Abstract
Traumatic brain injury is a serious medical condition that can be attributed to falls, motor vehicle accidents, sports injuries and acts of violence, causing a series of neural injuries and neuropsychiatric symptoms. However, limited accessibility to the injury sites, complicated histological and anatomical structure, intricate cellular and extracellular milieu, lack of regenerative capacity in the native cells, vast variety of damage routes, and the insufficient time available for treatment have restricted the widespread application of several therapeutic methods in cases of central nervous system injury. Tissue engineering and regenerative medicine have emerged as innovative approaches in the field of nerve regeneration. By combining biomaterials, stem cells, and growth factors, these approaches have provided a platform for developing effective treatments for neural injuries, which can offer the potential to restore neural function, improve patient outcomes, and reduce the need for drugs and invasive surgical procedures. Biomaterials have shown advantages in promoting neural development, inhibiting glial scar formation, and providing a suitable biomimetic neural microenvironment, which makes their application promising in the field of neural regeneration. For instance, bioactive scaffolds loaded with stem cells can provide a biocompatible and biodegradable milieu. Furthermore, stem cells-derived exosomes combine the advantages of stem cells, avoid the risk of immune rejection, cooperate with biomaterials to enhance their biological functions, and exert stable functions, thereby inducing angiogenesis and neural regeneration in patients with traumatic brain injury and promoting the recovery of brain function. Unfortunately, biomaterials have shown positive effects in the laboratory, but when similar materials are used in clinical studies of human central nervous system regeneration, their efficacy is unsatisfactory. Here, we review the characteristics and properties of various bioactive materials, followed by the introduction of applications based on biochemistry and cell molecules, and discuss the emerging role of biomaterials in promoting neural regeneration. Further, we summarize the adaptive biomaterials infused with exosomes produced from stem cells and stem cells themselves for the treatment of traumatic brain injury. Finally, we present the main limitations of biomaterials for the treatment of traumatic brain injury and offer insights into their future potential.
Keywords: bioactive materials, biomaterials, exosomes, neural regeneration, scaffolds, stem cells, tissue engineering, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a serious medical condition that can have detrimental effects on both the patient and society at large. TBI can be caused by various factors such as falls, car accidents, sports-related injuries, and/or acts of violence. There are several harmful effects of TBI. First, TBI can cause a range of physical disabilities including paralysis, loss of sensation, and impaired coordination and balance. Second, TBI can also cause cognitive impairments such as memory loss, difficulty concentrating, and impaired judgment (Fure et al., 2021; McGeary et al., 2022). These limitations and impairments may significantly affect a person’s capacity to carry out everyday tasks and participate in social interactions. Third, TBI can lead to certain emotional and behavioral changes such as depression, anxiety, aggression, and impulsivity, which can strain relationships with family and friends and lead to social isolation and other negative outcomes (Czeiter et al., 2020; Tarudji et al., 2021). Finally, TBI can result in significant economic costs including medical expenses, lost productivity, and long-term care needs, which can be a burden on individuals, families, and society as a whole (Lu et al., 2021).
TBI not only results in neural injury, which is always followed by a number of complex and neuropsychiatric symptoms such as seizures, memory loss, insomnia, nervousness, and depression but also damages the brain and triggers cerebral edema, ischemia, and inflammation that disrupt homeostasis and induces unusual biochemical, metabolic, circadian, and neural circuit changes (Campos-Pires et al., 2020; Killgore et al., 2020; Rowell et al., 2020; Mahncke et al., 2021; Roquilly et al., 2021). Moreover, because of the intricate histological and anatomical structure, cellular and extracellular environment, and insufficient capability of the central nervous system (CNS) for healing by endogenous cells, injured brain tissue and function can be difficult to repair. In addition, neuroinflammation, glial scar formation, and neurodegeneration in secondary injuries after TBI may characterize CNS damage (Lassarén et al., 2021). The slow infiltration of macrophages can make the region of the injury take a long time to heal (Ma et al., 2020a).
Following TBI, neurogenic inflammation may disrupt metabolic balance through biological stress, excitatory toxicity, and cell activation (Anderson et al., 2020). Proinflammatory conditions are characterized by an increase in cytokines, such as interleukins, tumor necrosis factor-α, and reactive oxygen species (ROS), which reduce healthy cell function while keeping tissues responsive (Ashina et al., 2020a; Bernard et al., 2022). Astrocyte reactivity is an important component in the vast majority of events that occur during CNS injury. However, there are dual aspects to astrocyte reactivity. On the one hand, activated astrocytes can result in the formation of glial scars, which help protect neurons by monitoring immune reactions and blood flow and preventing further damage. On the other hand, it can also cause blood-brain barrier (BBB) disruptions, persistent inflammation, neural toxic effects, and cell death in nearby areas (Galarza et al., 2020). Additionally, the activation of astrocytes may provide a significant therapeutic obstacle for CNS tissue restoration (Maas et al., 2022).
Based on these mechanisms, various therapeutic strategies have been developed; for example, traditional treatment methods such as drugs and surgery and emerging treatment methods such as tissue engineering (TE) and regenerative medicine, including bioactive materials, growth factors, scaffolds, stem cells, and stem cell-derived exosomes. However, traditional treatments can only relieve the discomfort and prevent the aggravation of the disease, they cannot improve prognosis (Nemkova, 2022). Biomaterials have demonstrated benefits in promoting brain growth, preventing the creation of glial scars, and offering a proper biomimetic neural microenvironment. For instance, stem cell-loaded bioactive scaffolds can offer a biocompatible and biodegradable environment (Tuladhar et al., 2020). Additionally, stem cell-derived exosomes combine the benefits of stem cells, reduce the chance of immune rejection, synergistically work with biomaterials to enhance their biological functions, and exert stable functions (Ghosh et al., 2020). As a result, they help TBI patients heal from their injuries by encouraging angiogenesis and nerve regeneration (Ma et al., 2020b; Fletcher et al., 2021). Therefore, by contrast, the emerging treatments overcome the limitations and have broad application prospects in the field of nerve regeneration. In this review, based on the mechanisms of TBI and the properties of various biomaterials, we discuss the role of biomaterials and TE in regulating biochemical stress, anti-neuroinflammation, neuroprotection, and neural and vascular regeneration in TBI patients (Figure 1).
