Abstract
BACKGROUND:
Acute cerebral infarction (ACI) is one of the most common ischemic cerebrovascular diseases in neurology, with high morbidity, mortality, and disability. Early thrombolytic treatment of ACI has significant efficacy, but intraprocedural complications of hypoxemia can significantly reduce the efficacy. This study aims to analyze the risk factors for intraprocedural hypoxemia in patients with ACI, so as to take effective measures in advance to reduce the likelihood of adverse patient outcomes.
METHODS:
We retrospectively analyzed a total of 238 patients with ACI treated with vascular interventions from May 2017 to May 2022. To assess and collate the patients’ characteristics, factors associated with the development of intraprocedural hypoxemia. The independent risk factors for the development of intraprocedural hypoxemia were analyzed by binary logistic regression.
RESULTS:
A total of 238 patients were included in this study. Of these, intraprocedural hypoxemia occurred in 89 (37.4%). The results showed that old age (odds ratio [OR] = 2.666, P = 0.009), obesity (OR = 3.029, P = 0.003), smoking history (OR = 2.655, P = 0.010), preoperative oxygen saturation (SpO2) (OR = 0.001, P = 0.042), preoperative C-reactive protein (OR = 1.216, P = 0.002), and time from puncture to vascular recanalization (OR = 1.135, P = 0.000) were independent risk factors for intraprocedural hypoxemia in patients. The prognosis of the patients was assessed according to the modified Rankin scale, and the prognosis of the nonhypoxemia group was significantly better than that of the hypoxemia group. Regression analysis showed that intraprocedural hypoxemia (OR = 0.360, P = 0.001), postoperative lower extremity vein thrombosis (OR = 0.187, P = 0.018), hydrocephalus (OR = 0.069, P = 0.015), intracranial hemorrhage (OR = 0.116, P = 0.002), and reocclusion (OR = 0.217, P = 0.036) were independent risk factors for poor prognosis.
CONCLUSIONS:
Currently, intravascular hypoxemia in patients with ACI has a serious impact on prognosis. Clinical work should attach great importance to the clinical characteristics of patients, identify relevant risk factors, and aggressively take personalized therapeutic actions to improve patients’ prognosis.
Keywords: Acute cerebral infarction, hypoxemia, retrospective study, vascular intervention
Introduction
Acute cerebral infarction (ACI) is a disease in which there is a sudden interruption of blood supply to the patient’s brain resulting in localized brain tissue necrosis.[1] It is one of the most common ischemic cerebrovascular diseases in neurology, with an incidence of approximately 70% in all stroke diseases.[2,3,4] It was estimated that the poststroke accumulated all-cause mortality was 10.5% within 30 days and 21.2% within 1 year.[5]
Cerebral infarction is a complex disease caused by multiple etiologies which is often clinically classified into cerebral atherosclerotic type, cardiogenic embolism type, small artery occlusion type, other definite cause type, and unknown cause type. The infarct sites can be classified as anterior circulation and posterior circulation.[6] According to the Oxfordshire Community Stroke Study Project, total anterior circulation infarction is characterized by a triad of signs: higher brain dysfunction, unilateral dyspraxia, and homonymous hemianopia; partial anterior circulation infarction presents with two of this triad, only higher neurological activity dysfunction, or a more limited range of sensory or motor deficits than lacunar infarction (LACI); LACI mainly presents as lacunar syndrome; posterior circulation infarction mainly presents as ipsilateral cranial nerve paresis and contralateral sensorimotor deficits, as well as bilateral sensorimotor deficits.[7]
Chinese and international studies have confirmed the significant efficacy of early thrombolysis in the treatment of ACI, and the earlier the treatment, the better the recovery of neurological function.[8] Among them, vascular intervention is an important method, which refers to the delivery of micro-catheters into the diseased vessel under the precise positioning of fluoroscopic monitoring, and direct drug delivery to the penumbra region in the ischemic zone to increase the local drug concentration so as to improve the thrombolytic effect. Interventional techniques result in that blood flow to the infarct is rapidly restored and brain tissue is reperfused, thus repairing the neurological function damage caused by local ischemia and improving clinical prognosis.[9,10]
Referring to the criteria of the Berlin definition of acute respiratory distress syndrome in 2010,[11] patients with a minimum oxygenation index (partial pressure of oxygen/fraction of inspired oxygen [PaO2/FiO2]) <200 mmHg in 48 h were defined as hypoxemia. Hypoxemia mainly manifests as a decrease in PaO2 and oxygen saturation (SpO2), which can directly affect the patient’s regression and prognosis, and even endanger the patient’s life safety.[12] Untimely management of hypoxemia may lead to severe respiratory failure syndrome as well as a high morbidity and mortality rate. One study[13] showed that the probability of intraprocedural hypoxemia was about 6.8%, with severe hypoxemic events of 2 consecutive minutes or longer occurring in about 3.5% of patients. 70% of hypoxemia occurred during the induction or awakening period, which accounted for 21% of overall surgical time. This study also found that intraprocedural hypoxemia had a significant impact on clinical outcomes. Although hypoxemia during vascular interventions in patients with ACI may have serious adverse consequences, there are few reports of risk factors of hypoxemia occurring during vascular interventions in such patients. This study aimed to analyze the risk factors for intraprocedural hypoxemia in patients with ACI undergoing vascular intervention, so as to take effective measures in advance to reduce the likelihood of adverse patient outcomes.
