ABSTRACT
Despite carbapenems not being used in animals, carbapenem-resistant Enterobacterales (CRE), particularly New Delhi metallo-β-lactamase-producing CRE (NDM-CRE), are prevalent in livestock. Concurrently, the incidence of human infections caused by NDM-CRE is rising, particularly in children. Although a positive association between livestock production and human NDM-CRE infections at the national level was identified, the evidence of direct transmission of NDM originating from livestock to humans remains largely unknown. Here, we conducted a cross-sectional study in Chengdu, Sichuan Province, to examine the prevalence of NDM-CRE in chickens and pigs along the breeding–slaughtering–retail chains, in pork in cafeterias of schools, and in colonizations and infections from children's hospital and examined the correlation of NDM-CRE among animals, foods and humans. Overall, the blaNDM increases gradually along the chicken and pig breeding (4.70%/2.0%) –slaughtering (7.60%/22.40%) –retail (65.56%/34.26%) chains. The slaughterhouse has become a hotspot for cross-contamination and amplifier of blaNDM. Notably, 63.11% of pork from the school cafeteria was positive for blaNDM. The prevalence of blaNDM in intestinal and infection samples from children's hospitals was 21.68% and 19.80%, respectively. whole genome sequencing (WGS) analysis revealed the sporadic, not large-scale, clonal spread of NDM-CRE along the chicken and pig breeding–slaughtering–retail chain, with further spreading via IncX3-blaNDM plasmid within each stage of whole chains. Clonal transmission of NDM-CRE is predominant in children's hospitals. The IncX3-blaNDM plasmid was highly prevalent among animals and humans and accounted for 57.7% of Escherichia coli and 91.3% of Klebsiella pneumoniae. Attention should be directed towards the IncX3 plasmid to control the transmission of blaNDM between animals and humans.
KEYWORDS: NDM-CRE, food chain, IncX3, livestock, child
Introduction
Carbapenems are among the most effective antimicrobial agents for treating human infections caused by extensively drug-resistant Enterobacterales, mainly E. coli and K. pneumoniae [1]. However, the intensive use of carbapenems in clinical settings has led to a gradual increase in carbapenem-resistant Enterobacterales (CRE), posing a significant threat to anti-infective treatment, especially among children [2,3]. Notably, carbapenems are not yet approved for use in the livestock industry; however, CRE was prevalent in these sectors [4]. Specifically, the New Delhi metallo-β-lactamase-producing (NDM)CRE has been found to be widely disseminated throughout the livestock industry chain [5–7]. Therefore, it is important to determine whether blaNDM can disseminate to human populations through the food chain and understand its transmission dynamics as the risk of antimicrobial-resistant bacteria and genes spreading among animals and human populations has been demonstrated previously [8].
Human infections caused by pathogens with antimicrobial-resistant genes (ARGs) originating from food-producing animals involve the transmission of ARGs or ARGs-carrying bacteria among multiple stages, including breeding, slaughtering, retail, intestinal colonization and ascending infection [9,10]. NDM-CRE has been reported to be detected at various stages, and its prevalence has remained high in certain stages, such as chicken farms, retail meat and healthcare settings [11–13]. Despite the acknowledged positive correlation between livestock production and NDM-CRE infections in humans at the national level [14], the majority of studies have primarily focused on fragmented surveillance and have not conducted overall correlation studies throughout the entire food production chain. Therefore, we selected and conducted a cross-sectional study in Chengdu, the provincial capital of Sichuan, one of the largest Chinese provinces for livestock production, with the high prevalence of NDM-CRE separately reported in animals (6.75%, [15]), foods (6.9%, [16]) and humans (4.5%, [17]). Herein, we aimed to explore the presence of NDM-CRE in the production, slaughter, sale of livestock as well as human carriages and infections and to assess the potential connections between these sectors. To do so, we examined the breeding–slaughtering–marketing chains of chickens and pigs as well as nurseries, primary and secondary schools, and a children's hospital within the designated area. We further analysedthe patterns of NDM-CRE transmission between animal and human populations at both bacterial and gene levels.
Methods
Study setting and design
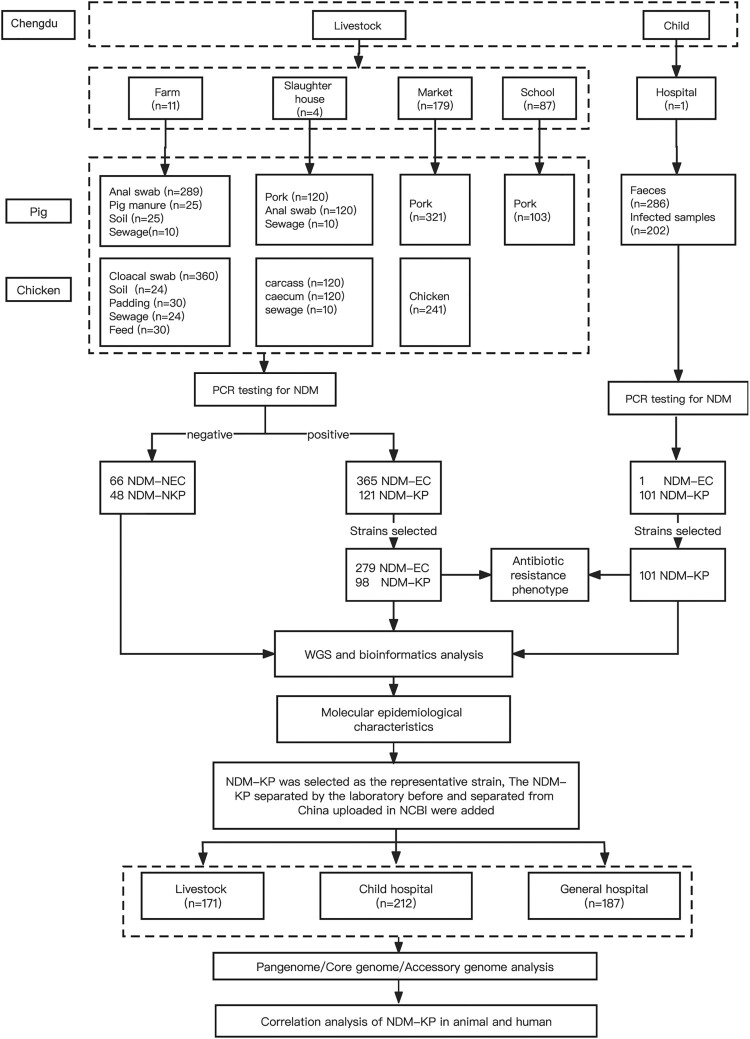
We conducted a cross-sectional study from August 2020 to March 2021 to investigate the prevalence of NDM-CRE isolates from livestock, poultry, animal-derived food products and humans in Chengdu, Sichuan Province, China. The sampling design was based on the potential transmission modes of antimicrobial-resistant bacteria/genes, covering the entire chain from livestock (breeding–slaughtering–retail) to humans (gut colonization-ascending infection) (Figure 1). The pork and chicken industry chains were selected from local enterprises in Chengdu, including two pork industry chains and two chicken industry chains. Within each industrial chain, the farms and slaughterhouses belonged to the same vertically integrated production system. The slaughtered pork and chicken were then transported to markets in Chengdu for sale. The study encompassed five large-scale pig farms, six large-scale broiler farms, two pig slaughterhouses, and two broiler slaughterhouses. For the sales procedure, we selected 179 markets in five major urban areas and surrounding regions of Chengdu. Additionally, we included 87 canteens of schools and nurseries in Chengdu and surrounding areas as well as one children's hospital in Chengdu. The geographic information, recorded using the global positioning system (GPS), was documented for all the aforementioned sample collection sites.