Figure 1.
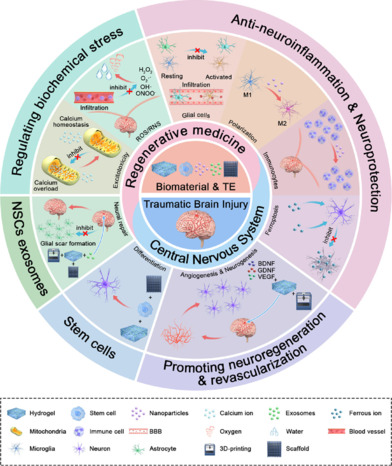
Tissue engineering and different types of biomaterials in TBI treatment.
After TBI, excitotoxicity caused by intracellular calcium overload and the increase of reactive oxygen species and reactive nitrogen species (ROS/RNS) leads to oxidative stress. Biomaterials can remodulate calcium homeostasis and promote ROS scavenging to regulate biological stress. Astrocyte reactivity is two-sided, with activated astrocytes leading to glial scar formation, disruption of the blood-brain barrier, persistent inflammation, neurotoxic effects, and cell death in nearby areas. There are two types of microglia, of which the M1 type is pro-inflammatory and the M2 type is anti-inflammatory. In addition, infiltration of inflammatory cells and neuronal ferroptosis contribute to increased neuroinflammation in TBI patients. Therefore, biomaterials can play an anti-inflammatory role and promote neural regeneration by inhibiting the activation and polarization of glial cells, reducing the infiltration of inflammatory cells, and restraining ferroptosis. In addition, the combination of 3D printing technology with biomaterials, growth factors, stem cells, and stem cell-derived exosomes can promote neurogenesis and angiogenesis, accelerate the differentiation of neurons, and facilitate neural repair. Created with CINEMA 4D and Adobe Photoshop. BDNF: Brain-derived neurotrophic factor; GDNF: glial-cell-derived neurotrophic factor; TBI: traumatic brain injury; TE: tissue engineering; VEGF: vascular endothelial growth factor.
Search Strategy
Studies cited in this review were obtained based on a PubMed search and were published between 2018 and 2023. The following search words appearing in the Title/Abstract were used: therapeutic strategies, neuroinflammation, and biochemical stress. Additionally, the following MeSH search terms were used: biocompatible materials, tissue engineering, nerve regeneration, tissue scaffolds, stem cells, exosomes, neuroprotection, and brain injuries. Identified articles were screened by title and abstract and articles that were inconsistent with our inclusion criteria were excluded from further analysis.
The Therapeutic Strategies and Challenges of Traumatic Brain Injury
Drugs, surgery, cell-based therapy, exosomes, and TE scaffolds are the most common treatment methods (Bailes and Borlongan, 2020; Nemkova, 2022; Zhu et al., 2022). Traditional therapeutic approaches slow the advancement of injury, while medications have thus far mostly focused only on pain management (Ashina et al., 2020b; Nilsson et al., 2020). The creation of a biomaterial that can effectively encourage neural stem cells (NSCs) to multiply and differentiate is therefore urgently needed (Li et al., 2019; Lainé et al., 2022). The complexity of the spatial organization and structure of CNS tissue, post-mitotic state of native cells, multiple routes of damage, and restricted window of potential for treatment are just some of the factors that hinder the validity of CNS treatments (Li et al., 2020a; Liao et al., 2021; Kolias et al., 2022).
A novel strategy for the regeneration of injured tissue and organs has evolved as a result of the advancement and growth of TE and regenerative science, surpassing the drawbacks of conventional therapies (Ghosh et al., 2020; Qian et al., 2021; Hasanzadeh et al., 2023). For example, multifunctional scaffolds that carry therapeutic compounds and cells, as well as hydrogels with fine material biochemical adaptations and the ability to carry cells, could be used to transport cells, therapeutic molecules, and proteins for the treatment of damaged brain tissue (Basit et al., 2021; Roh et al., 2023). Cyclosporine A can preserve the integrity of mitochondrial function, which might be a promising neuroprotective therapeutic strategy, but it has limited access to brain targets (Tuladhar et al., 2020). Surprisingly, a lipoprotein-biomimetic nanocarrier loaded with cyclosporine A in the core was proven to accomplish medication delivery that targeted the mitochondria and the TBI damage location (Sun et al., 2022b), which could effectively improve mitochondrial dysfunction, substantially ameliorate neural inflammation, minimize damage to neurons, and restore memory loss after TBI (Chen et al., 2020b).
The introduction of new biomolecules or mediators in certain prospective animal studies on CNS restoration has shown enhanced neural network repair, but when similar mediators were utilized in human clinical investigations of CNS regeneration, they showed poor regenerative potential. The challenges of transferring these therapies to clinical use are likely dependent on the intricate and unknown essential molecular and cellular functions, as well as the healing pathways, physical structure, and pathophysiological characteristics of the CNS (Jiang et al., 2021). Additionally, the BBB presents a significant obstacle, as it is a highly selective semipermeable membrane that limits the number of potential biomolecules for therapeutic use.
In conclusion, the only goals of conventional therapies are to reduce discomfort and halt the spread of disease. By efficiently promoting the proliferation and differentiation of neurons, fresh strategies for neural regeneration have been developed as a result of the development of TE and biomaterials, which go beyond the restrictions of conventional therapies. Furthermore, these innovative methods also offer a platform for creating effective treatments for nerve injuries, with the potential to restore nerve function, enhance patient outcomes, and lessen the need for drugs and invasive surgical procedures, which is accomplished by combining biomaterials, stem cells, growth factors, and scaffolds. The use of biomaterials in the field of nerve regeneration is promising because of their advantages in promoting neural development, preventing the growth of glial scars, and providing an appropriate biomimetic neural milieu (Figure 2).
Figure 2.