Methods
Research participants
A total of 250 patients with ACI experiencing intraprocedural hypoxemia who underwent intervention in our hospital from May 2017 to May 2022 were included in this study.
The inclusion criteria were as follows: (i) a diagnosis of ACI and confirmed by symptoms, signs, and imaging (magnetic resonance imaging [MRI] and computed tomography [CT]); (ii) meeting the indications for arterial thrombolysis, i.e., age 18–80 years old, onset to admission time of 6 h for anterior circulation patients, onset to admission time of ≥24 h for posterior circulation patients, NIHSS (National Institutes of Health stroke scale, NIHSS) score of 4–24, good control of blood glucose and blood pressure; (iii) time from onset to arterial puncture <6 h; and (iv) NIHSS ≥4 and the Glasgow Coma Scale ≤12.
The exclusion criteria were as follows: (i) contraindications to vascular intervention; (ii) intracranial hemorrhage on imaging; (iii) history of intracranial hemorrhage or history of head trauma or cerebral infarction within 3 months before admission; (iv) other intracranial lesions such as cerebrovascular malformations and aneurysms; (v) cardiopulmonary insufficiency; and (vi) time from onset to arterial puncture >6 h [Figure 1].
Figure 1.
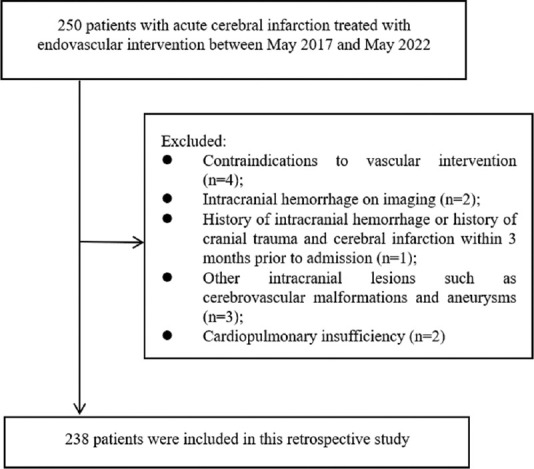
Flow chart of patient selection
In this study, all patients with ACI who received thrombolytic therapy in the interventional catheterization department of our hospital within 5 years were included, combining the results of previous studies by our group. According to the previous studies by our group, the probability of intraprocedural hypoxemia after ACI is 35%. A tolerance error of 3% and a confidence level of 1-α = 0.95 were specified, and we found that the sample size of cases to be investigated was 167 participants, as determined by the PASS 15 software. Assuming a nonresponse rate of 10% for the study population, the required sample size was found to be N = 167 × 1.1 = 184 cases. Assuming a 90% pass rate for the questionnaire, the total sample size required was n = 184 ÷ 0.9 = 205 cases. A total of 250 cases were proposed to be included after excluding patients who did not meet the study criteria. 12 patients were lost because of missed visits, and 238 patients were eventually admitted to this study.
The study was approved by the Ethics Committee of our hospital (No. LC-2022-019) and informed consent was taken from all the patients (or individual consent for this retrospective analysis was waived).
Clinical trial registry
This work is a retrospective analytical study. No clinical trials were involved.
General information questionnaire
The general information questionnaire included demographic data (e.g., gender, age, body mass index [BMI]) and clinical data (history of hypertension, history of hyperlipidemia, previous respiratory history, history of smoking, preoperative arterial SpO2, preoperative hemoglobin [Hb] concentration, preoperative prothrombin time, preoperative C-reactive protein [CRP] level, time from femoral artery puncture to revascularization, mode of intervention, site of cerebral infarction, presence of intraprocedural hypoxemia, and postoperative complications).
Patients grouping and surgical method
Diagnosis of acute cerebral infarction: (1) acute onset; (2) focal neurological deficit, and in a few cases, global neurological deficit; (3) symptoms or signs of unlimited duration, or for more than 24 h; (4) exclude nonvascular causes; (5) brain CT/MRI to exclude cerebral hemorrhage. The selection of patients for thrombolysis should refer to the indications and contraindications in addition to the above requirements. Hypoxemia, a decrease in the oxygen content of the blood, is mainly manifested as a decrease in PaO2 and SpO2. It is generally considered that hypoxemia occurs when the SpO2 is <92%,[14] and it is considered severe hypoxemia if SpO2 is <85%. Based on the above definitions, we defined hypoxemia as occurring if SpO2 < 92% was present during surgery and maintained for 1 min, and the patients were divided into hypoxemic and nonhypoxemic groups.