Figure 1.
Schematic diagram of the research workflow.
Sample collection and strain isolation
A total of 2470 non-duplicate samples were collected from the entire chain, including samples related to the pig industry chain (n = 1023), chicken industry chain (n = 959), and child-related samples (n = 488) (Table S1). The sampling of animals was conducted in accordance with the principles of the Beijing Municipality Review of Welfare and Ethics of Laboratory Animals (BAOLA 2005). The collection and use of faeces and infection samples from children were ethically approved by the Sichuan Provincial Maternity and Child Health Hospital.
The isolation and identification of all NDM-CRE from the samples followed a three-step process. Firstly, the samples were added to brain heart infusion (BHI) medium containing 0.5 μg/mL of meropenem (MEN) and 30 μg/mL of vancomycin and incubated in a 37°C constant temperature shaker for 24 h to complete the initial pre-growth step. For swab samples, 100 μL of the transfer liquid was drawn into 5 mL of BHI. For sewage samples, soil, bedding, feed, intestinal contents, and stool samples, 0.25 g to 5 mL of BHI were collected using an inoculation loop. For meat samples (50 g), they were macerated in 10 mL of BHI broth for 5 min in aseptic sampling bags (Hope Biol-Technology Co). The enrichments were then inoculated onto MacConkey agar (MIAC, Luqiao) plates containing 0.5 μg/mL of MEN, and red colonies were selected for further pure culture. Secondly, 100 μL of the enrichments was absorbed into 1 mL of BHI containing 0.5 μg/mL of MEN and 30 μg/mL of carbenicillin and incubated in a 37°C constant temperature shaker for 24 h to complete the second pre-growth step. The enrichments were then inoculated onto MIAC agar plates containing 0.5 μg/mL MEN, and pink colonies were selected for further pure culture. Thirdly, colonies on the agar were confirmed for the blaNDM gene by PCR, and the species were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonik).
Data collection and whole-genome sequencing
A total of 279 blaNDM-positive Escherichia coli (NDM-EC) and 197 blaNDM-positive Klebsiella pneumoniae (NDM-KP) isolates were selected for whole genome sequencing, including all NDM-CRE isolated from chicken and pig breeding, slaughtering, and children's hospital and some isolated from sales procedure (market and school). The selection criteria for isolates in the sales procedure is to select one NDM-EC and one NDM-KP according to the different animal species sources of each blaNDM-positive sampling site. The number of selected isolates in each procedure is shown in Table S2. Furthermore, 66 blaNDM-negative E. coli (NDM-NEC) and 48 blaNDM-negative K. pneumoniae (NDM-NKP) isolated from livestock were selected for whole genome sequencing. In addition, whole genome sequencing data of 374 NDM-KP isolates from China were collected from the laboratory and NCBI database, including 76 isolates collected from livestock, 111 isolates collected from a children’s hospital and 187 isolates collected from a general hospital.
Total DNA was extracted using a Magen Genomic DNA Purification Kit (Magen, Guangzhou, China) as per the manufacturer’s instructions. Indexed DNA libraries were prepared using a KAPA Hyper Prep Kit and sequenced on the Illumina HiSeq X Ten platform (Annoroad, Beijing, China). Genome assembly was conducted with SPAdes, version 3.9.0 [18]. Oxford nanopore MinION sequencing was conducted to obtain the complete genome sequences of isolates carrying the blaNDM gene, using the SQK-RBK004 sequencing kit and flowcell R9.5. A hybrid assembly of Illumina and nanopore sequencing reads was constructed using Unicycler v 0.3.0 [19]. All raw data in this study were deposited in GenBank under Bioproject accession no. PRJNA1037737.
Antimicrobial susceptibility testing
Minimum inhibitory concentration (MIC) of NDM-positive isolates against 15 commonly used antimicrobials (meropenem, imipenem, ertapenem, cefmetazole, ceftazidime, cefotaxime, piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime/avibactam, cefepime, colistin, tigecycline, ciprofloxacin, amikacin, and aztreonam) were determined by the microdilution broth method (Zhuhai DL Biotech). Results of MIC were interpreted according to the Clinical and Laboratory Standards Institute document M100-ED3015 [20] and the European Committee on Antimicrobial Susceptibility Testing [21]. Reference strain E. coli ATCC25922 served as a quality control.