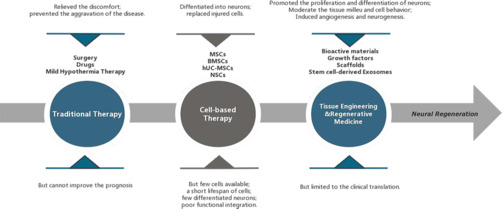
The development and characteristics of traumatic brain injury (TBI) treatment strategies.
The earliest treatment strategies are surgery, drugs, and mild hypothermia therapy, but they are mainly symptomatic treatments and cannot improve the prognosis (Ashina et al., 2020b; Nilsson et al., 2020). After a period of time, the emergence of cell therapy has made neural repair possible, but the use of stem cells alone is often limited by low cell survival and insufficient differentiation ability (Bonsack et al., 2020; Ruppert et al., 2020; Jha et al., 2021; Pischiutta et al., 2021). The development of tissue engineering and regenerative medicine has broken through the limitations of traditional treatment and cell therapy. Among them, stem cell-derived exosomes inherit the advantages of cell therapy, but also make up for the shortcomings of difficult cell survival and slow differentiation. In addition, they can regulate the tissue microenvironment and promote angiogenesis and neurogenesis. However, the challenge of clinical translation is a barrier that we need to break through in future research (Ghosh et al., 2020; Qian et al., 2021; Hasanzadeh et al., 2023).
Natural and Synthetic Biomaterials
The interaction between immune cells and biomaterials remains a challenge in tissue regeneration. The application of stem cells in therapy to treat a variety of illnesses has shown promise, but their low retention rate, viability, and differentiation have limited their efficacy. To overcome these drawbacks, biomaterials can serve as carriers and regulators to amplify the benefits of stem cells for healing (Jeong et al., 2021; Thomas et al., 2021). Effective biomaterials must regulate the tissue microenvironment and cell behavior to promote neural regeneration (Tuladhar et al., 2020; Shen et al., 2022). Adaptive biomaterials can also respond to unfavorable chemicals in the milieu to improve the viability and functionality of host cells, creating an adjustable regenerative physiological environment (Jiang et al., 2021).
Collagen, gelatin, laminin, silk fibroin, alginate, and other injectable hydrogels based on polysaccharides, such as chitosan and hyaluronic acid (HA), are examples of natural substances and derivates used in neural applications (Liu et al., 2020b; Nguyen et al., 2020; Lainé et al., 2022). These materials and derivatives aim to mimic extracellular matrix (ECM) properties such as viscoelasticity, biological degradation, and compatibility (Lim et al., 2020).
A potential hydrogel for neural TE was the methylcellulose/agarose composite thermosensitive hydrogel, a synthetic biomaterial that maintains the survival of neuronal cells. Another thermosensitive hydrogel was poly (N-isopropylacrylamide) (PNIPAAm), which might combine with polyethylene glycol to ensure excellent cell attachment and maintain growth factor production throughout protracted CNS treatment (Hasanzadeh et al., 2023).
Physical hydrogels are simple to produce and have low cytotoxicity, but they frequently become unstable in physiological settings and do not immediately react (Khan et al., 2022; Liang et al., 2023). Chemical hydrogels have had deleterious effects in vivo because of the chemical interactions, while having outstanding mechanical characteristics and remarkable stability (Finbloom et al., 2020; Townsend et al., 2020). Synthetic biomaterials used to create hydrogels showed great stability but low biological activity and compatibility (Macks et al., 2022; Park et al., 2022). Natural biomaterial-based hydrogels are distinguished by their excellent biocompatibility and biodegradability and low cytotoxicity, but their potential for use in medicine was constrained by the absence of physical rigidity (Xin et al., 2023). In light of these benefits, hybrid hydrogels assembled from both synthetic and natural components might provide a more promising avenue for therapeutic applications in CNS regrowth and engineering (Man et al., 2021; Park et al., 2021; Asghari et al., 2022). In summary, a potential biomaterial that helps to promote neurite outgrowth and 3D cell culture should have the best possible biodegradability, stability, biocompatibility, and mechanical properties (Bartlett et al., 2020; Ye et al., 2021; Table 1).
Table 1.
Natural and synthetic biomaterials and their derivatives for TBI treatment
| Material | Delivery method | Injury model | Treatment Time-point | Combination | Duration | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|
| Natural | Alginate | Injected | Weight-drop Rats | Immediate | BMSC/CoI/SDF-1 | 30 d | - Aided BMSCs in surviving, migrating, and differentiating into neurons in vitro; - Showed good biocompatibility and persistence of action; - Ameliorated the nervous system deficiencies, tension, and depressive-like symptoms; - Lowered the size of the lesion, prevented neuronal death, and eased cerebral edema; - Attenuated neuroinflammation and oxidative stress; - Boosted BMSC migration and survival, and improved neuronal regeneration. |
Ma et al., 2021 |
| Silk fibroin | Injected | CCI Mice | 1 h | H2S | 28 d | - Slowly and continuously released H2S and exerted biological effects; - Encouraged the formation of the ECM, provided a foundation for neuronal attachment, and directed the proliferation of neuronal sprouts; - Suppressed the expression of neuronal H2S synthase, pyroptosis, and inflammation brought on by TBI; - Alleviated neuronal apoptosis and brain edema from TBI; - Ameliorate memory impairments, anxiety-related behavior, and motor disorders brought on by TBI. |
Chen et al., 2022 | |
| Collagen | Grafted Scaffold | CCI Rats | Immediate | Heparin/VEGF | 60 d | - Promoted the recovery of motor and cognitive defects; - Alleviated brain edema and inflammatory reactions at the lesion sites; - Improved the remodeling of injured nerve tissue; - Expedited regrowth of synaptically connected neurons, nerve fibers, and myelin sheaths, as well as functional revascularization with unbroken endothelium, at the locations of damage. |
