Abstract
Virus infection of animal cells can induce intracellular antiviral responses mediated by the induction of interferon-regulatory transcription factors (IRFs), which bind to and control genes directed by the interferon-stimulated response element (ISRE). The purpose of this study was to determine whether adenovirus (Ad) induces IRFs during infection, because they might play a role in promoting viral pathogenesis. Here we show that after the late phase of infection, Ad induces a transcription factor related to the IRF family of factors. The IRF is induced shortly after Ad entry into late phase and is shown to stimulate ISRE-directed transcription, to require activation by protein tyrosine kinase signalling, and to be induced several hours prior to the inhibition of cell protein synthesis. Inhibition of tyrosine kinase activity blocks Ad induction and activation of the IRF. Attempts to identify the Ad-induced factor immunologically and by photo-UV cross-linking indicate that it is likely a novel member of the IRF family. Finally, several independent lines of evidence also suggest that Ad induction of the IRF might correlate with the ability of the virus to block host cell protein synthesis later during infection.
Infection of animal cells by many viruses induces a variety of interferon-regulatory factors (IRFs), which bind the interferon-stimulated response element (ISRE) of interferon-inducible genes, thereby activating or repressing transcription. IRFs comprise a growing family of factors induced by interferons, cytokines, virus infection, cell stress, or double-stranded RNA (dsRNA). The family of IRFs includes IRF-1, IRF-2, IRF-3, IRF-4/Pip/LSIRF/ICSAT, IRF-5, IRF-6, IRF-7, interferon-stimulated gene factor 3γ (ISGF-3γ/p48), interferon consensus sequence binding protein, and dsRNA-activated factors known as DRAFs (3, 8, 9, 12, 17, 30, 50–54, 56; reviewed in reference 49). Although some IRFs such as IRF-1 and -3 are involved in control of interferon-inducible genes (34, 39, 53), other functions have also been described, including control of cell proliferation and differentiation (49). In this regard, IRF-1 can act directly as an antiproliferation factor (26, 48), and it can also activate the dsRNA protein kinase known as PKR (5, 29), in turn inhibiting protein synthesis by inactivating translation factor eIF-2 (42). The induction of at least some IRFs by viruses mediates some antiviral responses by the infected cell (40). In other cases, viruses can exploit activation of IRFs for the regulation of their own genes or possibly to benefit their replication programs by altering the proliferative state of the cell (7, 35, 46, 56). We therefore investigated whether adenovirus (Ad) infection is strongly associated with induction and activation of IRFs, particularly during the late phase, during which profound cytopathic changes in cells are mediated.
The late phase of Ad infection is separated from the early phase by replication of the viral DNA genome and the synthesis of large amounts of virion structural polypeptides. As the late phase of Ad infection progresses, there is a marked inhibition of cellular protein synthesis and RNA transport to the cytoplasm, in contrast to the exclusive transport and translation of late-Ad mRNAs (reviewed in reference 42). Ad late mRNAs bear a common 5′ noncoding region known as the tripartite leader (42), which confers on mRNAs the ability to be selectively transported and translated during late Ad infection, despite inhibition of cellular RNA transport and protein synthesis (6, 10, 11, 22, 31). The inhibition of host cell protein synthesis by Ad involves a poorly understood virus-induced inhibition of translation initiation factor eIF-4E (20). Factor eIF-4E is a 28-kDa m7GTP (cap)-binding protein. Together with an RNA helicase known as eIF-4A, and a large adapter protein known as eIF-4G, the 4E-4A-4G complex comprises translation initiation factor eIF-4F (reviewed in references 15 and 43). eIF-4F mediates mRNA unwinding from the 5′ cap, promoting ribosome entry and translation initiation. Ad has been shown to block phosphorylation of the majority of eIF-4E during late infection, thereby impairing eIF-4F activity, although the mechanism by which dephosphorylation inhibits eIF-4E and 4F activity is not well understood. It is known that phosphorylation of eIF-4E is strongly associated with increased eIF-4F activity and hence enhanced translation initiation, whereas dephosphorylation has the opposite effect (reviewed in reference 43). Late-Ad mRNAs are able to translate despite inhibition of eIF-4E phosphorylation because the tripartite leader promotes an unusual form of translation initiation known as ribosome shunting, which permits ribosomes to initiate via a nonlinear mechanism despite the loss of eIF-4F unwinding activity (55).
The mechanism by which Ad infection blocks the phosphorylation of eIF-4E and cellular mRNA translation is poorly understood. It has been established that Ad must enter the late phase of its replication cycle to block cell protein synthesis (48), and it must activate the viral late transcription unit (57). These results implicate one or more late viral gene products in the inhibition of eIF-4E phosphorylation and host cell translation. It is also known that inhibition of cell protein synthesis only occurs many hours after Ad has entered the late phase of its replication cycle (57). Thus, the cell has ample opportunity to institute the induction of antiviral responses that might block translation as a protective response. For this reason we investigated whether Ad infection, particularly the late phase, is associated with the induction of IRF binding factors. Here we demonstrate that Ad induces a novel, transcriptionally active ISRE DNA-binding protein as it enters the late phase of infection. The ability of Ad to induce an ISRE binding protein was found to be correlated with its ability to activate tyrosine kinase signalling and inhibit cell protein synthesis.
MATERIALS AND METHODS
Viruses and cells.
Ad type 5 dl309 (Ad5dl309) is a phenotypically wild-type virus that contains a series of altered restriction enzyme cleavage sites (23). Ad5 ts125 (H5ts125) carries a temperature-sensitive mutation in the E2A-72-kDa DNA binding protein (13). 293 cells are a human embryonic kidney cell line transformed with the E1 region of Ad5 (16). HeLa and 293 cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum and 0.1 μg of gentamicin sulfate per ml. Recombinant virus Ad-E3L, which expresses the vaccinia virus E3L gene controlled by the cytomegalovirus promoter, was constructed as described previously (32, 47), in which the trans-gene replaces the Ad E1A and E1B regions. Titers of virus stocks were determined on 293 cells.
Analysis of polypeptides.
Cells were labeled by incubation for 1 h with [35S]methionine (NEN) at 50 μCi per ml in DMEM without methionine. Cells were lysed in 50 mM KCl–10 mM Tris-HCl (pH 7.4)–1 mM EDTA at 4°C by sonication and cleared of debris at 10,000 × g, and equal amounts of soluble S10 protein were subjected to sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography. Autoradiograms were quantitated with LinoColor data software.
Analysis of eIF-4E.
The state of eIF-4E phosphorylation was characterized by labeling cells in vivo with 100 μCi of carrier-free 32PO4 for 2 h at 37°C in 1 ml of phosphate-free DMEM per 6 cm plate of cells. Preparation of 32P-labeled extracts for cap affinity chromatography or immunoprecipitation with antibodies specific for eIF-4E was carried out as described previously (14). Equal amounts of eluted or immunoprecipitated protein were resolved by SDS-PAGE, visualized by autoradiography and quantitated with software as described above.
Analysis of protein kinase activities.
As a positive control for the activation of protein kinase C (PKC), cells were treated with 50 nM phorbol-13-myristate-12-acetate (PMA) for 30 min. PKC was inhibited by treatment of cells with 1 μM specific inhibitor calphostin C for 30 min prior to PMA treatment (27). Cytosolic and membrane fractions were prepared by centrifugation as described previously (28, 44). The soluble cytoplasmic fraction and the insoluble membrane fraction were resolved by SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose, and immunoblot analysis was performed with an affinity-purified antiserum directed against PKC α, β, or γ (a gift of A. Czernick, Rockefeller University). For analysis of tyrosine kinase activity, cells were treated with the specific inhibitor genistein (1) at the indicated concentrations. Ad-infected cells were treated prior to entry into late phase at 7 h or after entry at 12 h and then harvested at 22 h. Uninfected cells were treated for 10 h. The effect of genistein treatment on tyrosine kinase activity was determined by resolving equal amounts of cell lysates by SDS-PAGE, transferring them to a membrane, and immunoblotting with antiphosphotyrosine antibodies and enhanced chemiluminescence. The specific inhibitor H8 was used at 100 nM to block protein kinase A and cyclic-GMP-dependent kinases (19). Staurosporine was used at 500 nM to block PKC and partially block tyrosine kinases (9).