All patients were treated with arterial interventional thrombolytic therapy. 6F arterial sheath was replaced with guide wire and placed into a guide tube. The microcatheter was passed through the thromboembolism by coaxial catheter technique, and 1 mg of alteplase was taken from the distal end for injection, then the microcatheter was returned to the thrombus and 18 mg of alteplase was taken for continuous injection, and finally the microcatheter was pulled back to the proximal end for injection of 1 mg of alteplase, and then arterial thrombolysis was stopped. Monitoring nursing anesthesia was used during the operation. Imaging examination was performed after vascular recanalization, and angioplastic stent implantation was performed if the degree of vascular stenosis was more than 70%. 24 h after thrombolysis, aspirin was given orally at 100 mg/d once a day for 2 weeks.
Modified Rankin scale score
The modified Rankin scale (mRS) score is a scale used to evaluate the neurological recovery status of cerebral infarction patients. The mRS score includes 7 levels: 0 score is asymptomatic; 1 score is symptomatic, but does not affect daily life; 2 score is mildly disabled, but can perform daily tasks on his or her own; 3 score is moderately disabled, requiring assistance, but can walk independently; 4 score is moderately severely disabled, unable to walk independently, requiring assistance in daily life; 5 score is severely disabled, bedridden, incontinent, and completely dependent on others; 6 score is death. According to the mRS score, patients can be classified into a poor prognosis group (mRS ≥4 score) and a good prognosis group (mRS <4 score).[15]
Patients were followed up lasted for 3 months after discharge from the hospital; either as an outpatient or by telephone, and their prognosis at the last follow-up was assessed using the mRS score. The last follow-up visit was in August 2022.
Statistical analysis
The results of each scale were entered into a computer for score conversion and statistical analysis was performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA), with measured data expressed as mean and standard deviation and counted data expressed as frequencies and percentages. Statistical analysis between groups was performed using t-test and Chi-square test. Variables with differences after t-test or Chi-square test were included in binary logistics regression analysis was used to analyze the factors influencing the presence of intraprocedural hypoxemia and prognosis. A two-sided P < 0.05 was considered statistically significant.
SPSS 26.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis of the data, with measured data presented as mean ± standard deviation and counted data presented as frequencies and percentages. The t-test and Chi-square test were used for the analysis of between-group variability. Variables that differed by t-test or Chi-square test were included in a binary logistic regression analysis to analyze the presence of intraprocedural hypoxemia and factors influencing prognosis. A two-sided P < 0.05 was considered statistically significant.
Results
Baseline data
A total of 238 patients were included in this study. Among the patients with intraprocedural hypoxemia, 53 cases (59.6%) were elderly patients over 70 years of age, a total of 50 cases (56.2%) had a BMI ≥24 kg/m2, 41 cases (46.1%) had a history of smoking, the mean preoperative SpO2 was 84.98%±8.50%, the mean preoperative Hb was 115.74 ± 24.13 g/L, the mean preoperative CRP was 14.83 ± 3.06 mg/L, and the mean time from femoral artery puncture to revascularization was 129.60 ± 13.04 min. In contrast, in the nonhypoxemia group, a total of 55 cases (36.9%) were older than 70 years, a total of 56 cases (37.6%) had a BMI ≥24 kg/m2, 46 cases (30.9%) with a history of smoking, the mean preoperative SpO2 was 89.38%±7.47%, the mean preoperative Hb was 122.01 ± 19.59 g/L, the mean preoperative CRP was 13.60 ± 2.74 mg/L, and the mean time from femoral artery puncture to revascularization was 114.00 ± 10.35 min. In the nonhypoxemic group, there were significantly less patients who were aged over 70 years, had a BMI ≥24 kg/m2, and a history of smoking than there were in the hypoxemic group (P < 0.05); whereas the mean preoperative SpO2 and mean preoperative Hb were significantly higher than those in the hypoxemic group (P < 0.05) [Table 1].
Table 1.
