that does not express proinflammatory cytokines. Result is representative of three independent experiments
IFN-γ does not mediate the effects of proinflammatory cytokines on CD80 expression
To determine whether IFN-γ is the mediator of the effects of cytokines on CD80 expression, we stimulated regressor cell lines B6C3-4scid and B6D8-4scid with exogenous proinflammatory cytokines IL-1α (5,000 pg/ml), IL-6 (500 pg/ml), and GM-CSF (5,000 pg/ml) for 48 h and measured the changes in IFN-γ mRNA by RT-PCR. We were unable to detect IFN-γ mRNA in either cytokine-stimulated or unstimulated cell lines, suggesting that IFN-γ mRNA may not be the mediator of the down-regulation of CD80 expression by proinflammatory cytokines.
IFN-γ increases CD80 expression in HNSCC cell lines
Since IFN-γ is known to up-regulate CD80 expression, determining whether IFN-γ stimulates CD80 expression in our system of HNSCC lines will confirm that: (1) progressor cells have intact ability to process IFN-γ signal, and (2) progressor cells have retained the ability to modulate CD80 expression. Therefore, we treated our progressor and regressor HNSCC cell lines with exogenous IFN-γ and measured the changes in CD80 expression by flow cytometry. Progressor (B7E3-4scid, B4B8-4scid, B7E11-4scid) and regressor (B6C3-4scid, B6D8-4scid) HNSCC cell lines were treated with murine recombinant IFN-γ (500, 1,000, and 2,000 U/ml) for 12 and 24 h. Untreated cell lines were used as controls. Results of these experiments showed that IFN-γ (1,000–2,000 U/ml for 24 h) was able to up-regulate CD80 expression in 4/5 HNSCC cell lines (Table 2). However, significant up-regulation was not seen in the B7E11-4scid cell line that already expressed high constitutive CD80 levels prior to IFN-γ stimulation. This finding suggests that these HNSCC cells have retained the ability to modulate CD80 expression after malignant transformation, and confirms that IFN-γ can up-regulate CD80 expression.
Table 2.
IFN-γ stimulation results in an increase in CD80 expression in HNSCC. Twenty-four-hour stimulation of regressor and progressor cell lines with IFN-γ (1,000–2,000 U/ml) results in up-regulation of CD80+ expressing cells. Results are average of two independent experiments
| Cell lines | CD80 expression (%) | ||
|---|---|---|---|
| Constitutive | Average increase by IFN-γ | p probability | |
| B7E3-4scid | 2–6 | 34 | p<0.0001 |
| B4B8-4scid | 6–7 | 16 | p=0.535 |
| B7E11-4scid | 80 | 1.5 | p=0.107 |
| B6C3-4scid | 80–95 | 23 | p<0.001 |
| B6D8-4scid | 60–80 | 34 | p=0.080 |
Discussion
In the present study, we found that constitutive expression of one or more of the cytokines IL-1α, IL-6, and GM-CSF is associated with down-modulation of CD80 costimulatory molecule expression in oral squamous cell carcinoma cells in a syngeneic murine model. The HNSCC cell lines that exhibit a combination of constitutive cytokine expression and low CD80 expression also exhibit increased tumorigenic potential in immune-competent mice, as previously reported [10]. Recombinant IL-1α, IL-6, and GM-CSF and media containing these cytokines secreted by cytokine-expressing HNSCC could partially suppress expression of CD80 in HNSCCs that regress in immune-competent mice. Reduction of CD80 costimulatory molecule expression by proinflammatory cytokines IL-1α, IL-6, and GM-CSF has not been previously described. This decrease in CD80 expression during malignant progression of HNSCC may result in dysfunctional antitumor immunity, thereby promoting the progression of malignant growth. Importantly, this cytokine-induced down-regulation of CD80 expression appears to be independent of, and may be restored by, IFN-γ, a well-known inducer of CD80 expression. The ability of IFN-γ to restore CD80 expression in HNSCC cells suggests that incorporation of immune therapy strategies that include exogenous or endogenous IFN-γ could provide costimulatory molecule expression needed to prevent anergy and promote immune rejection. Consistent with this, we showed previously that IL-12–induced regression of murine oral SCC was abrogated in knockout mice deficient in IFN-γ [19].