Zhang et al., 2021a | |
| Chitosan | Grafted Scaffold | Drill-striking Rats | Immediate | BMExos/Collagen | 60 d | - Enhanced restoration of sensorimotor and cognitive function; - Encouraged the reconfiguration of neuronal networks; - Stimulated hemostasis, tissue regeneration, and the activity of neuronal proliferation; - Acted as an antibacterial agent to stop the growth of bacteria and fungi at the lesion sites. |
Liu et al., 2023b | |
| Hyaluronic acid | Grafted Scaffold | Weight-drop Mice | 7 d | Neurotrophic factors | 180 d | - Stimulated neuronal differentiation, evolution, and synaptic plasticity; - Provided strong adhesion qualities and a beneficial milieu for cells, and favorable dynamics for the rehabilitation of damaged brain tissue; - Boosted the lifespan and multiplication of grafted neurons. |
Mishchenko et al., 2022 | |
| Gelatin | Injected | Weight-drop Mice | Immediate | MA/PPS60/PC | 28 d | - Controlled the cells’ reaction to biochemical stress and minimized tissue loss; - Synergistically lowered ROS to act as a neurotrophic protective factor; - Preserved the integrity of the BBB and decreased brain edema; - Enhanced local anti-inflammatory activity and neural repair. |
Huang et al., 2022 | |
| Synthetic | Thermosensitive composition (CS-HEC-HA/GP) | Injected | Weight-drop Mice | 7 d | hUC-MSC | 35 d | - Encouraged injected hUC-MSC to remain alive, migrate, and be retained; - Accelerated the neurological motor function recovery; - Ameliorated the depression, learning and memory ability, and lesion volume after TBI; - Decreased neuron death, and boosted neuron longevity and multiplication by producing BDNF. |
Yao et al., 2019a |
| Biodegradable biopolymer (PLGA-COOH) | Injected | CCI Mice | 2–3 h 24 h 48 h | – | 240 d | - Transformed the quantity and phenotype of proinflammatory cells invading the brain to alter the general neuroinflammatory reactivity; - Affected immunocytes’ ability to maintain inflammation and regulate the mobilization of extra immune cells; - Decreased the quantity of infiltrative leukocytes, mDCs, and monocytes/macrophages at the injured sites; - Improved electrophysiologic visual function and continued motor behavior; - Ameliorated brain edema formation and lesion volumes. |
Sharma et al., 2020 | |
| Self-assembling peptide (SLanc) | Injected | FPI Rats | Immediate | – | 14 d | - Increased blood vessel density and endothelial cell adhesion in the injured brain; - Promoted angiogenesis, axonal outgrowth, and neuroprotection; - Created regenerative milieus for neuroprotection, potential neuronal regeneration, and a variety of other neural ischemic tissue diseases. |
Ma et al., 2020b | |
| Self-assembling peptide (IKVAV) | Injected | Craniotomy Mice | Immediate | NSC/Myoglobin | 28 d | - Strengthened differentiation and incorporation of NSC transplantation without overproliferation or obstruction to cell migration into the host parenchyma; - Effectively avoided unexpected cell death and immunological reactions, and synergistically promoted longevity and incorporation of NSC grafts inside the host brain; - Provided oxygen to stem cells and functionally relevant treatments while inducing tissue repair and rebuilding with metabolic needs. |
Wang et al., 2023 |
BBB: Blood-brain barrier; BDNF: brain derived neurotrophic factor; BMExos: BDNF-stimulated hUC-MSC-derived exosomes; BMSC: bone marrow-derived mesenchymal stem cell; CoI: collagen type I; CS-HEC-HA/GP: chitosan, hydroxyethyl cellulose, HA, and β-glycerophosphate; ECM: Extracellular matrix; HA: hyaluronic acid; hUC-MSC: human umbilical cord mesenchymal stem cell; MA/PPS60/PC: methacrylate-poly (propylene sulfide)60/procyanidin; mDCs: myeloid dendritic cells; NSC: neural stem cell; PLGA-COOH: poly(lactic-co-glycolic) acid; ROS: reactive oxygen species; SDF-1: stromal cell-derived factor-1; TBI: traumatic brain injury; VEGF: vascular endothelial growth factor.
Mechanisms and Regulation of Biochemical Stress
It is vulnerable to suffer from excitotoxicity due to the depletion of mitochondria and the disruption of mitochondrial calcium homeostasis. According to a recent study (Verma et al., 2022), PTEN-induced putative kinase 1 controlled the activity of the mitochondrial sodium calcium exchanger protein by phosphorylation, which controlled the regulation of mitochondrial calcium homeostasis and suppressed the expression of leucine-rich repeat kinase 2 mutants (G2019S or R1441C) by inhibiting mitochondrial calcium uniporter activity, reducing the Parkinson’s loss-of-function mutant, and preventing exposure to oligomeric amyloid-beta peptide (Chavez et al., 2020; Pandya et al., 2023). By normalizing mitochondrial calcium flow, a common group of methods that targeted the mitochondrial calcium homeostasis machinery could prevent excitatory mitochondrial toxicity and protect nerves (Du et al., 2022). Biomaterials combined with drugs based on the above mechanisms could be developed to prevent biochemical stress (Han et al., 2022; Mason et al., 2023).
Excessive levels of reactive oxygen species and reactive nitrogen species (ROS/RNS) by inflammatory cells can cause an oxidative microenvironment after initial brain damage caused by external stimuli (Fan et al., 2022). For patients with severe TBI, lowering ROS/RNS levels ameliorated prognosis by enhancing neuronal survival, reducing neuroinflammation, and facilitating recovery (Sun et al., 2022a). By encouraging the expression of cytokines, excessive ROS production in the milieu of brain injury might stimulate inflammation and the immune system, which can ultimately lead to neurological impairments (Takahashi et al., 2020; Waheed et al., 2022). Additionally, neuroinflammation, which is characterized by a mass of activated astrocytes and microglia in the damaged region, inhibits neuron regeneration and axon development (Zhang et al., 2020b). Therefore, it is critical to eliminate ROS, reduce neural inflammation, and accelerate neuronal regeneration following TBI (Sun et al., 2022a).
