Abstract
Purpose: Deficiency of the mannan-binding lectin (MBL) pathway of innate immunity is associated with increased susceptibility to infections. In patients with colorectal cancer (CRC), postoperative infection is associated with poor prognosis. The aim of the present study was to evaluate (1) the relation between the MBL pathway and postoperative infectious complications and survival of patients resected for CRC, and (2) the role of MBL in acute phase response compared to C-reactive protein (CRP). Methods: Preoperative MBL concentration, MBL-associated serine protease (MBL/MASP) activity and CRP were determined in serum from 611 patients and 150 healthy controls. The patients were observed for 8 years. Postoperative infections, recurrence and survival were recorded. Results: The MBL pathway components were increased in the patients compared with the healthy controls (p<0.0001). Low MBL levels were predictive of pneumonia (p=0.01), and pneumonia (n=87) was associated with poor survival (p=0.003; HR=1.5; 95% CI, 1.1 to 1.9). MBL and MBL/MASP activity showed no correlation with CRP (Spearman’s ρ=0.02; 95% CI, −0.06 to 0.10). Conclusion: Low preoperative MBL levels are predictive of pneumonia, which is associated with poorer survival. MBL concentration and MBL/MASP activity was not predictive of other postoperative infections or long-term prognosis, and showed no correlation with CRP.
Keywords: Colorectal cancer, Complement, Infectious complications, MBL, Prognosis, Survival
Introduction
Colorectal cancer (CRC) is the second leading cause of death from malignant disease, and the third most common cancer in the industrialised world. Nearly all patients are treated with surgery, and 80% are considered curatively resected. However, approximately 50% of these intended curatively resected patients would develop recurrent disease within the next 5 years. At present, the risk of recurrence after curative surgical intervention includes a variety of mechanisms and events including infectious complications. Thus, patients developing postoperative infectious complications are at increased risk of recurrence and poor long-term survival [3, 14, 22, 27], a consequence that has been underlined by reports of other solid tumours [6, 9, 32, 35]. At present, all patients are given preoperative antibiotic prophylaxis, but despite this, 5–20% of the patients undergoing elective operations and 15–40% of the patients undergoing emergency operations develop postoperative bacterial infectious complications. The mechanisms leading to development of postoperative infectious complications after major surgery are not yet known in detail, but impaired preoperative and postoperative immune competence, including impaired complement activation pathways [42], and possibly low mannan-binding lectin (MBL) levels [33] may play a role [2, 7, 12, 17, 40]. As postoperative infectious complications may identify a group of patients with increased risk of recurrent cancer and poor prognosis, reduction of the frequency of such events may improve patient outcome and thereby prognosis after operation for CRC.
The plasma protein MBL causes activation of the complement system. MBL circulates in the form of complexes with various MBL-associated serine proteases (MASP-1, MASP-2 and MASP-3), which are able to activate the complement system [8]. This activation is initiated by the binding of MBL to carbohydrate structures presented by e.g. microorganisms, and leading to the destruction of the microorganisms. Thus MBL is considered an important component of the innate immune defence system [29]. Individuals with low serum concentrations of MBL are more susceptible to infections than individuals with adequate concentrations [23, 30]. At present, it is unknown whether the development of postoperative bacterial infectious complications and the overall prognosis after resection of CRC may be related to impaired MBL pathway complement activation.
In a preliminary study [41], the MBL pathway was increased in patients with CRC (n=193) compared with healthy controls. The biological importance and the relation to postoperative complications of this finding are unknown. However, the increased MBL level may signify a cancer-related inflammatory response in these patients. The serum levels of another well-established acute phase reactant, C-reactive protein (CRP), correlates to survival in CRC, with increased levels associated with poor survival in curatively resected patients as well as in patients with advanced disease [19–21, 25, 39]. The increased CRP levels may be indicative of impaired immunity [26], and a concomitant rise in MBL and CRP could support the hypothesis of a role of MBL in the inflammatory response with additional prognostic information.
Therefore, the aim of the present study was to evaluate whether preoperative concentrations and activity of MBL hold prognostic information with respect to postoperative infectious complications and to the overall disease course and survival. Further, we analysed if MBL plays a part in the acute phase response by relating the components of the MBL pathway to the levels of the CRP, which is a significant prognostic marker.