Gel mobility shift electrophoresis.
Nuclear extracts were prepared as described previously (2). For ISRE binding, protein-DNA complexes were formed by incubating 5 μg of nuclear protein with 1 ng of a 32P-labeled dsDNA probe at 25°C for 30 min in a 10-μl reaction volume containing 12 mM HEPES (pH 8.0)–50 mM KCl–10 mM EDTA–5% glycerol–0.2 mM dithiothreitol–2 μg of poly(dI-dC). Protein-DNA complexes were separated from free DNA probe in a 5.3% polyacrylamide gel containing 18 mM Tris-borate (pH 8.0)–0.4 mM EDTA. The dsDNA oligonucleotide containing the alpha interferon ISRE binding site corresponded to the sequence 5′-GATCGGGAAAGGGAAACCGAACTGAAGCC-3′. Supershift analysis was performed by incubation of specific antibodies in the reaction mix. IRF antibody was a generous gift of R. Pine (Public Health Laboratories, New York, N.Y.). p48, STAT1, and specific IRF-1 antibodies were the gift of D. Levy New York University School of Medicine, New York, N.Y.). IRF-3 antibody was the gift of N. Reich (State University of New York, Stony Brook). IRF-2 antibody was the gift of J. Vilcek (New York University School of Medicine). All antibodies were used at the maximum concentration shown to effectively and specifically block formation of respective DNA binding complexes by the contributors.
Photo-UV cross-linking.
For UV cross-linking, nuclear extracts from 16-h Ad-infected 293 cells were subjected to electrophoretic mobility shift assay (EMSA) with a 32P-labeled ISRE oligonucleotide exposed to 304-nm UV light for 45 min. ISRE binding complex was identified by autoradiography, extracted, and resolved by SDS-PAGE. 32P-labeled polypeptides were detected by PhosphorImager analysis (Molecular Dynamics).
Transfection and CAT assays.
293 cells were transfected with 10 μg of DNA by the calcium phosphate precipitation technique, followed 18 h later by infection with Ad dl309. Cells were lysed at the indicated times, and assays were carried out as described by the manufacturer (Green-CAT; Molecular Probes).
RESULTS
Ad induces an ISRE DNA binding factor during entry into the late phase of infection.
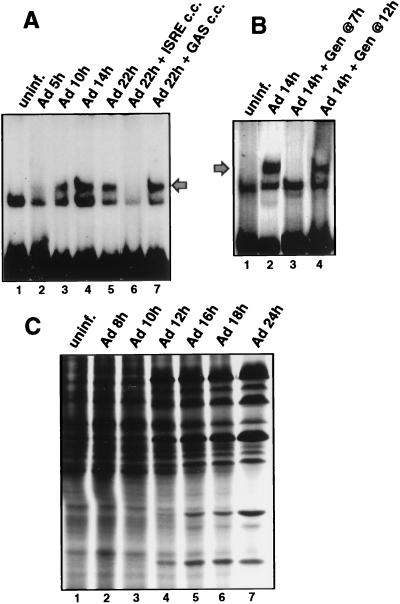
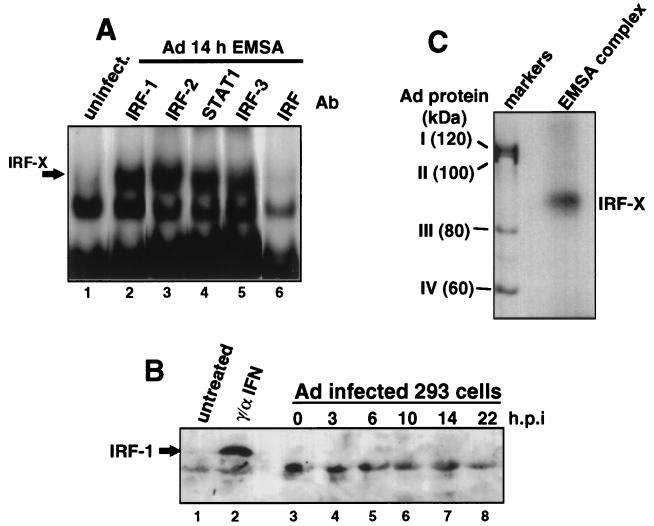
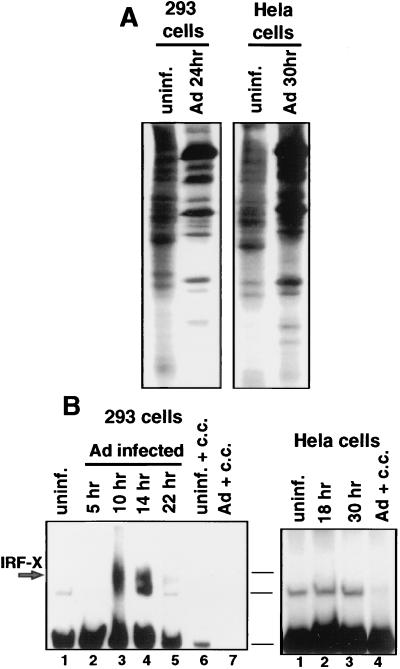
Reports have shown that dsRNA produced during viral infection (4, 8, 9, 51) and unknown stimuli of viral infection (7) can lead to the activation of transcription factors that bind to the alpha interferon ISRE in a tyrosine-kinase-dependent fashion. Although Ad has evolved several strategies to thwart some antiviral responses, such as E1A protein inhibition of ISGF3 activation or virion-associated RNA1 (VAI RNA) inhibition of the double-stranded RNA-activated protein kinase (PKR) (reviewed in reference 42), neither of these inhibitors excludes the possibility that the cell responds to Ad infection by activation of IRFs. We therefore determined whether an ISRE binding factor is activated during Ad infection. 293 cells were infected with wild-type Ad for various times, and nuclear extracts were prepared and examined for formation of ISRE DNA binding complexes (Fig. 1A). An EMSA was performed with nuclear extracts from Ad-infected cells with a 32P-labeled dsDNA oligonucleotide containing a single ISRE binding site as a probe. A binding protein was consistently induced by 10 h after Ad infection (lane 3). This corresponds to the first 1 to 2 h of the late phase of infection, determined by activation of viral DNA synthesis (data not shown) and appearance of late viral polypeptides (Fig. 1C), and it occurs many hours prior to the shutoff of cell protein synthesis. Induction of this factor (herein called IRF-X) is not a result of interferon production, because incubation of infected cells with blocking antibodies to alpha and beta interferons for the entire course of infection did not prevent formation of the IRF-X complex (data not shown). In addition, IRF-X was not induced upon cultivation of uninfected 293 cells with conditioned media from 16-h Ad-infected cells (data not shown), excluding the secretion of noninterferon cytokines as the source for induction of IRF-X. Previous studies showed that the cell response to dsRNA, some forms of stress, and interferon is transduced through tyrosine kinase signalling pathways, including the ability to induce or activate some IRFs (4, 9, 18, 41, 51). Inhibition of tyrosine kinase signalling was carried out by treating cells with genistein, a specific inhibitor of many tyrosine kinases (27), beginning just prior to viral entry into the late phase of infection. Genistein inhibited induction of the ISRE DNA binding activity mediated by Ad (Fig. 1B, lane 3). Genistein was used at a 100 μM concentration, which was shown later (Fig. 8) to suppress tyrosine kinase activity and is a level routinely used by others (27). If genistein was added to cells several hours after Ad entered late phase (Fig. 1B), it no longer blocked activation of ISRE DNA binding activity, indicating that induction of IRF-X occurs by stimulation of tyrosine kinase signalling shortly after Ad enters late phase. Ad was also found to induce factor IRF-X in cell lines other than 293 cells, such as Chang liver cells (data not shown), when the full viral infectious program was apparent. Therefore, induction of IRF-X by Ad is not likely to be cell type restricted and occurs independently of whether cells express the Ad E1A gene (in 293 cells). These data demonstrate that induction of an ISRE DNA binding factor by Ad occurs during the onset of the late phase of infection. Results presented later demonstrate that genistein treatment of infected cells, either before or after entry of the virus into late phase, does not significantly impair Ad replication or production of late viral polypeptides (Fig. 6).