Bioinformatics and statistical analysis
The geographical distribution of all 282 sampling sites was visualized using QGIS 3.16 based on GPS information. The multilocus sequence typing (MLST) of the isolates was determined using the SRST2 v0.2.0 calling function [22], and minimum-spanning trees were generated in BioNumerics v7.6 for all blaNDM-positive isolates. Prokka v1.5 [23] was utilized to annotate the de novo assemblies with predicted genes. The annotated assemblies were then input into Panaroo [24] for pan-genome analysis, using the default parameters and running the Panaroo pipeline in strict mode, with paralogs being split. Phylogenetic analysis of the core genome was conducted using Snippy (https://github.com/tseemann/snippy) based on single-nucleotide polymorphism (SNP) variants. Recombined regions were identified and excluded from the pseudo-genome alignment using Gubbins v1.4.10 [25]. The resulting alignment was used to construct a phylogenetic tree using FastTree v2.1.10 [26], with the tree being rooted at the midpoint and subsequently annotated in interactive tree of life (ITOL) [27]. Bayesian model-based population structures were determined using HierBAPS62 v6.0. discriminant analysis of principal components (DAPC) [28] was performed using the Adegenet R package, employing SNP variants of the core genome. The results were visualized using the ggplot R package. To investigate the possibility of accessory genome transfer between NDM-KP from different sources, AccNET [29] was used to construct a bipartite network connecting the genomic units and the PCs (protein clusters). The visual location of isolates in the network was determined using ForceAtlas2 [30] as a layout algorithm, which arranged the NDM-KP according to the set of shared proteins. Broad-range and narrow-range proteins tend to lie in the centre and outside of the network, respectively. Gephi [31] software was used for network visualization and manipulation. The assembled data were screened against the ARGannot database, PlasmidFinder database, and ISFinder database using abricate to detect acquired ARGs, plasmid replicons, and ISs in each genome. The results were transformed into a binary table in R v3.6.0 to analyse the presence/absence of acquired ARGs alleles and the prevalence of each gene in different isolates. Contigs lacking plasmid replicon markers were analysed using Bandage version 0.8.1 [32] to identify potential plasmid types. The prevalence rates of plasmids and ISs in NDM-KP isolates from human and animal sources were analysed using the Spearman rank-order correlation coefficient.
Results
NDM-CRE are widespread in Chengdu
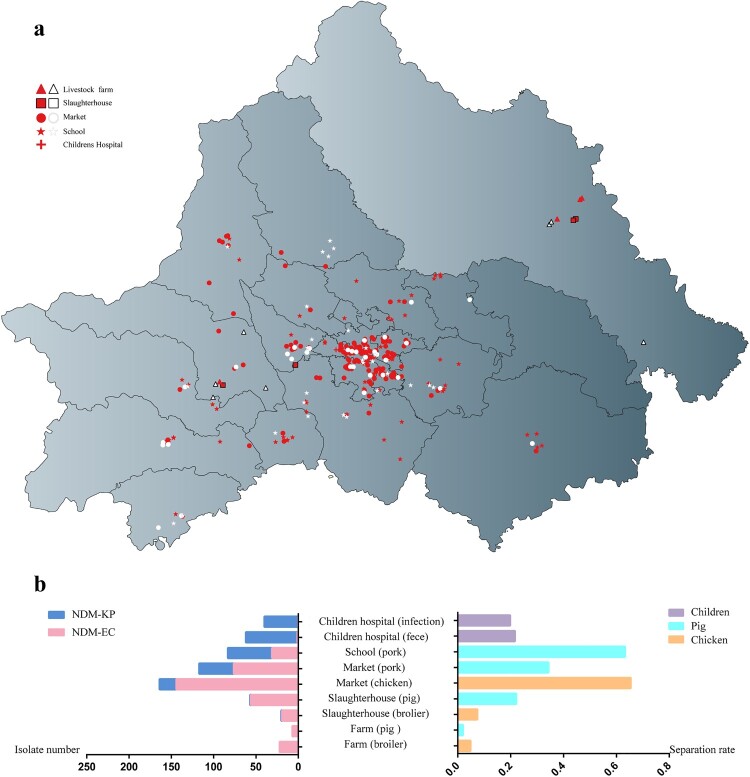
A total of 2470 samples from animal (n = 889), food (n = 905), human (n = 488) and environment (n = 188) were collected from 406 sites of farms, slaughterhouses, markets, schools and hospital across Chengdu (Figures 1 and 2(a)). The prevalence of blaNDM increases gradually along the livestock breeding–slaughtering–retail chains (Figure 2(b), Table S1). The prevalence of blaNDM-positive samples in chicken and pig farms was 4.70% (22/468) and 2.0% (7/349), respectively, with three chicken farms and four pig farms negative for blaNDM. The proportion of blaNDM-positive sample in slaughterhouses for chicken and pig was 7.60% (19/250) and 22.40% (22/250), respectively. The proportion of blaNDM-positive samples in chicken and pork from the market was 65.56% (158/241) and 34.26% (76/321), respectively, and in pork from school was 63.11% (65/103). In children's hospital, the prevalence of blaNDM-positive samples was 21.68% (62/286) in intestinal samples and 19.80% (40/202) in infection samples. Among these blaNDM-positive samples (471/2470), 366 NDM-EC and 222 NDM-KP were identified. NDM-KP was rarely found in the breeding and slaughtering stages of livestock, in which the NDM-EC was predominantly detected. A significant proportion of NDM-KP was observed in the retail stage. Conversely, NDM-KP was dominant in children's hospitals, while NDM-EC was infrequent.
Figure 2.
(a) Geographical distribution of farms, slaughterhouses, market, school and hospital collecting sample during from August 2020 to March 2021 in Chengdu, China. (b) Number of NDM-KP and NDM-EC isolates (left) and separation rate of blaNDM positive samples (right) in different procedures of food chain and children's hospital.