Materials and methods
Serum samples were obtained from 611 patients with primary CRC in different disease stages and from a control group of 150 healthy blood donors. At sample time, all patients were without clinical signs of infectious disease, and none had been treated with antibiotics apart from the standardised preoperative prophylaxis, or with immunosuppressive agents such as systemic steroids, antiviral drugs, or cytotoxic agents within 2 weeks prior to surgery. None of the patients received treatment with preoperative radiotherapy. Patients with severe concurrent illness other than colorectal carcinoma were not included. All patients included underwent elective surgery, thus none of the patients presented with bowel obstruction, perforation or other symptoms requiring emergency surgery. Data from the patients included age, gender, localisation of the tumour (colon or rectum) and disease stage according to Dukes’ classification with the addition of a D group that identifies patients with distant metastasis. The infections recorded are described at the end of this section.
Blood for serum preparation was collected just prior to skin incision. Serum from the blood donors was prepared by the blood bank. Health registrations of the blood donors in Denmark are taken prior to every blood donation, thus this group is well characterised, in particular the donors cannot give blood during ongoing infections. The Regional Ethics Committee approved the study, and all patients as well as donors gave informed consent to participate.
The MBL concentration was determined by a time-resolved immunofluorometric assay (TRIFMA) described in detail in previous studies using microtitre wells coated with mannan for catching MBL [38]. The principle of the assay is as follows: diluted serum is incubated in mannan-coated microtitre wells in the presence of Ca2+, which allows for the binding of MBL to mannan. Subsequently, europium-labelled antibodies detect MBL and are quantified by fluorometry. The serum dilution routinely applied (1/200) can detect MBL levels between 20 ng and 5 μg MBL/ml serum. For samples exceeding 5 μg/ml, assays were repeated at successively higher serum dilutions. MBL/MASP activity was determined also using a TRIFMA method, in which the principle is the detection of C4b bound to the mannan surface, a result of MBL/MASP-induced cleavage of C4. In this assay, diluted serum is incubated in mannan-coated microtitre wells followed by wash and incubation at 37°C with purified C4. Anti-C4 antibodies subsequently detect the resulting C4b deposition [28]. The values of this assay are given as arbitrary units with a standard pool of sera assigned the value of 1,154 mU/ml. The measuring range of the assay is between 0.5 and 57.7 mU.
Correspondingly, as the samples used in the present report were 200-fold diluted, the assay gives the correct activity values in samples from 100 to 11,540 mU/ml. Due to accuracy limitations of the functional assay at low MBL concentrations, no values are given for samples with MBL concentrations below 250 ng/ml. The serum CRP levels were determined using a nephelometric method, and the concentrations of CRP were expressed as milligram per litre with 2.6 mg/l as the lower detection limit. The upper normal limit of CRP was 10 mg/l.
Endpoints
Postoperative infectious complications and overall survival were the primary endpoints. The postoperative infectious complications were defined as follows: (1) Wound infection was defined as the presence of pus, either discharged spontaneously or requiring drainage. Wound infection included a subgroup of patients with perineal infection, following abdomino-perineal resection of the rectum. (2) Intra-abdominal abscess was verified by either surgical drainage or by ultrasonographically guided aspiration of pus. (3) Anastomotic leakage was defined as radiologically verified fistula to bowel anastomosis or diagnosed by relaparatomy. (4) Pneumonia was defined by fever above 38.5°C and a positive X-ray, and requirement of antibiotic treatment. (5) Septicaemia was defined by clinical symptoms combined with a positive blood culture. Nonsymptomatic or minor urinary tract infection was not recorded, and therefore only included if complicated by septicaemia. Registration of these complications was carried out on a daily basis.
After discharge, the patients were examined in the outpatient clinic every 3 months for up to 5 years with registration of local recurrent disease or metastasis, and thereafter when required. Overall survival was surveyed through the National Patient Registry.
The patient cohort is from the RANX05 trial [24], in which the patients were randomised to treatment with ranitidine or placebo in order to compare the effect on survival and the rate of postoperative infectious complications in patients electively resected for CRC. In addition, this study included the analysis of the effect of prognostic factors on treatment and postoperative complications.
Statistical analysis
The SAS software package (version 8.2; SAS Institute, Cary, NC, USA) was used for data management and statistical calculations. Levels of MBL and MBL/MASP activity are presented by the median, first and third quartiles and the range. Hypothesis tests on location were done using the Wilcoxon rank sum test. The associations between the variables were estimated by the Spearman rank correlation. Estimates of overall survival were done using the Kaplan–Meier method. The Cox proportional hazards model was used for multivariate analysis of curatively resected patients. The covariates included in the model are disease stage, localisation of the tumour, age, gender, infectious complications, perioperative blood transfusion as well as the markers included in this study. All p values less than 5% were considered significant.