FIG. 1.
Ad induces an ISRE DNA binding protein during the late phase of infection. 293 cells were infected with 20 PFU of Ad dl309 per cell, and nuclear extracts were prepared at the indicated times postinfection. Equal amounts of protein were used to measure ISRE DNA binding activity by EMSA with a 32P-labeled dsDNA oligonucleotide probe containing a consensus ISRE binding site. (A) Autoradiogram of EMSA during Ad infection. Cold competitor studies (c.c.) used a 100-fold molar excess of unlabeled dsDNA oligonucleotide containing one ISRE or gamma interferon-activated factor binding site (GAS). The fastest-migrating band is an unidentified unresponsive complex. (B) Ad-infected cells were treated with 100 μM genistein (Gen) at 7 h postinfection followed by EMSA as in panel A above. (C) Profile of Ad-mediated inhibition of host cell protein synthesis. 293 cells were labeled with [35S]methionine, and equal amounts of protein were resolved by SDS-PAGE and autoradiographed. uninf., uninfected.
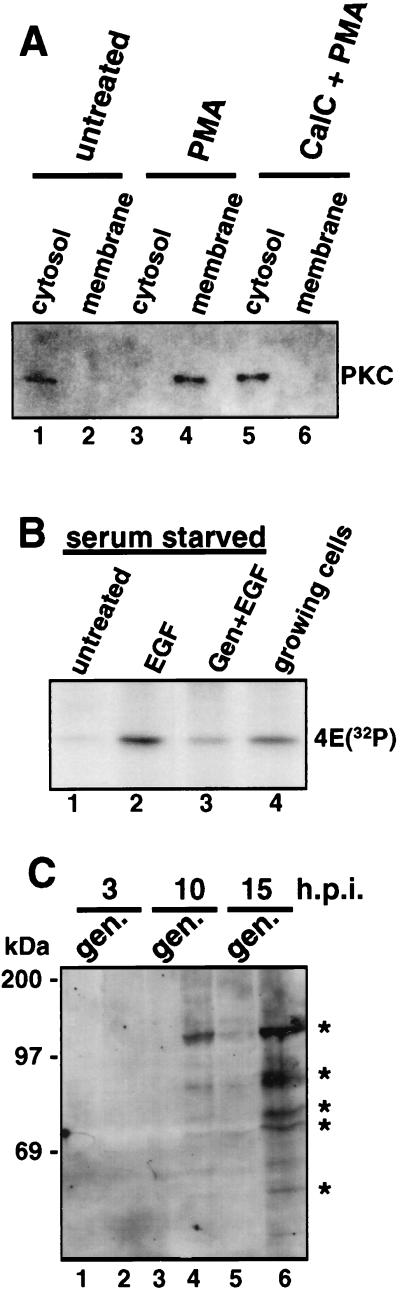
FIG. 8.
Control studies for effects of protein kinase inhibitors. (A) Uninfected 293 cells were treated with 50 nM PMA for 30 min with or without pretreatment with 1 μM calphostin C for 30 min. Cytosol and membrane fractions were prepared, and proteins were resolved by SDS-PAGE and immunoblotted with PKC-specific antibodies. (B) 293 cells were serum starved for 24 h by cultivation in DMEM with 0.5% serum, labeled with 32PO4 in phosphate-free DMEM, and then stimulated with 50 ng of human EGF per ml for the last 30 min of labeling with or without 100 μM genistein (Gen). 32P-labeled eIF-4E was immunoprecipitated and resolved by SDS-PAGE. (C) Ad-infected 293 cells were treated with 100 μM genistein (gen.) at the indicated times, and equal amounts of proteins from whole-cell lysates were resolved by SDS-PAGE, transferred to a membrane, and immunoblotted with antiphosphotyrosine antibodies.
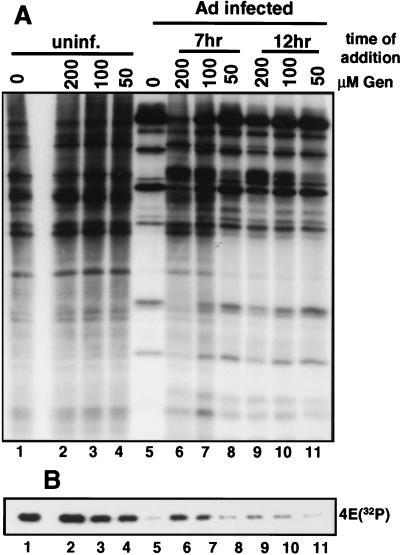
FIG. 6.
Effect of genistein (Gen) on Ad inhibition of host cell translation and block in eIF-4E phosphorylation. 293 cells were infected with 20 PFU of Ad dl309 per cell, and then uninfected (uninf.) and infected cells were treated with 0-, 50-, 100-, or 200-μM doses of genistein. Infected cells were treated with genistein at 7 h (lanes 6 to 8) or 12 h postinfection (lanes 9 to 11). Uninfected cells were treated for 10 h. Cells were labeled with [35S]methionine or 32PO4. (A) Equal amounts of [35S]methionine-labeled proteins were resolved by SDS-PAGE and autoradiographed. (B) Equal amounts of 32PO4-labeled proteins were subjected to cap affinity chromatography, and eIF-4E was eluted and resolved by SDS-PAGE and autoradiography.
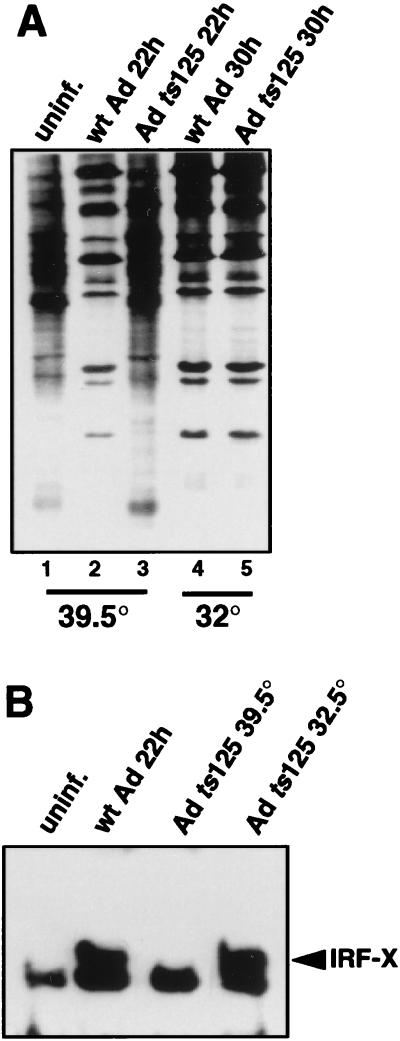
Infection of 293 cells with Ad DNA binding protein mutant ts125, which fails to enter the late phase of infection at the restrictive temperature of 39.5°C (Fig. 2A, compare lanes 3 and 6), also failed to induce the ISRE binding factor at the restrictive temperature (Fig. 2B). Infection of 293 cells with Ad ts125 at the nonrestrictive temperature of 32°C demonstrated that when the virus enters late phase, evidenced by exclusive late viral polypeptide synthesis, it induces IRF-X. These results confirm that Ad must enter the late phase of infection and express late viral genes to induce IRF-X.