Comparative analysis of antimicrobial resistance rates and resistance genes in NDM-CRE
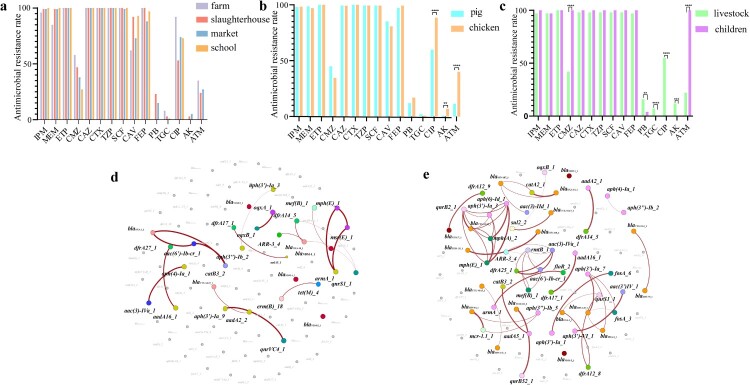
Along the livestock breeding–slaughtering–retail chains, NDM-EC displayed a declining trend in resistance rates to cefmetazole [farm:58% (MIC90/50 = 128/64 μg/mL), slaughterhouse:47% (MIC90/50 = 128/32 μg/mL), market:38% (MIC90/50 = 128/32 μg/mL) and school:27% (MIC90/50 = 64/32 μg/mL)], tigecycline [8% (≤0.25/≤0.25 μg/mL), 3% (≤0.25/≤0.25 μg/mL), 1% (0.5/≤0.25 μg/mL) and 0% (≤0.25/≤0.25 μg/mL)] and aztreonam [35% (32/8 μg/mL), 24% (64/≤4 μg/mL), 27% (64/≤4 μg/mL), and 0% (≤4/≤4 μg/mL)] (Figure 3(a)). In contrast, resistance rates to other β-lactams and β-lactamase inhibitors (meropenem, ertapenem, ceftazidime, cefotaxime, piperacillin/tazobactam, and cefoperazone/sulbactam) remained relatively stable (Figure S1, Table S3). NDM-EC from chicken and pig exhibited similar resistance rates to all tested antimicrobials (Figure 3(b) and Table S4), except for ciprofloxacin (88% in chicken vs. 60% in pig, p < 0.0001), aztreonam (40% vs. 11%, p < 0.0001) and amikacin (7% vs. 1%, p < 0.01). Given the limited number of chicken NDM-KP isolates, the resistance rates of NDM-KP isolates from livestock were compared with those from children (Figure 3(c), Table S3). Significant variations in resistance rates were observed in NDM-KP collected between livestock and children concerning cefmetazole (40% vs. 100%), aztreonam (22% vs. 100%), tigecycline (7% vs. 0%), ciprofloxacin (55% vs. 0%) and amikacin (12% vs. 0%).
Figure 3.
(a) Comparison of antimicrobial resistance rates for NDM-EC along the livestock breeding–slaughtering–retail chains. (b) Comparison of antimicrobial resistance rates for NDM-EC in pigs and chickens. (c) Comparison of antimicrobial resistance rates for NDM-KP in livestock and children. (d) Coexistence network of ARGs in NDM-EC from livestock. (e) Coexistence network of ARGs in NDM-KP from livestock.
The correlation analysis of all ARGs in NDM-CRE from livestock production chain was conducted by statistical analysis. Correlation was observed among several ARGs in NDM-EC, such as the coexistence of β-lactam resistance gene blaOXA-1, chloramphenicol resistance gene catB and aminoglycoside resistance gene aac(6')-Ib-cr (Figure 3(d), Figure S1a). A more frequency of ARGs coexistence was identified in NDM-KP (Figure 3(e), Figure S1b). For instance, a complex association network existed among aminoglycoside resistance genes aph(3’)-Ia and aph(6’)-Id, macrolide resistance genes mph(A) and mph(E), Plasmid-mediated quinolone resistance (PMQR) gene qnrB and extended-spectrum beta-lactamase (ESBL) gene blaCTX-M-65. We found that the edge count in ARGs-associated network was 62 and 20 for NDM-KP and NDM-EC, respectively, indicating NDM-KP in livestock presented a greater potential to become multidrug-resistant bacteria than NDM-EC.
Epidemiological characteristics of blaNDM-positive plasmids
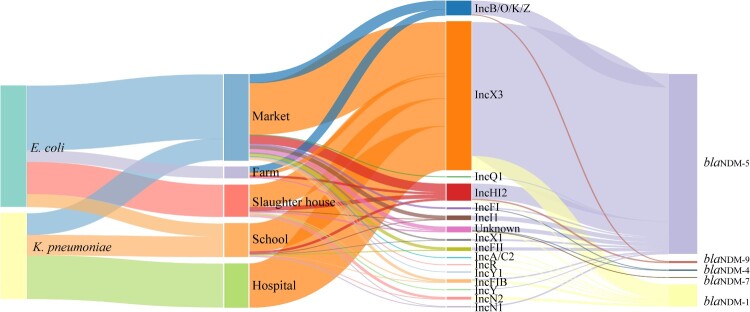
Among 476 sequenced CRE isolates, we determined 15 Inc-plasmid types associated with blaNDM in 463 isolates, except for 13 Inc-untypable isolates. The highest prevalent type is IncX3 plasmid (71.6%, n = 341, Figure 4), followed by IncHI2 (n = 39), IncB/O/K/Z (n = 34), IncI1 (n = 10), IncFIB (n = 8), IncFII (n = 8), IncN2 (n = 6), IncFI (n = 4), IncX1 (n = 4), IncN1 (n = 3), IncY (n = 2), and one for each of IncA/C2, IncQ1, IncR, and IncY1. We identified 13 and 6 types of blaNDM-carrying plasmids in NDM-EC (n = 267) and NDM-KP (n = 196), respectively. Among them, blaNDM-IncX3 is the most prevalent plasmid accounted for 57.7% (n = 161) in NDM-EC and 91.3% (n = 180) in NDM-KP, exhibiting an increasing proportion of all blaNDM-Inc plasmids along the chain: 22.20% in farm, 68.90% in slaughterhouse, 60.40% in market, 83.10% in school and 100% in hospital. blaNDM-5 gene was exclusively carried by IncX3 plasmid (n = 309), whilst blaNDM-1 was harboured by IncX3 (n = 32), IncN2 (n = 6), IncFIB (n = 5), IncY (n = 2), IncA/C2 (n = 1), IncFII (n = 1), IncHI2 (n = 1) and IncR (n = 1) plasmids.
Figure 4.
The distribution and linkages among isolate species, isolate sources, blaNDM-carrying plasmid types and NDM protein subtypes.