Results
The group of patients included 240 women and 371 men with histologically verified primary CRC. The median age at the time of tumour resection was 68 years (range 33–90 years). The tumour was located in the colon in 345 patients, and in the rectum in 266 patients. None of the patients presented with bowel obstruction or perforation, as only patients undergoing elective surgery were included.
Of the patients, 10% were staged Dukes’ A, 38% Dukes’ B, 29% Dukes’ C and 23% Dukes’ D. The stage D patients included 11 with liver metastases that were radically resected during the primary operation and were therefore classified as curatively treated when performing separate statistical analyses for the group of curatively treated patients.
Overall, the MBL concentration and MBL/MASP activity were significantly higher (both p<0.0001) among patients compared with the concentrations in healthy blood donors (see Table 1). As shown in Table 1, the levels of MBL concentration and MBL/MASP activity were independent of gender, tumour location in colon or rectum, and stage of disease. Likewise, the levels were independent of age, with the Spearman correlation coefficient for MBL vs age: r=0.02 (p=0.59), and for MBL/MASP activity vs age: r=−0.12 (p=0.68).
Table 1.
MBL concentrations and MBL/MASP activity in the patients and healthy controls given by median levels, interquartile range () and total range [ ]
| MBL concentration (ng/ml) | p-Value | MBL/MASP activity (mU/ml) | p-Value | |
|---|---|---|---|---|
| Patients | ||||
| All patients (n=611) | 1208 (364–2,322) [0–11,103] | 424 (170–831) [0–3,046] | ||
| Gender | ||||
| M (n=371) | 1206 (364–2,372) [0–11,103] | 0.90 | 414 (184–861) [0–2,821] | 0.64 |
| F (n=240) | 1244 (361–2,282) [0–6,144] | 441 (157–797) [0–3,046] | ||
| Tumour localisation | ||||
| Colon (n=345) | 1,234 (366–2,412) [0–11,103] | 0.40 | 429 (179–837) [0–2,821] | 0.60 |
| Rectum (n=266) | 1,207 (358–2,230) [0–7,130] | 412 (165–819) [0–3,046] | ||
| Dukes’ stage | ||||
| A (n=63) | 1,030 (170–2,064) [0–3,588] | 0.18 | 354 (81–657) [0–1,305] | 0.15 |
| B (n=230) | 1,351 (382–2,286) [0–11,103] | 436 (189–894) [0–2,743] | ||
| C (n=180) | 1,164 (394–2,304) [0–10,074] | 437 (184–807) [0–3,046] | ||
| D (n=138) | 1,220 (358–2,552) [0–5,418] | 395 (166–845) [0–2,432] | ||
| Donors | ||||
| All donors (n=150) | 924 (230–1,476) [0–4,474] | 319 (0–684) [0–1,934] | ||
| Gender | ||||
| M (n=89) | 1,028 (188–1,420) [0–3,994] | 0.37 | 412 (0–748) [0–1,498] | 0.19 |
| F (n=61) | 702 (288–1,410) [0–4,474] | 287 (134–452) [0–1,934] | ||