FIG. 2.
Analysis of ISRE DNA binding activity in the absence of the late phase of Ad infection. 293 cells were infected with 20 PFU of Ad dl309 per cell at 37°C for 22 h or with Ad ts125 at a restrictive (39.5°C) or a nonrestrictive temperature (32°C) for 30 or 22 h, respectively. (A) Cells were labeled with [35S]methionine for the last hour of infection, and equal amounts of proteins were resolved by SDS-PAGE and autoradiography. (B) Nuclear extracts were prepared, and EMSA was performed with a 32P-labeled ISRE dsDNA oligonucleotide as described in the legend to Fig. 1. uninf., uninfected; wt, wild type.
The Ad-induced IRF-X factor is a novel ISRE-binding protein.
The possibility that IRF-X may be a known ISRE binding protein was investigated. In order to identify the component(s) of the Ad-induced factor, antibodies to known ISRE binding factors were examined for their ability to ablate IRF-X DNA binding (Fig. 3A). Antibodies directed to the STAT1 component of ISGF3 were unable to compete or supershift the Ad-induced ISRE-IRF complex. Antibodies that specifically recognize IRF-1, -2, and -3 also had no effect on the formation of the Ad-induced ISRE complex. Specificity of several antibodies was verified by the ability to ablate or supershift complexes induced by alpha and gamma interferons (anti-IRF-1 and anti-STAT-1) or to supershift complexes in untreated cells (anti-IRF-2) (data not shown). However, a polyclonal antiserum prepared against IRF-1 which predominantly recognizes the common ISRE binding motif shared by IRFs (37) was able to ablate formation of the Ad-induced DNA binding complex (Fig. 3A, lane 6). These results suggest that the Ad-induced DNA binding factor might be an IRF. Immunoblot analysis confirmed that the Ad-induced factor is not IRF-1. Studies have shown that IRF-1 is typically induced by new synthesis of IRF-1 protein, which is undetectable in uninduced cells (37). The 56-kDa IRF-1 protein was not detectable by immunoblot analysis at any time point examined during the entire Ad infectious cycle, whereas it was easily identified in uninfected HeLa cells treated with alpha and gamma interferons (Fig. 3B, compare lane 2 to lanes 3 to 8). The lower molecular weight band is a cross-reactive polypeptide observed previously (37).
FIG. 3.
Composition of Ad-induced ISRE DNA binding complex. (A) 293 cells were infected with 20 PFU of Ad dl309 per cell or left uninfected (uninfect.). Nuclear extracts were prepared, and ISRE DNA binding activity was determined by EMSA with a 32P-labeled dsDNA ISRE oligonucleotide. Specific antibodies were added to binding reactions as indicated with concentrations shown in previous reports to block respective DNA binding complexes. Ab, antibody. (B) 293 cells were infected with 20 PFU of Ad dl309 per cell for the times indicated (hours postinfection [h.p.i.]) or left untreated. HeLa cells were treated with alpha and gamma interferons as described in Materials and Methods. Equal amounts of whole-cell protein were resolved by SDS-PAGE and then immunoblotted with antibodies specific for IRF-1. The faster-migrating lower band is a cross-reacting polypeptide observed previously to bind IRF-1 antisera (37). (C) The ISRE DNA binding complex from 16-h Ad-infected 293 cells was irradiated for 45 min at 304 nm while in the gel and then extracted and subjected to SDS-PAGE and autoradiography. [35S]methionine-labeled late-Ad polypeptides were used as molecular size markers.
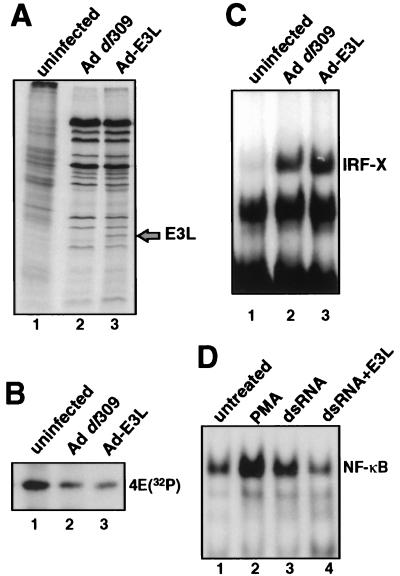
The Ad-induced IRF complex was further characterized by determining the approximate molecular weight of the DNA binding component. To do so, EMSA was performed with 16-h Ad-infected cells, the IRF-X protein-DNA complex was photo-UV cross-linked to the 32P-labeled ISRE probe, and the IRF-X complex was detected by autoradiography, extracted from the gel, and resolved by SDS-PAGE. [35S]methionine-labeled Ad-late proteins were coelectrophoresed in an adjacent lane as molecular weight markers (Fig. 3C). A protein was consistently cross-linked to the ISRE, made visible due to its covalent attachment to the 32P-labeled DNA. The unbound 32P-dsDNA probe migrates at 20 kDa in this system (data not shown), indicating that the IRF-X DNA binding protein is approximately 70 to 75 kDa in size. This molecular size is similar to the approximate molecular size of the smaller dsRNA-induced DRAFs (8, 9, 51). However, comparison of the electrophoretic migration of the IRF-X DNA complex with that of DRAF-1 and -2 (DRAF extract provided by N. Reich) showed that they do not have the same electrophoretic mobility (data not shown). DRAF-1 is now known to consist of IRF-3 and the p300 coactivator protein (51), and IRF-X clearly possesses a different molecular weight from that of either of these two DNA binding factors. In addition, we have not been able to detect induction of the DRAFs in wild-type Ad-infected cells. It is known that E1A blocks DRAF induction during infection, which is typically observed by infecting with Ad E1A mutant dl312 (9). The inability to observe DRAF-1 or -2 during wild-type Ad infection of 293 cells (which express viral E1A) is therefore not surprising. Further evidence that IRF-X is not a dsRNA-activated factor was provided by construction of a recombinant Ad virus which expresses large amounts of the vaccinia virus dsRNA scavenger E3L protein (Ad-E3L) (Fig. 4A to D). Comparison of 293 cells infected with wild-type Ad or Ad-E3L demonstrated no significant difference in entry into late phase, as shown by inhibition of cell protein synthesis and selective translation of late viral mRNAs (Fig. 4A) and dephosphorylation of eIF-4E (Fig. 4B). Ad-E3L still induced IRF-X by 14 h postinfection (Fig. 4C), again excluding viral dsRNA as the activator of IRF-X. As an important control, E3L protein was found to effectively inhibit the ability of dsRNA to induce the activation of NF-κB. This was shown by cotransfection of an E3L expression plasmid with dsRNA into 293 cells (Fig. 4D). In the absence of E3L expression, strong NF-κB DNA binding activity was detected in cells transfected with 20 μg of poly(rI-rC) per ml (Fig. 4D, lane 3), whereas with coexpression of E3L protein the activation of NF-κB by dsRNA was largely reduced (lane 4) and similar to that of untreated control cells (lane 1). The dsRNA activation assay could not be carried out on Ad-infected cells because Ad VAI RNA itself blocks dsRNA activation of NF-κB independently of E3l protein. It is therefore unlikely that IRF-X is induced by dsRNA derived from opposing Ad transcription units.
FIG. 4.