blaNDM can spread through cloning and horizontal transfer along the food chain
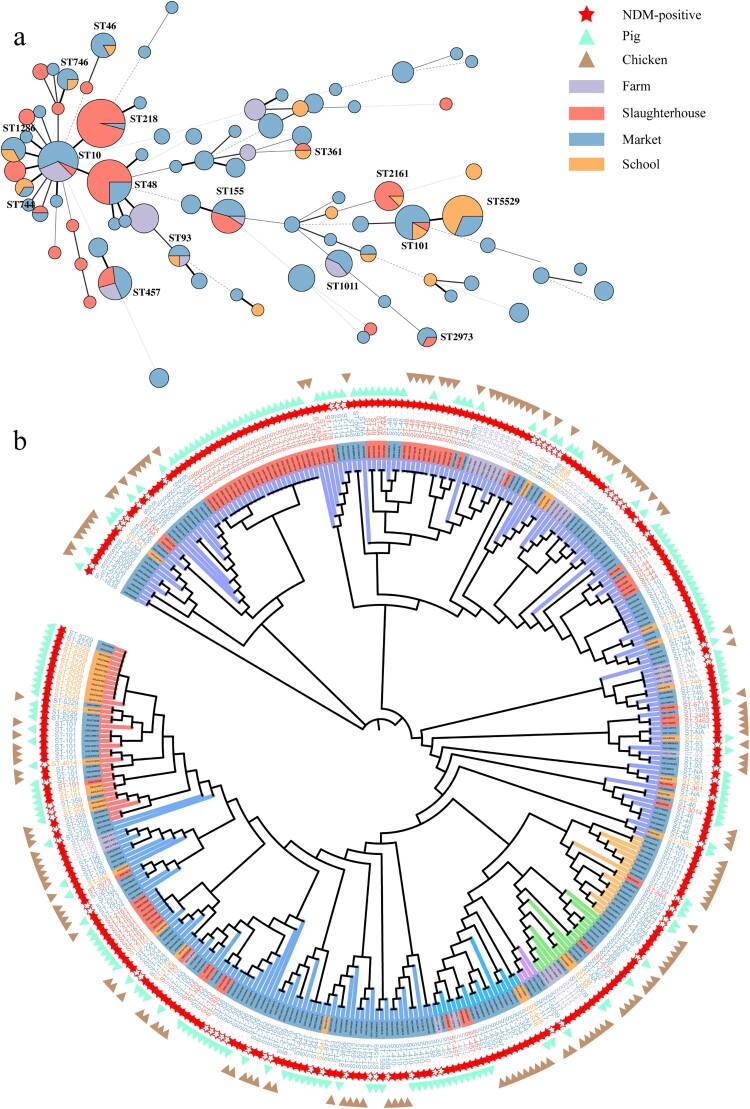
The MLST analysis revealed 73 known sequence types (ST) in 279 sequenced NDM-EC isolates from the livestock production chain. Among them, ST218 (n = 24, 8.6%), ST48 (n = 18, 6.5%), ST10 (n = 16, 5.7%) and ST5529 (n = 16, 5.7%) were the most prevalent (Figure 5(a)). Certain NDM-EC isolates with high genetic similarity were present throughout each stage (breeding, slaughtering and retail) of the chicken and pig production chains, as evidenced by the presence of ST10, ST457, ST48 and ST155 (Figure 5(b)). In the slaughter stage, some ST2161 and ST48 NDM-EC isolates from anal of slaughtered pigs and pork of processed pigs exhibited high genetic similarity, suggesting that pork in slaughterhouse was commonly contaminated by NDM-positive pigs from different farms. Moreover, some ST5529, ST101, and ST359 isolates from pork in markets and school cafeterias exhibited high genetic similarity, suggesting that NDM-EC could disseminate from markets to school cafeterias. However, NDM-EC isolates obtained from pork and chicken within the same market showed limited genetic relatedness, indicating that NDM-EC in markets had limited cross-contamination between different types of meat. In addition, we collected 66 NDM-NEC isolates from the livestock production chain (Figure 5(b)) and observed a high genetic similarity between certain NDM-NEC and NDM-EC isolates from the same procedure (e.g. ST410 acquired blaNDM-5-IncX3 in market), suggesting that NDM-NEC may convert to NDM-EC through the acquisition of blaNDM carrying plasmids. All these results revealed that NDM-EC could be presented and transmited through the livestock production chain.
Figure 5.
(a) MLST of NDM-CRE from livestock sources. (b) Phylogenic tree of E. coli from livestock based on core-genome SNPs.
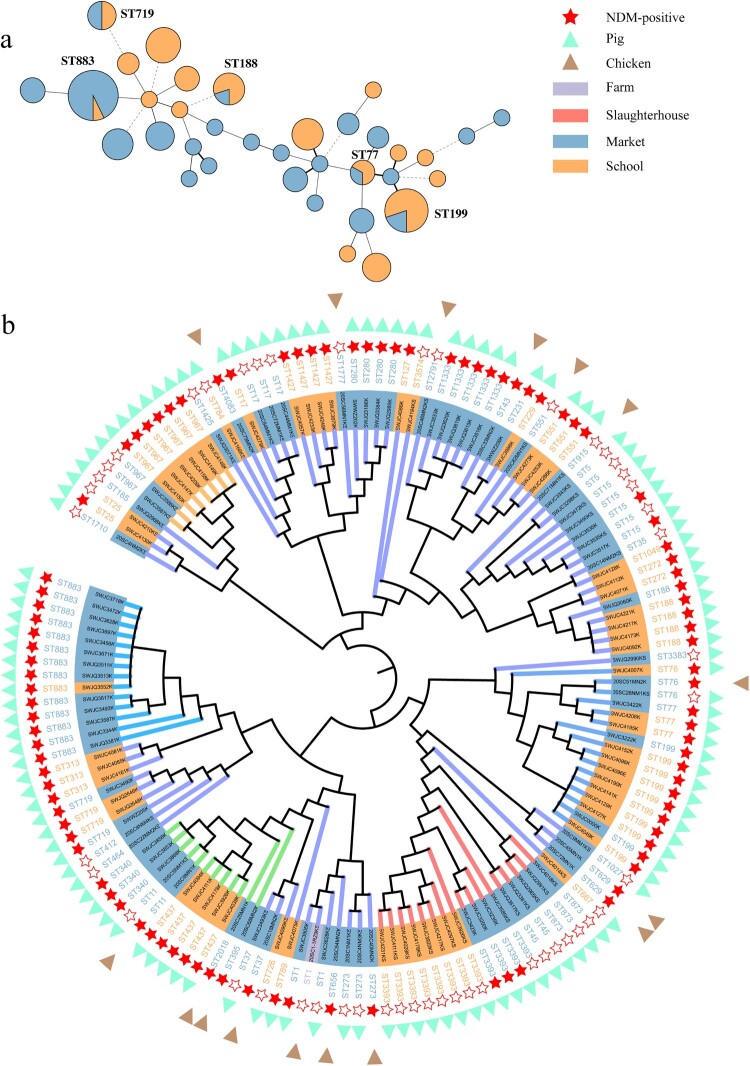
Ninety-six sequenced NDM-KP isolates obtained from livestock exhibited 36 STs. Among these, ST883 (n = 13, 13.3%) and ST199 (n = 10, 10.2%) were the most prevalent ST in livestock production chain (Figure 6(a)). Certain NDM-KP isolates with high genetic similarity presented at the stages of retail and school in chicken and pig production chains, as evidenced by ST883, ST199 and ST188 (Figure 6(b)). Meanwhile, ST789 (n = 99, 98.0%) is the predominant ST among 101 sequenced NDM-KP isolates from children carriage (n = 61) and infection (n = 40), showing genetic uniformity with SNPs ranging from 0 to 98, suggesting the potential intestinal colonization and clonal transmission of ST789 within the children hospital. No apparent association at the ST level was observed between NDM-KP from livestock and humans. In addition, we collected 48 NDM-NKP isolates from the livestock production chain (Figure 6(b)) and observed a high genetic similarity between certain NDM-NKP and NDM-KP isolates from the same procedure (e.g. ST15 acquired blaNDM-5-IncX3 in the market), suggesting that NDM-NKP may convert to NDM-KP through the acquisition of blaNDM carrying plasmids.