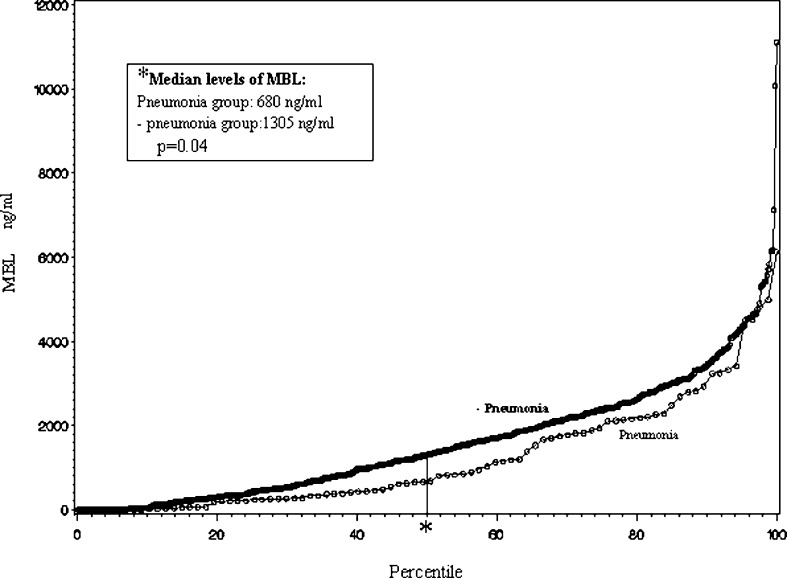
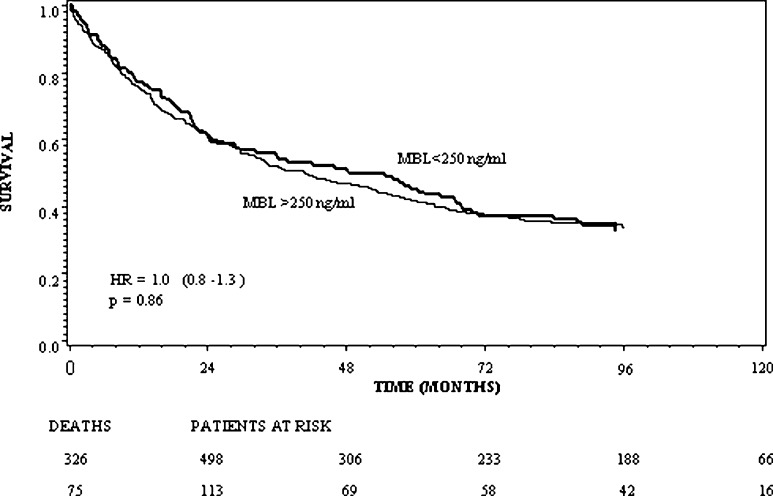
In the patient group, a significant association between pneumonia (n=87), and the MBL concentration (p=0.01) and MBL/MASP activity (p=0.004), respectively, was found, with low levels of MBL and low MBL/MASP activity associated with increased risk of pneumonia. The association between the MBL concentration and the frequency of pneumonia is illustrated in Fig. 1. Patients developing postoperative pneumonia had an increased rate of local recurrence (p<0.01; HR=1.8; 95% CI: 1.2 to 2.9) as well as decreased overall survival (p=0.003; HR=1.5; 95% CI: 1.1 to 1.9). However, the preoperative levels of serum MBL or MBL/MASP activity were not significantly associated with overall postoperative infectious complications (p=0.28 and p=0.19, respectively). Table 2 presents the frequency of the various infectious complications. For all infections other than pneumonia, the association with MBL levels was not significant. Neither the MBL level nor the MBL/MASP complex activity was related to the prognosis with respect to survival (p=0.87), as illustrated for MBL by the Kaplan–Meier plot in Fig. 2.
Fig. 1.
MBL concentration vs percentile for patients developing pneumonia (n=87) and those not developing pneumonia (n=524). *Median levels for each group are also shown (p=0.04).
Table 2.
Frequencies of all infections and association (p values) to MBL deficiency defined as MBL concentration <250 ng/ml serum; 118 patients are registered with one infection, 16 with two, 6 with three, and 4 with four
| Infection | Frequency | p Value |
|---|---|---|
| Perineal (rectal amputation only) | 19/95 | 0.36 |
| Intra-abdominal abscess | 21/611 | 0.95 |
| Anastomotic leakage | 37/611 | 0.71 |
| Septicaemia | 20/611 | 0.68 |
| Wound | 34/611 | 0.34 |
| Pneumonia | 87/611 | 0.04 |
| Pneumoniaa | 87/611 | 0.03 |
a Adjusted for Dukes’ stage and localisation
Fig. 2.
Overall survival in CRC patients stratified by the preoperative MBL concentration dichotomised at 250 ng/ml. The numbers of patients at risk at the time of surgery, and 24, 48, 72 and 96 months after, are shown below the X-axis.
Dichotomising at an MBL concentration of 250 ng/ml, the frequency of individuals with MBL concentration lower than 250 ng/ml in the patient and control group, respectively, differed, with 113 of 611 (18%) in the patient group and 40 of 110 (36%) in the group of blood donors (p=0.03, Fisher exact test). There was no sign of association between age and MBL concentration (r=0.02; p=0.70) or between age and MBL/MASP activity (r=−0.02; p=0.67).