Viral dsRNA does not induce formation of IRF-X or dephosphorylation of eIF-4E. A recombinant Ad virus was developed which expresses the vaccinia virus E3L dsRNA scavenging protein as a left-end substitution for the Ad E1 region (Ad-E3L; see Materials and Methods). 293 cells were infected with 20 PFU of either Ad dl309 or Ad-E3L per cell. (A) Cells were labeled at 22 h postinfection with [35S]methionine, and equal amounts of protein were resolved by SDS-PAGE and autoradiography. (B) Cells were labeled with 32PO4 at 22 h postinfection, and eIF-4E was purified by immunoprecipitation with specific antibodies and resolved by SDS-PAGE and autoradiography. (C) EMSA was performed with nuclear extracts from cells infected for 16 h with Ad dl309 or Ad-E3L and a 32P-labeled ISRE probe. The position of IRF-X is identified. (D) 293 cells were transfected with 20 μg of poly(rI-rC) dsRNA per ml with or without cotransfection of a plasmid expressing E3L under the control of the cytomegalovirus promoter. Eighteen hours later, nuclear extracts were prepared, and NF-κB DNA binding activity was determined by EMSA with a 32P-labeled dsDNA oligonucleotide probe, as described previously (45). PMA was used at 50 nM for 30 min.
IRF-X is a transcriptional activator.
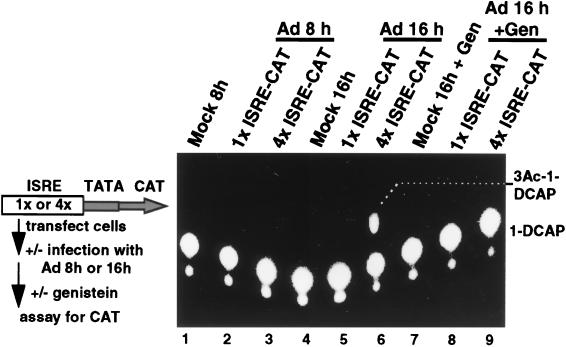
The potential function of IRF-X was assessed by determining whether it is a positively or negatively acting transcription factor. 293 cells were transfected with a CAT reporter driven by one or four copies of the ISRE and a basic TATA element (1×ISRE-CAT or 4×ISRE-CAT, respectively) to determine the strength of IRF-dependent DNA binding activation. Cells were infected with Ad, and transcriptional activation was determined during the period of Ad infection. Early virus infection (8 h) had no stimulatory effect on IRF-directed transcription (Fig. 5, lanes 2 and 3), whereas late Ad infection (assayed at 16 h) induced a marked activation of transcription from the 4×ISRE-CAT construct (lane 6). Treatment of infected cells with genistein from 7 h postinfection blocked IRF-directed transcription (lane 9), in accord with the inhibition of IRF-X DNA binding activity shown earlier (Fig. 1). No activation was observed during late Ad infection from the reporter containing one copy of the ISRE, indicating that IRF-X is a moderate activator of transcription. Since the only ISRE DNA binding factor which is induced during Ad infection is IRF-X, transcriptional activation of the 4×ISRE-CAT reporter must correspond to binding and activation by IRF-X. Therefore, these results demonstrate that Ad activates a modestly strong ISRE-directed transcription factor during the late phase of infection.
FIG. 5.
Ad-induced IRF-X activates transcription directed by the ISRE. 293 cells were transfected with plasmids encoding the CAT reporter controlled by a basic TATA element and one or four copies of the ISRE inserted immediately upstream of the core promoter. Cells were infected 18 h later with 20 PFU of Ad dl309 per cell, and the level of CAT activity was determined at either 8 or 16 h postinfection. Cells were treated with 100 μM genistein (Gen) at 7 h postinfection and harvested at 16 h. CAT activity was determined with a fluorescent substrate as described by the manufacturer (Molecular Probes). Lanes labeled mock were transfected with the 4×ISRE-CAT construct. Typical results are shown from three independent assays.
Possible role for induction of IRF-X.
The specific tyrosine kinase inhibitor genistein was found to block IRF-X induction by Ad (Fig. 1). In the course of performing control studies to determine the effect of genistein treatment on Ad infection, we serendipitously found that inhibition of tyrosine kinase signalling also blocked the ability of Ad to inhibit cell protein synthesis without significantly impairing Ad replication or late gene expression, suggesting but not providing a potential link between the two events. Cells were infected with wild-type Ad dl309 and treated with various concentrations of genistein at either 7 or 12 h postinfection and then allowed to proceed fully into the late phase of infection (22 h). During late phase, cells were metabolically labeled with [35S]methionine to determine the extent of translation shutoff or with 32PO4 to examine the level of eIF-4E phosphorylation. As shown in Fig. 6A, genistein treatment of cells at 7 h postinfection (2 to 3 h before virus entry into late phase; Fig. 1C) largely prevented Ad-induced shutoff of host cell translation in a dose-dependent manner, without strongly impairing the synthesis of late viral polypeptides (three- to fourfold reduction; lanes 5 to 7). The dephosphorylation of eIF-4E tracked with the extent of host translation shutoff (Fig. 6B, lanes 5 to 7), which was largely but not completely blocked in infected cells treated with genistein prior to Ad entry into late phase. Genistein treatment of infected cells reduced only slightly the level of viral DNA replication (approximately threefold), in accord with the level of late-Ad polypeptide synthesis (data not shown). The slight decrease in viral replication and late polypeptide synthesis caused by genistein treatment is unlikely to be responsible for the failure to inhibit eIF-4E phosphorylation, because it is not a significant reduction compared to the normal variation in viral replication levels in which shutoff occurs. When genistein was added 2 to 3 h after Ad entry into late phase (12 h postinfection), there was only a slight reduction in the Ad-induced block to eIF-4E phosphorylation and cell translation (Fig. 6A and B, lanes 9 to 11) and no detectable effect on viral replication levels (data not shown). These results indicate that induction of tyrosine kinase activity shortly after Ad entry into the late phase of infection is required for activation of IRF-X DNA binding activity and for inhibition of cell protein synthesis, although the two events may not be directly coupled.
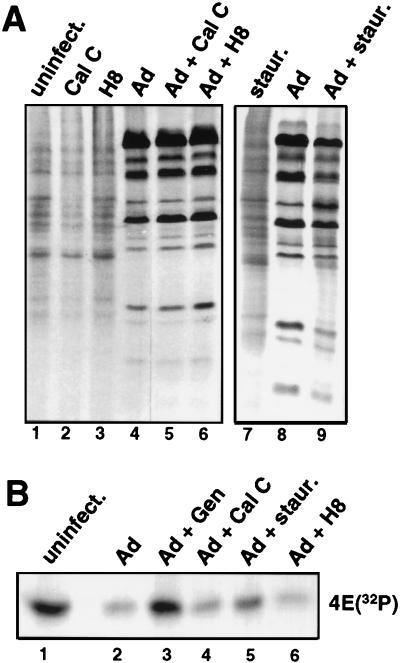
The role of other known classes of protein kinases, such as PKC, PKA, and cyclic-GMP-dependent protein kinases, was determined by using specific chemical inhibitors. Calphostin C is a specific inhibitor of PKC, H8 inhibits both PKA and cyclic-GMP-dependent kinases, and staurosporine can inhibit PKC at low doses and tyrosine kinases to some extent at high doses. Parallel plates of Ad-infected cells were treated with established effective concentrations of the various kinase inhibitors (1 μM calphostin C, 100 μM H8, 500 nM staurosporine) at 7 h postinfection and labeled at late times with 32PO4, followed by cap affinity purification, or with [35S]methionine, followed by resolution of proteins by SDS-PAGE. None of these inhibitors had any effect on Ad-induced shutoff of host cell protein synthesis (Fig. 7A) or dephosphorylation of eIF-4E (Fig. 7B). The exception was a high dose of staurosporine, which had a moderate inhibitory effect on shutoff of host translation and dephosphorylation of eIF-4E. This effect is probably related to the ability of high doses of staurosporine to partially inhibit tyrosine kinases (9).