We previously reported that several cytokines, including IL-1α, IL-6, and GM-CSF, are detectable in tumor homogenates and cell lines from patients with HNSCC [11, 12, 13], and that a homologous repertoire of cytokines is expressed in murine squamous cell carcinoma [11]. The expression of these cytokines by HNSCCs and other epithelial tumors seems paradoxical however, since IL-1α, IL-6, and GM-CSF can activate a variety of host inflammatory responses, and have been demonstrated to have antitumor activity in certain experimental and human tumors [20, 21, 22]. Although all of the pathogenic or protective effects of expression of this group of cytokines in HNSCC are not yet clear, they are commonly expressed by aggressive human and murine squamous cell carcinomas [11, 12, 13]. The apparent association between tumor promotion and inflammation and the prevalence of tumors that constitutively express proinflammatory cytokines, suggests that expression of proinflammatory cytokines may provide tumors with a survival or growth advantage. IL-1α has been shown to promote proliferation of HNSCC [23], and GM-CSF–expressing HNSCCs have been shown to induce cells of the granulocyte lineage that suppress lymphocyte-mediated immunity [24]. In immune-competent hosts, it is possible that chronic IL-1α and GM-CSF exposure could provide an immunologically mediated survival advantage, such as preferential induction of nonprotective T-helper type 2 responses, or exhaustion of dendritic cells necessary for antigen presentation. Recent evidence has also shown an immunosuppressive role of IL-6 in multiple myeloma patients by inhibiting the development of dendritic cells (DCs). IL-6 inhibited the colony growth of CD34+ DC progenitors thereby exerting low antigen-presentation capacity to these cells [25]. Moreover, peripheral blood DCs of these myeloma patients showed a decrease in MHC class II, CD40, and CD80 costimulatory molecules that was responsible for defective induction of T-cell proliferation [25].
The mechanisms by which proinflammatory cytokines induce down-regulation of CD80 expression in our system are unclear and are presently being investigated. Cytokines may regulate CD80 expression by controlling the CD80 promoter. The mouse CD80 promoter contains an enhancer element with sequence similarities to the consensus NF-κB motif [26], making this enhancer element a potential site of inhibitory action in IL-6–induced CD80 modulation. CD80 expression has been shown to be modulated by a variety of stimuli including IFN-γ, lipopolysaccharide (LPS), CD40 cross-linking, TNF-α, IL-10, and IL-4 [27, 28, 29, 30, 31, 32]. We did not detect expression of IFN-γ or changes in its expression following stimulation by proinflammatory cytokines. This suggests that IFN-γ up-regulates rather than down-regulates CD80 expression in the presence of proinflammatory cytokines in our system. Moreover, the difference in the extent of cytokine-induced down-regulation between the two regressor HNSCC lines could be the result of differences in expression of cytokine receptors. Preliminary studies on cytokine-receptor expression by HNSCC lines have shown reduced expression of IL-6 gp130 receptor subunits in the cell line showing decreased cytokine-induced CD80 modulation compared with the cell line showing significant CD80 modulation (Thomas et al., unpublished data).
We are presently studying the effects of cytokine-induced down-regulation of CD80 molecule expression in vivo. Until these studies are completed, we cannot exclude other possible explanations for differences in growth in immune-competent mice. Differences in immune recognition can result from immunodominance of certain tumor antigens, loss of expression of tumor-specific antigens, MHC I, or immune costimulatory molecules (reviewed in [33]). However, in previous studies, we did not detect a decrease in murine MHC class I proteins in the HNSCC progressor lines [10], and we were able to induce immunity to, and regression of, the progressor B7E11-4 by treating animals with IL-12 [19], suggesting that tumor-specific antigens are expressed on these tumor cells.