The frequency of pneumonia was significantly (p=0.04) higher among the patients with MBL concentration <250 ng/ml (23/113=20%) than in patients with higher MBL levels (64/498=13%), and remained significant when adjusted for Dukes’ stage, localisation, age and gender. For all other infections this association was not detected. MBL deficiency was not statistically significant for survival in curatively treated patients (Dukes’ A, B and C, n=461) using a multivariate Cox model including disease stage, localisation of the tumour, gender, age at operation as well as postoperative complications (Table 3). Including treatment, ranitidine vs placebo stratified by postoperative complications [24] did not demonstrate a significant effect of MBL deficiency (p=0.97). Including CRP (dichotomised at 10 mg/l) in the multivariate model shows that elevated levels of CRP correlate significantly with respect to poor survival (p=0.001; HR=1.5; 95% CI: 1.2 to 2.0; n=457).
Table 3.
Results of multivariate analysis estimating overall survival using the Cox proportional hazards model. N=461; 257 deaths. Curatively treated patients including 11 Dukes’ D
| Covariate | p Value | HR | 95% CI |
|---|---|---|---|
| Dukes’ B vs Dukes’ A | 0.002 | 2.2 | 1.3–3.7 |
| Dukes’ C + D vs Dukes’ A | <0.0001 | 4.9 | 3.0–8.2 |
| Rectum vs colon | <0.0001 | 1.7 | 1.3–2.2 |
| Infection (other than pneumonia) | 0.31 | 1.2 | 0.8–1.8 |
| Pneumonia | 0.007 | 1.6 | 1.1–2.2 |
| Transfusion vs none | 0.52 | 1.1 | 0.8–1.4 |
| Gender (male vs female) | 0.02 | 1.4 | 1.0–1.8 |
| Age in years | <0.0001 | 1.4 | 1.3–1.6a |
| MBL deficiency (<250 ng/ml) | 0.94 | 1.0 | 0.7–1.4 |
aIn 10-year increment
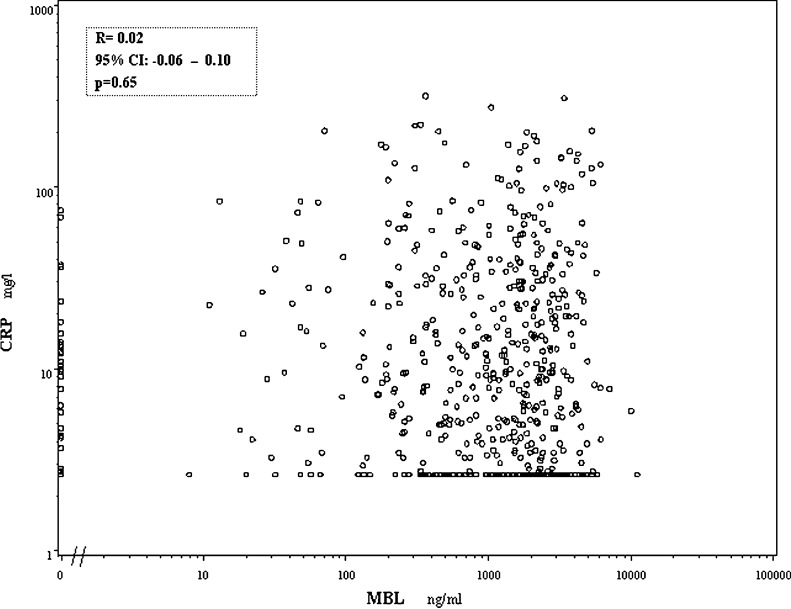
A correlation analysis between preoperative MBL, MBL/MASP activity and CRP (n=603) showed that none of the parameters of the MBL pathway correlated with CRP (Spearman’s ρ=0.02; 95% CI: −0.06 to 0.10; p=0.65; and ρ=0.06; 95% CI: −0.02 to 0.14; p=0.15). This is illustrated in a scatter plot showing the CRP levels vs the MBL concentration in Fig. 3.
Fig. 3.
Scatter plot of the preoperative levels of CRP and MBL. The Spearman rank correlation coefficient between CRP and MBL is shown. The lower detection limit of the CRP assay was 2.6 mg/l.
Discussion
Low serum levels of MBL are known to imply an increased susceptibility of infections [11, 13, 23, 30, 34]. The aim of the present study was to evaluate the risk of postoperative infectious complications in relation to the function of the MBL pathway in patients resected for CRC. Overall, the levels of MBL and MBL pathway activity were increased in the patient group compared with the healthy controls. This is in accordance with the findings of a recent study [41] and could point to MBL playing a role as marker of inflammatory reactions related to cancer. The results suggest that preoperative low levels of MBL are predictive of the development of postoperative pneumonia in patients with CRC, and furthermore that the patients developing pneumonia have increased recurrence rates and poorer survival. For the other postoperative infectious complications, these associations were not found.