Figure 6.
(a) MLST of NDM-KP from livestock sources. (b) Phylogenic tree of K. pneumoniae from livestock based on core-genome SNPs.
Genomic association among NDM-KP between animals and humans
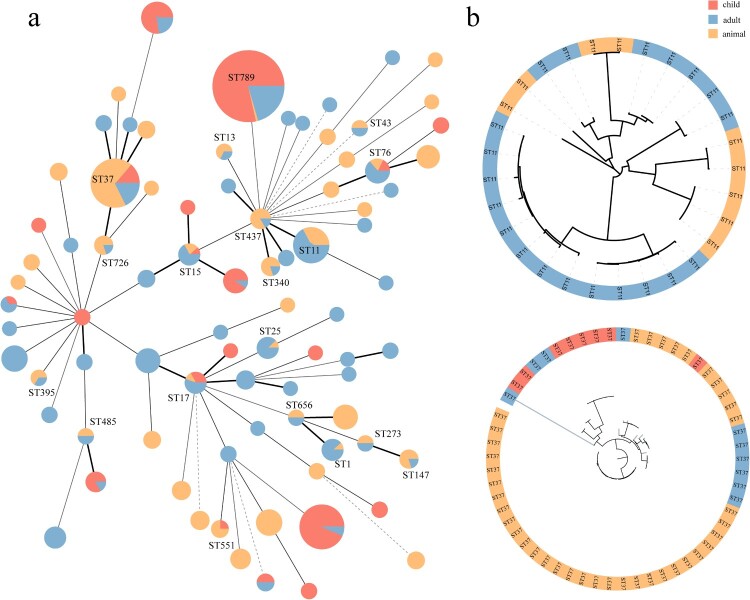
To better understand the association of NDM-KP derived from animals and humans, we collected 373 NDM-KP isolates (75 from animals, 111 from children and 187 from adults) across China and in combination with 197 NDM-KP isolates in this study. Genomic analysis of 570 NDM-KP isolates revealed a total of 18,521 genes, comprising 3920 core genes and 14,601 accessory genes (Figure S2). The MLST results revealed a total of 83 STs, with 19 STs (22.9%) being shared between livestock and human sources (Figure 7(a)): ST37 (human: n = 18, animal: n = 39), ST11 (n = 20:9), ST789 (n = 130:1), ST1 (n = 8:1), ST13 (n = 1:2), ST15 (n = 7:2), ST17 (n = 8:1), ST25 (n = 7:1), ST43 (n = 1:1), ST76 (n = 9:2), ST147 (n = 1:4), ST273 (n = 1:1), ST340 (n = 1:4), ST395 (n = 1:2), ST437 (n = 1:5), ST485 (n = 1:1), ST551 (n = 1:3), ST656 (n = 1:1) and ST726 (n = 1:4). However, NDM-KP from humans and animals with the same STs (e.g. ST11 and ST37) exhibited differences in core genome (Figure 7(b)). The DAPC results (Figure S3) based on core genome SNPs revealed that NDM-KP from adults, children and animals formed three distinct clusters, with the overlapped clusters from children and adult NDM-KP, which differed from that from animals. To further investigate the potential association of accessory genomes between NDM-KP isolates from different sources, we constructed a bipartite network connecting accessory genome-encoded homologous protein clusters (HPCs) with all NDM-KP. This network arranged the isolates according to the shared set of HPCs (Figure S4). Isolates from adult, child and animals showed an even distribution, with no host specific cluster among the bipartite network, indicating a similar accessory genome of isolates from humans and animals. Consequently, we identified mobile genetic elements associated with ARGs transfer in accessory genomes and found 53 plasmid types and 44 IS types (Figure S5). Spearman correlation analysis revealed a strong correlation in the separation rates of plasmids carried by NDM-KP from humans and animals (R2 = 0.7308, P < 0.0001). The blaNDM-5-IncX3 was identified in 467 isolates and preformed as the most prevalent plasmid in China. Additionally, a correlation was observed in the separation rates of IS elements carried by NDM-KP from humans and animals (R2 = 0.3563, P < 0.0001), with IS26 frequently located in IncX3 plasmid being the most common and observed in 278 NDM-KP isolates.
Figure 7.
(a) MLST of NDM-KP from livestock, child and adult sources. (b) Phylogenic tree of NDM-KP ST11 and ST37 based on core-genome SNPs.
Discussion
NDM-CRE continues to be highly prevalent in the livestock production chain (production –slaughter –retail) as well as in hospitalized children (intestinal tract – infection). Compared to previous studies conducted in Sichuan [16,17], we observed higher detection rates of NDM-CRE in retail meat (34.26–65.56% vs. 6.9%) and humans (19.80–20.68% vs. 4.5%). This can be attributed to the use of two selective pre-augmentation steps during our isolation process, also suggesting that the prevalence of blaNDM from both livestock and humans might be underestimated. NDM-CRE exhibited a significant increase in detection rate within the livestock production chain, in which the slaughterhouses are the hotspots for cross-contamination of NDM-CRE. Slaughterhouse was proved to play a significant role in the contamination and dissemination of various pathogens including Salmonella [33], Listeria monocytogenes [34] and particularly the antimicrobial resistance (AMR) strains [35]. Here, in this study, although NDM-negative isolates presented in NDM-free chicken and pig farms, the livestock from these NDM-free farms are transported to the slaughterhouses, where the livestock from NDM-positive farms entered and slaughtered as well, cross-contaminated with NDM-CRE in meat processing. Furthermore, the transportation of NDM-CRE-carrying processed meat from slaughterhouses to markets across different regions in Chengdu has contributed to the widespread of NDM-CRE in retail meat. These results indicated that the slaughtering and processing procedures may act as an “amplifier” for NDM-CRE, and solely blocking of NDM-CRE on livestock farms was insufficient.