In contrast to traditional systemic drug treatment, which is currently unable to effectively deliver the medicine to the targeted region in the early stages of TBI and might instead have systemic adverse reactions owing to the existence of the BBB, gelatin methacrylate-propylene sulfide/procyanidin (GelMA-PPS/PC) has provided an immediate approach to the surface of brain tissue and concentrated on the injured region (Huang et al., 2022). As a result of the involvement of the hydrogen peroxide (H2O2)-responsive material of hydrophobic poly (propylene sulfide) 60 (PPS60), PPS60 might additionally trigger hydrogel construction to breakdown and expel the procyanidins it contains. In addition, PC could modulate the oxidative stress response in the cells, work in concert to deplete ROS, and raise the level of brain-derived neurotrophic factor (BDNF), so that could exert a neurotrophic protective function, and inhibit the expression of ionized calcium-binding adapter molecule 1 (Iba-1), glial fibrillary acidic protein, chemokine (C-X-C motif) ligand 1, tumor necrosis factor-α, interleukin-1β (IL-1β), and IL-8 to reduce inflammation (Grebenik et al., 2020). Triglycerol monostearate-loaded PC (TM/PC) was another novel ROS depletion hydrogel. According to a study, PPS120 and curcumin (Cur) might be enclosed within the hydrophobic core of TM lamellae, which could be self-assembled into a hydrogel to create a TM/PC hydrogel. Upon direct injection into the TBI wound cavity, TM decomposed, and PPS120 scavenged ROS to release Cur, thereby decreasing neural inflammation and subsequent damage spread. In addition, enhanced neuronal growth and regeneration as well as migration after TM/PC treatment could be observed by more powerful DCX expression. Moreover, a lower dichlorodihydrofluorescein fluorescence intensity after treatment showed stronger ROS-scavenging ability of the TM/PC hydrogel (Qian et al., 2021; Figure 3).
Figure 3.
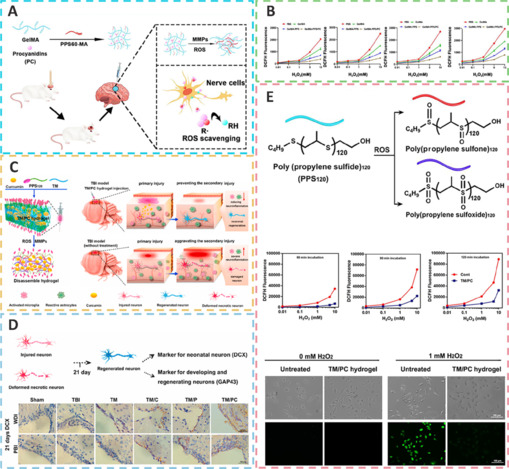
ROS-scavenging functional hydrogels treat TBI through neuroprotection, pro-nerve regeneration, and anti-neuroinflammatory effects.
(A) The preparation process and mechanism of gelatin methacrylate-propylene sulfide/procyanidin (GelMA-PPS/PC). Reprinted from Huang et al. (2022) with permission from American Chemical Society under CC BY license. (B) The dichlorodihydrofluorescein (DCFH) fluorescence intensity changed with hydrogen peroxide (H2O2) concentration. Reprinted from Huang et al. (2022) with permission from American Chemical Society under CC BY license. (C) The preparation process and mechanism of action and effect of triglycerol monostearate-loaded PC (TM/PC). Reprinted from Qian et al. (2021) with permission from Elsevier. (D) Cellular characteristics of regenerated tissue from different treatment groups in TBI. Reprinted from Qian et al. (2021) with permission from Elsevier. (E) TM/PC hydrogel reaction with the ROS and DCFH-DA assay, and DCFH fluorescence exposed to H2O2 in different treatment groups. Reprinted from Qian et al. (2021) with permission from Elsevier. ROS: Reactive oxygen species; TBI: traumatic brain injury.
In a recent study, Zhang et al. (2022b) developed a novel injectable, ROS-scavenging, and biocompatible gallic acid-conjugated gelatin/oxidized dextran hydrogel to provide protection from the harmful microenvironment by neutralizing free radicals and aid in neural healing by suppressing oxidative stress and inflammation.
In addition to being safe for neurons under physiological conditions, carbogenic nanozymes that are tiny multienzyme mimics also showed powerful scavenging capacity for redundant ROS and RNS, thereby offering neuroprotection (Mu et al., 2019; Zhang et al., 2021d; Tarudji et al., 2023). Moreover, the carbogenic nanozyme could decrease the level of matrix metalloproteinases in TBI patients, contributing to effective repair of BBB disruption and a reduction in cerebral edema (Zhai et al., 2021). In addition, nanozymes could reduce proinflammatory immune responses by limiting the proliferation of microglia and astrocytes, which could result in strong neuronal inflammation. The nanozyme could also increase the activity of superoxide dismutase and decrease the amount of H2O2 and the expression levels of lipid peroxidation and glutathione disulfide (GSSG), which could alleviate severe oxidative stress (He et al., 2020). Additionally, the nanozyme might efficiently boost the capacity of spatial memory. Carbon dots (CDs), a new type of carbon-based nanomaterial, has recently emerged as a valid new method to permeate the BBB and enter the brain parenchyma (Zhang et al., 2022c). Given their vast and unique surface area and abundance of functional groups, carbon dots offer the benefits of outstanding solubility, superior biocompatibility, simple functionalization, and tremendous drug-loading ability to deliver therapeutic medicines. More significantly, carbon dots can maintain or even increase the biological activity of their precursors (Ouyang et al., 2020).
Strategies for Anti-Neuroinflammation and Neuroprotection
The prevention of neuroinflammation and pyroptosis was thought to be a potential therapeutic method for TBI. In recent studies, a surface-fill hydrogen-sulfide (H2S)-releasing silk fibroin (SF) hydrogel (H2S@SF hydrogel) was shown to be neuroprotective following TBI (Liu et al., 2023e). On the one hand, the H2S@SF hydrogel most significantly inhibited the activation of H2S synthase, and the expression of neuronal NOD-like receptor protein 3, gasdermin D, cysteinyl aspartate specific proteinase-1 (caspase-1), apoptosis-associated speck-like protein containing CARD, IL-1β, and tumor necrosis factor-α, and inhibited TBI-induced pyroptosis and necroptosis-related protein (receptor interacting protein-1; Wehn et al., 2021; Chen et al., 2022; López-Preza et al., 2023). H2S@SF hydrogel, on the other hand, appeared to slow the decrease in the level of glutathione following moderate TBI, demonstrating that it might protect against mitochondrial dysfunction and oxidative stress in this scenario (Kwiecien, 2021). In addition, H2S@SF could reduce neuroinflammation by inhibiting the proliferation and activation of reactive astrocytes and activated microglia, and the expression of glial fibrillary acidic protein and Iba-1 in these glial cells (Zhang et al., 2022a; Figure 4).