Baseline data of included patients
| Projects | Nonhypoxemia group | Hypoxemia group | χ2/t | P |
|---|---|---|---|---|
| Age (years), n (%) | ||||
| <70 | 94 (63.1) | 36 (40.4) | 11.520 | 0.001 |
| ≥70 | 55 (36.9) | 53 (59.6) | ||
| Gender, n (%) | ||||
| Male | 79 (53.0) | 45 (50.6) | 0.135 | 0.713 |
| Female | 70 (47.0) | 44 (49.4) | ||
| BMI (kg/m2), n (%) | ||||
| <24 | 93 (62.4) | 39 (43.8) | 7.800 | 0.005 |
| ≥24 | 56 (37.6) | 50 (56.2) | ||
| Hypertension or not, n (%) | ||||
| Yes | 68 (45.6) | 40 (44.9) | 0.011 | 0.917 |
| No | 81 (54.4) | 49 (55.1) | ||
| Hyperlipidemia or not, n (%) | ||||
| Yes | 43 (28.9) | 31 (34.8) | 0.928 | 0.335 |
| No | 106 (71.1) | 58 (65.2) | ||
| Previous respiratory disease history, n (%) | ||||
| Yes | 47 (31.5) | 29 (32.6) | 0.028 | 0.868 |
| No | 102 (68.5) | 60 (67.4) | ||
| Smoking history, n (%) | ||||
| Yes | 46 (30.9) | 41 (46.1) | 5.547 | 0.019 |
| No | 103 (69.1) | 48 (53.9) | ||
| Preoperative SpO2 (%), mean±SD | 89.38±7.47 | 84.98±8.50 | 4.174 | 0.000 |
| Preoperative Hb (g/L), mean±SD | 122.01±19.59 | 115.74±24.13 | 2.186 | 0.030 |
| Preoperative PT (s), mean±SD | 13.31±3.45 | 12.60±3.41 | 1.550 | 0.123 |
| Preoperative CRP (mg/L), mean±SD | 13.60±2.74 | 14.83±3.06 | −3.219 | 0.001 |
| Time from puncture to vascular recanalization (min), mean±SD | 114.00±10.35 | 129.60±13.04 | −9.616 | 0.000 |
| Interventional ways, n (%) | ||||
| Thrombolysis | 51 (34.2) | 25 (28.1) | 1.576 | 0.665 |
| Stent thrombectomy | 60 (40.3) | 43 (48.3) | ||
| Balloon dilatation and stent placement | 16 (10.7) | 9 (10.1) | ||
| Remedial balloon dilatation and stent placement after stent thrombolysis | 22 (14.8) | 12 (13.5) | ||
| Cerebral infarction site, n (%) | ||||
| Anterior cerebral artery | 48 (32.2) | 29 (32.6) | 3.351 | 0.341 |
| Middle cerebral artery | 51 (34.2) | 39 (43.8) | ||
| Vertebral artery | 24 (16.1) | 9 (10.1) | ||
| Basilar artery | 26 (17.4) | 12 (13.5) |
BMI: Body mass index, SpO2: Oxygen saturation, SD: Standard deviation, Hb: Hemoglobin, PT: Prothrombin time, CRP: C-reactive protein
Binary logistic regression analysis of intraprocedural hypoxemia
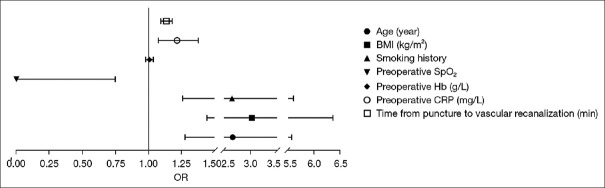
The results showed that age over 70 years, BMI ≥24 kg/m2, history of smoking, low preoperative SpO2, high preoperative CRP, and a prolonged duration from femoral artery puncture to revascularization were independent risk factors for intraprocedural hypoxemia in patients (P < 0.05) [Table 2 and Figure 2].
Table 2.
Risk factors of intraprocedural hypoxemia analyzed by binary logistic regression models
| Parameters | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Upper | Lower | ||||||
| Age (years) | 0.980 | 0.376 | 6.799 | 0.009 | 2.666 | 1.276 | 5.570 |
| BMI (kg/m2) | 1.108 | 0.379 | 8.568 | 0.003 | 3.029 | 1.442 | 6.363 |
| Smoking history | 0.976 | 0.381 | 6.560 | 0.010 | 2.655 | 1.258 | 5.605 |
| Preoperative SpO2 | −7.590 | 3.724 | 4.154 | 0.042 | 0.001 | 0.000 | 0.748 |
| Preoperative Hb (g/L) | 0.007 | 0.014 | 0.223 | 0.637 | 1.007 | 0.979 | 1.036 |
| Preoperative CRP (mg/L) | 0.195 | 0.063 | 9.557 | 0.002 | 1.216 | 1.074 | 1.376 |
| Time from puncture to vascular recanalization (min) | 0.127 | 0.019 | 43.202 | 0.000 | 1.135 | 1.093 | 1.179 |
SE: Standard error, OR: Odds ratio, CI: Confidence interval, BMI: Body mass index, SpO2: Oxygen saturation, Hb: Hemoglobin, CRP: C-reactive protein
Figure 2.
Binary logistic regression of intraprocedural hypoxemia. OR: Odds ratio, BMI: Body mass index, SpO2: Oxygen saturation, Hb: Hemoglobin, CRP: C-reactive protein
Comparison of postoperative complications after endovascular intervention between two groups
In the group with intraprocedural hypoxemia, a total of 8 cases (9.0%) had postoperative lower limb venous thrombosis, 7 cases (7.9%) had postoperative cerebral edema, and 9 cases (10.1%) had postoperative reocclusion. In contrast, in the nonhypoxemia group, a total of 4 cases (2.7%) had postoperative lower limb venous thrombosis, 3 cases (2.0%) had postoperative cerebral edema, and 4 cases (2.7%) had reocclusion after surgery, all of which were significantly lower than those in the hypoxemia group (P < 0.05) [Table 3].