The assay applied to determine MBL/MASP activity in the present study detects complement activation only for MBL concentrations above 250 ng/ml, suggesting deficiency of the MBL pathway for lower concentrations. We therefore considered this value a relevant threshold in relation to the risk of infections. Dichotomising at the level of 250 ng/ml revealed a significant association between the low MBL concentrations and an increased risk of pneumonia, with pneumonia being the only significant endpoint. For other infections we could not demonstrate this association with MBL levels, which is in contrast to the general finding of increased susceptibility to infections at MBL deficiency, and to the findings of a recent study [33]. In that study, the MBL concentration in a cohort (n=156) of patients undergoing elective gastrointestinal surgery for malignant disease was determined. The study suggested an association between low preoperative levels of MBL and the occurrence of postoperative infections. The patient group in that study, though, is less homogenous than the cohort in the present study due to the inclusion of patients with various abdominal malignancies. Furthermore, in both studies the number of patients developing postoperative infectious complications other than pneumonia was quite small, thus the conflicting results may be due to lack of statistical power. At present, no precise level for MBL deficiency is established. In the literature, MBL deficiency has thus been defined according to the detection limits of the assays used [1, 11] or to homozygozity and heterozygozity for mutations on the MBL gene, as these mutations are responsible for insufficient MBL levels in serum [4, 5, 15, 34]. Correlation studies based solely on gene analysis should be viewed with caution, since individuals with identical MBL genotypes may vary somewhat in MBL concentrations. Our use of an MBL concentration of 250 ng/ml as a threshold value defining MBL deficiency is also arbitrary, as it was selected on the basis of the sensitivity of our functional assay carried out in diluted serum. Only two studies attempt to define a threshold based on risk of infection in defined patient groups and note 0.5 μg MBL/ml and 1 μg/ml, respectively. The threshold value may well differ for different indications. There was no difference in the frequency of MBL deficiency between the patients and the healthy controls in the present study.
To investigate the role of MBL in the inflammatory response, we tested for the correlation between CRP and MBL concentration and activity.
C-reactive protein correlates to survival in colorectal cancer, with preoperatively increased levels of CRP being associated with poor prognosis [18, 25, 39]. As the increased CRP levels may be indicative of impaired immunity [26], a concomitant rise in MBL and CRP could support the hypothesis of a role of MBL in the inflammatory response, with additional prognostic information.
None of the parameters of the MBL pathway correlated with CRP though (Spearman’s ρ=0.02; 95% CI: −0.06 to 0.10). Considering the low correlation coefficients, and the narrow confidence intervals including zero, and given the high number of patients, this strongly suggests that there is no association between these parameters. Thus MBL is not a surrogate for CRP. Yet, the levels of CRP and of MBL are increased as well, which may reflect different states of chronic phase reactions. CRP is a well-established marker for acute phase reactions with a rapid increase of the level up to 100-fold or more in relation to trauma [31] and inflammation [10] and a subsequent drop after trauma [31] and at declining inflammation. This is apparently not the case in cancer where a prolonged increased level may be observed [18]. MBL, in contrast, during an acute phase reaction increases sluggishly up to twofold or threefold and then decreases again over 1–2 weeks [16, 37].
Testing for possible confounders, no association between age and the MBL concentration or between gender and MBL concentration was found. This is in accordance with the findings of a large population-based study [36], in which no dependence of MBL concentrations on gender, and only small variations of the MBL concentration in adult life were detected.
As described, the patient group is part of the RANX05 study in which randomisation to receive ranitidine or placebo was performed. No interaction between the treatment and MBL deficiency (i.e. MBL concentration <250 ng/ml) was detected, suggesting that the apparent effect of ranitidine on survival when stratifying by transfusion and infection as described previously [24] is not an effect involving the MBL pathway.
In conclusion, an insufficient function of the MBL pathway seems to be of significance for the development of postoperative pneumonia in patients with CRC, and the development of postoperative pneumonia is associated with increased recurrence rates and poorer long-term survival. Applying an MBL concentration of 250 ng/ml as threshold value defining MBL deficiency, low MBL level was the only significant predictor of postoperative pneumonia. There is no sign of association between the levels of MBL and CRP, suggesting that MBL is not part of the acute phase response in CRC patients.