Taken together, our data suggest that down-regulation of the expression of CD80 costimulatory molecules during tumor formation by cytokines IL-1α, IL-6, and GM-CSF could contribute to the decreased immune responsiveness in patients and animals bearing HNSCC. The studies presented here provide evidence of potential novel mechanisms of CD80 regulation and its restoration by IFN-γ. Moreover, the steps in malignant progression observed in our in vivo studies in immunosuppressed and competent mice may enable analysis of the role of cell and host factors in modulation of CD80 costimulatory molecules occurring in squamous tissue on exposure to environmental carcinogens.
References
- 1.Lenschow Ann Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Sperling Immunol Rev. 1996;153:155. doi: 10.1111/j.1600-065x.1996.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 3.Chambers Curr Opin Immunol. 1997;9:396. doi: 10.1016/S0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz J Exp Med. 1996;184:1. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzolino J Exp Med. 1987;166:303. doi: 10.1084/jem.166.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkey Arch Otolaryngol Head Neck Surg. 1991;117:1281. doi: 10.1001/archotol.1991.01870230097016. [DOI] [PubMed] [Google Scholar]
- 7.Bennet Cancer. 1971;28:1255. [Google Scholar]
- 8.Lang Cellular Immunol. 2000;201:132. doi: 10.1006/cimm.2000.1651. [DOI] [PubMed] [Google Scholar]
- 9.Lang Arch Otolaryngol Head Neck Surg. 1999;125:82. doi: 10.1001/archotol.125.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Thomas Int J Cancer. 1999;82:377. doi: 10.1002/(SICI)1097-0215(19990730)82:3<377::AID-IJC11>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith Clin Exp Metastasis. 1998;16:655. doi: 10.1023/A:1006559811429. [DOI] [PubMed] [Google Scholar]
- 12.Chen Clin Cancer Res. 1999;5:1369. [Google Scholar]
- 13.Van Waes C, Chen Z, Callister M, Colon I, Ortiz N, Smith C, Thomas GR, Dong G (2000) Cytokines in the immune pathogenesis and therapy of head and neck cancer. In: Veldman JE, Passali D, Lim DJ (eds) New frontiers in immunobiology. Kugler, Netherlands, p 233
- 14.Woodworth Proc Natl Acad Sci U S A. 1995;92:2840. [Google Scholar]
- 15.Stevenson Proc Natl Acad Sci U S A. 1997;94:508. doi: 10.1073/pnas.94.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loukinova Oncogene. 2000;19:3477. doi: 10.1038/sj.onc.1203687. [DOI] [PubMed] [Google Scholar]
- 17.Pak Clin Cancer Res. 1995;1:95. [Google Scholar]
- 18.Cheng J Immunol. 1998;160:2735. [PubMed] [Google Scholar]
- 19.Thomas Int J Cancer. 2000;86:368. doi: 10.1002/(sici)1097-0215(20000501)86:3<368::aid-ijc11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.DeVita VT, Hellman S, Rosenberg SA (eds) (1995) Biologic therapy of cancer. Lippincott, Philadelphia
- 21.Blankenstein Curr Opin Immunol. 1991;3:694. doi: 10.1016/0952-7915(91)90098-l. [DOI] [PubMed] [Google Scholar]
- 22.Paul WE (ed) (1993) Fundamental immunology, 3rd edn. Raven Press, New York
- 23.Wolf Clin Cancer Res. 2001;7:1812. [PubMed] [Google Scholar]
- 24.Young Int J Cancer. 1997;74:69. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Ratta Blood. 2002;100:230. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 26.Jennings Immunogenetics. 1999;50:109. doi: 10.1007/s002510050697. [DOI] [PubMed] [Google Scholar]
- 27.Foss Infect Immun. 1999;67:5275. doi: 10.1128/iai.67.10.5275-5281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stack J Immunol. 1994;152:5723. [PubMed] [Google Scholar]
- 29.Hatcock J Exp Med. 1994;180:631. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caux J Exp Med. 1994;180:1263. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranheim Cell Immunol. 1995;161:226. doi: 10.1006/cimm.1995.1031. [DOI] [PubMed] [Google Scholar]
- 32.Friedman Cell Immunol. 1991;137:429. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber Semin Cancer Biol. 2002;12:35. doi: 10.1006/scbi.2001.0401. [DOI] [PubMed] [Google Scholar]