Table 3.
Comparison of postoperative complications after endovascular intervention in two groups of patients with acute cerebral infarction
| Items | Lower extremity vein thrombosis | Hydrocephalus | Intracranial hemorrhage | Wound bleeding | Subcutaneous hematoma | Re-occlusion of blood vessels | Total |
|---|---|---|---|---|---|---|---|
| Hypoxemia group, n (%) | 8 (9.0) | 7 (7.9) | 7 (7.9) | 6 (6.7) | 5 (5.6) | 9 (10.1) | 42 (17.6) |
| Nonhypoxemia group, n (%) | 4 (2.7) | 3 (2.0) | 8 (5.4) | 4 (2.7) | 3 (2.0) | 4 (2.7) | 26 (10.9) |
| χ 2 | 4.625 | 4.740 | 0.588 | 2.278 | 2.229 | 5.953 | 24.150 |
| P | 0.032 | 0.029 | 0.443 | 0.131 | 0.135 | 0.015 | 0.000 |
Comparison of mRS after endovascular interventions between two groups
In the group with intraprocedural hypoxemia, the largest number of patients with a score of 4 was 36 (40.4%), followed by 21 patients (23.6%) with a score of 3, 38 patients (42.7%) with a score of <4, and 51 patients (57.3%) with a score of ≥4, which were evaluated according to the mRS. In contrast, in the nonhypoxemia group, the largest number of patients with a score of 3 was found, totaling 42 cases (28.2%), followed by a total of 41 (27.5%) with a score of 2 [Table 4 and Figure 3].
Table 4.
Modified Rankin Scale score after endovascular interventions in two groups of patients with acute cerebral infarction
| Items | 0 score | 1 score | 2 score | 3 score | 4 score | 5 score | 6 score | <4 score | ≥4 score |
|---|---|---|---|---|---|---|---|---|---|
| Hypoxemia group, n (%) | 0 | 1 (1.1) | 16 (18.0) | 21 (23.6) | 36 (40.4) | 7 (7.9) | 8 (9.0) | 38 (42.7) | 51 (57.3) |
| Nonhypoxemia group, n (%) | 8 (5.4) | 16 (10.7) | 41 (27.5) | 42 (28.2) | 22 (14.8) | 14 (9.4) | 6 (4.0) | 107 (71.8) | 42 (28.2) |
| Total | 8 | 17 | 57 | 63 | 58 | 21 | 14 | 145 | 93 |
Figure 3.
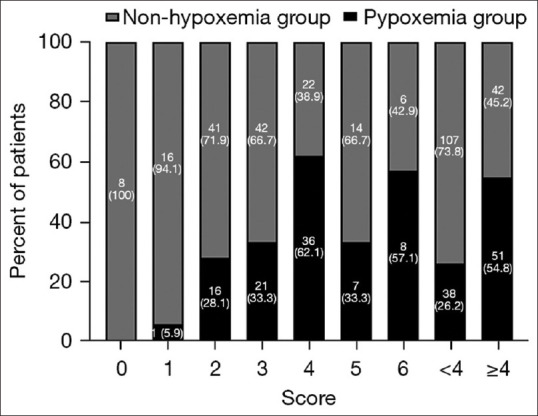
Modified Rankin scale score after endovascular interventions in two groups of patients with acute cerebral infarction. mRS: modified Rankin scale, ACI: Acute cerebral infarction
The Chi-square test showed that there was a significant difference between the hypoxemia and nonhypoxemia groups in terms of whether the prognosis was good (P < 0.05) [Table 5].
Table 5.
Comparison of prognosis after endovascular intervention in two groups of patients with acute cerebral infarction
| Item | mRS score <4 | mRS score ≥4 score |
|---|---|---|
| Hypoxemia group, n (%) | 51 (54.8) | 38 (26.2) |
| Nonhypoxemia group, n (%) | 42 (45.2) | 107 (73.8) |
| χ 2 | 19.840 | |
| P | 0.000 | |
mRS: Modified Rankin Scale
Binary logistic regression analysis of poor prognosis
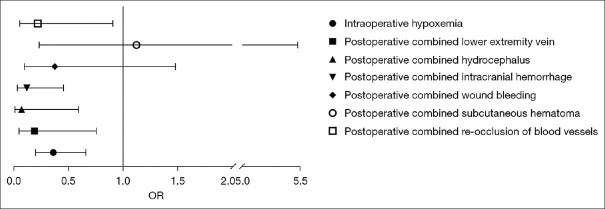
The results showed that intraprocedural hypoxemia, postoperative lower limb venous thrombosis, postoperative cerebral edema, postoperative intracranial hemorrhage, and postoperative reocclusion were independent risk factors for poor prognosis (P < 0.05) [Table 6 and Figure 4].