Acknowledgements
The authors thank Birgitte Sander Nielsen, Anette Hansen and Lisbeth Jensen for inspiration and skillful work during handling and analysis procedures. Furthermore, we wish to thank the Department of Clinical Biochemistry for performing the CRP analyses. The study received financial support from The Kornerup Fund, The Hede-Nielsen Family Fund, The Walter and O. Kristiane Christensen Fund, The Sven and Ina Hansen Fund, The Beckett Fund, The Aage and Johanne Louis-Hansen Fund, The Ingeborg Roikjer Fund, The Terese Maria Hansen Fund, The Sophus and Astrid Jacobsen Fund, The Karen A. Tolstrup Fund, The Ragnhild Ibsen Fund, The Lily Benthine Lund Fund, The Danish Pharmacy Foundation of 1991, The Danish Medical Research Fund, and The Danish Cancer Society (grant #DP 02012).
References
- 1.Aittoniemi J, Rintala E, Miettinen A, et al. Serum mannan-binding lectin (MBL) in patients with infection: clinical and laboratory correlates. APMIS. 1997;105:617–622. doi: 10.1111/j.1699-0463.1997.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 2.Faist E. The mechanisms of host defense dysfunction following shock and trauma. Curr Top Microbiol Immunol. 1996;216:259–274. doi: 10.1007/978-3-642-80186-0_12. [DOI] [PubMed] [Google Scholar]
- 3.Fujita S, Teramoto T, Watanabe M, et al. Anastomotic leakage after colorectal cancer surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol. 1993;23:299–302. [PubMed] [Google Scholar]
- 4.Garred P, Thiel S, Madsen HO, et al. Gene frequency and partial protein characterization of an allelic variant of mannan binding protein associated with low serum concentrations. Clin Exp Immunol. 1992;90:517–521. doi: 10.1111/j.1365-2249.1992.tb05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garred P, Strom J, Quist L, et al. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis. 2003;188:1394–1403. doi: 10.1086/379044. [DOI] [PubMed] [Google Scholar]
- 6.Grandis JR, Snyderman CH, Johnson JT, et al. Postoperative wound infection. A poor prognostic sign for patients with head and neck cancer. Cancer. 1992;70:2166–2170. doi: 10.1002/1097-0142(19921015)70:8<2166::aid-cncr2820700826>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Herroeder S, Durieux ME, Hollmann MW. Inflammatory responses after surgery. Hosp Med. 2002;63:99–103. doi: 10.12968/hosp.2002.63.2.2088. [DOI] [PubMed] [Google Scholar]
- 8.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 9.Jackson RM, Rice DH. Wound infections and recurrence in head and neck cancer. Otolaryngol Head Neck Surg. 1990;102:331–333. doi: 10.1177/019459989010200405. [DOI] [PubMed] [Google Scholar]
- 10.Johnson HL, Chiou CC, Cho CT. Applications of acute phase reactants in infectious diseases. J Microbiol Immunol Infect. 1999;32:73–82. [PubMed] [Google Scholar]
- 11.Kakkanaiah VN, Shen GQ, Ojo-Amaize EA, et al. Association of low concentrations of serum mannose-binding protein with recurrent infections in adults. Clin Diagn Lab Immunol. 1998;5:319–321. doi: 10.1128/cdli.5.3.319-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehlet H, Nielsen HJ. Impact of laparoscopic surgery on stress responses, immunofunction, and risk of infectious complications. New Horiz. 1998;6:S80–S88. [PubMed] [Google Scholar]
- 13.Kilpatrick DC. Mannan-binding lectin and its role in innate immunity. Transfus Med. 2002;12:335–352. doi: 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 14.Kressner U, Graf W, Mahteme H, et al. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316–321. doi: 10.1007/s10350-004-6174-4. [DOI] [PubMed] [Google Scholar]
- 15.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Jensen L, Hansen S, et al. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–497. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 17.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/S1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 18.McMillan DC, Wotherspoon HA, Fearon KC, et al. A prospective study of tumor recurrence and the acute-phase response after apparently curative colorectal cancer surgery. Am J Surg. 1995;170:319–322. doi: 10.1016/S0002-9610(99)80296-7. [DOI] [PubMed] [Google Scholar]
- 19.McMillan DC, Elahi MM, Sattar N, et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64–69. doi: 10.1207/S15327914NC41-1&2_8. [DOI] [PubMed] [Google Scholar]