FIG. 7.
Effect of serine-threonine protein kinase inhibitors on Ad shutoff of cell translation and eIF-4E phosphorylation. Uninfected 293 cells or cells infected with 20 PFU of Ad dl309 per cell or left uninfected (uninfect.) were treated at 7 h postinfection with 1 μM calphostin C, 100 μM H8, or 500 nM staurosporine (staur.) and then labeled with [35S]methionine or 32PO4 at 22 h after infection. (A) Equal amounts of [35S]methionine-labeled proteins were resolved by SDS-PAGE and autoradiographed. (B) Equal amounts of 32PO4-labeled proteins were subjected to cap affinity chromatography, and eIF-4E was eluted and resolved by SDS-PAGE and autoradiography.
Control studies demonstrated that calphostin C and genistein were used at specific inhibitory concentrations. Active PKC is localized to the cell membrane, whereas the inactive form resides in the cytoplasm. As shown in Fig. 8A, PKC was mobilized from the cytoplasm (lane 1) to the membrane (lane 4) upon stimulation with phorbol ester, which is blocked by calphostin C treatment (lanes 5 and 6). Genistein effectiveness was assessed by the ability to block epidermal growth factor receptor tyrosine kinase stimulation of eIF-4E phosphorylation (14). As shown in Fig. 8B, epidermal growth factor stimulation led to increased eIF-4E phosphorylation, which was blocked by pretreatment with genistein (lanes 2 and 3). The ability of H8 to inhibit PKA was assayed with a commercial peptide phosphorylation kit (Upstate Biotechnology Inc.) (data not shown). These results indicate that the concentrations of agents used in this study inhibited their respective protein kinase families but that only inhibition of tyrosine kinase activity both blocked induction of IRF-X and inhibited the shutoff of cell protein synthesis by Ad.
Activation of protein tyrosine kinase activity during Ad infection resulted in tyrosine phosphorylation of several proteins in infected cells. Tyrosine phosphorylated proteins were detected during Ad infection by Western immunoblot analysis with antiphosphotyrosine antibodies (Fig. 8C). By 10 h postinfection, Ad induced strong tyrosine phosphorylation of two proteins with molecular masses of ∼120 and ∼85 kDa, which were blocked by prior genistein treatment. By 15 h postinfection, several additional tyrosine-phosphorylated proteins were detected. The identities of the tyrosine-phosphorylated proteins are under investigation. However, these results indicate that strong tyrosine kinase activity is induced during the onset of the late phase of Ad infection, which occurs coincident with activation of IRF-X and might be associated with the ability of the virus to block eIF-4E phosphorylation and host cell protein synthesis.
Certain lines of cells, including some HeLa cell lines, are resistant to Ad-mediated shutoff of host protein synthesis (21, 36, 56). These cells do not undergo dephosphorylation of eIF-4E, despite entry into the late phase of infection and synthesis of the full repertoire of late viral polypeptides (56). One of these lines of HeLa cells was used as an independent approach toward examining a possible link between activation of tyrosine kinase activity, induction of IRF-X, and inhibition of cell protein synthesis. 293 cells or HeLa cells that are resistant to Ad shutoff (21, 36, 56) were infected with Ad and labeled with [35S]methionine during late phase, and proteins were resolved by SDS-PAGE (Fig. 9A). As expected, Ad failed to block cell protein synthesis in these HeLa cells, even during very late times of infection (the infection rate is slower in HeLa cells than in 293 cells). The ability of Ad to induce IRF-X DNA binding activity in HeLa cells was examined in the absence of shutoff of host protein synthesis (Fig. 9B). Ad induced strong IRF-X DNA binding activity in 293 cells by 10 h after infection but not in HeLa cells shortly after entry into late phase (18 h) or thereafter (30 h). Taken collectively, the results presented in Fig. 8 and 9 provide two independent lines of evidence which suggest that the ability of Ad to induce tyrosine kinase activity and IRF-X DNA binding activity shortly after entry into late phase correlates with the ability to block host cell protein synthesis by impairing the phosphorylation of eIF-4E.
FIG. 9.
Analysis of Ad-induced ISRE DNA binding activity in HeLa cells resistant to Ad inhibition of host protein synthesis. 293 cells or a line of HeLa cells which are resistant to Ad inhibition of cell protein synthesis (36, 57) were infected with 20 PFU of Ad dl309 per cell for 22 h (293 cells) or 30 h (HeLa cells). (A) Cells were labeled with [35S]methionine, and equal amounts of whole-cell protein were resolved by SDS-PAGE and autoradiography. (B) EMSA was performed with a 32P-labeled ISRE oligonucleotide and equal amounts of nuclear extracts from uninfected (uninf.) or infected 293 or HeLa cells. Cold competitor (c.c.) inhibition was carried out with a 100-fold molar excess of unlabeled dsDNA ISRE probe. The fastest-migrating band is an unresponsive and unknown binding complex. Horizontal lines indicate the positions of the same complexes in both gels.
DISCUSSION
IRF-X is a new IRF-related factor.
This study has identified a novel ISRE binding protein, referred to as IRF-X, which is induced by Ad as the virus enters the late phase of its infectious cycle (Fig. 1). Induction of the DNA binding activity of IRF-X requires that Ad activate and express its late transcription unit (Fig. 2). Prolonged propagation of mutant Ad ts125, which remains in the early phase of the infectious cycle at the restrictive temperature of 39.5°C, failed to induce IRF-X binding to the ISRE (Fig. 2). Induction of IRF-X DNA binding activity is therefore linked to the expression of one or more Ad late genes or to a downstream effect of late viral replication. Characterization of IRF-X has shown that it is probably related to the IRF family of factors, all of which bind to the alpha interferon ISRE. Antisera specific for known ISRE binding proteins all failed to block or supershift binding of the Ad-induced factor as determined by EMSA (Fig. 3A). However, a polyclonal antiserum prepared against IRF-1 which also recognizes the common IRF DNA binding motif (37) did prevent formation of the Ad-induced ISRE binding protein. In addition, Western immunoblot analysis indicated that the IRF-1 protein is not detectably induced during Ad infection (Fig. 3B). The DNA binding component of IRF-X possesses a predicted molecular size of 70 to 75 kDa based on photo-UV cross-linking to a 32P-labeled ISRE (Fig. 3C). This molecular size excludes most known ISRE binding proteins, including IRF-1 (56 kDa), DRAF-1 (IRF-3/p300), and the vesicular stomatitis virus-induced factor known as VIBP (7). However, the trimeric factor IRF-7 contains three DNA binding polypeptides of 69, 67, and 23 kDa (IRF-7A, -7B, and -7C, respectively) (35, 56), the largest of which might be a presumptive candidate. Nevertheless, IRF-X is probably not IRF-7 for several reasons. First, we did not detect multiple ISRE DNA binding proteins, typical of IRF-7, by photo-UV cross-linking analysis but rather, a single 70- to 75-kDa polypeptide. Second, IRF-7 is thought to be tissue restricted, found predominantly in B cells, peripheral blood leukocytes, spleen, and thymus (56). Our studies were carried out with kidney and liver cells. Third, IRF-7 is interferon inducible and parallels activation of the interferon-stimulated gene 54 (ISG-54) (35). On the other hand, we could never detect activation of ISG-54 or -15 during late Ad infection (14a). These data therefore exclude the possibility that IRF-X is IRF-7, IRF-3, or other IRFs that activate ISG-15 or -54. Fourth, IRF-7 has been shown to be a transcription repressor upon binding to the ISRE (56), whereas IRF-X activates ISRE-directed transcription. Therefore, by all measures the Ad-induced IRF-X appears to be a novel and positively acting IRF. Definitive identification of IRF-X will require its cloning and sequencing.
IRF-X is probably not induced by dsRNA.