Figure 4.
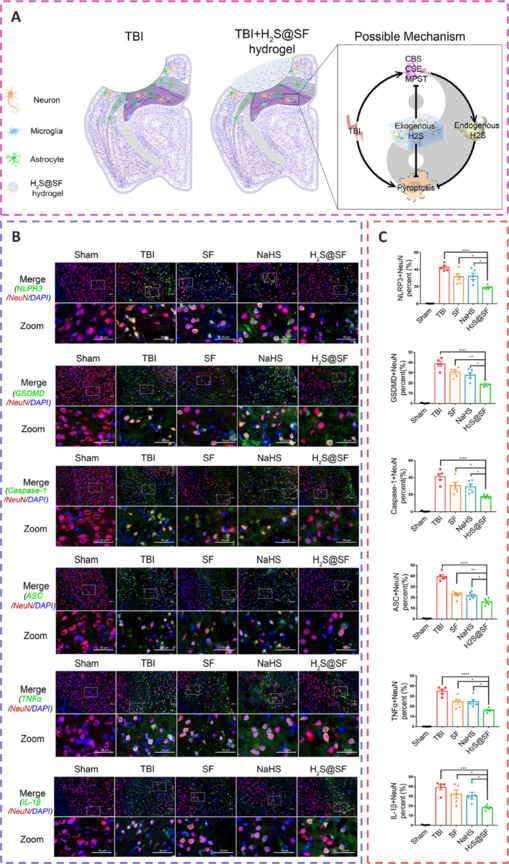
H2S-releasing SF hydrogel (H2S@SF hydrogel) inhibits neuronal pyroptosis and inflammation, and exerts a neuroprotective impact by inhibiting the expression of pyroptosis-related proteins, H2S synthase, and proinflammatory factors.
(A) The effects and mechanisms of the H2S@SF hydrogel and its synthase on neurons. (B) Fluorescence microscopy of pyroptosis-related proteins and pro-inflammatory factors. (C) Quantitative analysis of fluorescence images in each treatment group. Reprinted from Chen et al. (2022) with permission from Elsevier. DAPI: 4′,6-Diamidino-2-phenylindole; H2S: hydrogen-sulfide; IL: interleukin; NeuN: neuronal nuclei antigen; SF: silk fibroin; TBI: traumatic brain injury; TNF-α: tumor necrosis factor-α.
Bioactive multifunctional nanocomposites (ANG-MnEMNPs-Cur [AMEC]) can regulate antioxidation and anti-neuroinflammation for targeted TBI therapies by integrating the neuroprotective drug Cur with angiopep-2 functionalities and manganese-doped eumelanin-like nanoparticles (Fyfe, 2020; Sun et al., 2022a). Mechanistically, angiopep-2 could bind to low-density lipoprotein receptor-related protein-1 and help cross the BBB into TBI lesions and enhance drug accumulation (Bechinger et al., 2023). Melanin-like nanoparticles had powerful and extensive scavenging capabilities against an array of ROS (Zinger et al., 2021a). Cur, a specific kind of plant polyphenol, offered several pharmacological benefits, including antioxidative, anti-inflammatory, and antiapoptotic effects (Dong et al., 2022). The functional moieties of Cur and eumelanin, when combined, work cooperatively to increase the effectiveness of AMEC at the targeted site. This was accomplished by reducing oxidative stress, preventing neurological inflammation by converting M1 macrophages to M2 macrophages, and promoting neuronal regeneration (Hong et al., 2020; Wu et al., 2021; Figure 5).
Figure 5.
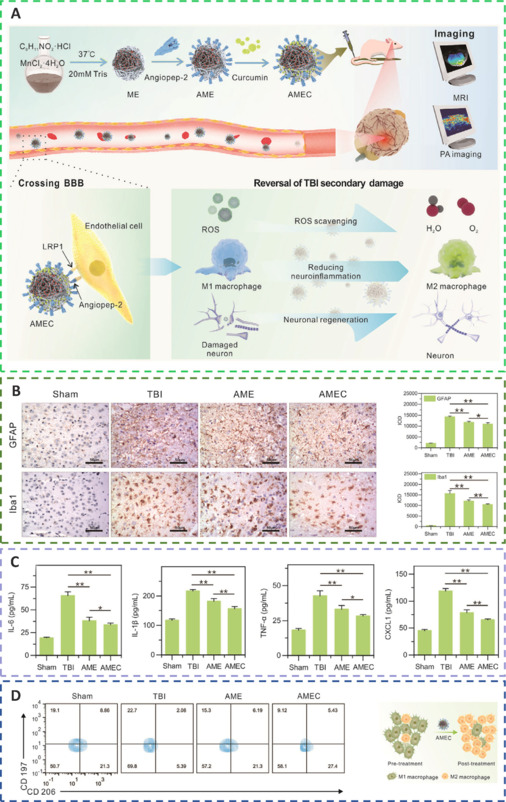
By preventing the appearance of activated astrocytes and microglia, reducing inflammatory cytokine levels, and activating M1-to-M2 macrophage reprogramming, AMEC significantly reduces oxidative stress, attenuates neural inflammation, and prevents the subsequent spread of damage following TBI.
(A) The major AMEC synthesis pathways and therapeutic mechanisms. (B) Representative immunohistochemical images and quantification of glial fibrillary acidic protein (GFAP) in astrocytes and ionized calcium-binding adapter molecule 1 (Iba-1) in microglia from injured tissues. (C) Serum interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), and chemokine (C-X-C motif) ligand 1 (CXCL1) levels in each treatment group. (D) Phenotyping of macrophages in the brains of different treatment groups and the process of macrophage repolarization through AMEC. Reprinted from Sun et al. (2022a) with permission from John Wiley and Sons. AME: ANG-MnEMNPs; AMEC: ANG-MnEMNPs-Cur (angiopep-2 -manganese doped eumelanin nanoparticles-curcumin); BBB: blood-brain barrier; ROS: reactive oxygen species; TBI: traumatic brain injury.