Although we missed the children-derived isolates collected from communities and schools in the entire food chain, and despite the limited clinical data, and might overlook potential cross-contamination during the strain collection process and additional data on factors influencing antimicrobial resistance development and spread (e.g. antimicrobial usage practices, biosecurity measures and meat consumptions patterns), we demonstrated the significant role of plasmids, not clonal strains, in the transmission of blaNDM across the chain with following reasons. First, both NDM-EC and NDM-KP in the chain exhibit significant strain diversity (73 STs for NDM-EC and 36 STs for NDM-KP), lacking apparent dominant clones, even the relatively common NDM-EC ST48 and ST10 have been extensively documented as carrying various resistance genes such as mcr-1, tet(X), ESBL, and PMQR [36–41]. Second, clonal transmission is often restricted to specific sectors of the entire chain from livestock to human, including hospitals and slaughterhouses, rarely forming a continuous transmission pattern across different sectors of the chain. Third, the clonal isolates either positive or negative for blaNDM-5-IncX3 and the robust stability of IncX3 plasmid in E. coli [42] support the transmission of blaNDM-5 via horizontal plasmid transfer. Fourth, the blaNDM-5-IncX3 plasmid was frequently identified from NDM-KP and NDM-EC in the same retail meat from various markets. The ability of IncX3 plasmid to cross different bacterial species, genus and phyla [42–44] facilitated the transmission of the blaNDM gene across species. Fifth, nearly all blaNDM variants resided on transferable Inc plasmids, both IncX3 and IncHI2 plasmids, were identified throughout the entire chain. Lastly, NDM-KP from animals and humans exhibited distinctions at the core genome level but showed similarity within the accessory genome level. The IncX3 plasmid in the accessory genome emerged as the most critical mobile element for the blaNDM gene to overcome barriers between animals and humans.
To address the transmission of NDM-CRE between animals and humans from the “One Health” perspective, the following measures should be taken into account: (1) The food chain plays a pivotal role in the transmission of AMR. Comprehensive monitoring across the entire chain, involving animals, animal-derived food, environment of animal farming and food processing and humans, is essential for understanding the key aspects of NDM-CRE transmission. (2) Slaughterhouses, acting as hub centres for livestock, played a significant role in the transmission of NDM-CRE. Efficient cleaning and disinfection protocols (e.g. optimizing slaughter processes and disinfection procedures, as well as standardizing personnel operations) need to be established in slaughterhouses to minimize NDM-CRE contamination in meat products in markets and schools. (3) Screening for compounds or materials that can inhibit IncX3 conjugative transfer [45] and promote plasmid elimination would be an effective strategy to limit the spread of NDM-CRE.
Conclusion
We revealed the high prevalence of blaNDM genes in the entire food chain, encompassing animals, food and humans, and the predominant transmission route via IncX3 plasmids. It also emphasized the critical role of slaughterhouses in the contamination and dissemination of blaNDM. Our research highlights the imperative need for comprehensive surveillance to kerb the spread of NDM-CRE. The surveillance should cover not just identifying the positive strain but also thoroughly examining the transmission dynamics of blaNDM-carrying plasmids. Special attention should be directed towards the IncX3-type plasmids to control the transmission of blaNDM from animals to human populations.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China [Grant Number 32141002; 81991535; 32202863].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Guh AY, Bulens SN, Mu Y, et al. . Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479–1487. doi: 10.1001/jama.2015.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu B, Yin D, Sun C, et al. . Clonal and horizontal transmission of blaNDM among Klebsiella pneumoniae in children’s intensive care units. Microbiol Spectr. 2022;10:e01574–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David S, Reuter S, Harris SR, et al. . Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4:1919–1929. doi: 10.1038/s41564-019-0492-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köck R, Daniels-Haardt I, Becker K, et al. . Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect. 2018;24:1241–1250. doi: 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang R, Li J, et al. . Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol. 2017;2:16260. doi: 10.1038/nmicrobiol.2016.260 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Lv L, Huang X, et al. . Rapid increase in carbapenemase-producing Enterobacteriaceae in retail meat driven by the spread of the blaNDM-5-carrying IncX3 plasmid in China from 2016 to 2018. Antimicrob Agents Chemother. 2019;63. doi: 10.1128/aac.00573-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Liu Y, Zhang Q, et al. . The prevalence of colistin resistance in Escherichia coli and Klebsiella pneumoniae isolated from food animals in China: coexistence of mcr-1 and bla NDM with low fitness cost. Int J Antimicrob Agents. 2018;51:739–744. doi: 10.1016/j.ijantimicag.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 8.Richardson EJ, Bacigalupe R, Harrison EM, et al. . Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat Ecol Evol. 2018;2:1468–1478. doi: 10.1038/s41559-018-0617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakradhar S. A curious connection: teasing apart the link between gut microbes and lung disease. Nat Med. 2017;23:402–404. doi: 10.1038/nm0417-402 [DOI] [PubMed] [Google Scholar]
- 10.Economou V, Gousia P.. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist. 2015;8:49–61. doi: 10.2147/IDR.S55778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai R, Fu B, Shi X, et al. . Contaminated in-house environment contributes to the persistence and transmission of NDM-producing bacteria in a Chinese poultry farm. Environ Int. 2020;139:105715. [DOI] [PubMed] [Google Scholar]
- 12.Sadek M, Poirel L, Nordmann P, et al. . Genetic characterisation of NDM-1 and NDM-5-producing enterobacterales from retail chicken meat in Egypt. J Glob Antimicrob Resist. 2020;23:70–71. doi: 10.1016/j.jgar.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 13.Hu F-P, Guo Y, Zhu D-M, et al. . Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22:S9–S14. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Hu F, Wang Y, et al. . Transmission of carbapenem resistance between human and animal NDM-positive Escherichia coli strains. Engineering. 2022;15:24–33. doi: 10.1016/j.eng.2021.07.030 [DOI] [Google Scholar]
- 15.Wen R, Wei H, Zhang T, et al. . Epidemiological characterisation of blaNDM-positive enterobacterales from food-producing animal farms in Southwest China. Microorganisms. 2023;11:2304. doi: 10.3390/microorganisms11092304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Xiang Q, Ma J, et al. . Characterization of carbapenem-resistant Enterobacteriaceae cultured from retail meat products, patients, and porcine excrement in China. Front Microbiol. 2021;12:743468. doi: 10.3389/fmicb.2021.743468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhu S, Wei L, et al. . Arm race among closely-related carbapenem-resistant Klebsiella pneumoniae clones. ISME Commun. 2022;2:76. doi: 10.1038/s43705-022-00163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bankevich A, Nurk S, Antipov D, et al. . SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wick RR, Judd LM, Gorrie CL, et al. . Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. Plos Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CLSI . Performance standards for antimicrobial susceptibility testing. 31th ed CLSI supplement M100. Wayne (PA: ): Clinical and Laboratory Standards Institute; 2021. [Google Scholar]
- 21.EUCAST . The European committee on antimicrobial susceptibility testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0. 2020.