- 20.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 21.McMillan DC, Canna K, McArdle CS. The effect of deprivation and the systemic inflammatory response on outcome following curative resection for colorectal cancer. Br J Cancer. 2003;89:612–614. doi: 10.1038/sj.bjc.6601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mynster T, Christensen IJ, Moesgaard F, et al. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX. 2000;05(Colorectal Cancer Study Group. Br J Surg 87):1553–1562. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 23.Neth O, Hann I, Turner MW, et al. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–618. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen HJ, Christensen IJ, Moesgaard F, et al. Ranitidine as adjuvant treatment in colorectal cancer. Br J Surg. 2002;89:1416–1422. doi: 10.1046/j.1365-2168.2002.02223.x. [DOI] [PubMed] [Google Scholar]
- 25.Nozoe T, Matsumata T, Kitamura M, et al. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–338. doi: 10.1016/S0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 26.Nozoe T, Matsumata T, Sugimachi K. Preoperative elevation of serum C-reactive protein is related to impaired immunity in patients with colorectal cancer. Am J Clin Oncol. 2000;23:263–266. doi: 10.1097/00000421-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Petersen S, Freitag M, Hellmich G, et al. Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. Int J Colorectal Dis. 1998;13:160–163. doi: 10.1007/s003840050158. [DOI] [PubMed] [Google Scholar]
- 28.Petersen SV, Thiel S, Jensen L, et al. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/S0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 29.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–149. doi: 10.1016/S0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 30.Peterslund NA, Koch C, Jensenius JC, et al. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–638. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen LA, Nielsen HJ, Sorensen S, et al. Ranitidine reduces postoperative interleukin-6 induced C-reactive protein synthesis. J Am Coll Surg. 1995;181:138–144. [PubMed] [Google Scholar]
- 32.Rodrigo JP, Suarez C. Prognostic significance of postoperative wound infection on head and neck cancer. Otolaryngol Head Neck Surg. 1998;118:272–275. doi: 10.1016/S0194-5998(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 33.Siassi M, Hohenberger W, Riese J. Mannan-binding lectin (MBL) serum levels and post-operative infections. Biochem Soc Trans. 2003;31:774–775. doi: 10.1042/BST0310774. [DOI] [PubMed] [Google Scholar]
- 34.Summerfield JA, Sumiya M, Levin M, et al. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Br Med J. 1997;314:1229–1232. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swisher SG, Holmes EC, Hunt KK, et al. Perioperative blood transfusions and decreased long-term survival in esophageal cancer. J Thorac Cardiovasc Surg. 1996;112:341–348. doi: 10.1016/S0022-5223(96)70260-X. [DOI] [PubMed] [Google Scholar]
- 36.Terai I, Kobayashi K, Fujita T, et al. Human serum mannose binding protein (MBP): development of an enzyme-linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem Med Metab Biol. 1993;50:111–119. doi: 10.1006/bmmb.1993.1052. [DOI] [PubMed] [Google Scholar]
- 37.Thiel S, Holmskov U, Hviid L, et al. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–35. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiel S, Moller-Kristensen M, Jensen L, et al. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology. 2002;205:446–454. doi: 10.1078/0171-2985-00145. [DOI] [PubMed] [Google Scholar]
- 39.Wigmore SJ, McMahon AJ, Sturgeon CM, et al. Acute-phase protein response, survival and tumour recurrence in patients with colorectal cancer. Br J Surg. 2001;88:255–260. doi: 10.1046/j.1365-2168.2001.01669.x. [DOI] [PubMed] [Google Scholar]
- 40.Windsor AC, Klava A, Somers SS, et al. Manipulation of local and systemic host defence in the prevention of perioperative sepsis. Br J Surg. 1995;82:1460–1467. doi: 10.1002/bjs.1800821106. [DOI] [PubMed] [Google Scholar]
- 41.Ytting Scand J Gastroenterol. 2004;39:674. doi: 10.1080/00365520410005603. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann-Nielsen E, Baatrup G, Thorlacius-Ussing O, et al. Complement activation mediated by mannan-binding lectin in plasma from healthy individuals and from patients with SLE, Crohn’s disease and colorectal cancer. Suppressed activation by SLE plasma. Scand J Immunol. 2002;55:105–110. doi: 10.1046/j.1365-3083.2002.01035.x. [DOI] [PubMed] [Google Scholar]