Immunomodulatory nanoparticles (IMPs) are highly negatively charged, 500 nm-diameter particles composed of the biodegradable biopolymer carboxylated poly(lactic-co-glycolic) acid (PLGA-COOH) (Sharma et al., 2020; Xu et al., 2022). The quantity of proinflammatory cells that infiltrates the brain and their phenotype can both be changed by IMPs. To alter the general neuroinflammatory reaction, IMP treatment drastically reduced the quantity of monocytes/macrophages, myeloid dendritic cells, and permeating leukocytes in the region of injury (Blanco-Ocampo et al., 2020). This had an effect on these cells’ ability to endure inflammatory reaction and guide the recruitment of extra immune cells. According to a study, R2* sequences were used to assess brain edema at 24 hours, whereas ventricular volume was used to assess intracranial pressure because brain edema caused ventricular compression and thus elevated intracranial pressure. The R2* hyperintensity volume was smaller in the IMP treatment group than in the vehicle treatment group, and the ventricular volume was larger in the IMP treatment group than in the vehicle treatment group, indicating that IMPs were effective in reducing brain edema and significantly reduced the intracranial pressure, thereby preserving brain tissue and significantly maintaining the anatomic and neurologic function (Sharma et al., 2020; Figure 6).
Figure 6.
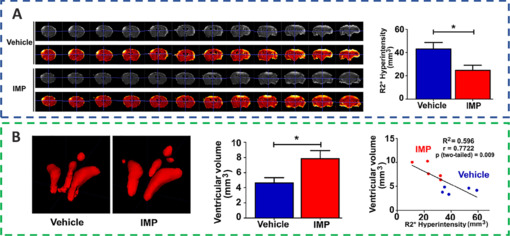
IMPs might ameliorate acute brain edema and lower intracranial pressure to provide neuroprotection.
(A)The quantitative magnetic resonance imaging R2* sequence and the total amount of R2* hyperintense volume of vehicle- and IMP-treated groups. (B) Quantitative analysis of the ventricular volumes in the groups that received IMP and the vehicle. Reprinted from Sharma et al. (2020) with permission from John Wiley and Sons. IMPs: Immunomodulatory nanoparticles.
Following TBI, ferroptosis was confirmed by iron buildup, higher transferrin expression, decreased glutathione peroxidase activity, lipid ROS accumulation, and shrinking mitochondria (Rui et al., 2021; Cheng et al., 2023; Li and Jia, 2023; Yang et al., 2023a). An unusually excessive iron buildup in chronic TBI patients might be present, and this was strongly connected with cognitive deficits (Xie et al., 2019; Wang et al., 2022a). Transferrin was essential for the induction of ferroptosis and might reduce cell death and lipid ROS accumulation when it was knocked out (Jia et al., 2023). When siramesine and lapatinib were used as treatments, the overexpression of ferroportin could lower ROS and cell death, while ferroportin knockout could increase ROS and cell death (Cheng et al., 2022). A significant regulator of ferroptosis was glutathione peroxidase 4, which could catalyze the decrease in lipid peroxides (Fang et al., 2023). In addition, ferrostatin-1 inhibited cell death, reduced glutamate-induced ferroptosis and iron accumulation, and attenuated neuronal degeneration (Ge et al., 2022). Ferrostatin-1 treatment might lessen neuronal cell loss and enhance cognitive and motor function over the long run.
Promotion of Neuroregeneration and Revascularization
Neuronal damage occurs immediately after TBI and is further aggravated in the chronic phase, leading to progressive neuronal death. Neuroplasticity goes some way toward minimizing the consequences of neuronal damage after TBI, but given that most such patients are adults, a critical question is whether it is possible to generate new neurons in the adult brain. Therefore, to prevent and improve this outcome, it is crucial to confirm the presence of adult neurogenesis.
For decades, it has been believed that neurogenesis occurs only during embryonic development and that new neurons do not appear in the adult brain (Ramon y Cajal and May, 1928; Ming and Song, 2005). Altman et al., however, disproved this theory in 1962 by proving that the adult rodent brain could reproduce new neurons (Altman and Das, 1965; Altman, 1969). This occurrence of neurogenesis in the adult rodent brain was further evidenced in the 1990s with the fast development of new technologies, including 5-bromo-2′-deoxyuridine labeling and antibody-based biomarker detection approaches (Ming and Song, 2005; Jurkowski et al., 2020). In the adult rodent brain, subventricular zone astrocytes were discovered to be NSCs around the year 2000 (Doetsch et al., 1999); in subsequent animal studies, Lindvall and Kokaia (2015) found the feasibility of stimulating endogenous neurogenesis in the subventricular zone to promote neural repair in the striatum and cortex.
In the course of progress or following injury and the onset of disease, BDNF, which exclusively integrates with the tyrosine kinase B (TrkB) receptor, can stimulate neuronal differentiation, evolution, and synaptic plasticity in the CNS (Edelbrock et al., 2018; Fletcher et al., 2021; Zhao et al., 2021). It has been reported that a supramolecular peptide amphiphile (PA) nanostructure incorporated in a BDNF mimetic sequence could activate the BDNF receptor TrkB and its downstream signaling pathways in primary cortical neurons (Mishchenko et al., 2022; Wu et al., 2023b). Next, the activated TrkB signaling pathways increased neuronal maturation. Then, the BDNF@PA nanostructures encouraged cell infiltration and increased functional maturation (Wu et al., 2023a). In summary, the BDNF-mimetic PA nanostructure produced an extremely bioactive matrix that could activate the TrkB receptor and its subsequent signaling cascades, which was necessary for cell viability, development, and functional maturation in 2D cultures, and could stimulate neuron infiltration in 3D while boosting maturity and functional electrical activity, which aided in the promotion of the regeneration or integration of transplanted cells in the CNS (Nam et al., 2020; Yin et al., 2020a; Liu et al., 2023b, d).