Table 6.
Binary logistic regression for poor prognosis of acute cerebral infarction
| Items | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Upper | Lower | ||||||
| Intraprocedural hypoxemia | −1.021 | 0.308 | 11.010 | 0.001 | 0.360 | 0.658 | 0.197 |
| Postoperative lower extremity vein thrombosis | −1.678 | 0.712 | 5.555 | 0.018 | 0.187 | 0.754 | 0.046 |
| Postoperative hydrocephalus | −2.677 | 1.097 | 5.955 | 0.015 | 0.069 | 0.590 | 0.008 |
| Postoperative intracranial hemorrhage | −2.150 | 0.692 | 9.651 | 0.002 | 0.116 | 0.452 | 0.030 |
| Postoperative wound bleeding | −0.984 | 0.702 | 1.963 | 0.161 | 0.374 | 1.480 | 0.094 |
| Postoperative subcutaneous hematoma | 0.116 | 0.808 | 0.021 | 0.886 | 1.123 | 5.476 | 0.230 |
| Postoperative re-occlusion of blood vessels | −1.529 | 0.729 | 4.392 | 0.036 | 0.217 | 0.906 | 0.052 |
SE: Standard error, OR: Odds ratio, CI: Confidence interval
Figure 4.
Binary logistic regression of poor prognosis. OR: Odds ratio
Discussion
ACI is considered the most common acute cerebrovascular disease in the world.[16] A study[17] has shown that ACI has become 1 of the 3 leading causes of human death. It is generally believed that following cerebral ischemia, there is activation of the immune system, and the massive production of chemokines and cytokines causes leukocytes to infiltration in ischemic tissues, microvascular damage, and blood–brain barrier damage, which ultimately aggravates brain injury.[18] The lack of effective and timely therapeutic measures at this time can lead to impaired cognitive function in patients. Mild cognitive function tends to cause vascular dementia, which not only impairs patients’ physical activity but also leads to a reduced quality of life.[19] Currently, intravenous thrombolysis and mechanical embolization are considered effective treatments for ACI.[20,21,22] Cerebral artery thrombectomy is a minimally invasive procedure in which the femoral artery is punctured with the aid of digital subtraction angiography technique, contrast is injected to form the neck and intracranial vessels, and guide wires and stents are delivered to the diseased vessel. Then, the embolus is removed from the body as much as possible.[23] It has been clinically found that a subset of patients are prone to intraprocedural hypoxemia and this subset of patients often have more severe clinical symptoms. However, whether there is a relationship between intraprocedural hypoxemia and patient prognosis has not been elucidated in detail in any study.
According to our study, the incidence of intraprocedural hypoxemia is as high as 37.4%. Two large academic medical centers studies suggest that hypoxemia still has a high incidence after surgery.[13]
This study showed that age, BMI, smoking history, preoperative arterial SpO2, preoperative Hb concentration, preoperative CRP level, and femoral artery puncture to revascularization time were significantly different between the hypoxemic and nonhypoxemic groups. Moreover, it also showed that age, BMI, smoking history, preoperative arterial SpO2, preoperative CRP level, and femoral artery puncture to revascularization time were independent risk factors for the development of intraprocedural hypoxemia. Khirfan et al.[24] reported that hypoxemia was more widely distributed in people with a high BMI. The reason for this may be that overweight patients with a BMI ≥24 kg/m2 usually have a thick chest wall, poor lung compliance, need to overcome more resistance to breathing, and consume more energy than normal people. During the operation, patients under general anesthesia have weaker cardiopulmonary function and can hardly withstand their previous load, so they are more likely to experience hypoxemia. In addition, the harmful substances in tobacco irritate the respiratory mucosa, causing increased secretions, weakened respiratory cilia movement, and even airway narrowing.[25] Long-term preoperative smoking affects the clearance function of the respiratory mucosa, resulting in the difficulty of discharging secretions in the airway, thus predisposing the airway to spasm and endocrine blockage, causing respiratory distress, which may also be the reason why a long-term smoking history predisposes to intraprocedural hypoxemia. Duan et al.[26] suggested that serum CRP levels are associated with the occurrence of hypoxemia. The systemic inflammatory response puts high CRP levels at increased risk with hypoxemia. A higher CRP count is associated with a stronger inflammatory response, which may lead to respiratory dysfunction and hypoxemia.[27]