Studies have shown that Ad might produce dsRNA from opposing transcription units after it enters the late phase of infection (33). Ad encodes two small RNA polymerase III-transcribed RNAs, known as VA RNAs I and II, that counter the antiviral effects triggered by viral dsRNA (reviewed in reference 42). In the absence of VAI RNA, PKR is activated and translation is inhibited by phosphorylation of the eIF-2α subunit (42). VAI RNA is a very effective inhibitor of PKR activation, although a slight activation might still occur during late Ad infection (21, 36). The potential presence of viral dsRNA during late Ad infection and the reported ability of transfected dsRNA to induce the ISRE binding proteins DRAF-1 and -2 (8, 9, 51) led us to investigate the possibility that IRF-X might be dsRNA induced and perhaps identical to the smaller DRAF-2 factor. However, by a number of criteria it is apparent that IRF-X is neither a dsRNA-induced factor nor a DRAF. First, DRAF induction during Ad infection is observed only if an E1A-deleted Ad mutant is used (8), consistent with our inability to observe induction during wild-type Ad infection. In addition, IRF-X was detected irrespective of E1A expression. Second, the EMSA profiles for IRF-X and DRAF-1 and -2 are not similar (14a). Third, overexpression of the vaccinia virus E3L dsRNA scavenging protein did not impair induction of IRF-X (Fig. 4). We therefore conclude that while Ad probably generates dsRNA during the late phase of infection, it is unlikely to be the activator of IRF-X, and it is not likely that IRF-X is a DRAF.
IRF-X is induced by Ad by tyrosine kinase activation.
A variety of stimuli can activate or induce some ISRE-binding proteins such as ISGF3γ and DRAF-1 and -2, all of which require tyrosine phosphorylation events (4, 9, 18, 24, 38). We therefore investigated whether Ad induction of IRF-X utilized a tyrosine kinase signalling pathway. Specific inhibition of tyrosine kinase signalling with the agent genistein blocked Ad induction of IRF-X, but only when it was added prior to viral entry into the late phase of infection (Fig. 1B). We interpret these results as indicating that activation or new induction of IRF-X by a tyrosine kinase signalling pathway occurs very rapidly upon viral entry into late phase. The kinetics for Ad entry into late phase in 293 cells, determined by expression of late viral genes (Fig. 1C and references 57 and 58), and induction of IRF-X DNA binding activity, occur between 9 and 10 h postinfection (Fig. 1A). We presume that the critical tyrosine kinase event involves upstream induction of IRF-X rather than direct tyrosine phosphorylation of this factor because anti-tyrosine phosphate antibody could not supershift the IRF-X–ISRE complex, whereas it could supershift ISGF3 (data not shown). We do not know the importance of the several polypeptides which become detectably tyrosine phosphorylated concomitant with Ad entry into late phase and induction of IRF-X DNA binding activity at 10 h postinfection (Fig. 8). It is also not yet known whether these are cellular and/or viral polypeptides.
Ad induction of IRF-X might be related to inhibition of cell protein synthesis.
Two independent lines of evidence suggest a possible connection between induction of IRF-X by Ad and inhibition of eIF-4E phosphorylation and cell mRNA translation. First, a line of HeLa cells which are resistant to Ad shutoff of cell translation (21, 36, 57) also do not demonstrate inducible IRF-X (Fig. 9). Ad replication levels and late viral polypeptide synthesis are normal in these cells. The genetic lesion in these cells which prevents Ad shutoff of translation is not known, but it is intriguing that these cells lack activated PKR (21, 36). The PKR signalling pathway has been strongly implicated as an essential component for induction or activation of IRF-1 (25, 29). This raises the possibility that induction of IRF-X might similarly utilize components of this pathway. However, inhibition of dsRNA signalling by overexpression of the E3L protein had no effect on Ad induction of IRF-X or shutoff of cell protein synthesis. This therefore argues that PKR itself is unlikely to be vital for inhibition of cell protein synthesis, but perhaps other components of the dsRNA-PKR signalling pathway are critical. The second line of evidence consists of the similar requirements for Ad induction of IRF-X DNA binding activity and eIF-4E dephosphorylation. Ad activation of tyrosine kinase signalling early during entry into the late phase of infection is essential to induce both IRF-X and eIF-4E dephosphorylation. Inhibition of tyrosine kinase activation must occur prior to entry into late phase to effectively block both, indicating that IRF-X induction and later dephosphorylation of eIF-4E involve very early activation of tyrosine kinase activity. Inhibition of other protein kinases had no effect on the ability of Ad to induce IRF-X or dephosphorylate eIF-4E. It is possible that Ad induction of IRF-X has no causal relationship to the later inhibition of eIF-4E phosphorylation. On the other hand, given the convergence of independent lines of evidence, it is also possible that the two events might be associated.
In summary, we have identified a novel IRF which is induced by Ad as it enters the late phase of infection. This factor appears to be a transcription activator, and it might be involved in viral inhibition of cell protein synthesis. We do not believe that IRF-X itself plays a direct role in Ad late gene expression, because the virus replicates normally and expresses late genes in the absence of IRF-X induction. We do not know whether induction of IRF-X is unique to Ad-infected cells. Although activation of certain IRF-like factors may be restricted to infection by specific viruses, such as VIBP in vesicular stomatitis virus-infected cells (7), in other cases virus-infected cells contain several commonly induced IRFs. This is clearly the case in Epstein-Barr virus-infected cells in which IRF-1 and -7 are induced (35, 56). Stress itself can induce IRF-like factors in the case of explanted herpes simplex virus-infected neurons, in which a large number of mRNAs which correspond to ISG-stimulatory factors and IRFs are induced (46). Our studies are now directed to the molecular identification of IRF-X and to determining how its induction is likely coupled to the Ad-mediated block to cell protein synthesis.
ACKNOWLEDGMENTS
We thank D. Levy (NYU) for the ISRE expression plasmids and antibodies to STATs and IRF-1, N. Reich (SUNY) for DRAF binding extracts and IRF-3 antibodies, and R. Pine (Public Health Research Institute) for IRF antisera. Many thanks to G. Laroia and R. Cuesta of this lab for critical review of the manuscript.
This work was supported by a grant from the National Institutes of Health to R.J.S. (CA 42357) and to the Kaplan Cancer Center core facility.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Andrews N C, Faller D V. A rapid micropreparation technique for extraction of DNA binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au W C, Moore P A, Lowther W, Juang Y T, Pitha P M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S K, Leonard G T, Bandyopadhyay T, Stark G R, Sen G C. Transcriptional induction by doublestranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J Biol Chem. 1995;270:19624–19629. doi: 10.1074/jbc.270.33.19624. [DOI] [PubMed] [Google Scholar]
- 5.Beretta L, Gabbay M, Berger R, Hanash S M, Sonenberg N. Expression of the protein kinase PKR is modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene. 1996;12:1593–1596. [PubMed] [Google Scholar]
- 6.Berkner K E, Sharp P A. Effect of tripartite leader on synthesis of a non-viral protein in an adenovirus 5′ recombinant. Nucleic Acids Res. 1985;13:841–857. doi: 10.1093/nar/13.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bovolenta C, Lou J, Kanno Y, Park B-K, Thornton A M, Coligan J E, Schubert M, Ozato K. Vesicular stomatitis virus infection induces a nuclear DNA-binding factor specific for the interferon-stimulated response element. J Virol. 1995;69:4173–4181. doi: 10.1128/jvi.69.7.4173-4181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly C, Reich N C. Characterization of specific DNA-binding factors activated by double-stranded RNA as positive regulators of interferon α/β-stimulated genes. J Biol Chem. 1995;270:23739–23746. doi: 10.1074/jbc.270.40.23739. [DOI] [PubMed] [Google Scholar]
- 9.Daly C, Reich N C. Double-stranded RNA activates novel factors that bind to the interferon-stimulated response element. Mol Cell Biol. 1993;13:3756–3764. doi: 10.1128/mcb.13.6.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolph P J, Huang J, Schneider R J. Translation by the adenovirus tripartite leader: elements which determine independence from cap-binding protein complex. J Virol. 1990;64:2669–2677. doi: 10.1128/jvi.64.6.2669-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolph P J, Racaniello V, Villamarin A, Palladino F, Schneider R J. The adenovirus tripartite leader eliminates the requirement for cap binding protein during translation initiation. J Virol. 1988;62:2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenbeis C F, Singh H, Storb U. Pip, a novel IRF family member is a lymphoid specific PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 13.Ensinger M, Ginsberg H. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972;10:328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feigenblum D, Schneider R J. Cap-binding protein (eukaryotic initiation factor 4E) and 4E-inactivating protein BP-1 independently regulate cap-dependent translation. Mol Cell Biol. 1996;16:5450–5457. doi: 10.1128/mcb.16.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Feigenblum, D., and R. J. Schneider. Unpublished results.