- 22.Inouye M, Dashnow H, Raven L-A, et al. . SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6:90. doi: 10.1186/s13073-014-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 24.Tonkin-Hill G, MacAlasdair N, Ruis C, et al. . Producing polished prokaryotic pangenomes with the panaroo pipeline. Genome Biol. 2020;21:180. doi: 10.1186/s13059-020-02090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croucher NJ, Page AJ, Connor TR, et al. . Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15–e15. doi: 10.1093/nar/gku1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price MN, Dehal PS, Arkin AP.. Fasttree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letunic I, Bork P.. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021: gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jombart T, Devillard S, Balloux F.. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 2010;11:94–94. doi: 10.1186/1471-2156-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza VF, Baquero F, Cruz Fdl, et al. . AcCNET (accessory genome constellation network): comparative genomics software for accessory genome analysis using bipartite networks. Bioinform (Oxf, Engl). 2016;33:283–285. [DOI] [PubMed] [Google Scholar]
- 30.Jacomy M, Venturini T, Heymann S, et al. . Forceatlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One. 2013;9:e98679. doi: 10.1371/journal.pone.0098679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastian M, Heymann S, Jacomy M.. Gephi: an open source software for exploring and manipulating networks. Proc Int AAAI Conf Web Soc Media. 2009;3:361–362. doi: 10.1609/icwsm.v3i1.13937 [DOI] [Google Scholar]
- 32.Wick RR, Schultz MB, Zobel J, et al. . Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31:3350–3352. doi: 10.1093/bioinformatics/btv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang K, Wei B, Jang H-K, et al. . Phenotypic characteristics and genotypic correlation of antimicrobial resistant (AMR) Salmonella isolates from a poultry slaughterhouse and its downstream retail markets. Food Control. 2019;100:35–45. doi: 10.1016/j.foodcont.2018.12.046 [DOI] [Google Scholar]
- 34.Guidi F, Centorotola G, Chiaverini A, et al. . The slaughterhouse as hotspot of CC1 and CC6 listeria monocytogenes strains with hypervirulent profiles in an integrated poultry chain of Italy. Microorganisms. 2023;11:1543. doi: 10.3390/microorganisms11061543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Díez J, Saraiva S, Moura D, et al. . The importance of the slaughterhouse in surveilling animal and public health: a systematic review. Vet Sci. 2023;10:167. doi: 10.3390/vetsci10020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agabou A, Lezzar N, Ouchenane Z, et al. . Clonal relationship between human and avian ciprofloxacin-resistant Escherichia coli isolates in North-Eastern Algeria. Eur J Clin Microbiol Infect Dis. 2016;35:227–234. doi: 10.1007/s10096-015-2534-3 [DOI] [PubMed] [Google Scholar]
- 37.Cui C-Y, Chen C, Liu B-T, et al. . Co-occurrence of plasmid-mediated tigecycline and carbapenem resistance in acinetobacter spp. from waterfowls and their neighboring environment. Antimicrob Agents Ch. 2020. doi: 10.1128/aac.02502-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paiva Y, Nagano DS, Cotia ALF, et al. . Colistin-resistant Escherichia coli belonging to different sequence types: genetic characterization of isolates responsible for colonization, community- and healthcare-acquired infections. Rev Inst Med Trop São Paulo. 2021;63:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peirano G, Asensi MD, Pitondo-Silva A, et al. . Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli from Rio de Janeiro, Brazil. Clin Microbiol Infect. 2011;17:1039–1043. doi: 10.1111/j.1469-0691.2010.03440.x [DOI] [PubMed] [Google Scholar]
- 40.Tang B, Ma Y, He X, et al. . Similar antimicrobial resistance of Escherichia coli strains isolated from retail chickens and poultry farms. Foodborne Pathog Dis. 2021;18:489–496. doi: 10.1089/fpd.2021.0019 [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Xu G, Ruan Z, et al. . Genomic characterization of a multidrug-resistant Escherichia coli isolate Co-carrying blaNDM-5 and blaCTX-M-14 genes recovered from a pediatric patient in China. Infect Drug Resist. 2022;15:6405–6412. doi: 10.2147/IDR.S388797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma T, Fu J, Xie N, et al. . Fitness cost of blaNDM-5-carrying p3R-IncX3 plasmids in wild-type NDM-free Enterobacteriaceae. Microorganisms. 2020;8:377. doi: 10.3390/microorganisms8030377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Tong M-K, Chow K-H, et al. . Occurrence of highly conjugative IncX3 epidemic plasmid carrying blaNDM in Enterobacteriaceae isolates in geographically widespread areas. Front Microbiol. 2018;9:2272. doi: 10.3389/fmicb.2018.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang QE, Ma X, Zeng L, et al. . Interphylum dissemination of NDM-5-positive plasmids in hospital wastewater from Fuzhou, China: a single-centre, culture-independent, plasmid transmission study. Lancet Microbe. 2023. doi: 10.1016/s2666-5247(23)00227-6 [DOI] [PubMed] [Google Scholar]
- 45.Lopatkin AJ, Meredith HR, Srimani JK, et al. . Persistence and reversal of plasmid-mediated antibiotic resistance. Nat Commun. 2017;8:1689. doi: 10.1038/s41467-017-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.