In addition, this study also found a significant difference in the incidence of postoperative complications between the hypoxemic and nonhypoxemic groups. Logistic regression results showed that intraprocedural hypoxemia was an independent risk factor for poor prognosis. Hypoxemia is associated with cardiac arrest, arrhythmias, postoperative infections and impaired wound healing, cognitive decline, and delirium.[14] Bashar et al.[28] found that severe hypoxemia can affect the prognosis of surgery and quality of life of patients, and even endanger their lives if not properly managed. After cerebral infarction, the brain undergoes ischemia and hypoxia, which leads to irreversible damage to the brain tissue by the following mechanisms: (i) the pathology of cerebral infarction is characterized by inflammation and oxidative stress, which triggers endothelial cell dysfunction, promotes smooth muscle migration and proliferation, and ultimately leads to brain tissue damage;[29] (ii) hypoxia and acidosis damage the vascular endothelium and increase its permeability, resulting in cerebral interstitial edema; (iii) hypoxia reduces adenosine triphosphate (ATP) production in brain cells and affects the function of sodium pump on brain cell membrane, causing intracellular water and sodium retention, resulting in brain cell edema; (iv) in the case of hypoxia, the anaerobic metabolism of a large amount of glucose in the brain leads to the production and accumulation of a large number of harmful substances such as lactic acid, which intensifies the damage of brain tissue; and (v) the decrease in cerebrospinal fluid pH during hypoxia leads to intracellular acidosis, which can enhance brain glutamate decarboxylase activity, then the increased production of Y-aminobutyric acid leads to a strong central inhibitory effect on the nervous system, causing patients to fall into deep sleep, and produces side effects such as nervous fatigue, lethargy, lethargy, and coma. In addition, Vascular recanalization after cerebral infarction, opening of collateral circulation as well as some thrombolytic and anticoagulant drugs applied are prone to cause cerebral ischemia and reperfusion injury and therefore lead to cerebral edema, cerebral hemorrhage and neurological death.[30] Therefore, the occurrence of hypoxemia can cause complications such as cerebral edema and cerebral hemorrhage, which further aggravate the progression of the disease and seriously affect the patient’s prognosis. Therefore, we can predict the possibility of intraprocedural hypoxemia in patients based on the above results and focus on key populations to reduce the occurrence of hypoxemia, thus improving the prognosis and the quality of life of patients after surgery.
The main drawback of this study is that the follow-up period is relatively short and the postoperative survival rate of the two groups has not been studied. In addition, infarction size was not assessed among the assessment factors. It is suggested that the influencing factors should be supplemented and improved in future studies, and the follow-up time should be extended.
Conclusions
Currently, intravascular hypoxemia in patients with ACI has a serious impact on prognosis. Clinical work should attach great importance to the clinical characteristics of patients, identify relevant risk factors, and aggressively take personalized therapeutic actions to improve patients’ prognosis.
Author contributions
(I) Conception and design: Z Gu; (II) Administrative support: L Yin; (III) Provision of study materials or patients: Z Gu, A Yin, L Lu, Y Lu, B Jiang; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was provided by all patients. The study was approved by Ethics Committee of our hospital (No. LC-2022-019, dated on January 31st, 2017) and individual consent for this retrospective analysis was waived.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zheng D, Li X, Fu Y. Risk factors of acute cerebral infarction in patients with primary hypertension. Ir J Med Sci. 2023;192:2441–5. doi: 10.1007/s11845-022-03206-4. [doi:10.1007/s11845-022-03206-4] [DOI] [PubMed] [Google Scholar]
- 2.Xin M, Hao Y, Huang G, Wang X, Liang Z, Miao J, et al. The efficacy and safety of salvianolic acids on acute cerebral infarction treatment:A protocol for systematic review and meta analysis. Medicine (Baltimore) 2020;99:e20059. doi: 10.1097/MD.0000000000020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Liu J, Liu M, Lu C, Brainin M, Zhang J. Patterns of stroke between university hospitals and nonuniversity hospitals in Mainland China:Prospective multicenter hospital-based registry study. World Neurosurg. 2017;98:258–65. doi: 10.1016/j.wneu.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Methods in Medicine CAM. Retracted:Effect of new nursing on patients with acute cerebral infarction. Comput Math Methods Med. 2022;2022:9898723. doi: 10.1155/2022/9898723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi EY, Nieves GA, Jones DE. Acute stroke diagnosis. Am Fam Physician. 2022;105:616–24. [PubMed] [Google Scholar]
- 6.Lai J, Harrison RA, Plecash A, Field TS. A narrative review of persistent post-stroke headache –A new entry in the international classification of headache disorders, 3rd edition. Headache. 2018;58:1442–53. doi: 10.1111/head.13382. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Dennis MS, Lindley RI, Sellar RJ, Warlow CP. The validity of a simple clinical classification of acute ischaemic stroke. J Neurol. 1996;243:274–9. doi: 10.1007/BF00868526. [DOI] [PubMed] [Google Scholar]