- 15.Frederickson R M, Sonenberg N. eIF-4E phosphorylation and the regulation of protein synthesis. In: Ilan J, editor. Translational regulation of gene expression. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 143–162. [Google Scholar]
- 16.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 17.Grant C E, Vasa M Z, Deeley R G. cIRF3, a new member of the interferon regulatory factor (IRF) family that is rapidly and transiently induced by dsRNA. Nucleic Acids Res. 1995;23:2137–2146. doi: 10.1093/nar/23.12.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutch M J, Daly C, Reich N C. Tyrosine phosphorylation is required for activation of an α interferon-stimulated transcription factor. Proc Natl Acad Sci USA. 1992;89:11411–11415. doi: 10.1073/pnas.89.23.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidaka H, Inagaki M, Kawamoto S, Sasari Y. Isoquinoline sulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1989;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Schneider R J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap binding protein. Cell. 1991;65:271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Schneider R J. Adenovirus inhibition of cellular protein synthesis is prevented by the drug 2-aminopurine. Proc Natl Acad Sci USA. 1990;87:7115–7119. doi: 10.1073/pnas.87.18.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Flint S J. The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA transport. J Virol. 1998;72:225–235. doi: 10.1128/jvi.72.1.225-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 24.Kessler D S, Levy D E. Protein kinase activity required for an early step in interferon-alpha signaling. J Biol Chem. 1991;266:23471–23476. [PubMed] [Google Scholar]
- 25.Kirchoff S, Koromilas A E, Schaper F, Martina G, Sonenberg N, Hauser H. IRF-1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase PKR. Oncogene. 1995;11:439–445. [PubMed] [Google Scholar]
- 26.Kirchoff S, Schaper F, Hauser H. Interferon regulatory factor 1 (IRF-1) mediates cell growth inhibition by transactivation of downstream target genes. Nucleic Acids Res. 1993;21:2881–2889. doi: 10.1093/nar/21.12.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C, a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 28.Kraft A S, Anderson W B. Phorbol esters increase the amount of CA2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Yang Y-L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin R, Heylbroeck C, Pitha P M, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan J, Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci USA. 1984;81:3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucito R, Schneider R J. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J Virol. 1992;66:983–991. doi: 10.1128/jvi.66.2.983-991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maran A, Mathews M B. Characterization of the double-stranded RNA implicated in inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA I. Virology. 1988;164:106–113. doi: 10.1016/0042-6822(88)90625-3. [DOI] [PubMed] [Google Scholar]
- 34.Matsuyama T, Grossman A, Mittrucker H W, Siderovski D P, Kiefer F, Kawakami T, Richardson C D, Taniguchi T, Yoshinaga S K, Mak T W. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE) Nucleic Acids Res. 1995;23:2127–2136. doi: 10.1093/nar/23.12.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nonkwelo C, Ruf I K, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Malley R P, Duncan R F, Hershey J W B, Mathews M B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus infected cells. Virology. 1989;168:112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- 37.Pine R, Decker T, Kessler D S, Levy D E, Darnell J E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990;10:2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reich N C, Pfeffer L M. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc Natl Acad Sci USA. 1990;87:8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis L F L, Harada H, Wolchok J D, Taniguchi T, Vilcek J. Critical role of common transcription factor IRF-1 in the regulation of IFN-β and IFN-inducible genes. EMBO J. 1992;11:185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiss L F L, Ruffner H, Stark G, Aguet M, Weissmann C. Mice devoid of interferon regulatory factor 1 (IRF-1) show normal expression of type 1 interferon genes. EMBO J. 1994;13:4798–4806. doi: 10.1002/j.1460-2075.1994.tb06805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler C, Shuai K, Prezioso V, Darnell J E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 42.Schneider R J. Adenovirus and vaccinia virus translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 575–605. [Google Scholar]
- 43.Sonenberg N. mRNA 5′ cap-binding protein eIF-4E and control of cell growth. In: Hershey J W B, Mathews M, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 245–270. [Google Scholar]
- 44.Struloici B, Daniel-Issakani S, Baxter G, Knopf J, Sultzman L, Cherwinski H, Nester J, Webb D R, Ransom J. Distinct mechanisms of regulation of protein kinase C by hormones and phorbol diesters. J Biol Chem. 1991;266:168–173. [PubMed] [Google Scholar]
- 45.Su F, Schneider R J. Hepatitis B virus HBx protein activates transcription factor NF-κB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol. 1996;70:4558–4566. doi: 10.1128/jvi.70.7.4558-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tal-Singer R, Podrzucki W, Lasner T M, Skokotas A, Leary J J, Fraser N W, Berger S L. Use of differential display reverse transcription-PCR to reveal cellular changes during stimuli that result in herpes simplex virus type 1 reactivation from latency: upregulation of immediate-early cellular response genes TIS7, interferon, and interferon regulatory factor-1. J Virol. 1998;72:1252–1261. doi: 10.1128/jvi.72.2.1252-1261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thimmapaya B, Weinberger C, Schneider R J, Shenk T. Adenovirus VA1 RNA is required for efficient translation of viral mRNA at late times after infection. Cell. 1982;31:543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- 48.Thomas G P, Mathews M B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980;22:523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- 49.Vaughan P S, van Wijnen A J, Stein J L, Stein G S. Interferon regulatory factors: growth control and histone gene regulation—it’s not just interferon anymore. J Mol Med. 1997;75:348–359. doi: 10.1007/s001090050120. [DOI] [PubMed] [Google Scholar]
- 50.Veals S A, Schindler C, Leonard D, Fu X-Y, Aebersold R, Darnell J E, Jr, Levy D E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992;12:3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaver B K, Kumar K P, Reich N C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. J Virol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisz A, Marx P, Sharf R, Appella E, Driggers P H, Ozato K, Levi B Z. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992;267:25589–25596. [PubMed] [Google Scholar]
- 53.Yamagata T, Nishida J, Tanaka T, Sakai R, Mitani K, Yoshida M, Taniguchi T, Yazaki Y, Hirai H. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol Cell Biol. 1996;16:1283–1294. doi: 10.1128/MCB.16.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct activation of type I interferon system by virus infection: activation of a transcription factor complex containing IRf-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yueh A, Schneider R J. Selective translation by ribosome jumping in adenovirus infected and heat shocked cells. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Feigenblum D, Schneider R J. A late adenovirus factor induces eIF-4E dephosphorylation and inhibition of cell protein synthesis. J Virol. 1994;68:7040–7050. doi: 10.1128/jvi.68.11.7040-7050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Schneider R J. Adenovirus inhibition of cell translation facilitates release of virus particles and enhances degradation of the cytokeratin network. J Virol. 1994;68:2544–2555. doi: 10.1128/jvi.68.4.2544-2555.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]