- 8.Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen gas inhalation treatment in acute cerebral infarction:A randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017;26:2587–94. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Yamagami H, Yoshimoto T, Uchida K, Morimoto T, Toyoda K, et al. Endovascular therapy for acute ischemic stroke in patients with prestroke disability. J Am Heart Assoc. 2021;10:e020783. doi: 10.1161/JAHA.121.020783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva GS, Nogueira RG. Endovascular treatment of acute ischemic stroke. Continuum (Minneap Minn) 2020;26:310–31. doi: 10.1212/CON.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 11.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome:The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Scott JB, Jing G, Li J. Management of postoperative hypoxemia. Respir Care. 2021;66:1136–49. doi: 10.4187/respcare.08929. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenfeld JM, Funk LM, Van Schalkwyk J, Merry AF, Sandberg WS, Gawande A. The incidence of hypoxemia during surgery:Evidence from two institutions. Can J Anaesth. 2010;57:888–97. doi: 10.1007/s12630-010-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg SM, Nair B, Vavilala MS, Horibe M, Eisses MJ, Adams T, et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng. 2018;2:749–60. doi: 10.1038/s41551-018-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Kim DY, Sohn MK, Lee J, Lee SG, Shin YI, et al. Determining the cut-off score for the modified Barthel index and the modified Rankin scale for assessment of functional independence and residual disability after stroke. PLoS One. 2020;15:e0226324. doi: 10.1371/journal.pone.0226324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Y, Zhang Y, Wu R, Dou L, Cao F, Yan Y, et al. Network pharmacology approach to investigate the multitarget mechanisms of Zhishi Rhubarb soup on acute cerebral infarction. Pharm Biol. 2022;60:1394–406. doi: 10.1080/13880209.2022.2103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo Z, Tang C, Li H, Lei J, Zhu L, Kou L, et al. Eicosapentaenoic acid prevents inflammation induced by acute cerebral infarction through inhibition of NLRP3 inflammasome activation. Life Sci. 2020;242:117133. doi: 10.1016/j.lfs.2019.117133. [DOI] [PubMed] [Google Scholar]
- 18.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation:Friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YM, Zhao ZM, Wang W, Dong FM, Wang PP, Jia YJ, et al. Trends in cognitive function assessed by a battery of neuropsychological tests after mild acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:104887. doi: 10.1016/j.jstrokecerebrovasdis.2020.104887. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CS, Huang Y, Lindley RI, Chen X, Arima H, Chen G, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED):An international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019;393:877–88. doi: 10.1016/S0140-6736(19)30038-8. [DOI] [PubMed] [Google Scholar]
- 21.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Liu B, Rane N, Mitchell P, Dowling R, Yan B. Correlation between CT angiography and digital subtraction angiography in acute ischemic strokes. Clin Neurol Neurosurg. 2021;200:106399. doi: 10.1016/j.clineuro.2020.106399. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Song Y, Yin X, Wu W, Zhang L, Chen Y, et al. Deep learning-based digital subtraction angiography image generation. Int J Comput Assist Radiol Surg. 2019;14:1775–84. doi: 10.1007/s11548-019-02040-x. [DOI] [PubMed] [Google Scholar]
- 24.Khirfan G, Naal T, Abuhalimeh B, Newman J, Heresi GA, Dweik RA, et al. Hypoxemia in patients with idiopathic or heritable pulmonary arterial hypertension. PLoS One. 2018;13:e0191869. doi: 10.1371/journal.pone.0191869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong M, Wu Z, Xu S, Li L, Wang X, Guan X, et al. Increased risk for the development of postoperative severe hypoxemia in obese women with acute type a aortic dissection. J Cardiothorac Surg. 2019;14:81. doi: 10.1186/s13019-019-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan XZ, Xu ZY, Lu FL, Han L, Tang YF, Tang H, et al. Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection. J Thorac Dis. 2018;10:1628–34. doi: 10.21037/jtd.2018.03.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Ding X, Su Y, Yang P, Du X, Sun M, et al. Incidence, risk factors, and outcomes of severe hypoxemia after cardiac surgery. Front Cardiovasc Med. 2022;9:934533. doi: 10.3389/fcvm.2022.934533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bashar FR, Vahedian-Azimi A, Farzanegan B, Goharani R, Shojaei S, Hatamian S, et al. Comparison of non-invasive to invasive oxygenation ratios for diagnosing acute respiratory distress syndrome following coronary artery bypass graft surgery:A prospective derivation-validation cohort study. J Cardiothorac Surg. 2018;13:123. doi: 10.1186/s13019-018-0804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaeidi A, Hajializadeh Z, Jahandari F, Fatemi I. Leptin attenuates oxidative stress and neuronal apoptosis in hyperglycemic condition. Fundam Clin Pharmacol. 2019;33:75–83. doi: 10.1111/fcp.12411. [DOI] [PubMed] [Google Scholar]
- 30.Tang N, Wu J, Zhu H, Yan H, Guo Y, Cai Y, et al. Genetic mutation of GluN2B protects brain cells against stroke damages. Mol Neurobiol. 2018;55:2979–90. doi: 10.1007/s12035-017-0562-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.