HA, a natural polymer, is the major substance of the brain ECM, with the advantages of a lower cytotoxicity level, a flexible biostructure, and a controllable rate of biodegradation (Li et al., 2020b; Yang et al., 2023b). Because of the high hydrophilicity of HA, scaffolds made on its foundation could offer strong adhesion qualities for cells and provide a beneficial milieu for them (Ngo et al., 2020; Satish et al., 2021). Glial-cell-derived neurotrophic factor (GDNF) can boost the lifespan and multiplication of grafted neurons, promote the renewal of nerves, and offer favorable dynamics for the rehabilitation of damaged brain tissue (Selvakumar et al., 2020; Torres-Ortega et al., 2022; Wang et al., 2022b). GDNF-loaded scaffolds were better than BDNF in that they sustain long-term memory while also reducing intense neurological impairments and noticeable alterations in motor and orienting-exploratory activity (Mishchenko et al., 2022). The 3D extrusion printing technique has the advantages of rapid fabrication and faster deposition. Therefore, the implantation of 3D-printed HA hydrogel scaffolds impregnated with the neurotrophic factor GDNF can be a promising therapeutic strategy for morphological and functional nerve tissue restoration after TBI (Rouleau et al., 2020).
Angiogenesis initiates a vascular network in the growing body that results in the growth and maturation of the CNS (Ma et al., 2020b). Neogenesis/maturity, permeability, and the capacity to revascularize therapeutic regions of ischemia are crucial for the development of angiogenesis. SLanc, an angiogenic self-assembling peptide hydrogel, could accelerate endothelial cell attachment and angiogenesis by greatly increasing the recruitment of von-Willebrand factor, which plays an important role in CNS neuroprotection and angiogenesis (Ma et al., 2020b; Figure 7). The most important angiogenesis-inducing growth factor, vascular endothelial growth factor (VEGF), is associated with increased BBB opening, brain edema, and neurological impairments. In a recent study, a VEGF-containing hydrogel was injected directly into the region of damage to stimulate the creation of a vascular and neuronal structure that led to behavioral improvement (Zhang et al., 2021a). In the injured area, HA gel and high cluster VEGF might encourage the development of a strong, mature, and well-established vascular bed and accelerate axonal extention alongside these blood vessels. Additionally, this hydrogel could encourage the inward migration of embryonic neurons from the subventricular zone, decrease the activation of microglia and the extent of the reactive astrocyte boundary, and change the surrounding peri-infarct tissue (Ma et al., 2020a). Meanwhile, numerous studies have proven that heparin exerted anti-inflammatory effects by blocking leukocyte adhesion to the vessel wall and stabilizing chemokine and growth factor gradients (Schirmer et al., 2020; Zhang et al., 2021a; Liu et al., 2022b). VEGF delivery was related to increased inflammation, which could weaken the efficacy and strengthen the adverse effects. Surprisingly, the proinflammatory action of VEGF could be counteracted by bare heparin particles, which also provided a pro-repair milieu that promoted the proliferation of fresh neural tissue (Jiang et al., 2020; Figure 8). Hence, the combination of the rapid development of a neurovascular network in the damaged location and the control of responses to inflammation, scars, and NSC in the neighboring brain position might pave the way for a new approach in the field of neural healing following TBI (Nih et al., 2018).
Figure 7.
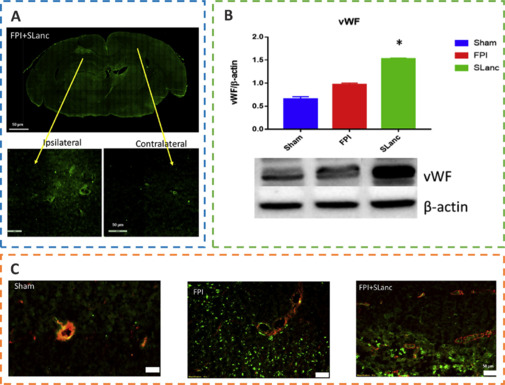
SLanc, an angiogenic self-assembling peptide hydrogel, accelerates ischemic tissue revascularization by improving endothelial cell adhesion and angiogenesis.
(A) Representative immunofluorescence staining of von-Willebrand factor (vWF) and endothelial cell adhesion after SLanc treatment. (B) Western blot assay reflecting vWF in different treatment groups. (C) Immunofluorescence staining of vWF and α-SMA in different treatment groups. Reprinted from Ma et al. (2020b) under CC BY license. FPI: Fluid percussion injury.
Figure 8.
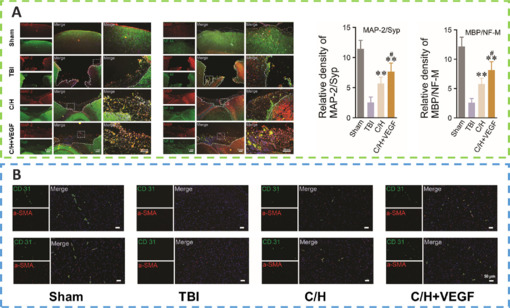
C/H scaffolds combined with VEGF can promote the regeneration of axons and myelin sheaths and promote angiogenesis after TBI.
(A) The expression of microtubule-associated protein 2/synaptophysin (MAP2/Syp) and neurofilament/myelin basic protein (NF-M/MBP). (B) The number of positive blood vessels detected by CD31/α-smooth muscle actin (α-SMA) double immunofluorescence staining in different treatment groups at 1 month and 2 months after TBI was compared. Reprinted from Zhang et al. (2021a) with permission from Royal Society of Chemistry. TBI: Traumatic brain injury; VEGF: vascular endothelial growth factor.
Biomaterial Scaffolds Carried with Stem Cells
Mesenchymal stem cells (MSCs) are capable of differentiating into neurons, replacing injured cells, and secreting paracrine hormones, making them a viable therapy for brain injury (Xu et al., 2020a; Sharma et al., 2021; Viet et al., 2022; Table 2). However, there has been little success and many problems with MSCs, namely limited cell availability, short cellular lifespan, few differentiated neurons, and poor functional integration (Bonsack et al., 2020; Ruppert et al., 2020; Jha et al., 2021; Pischiutta et al., 2021). Thus, encapsulation of transplanted cells with biomaterials might provide a suitable milieu for the growth and proliferation of these cells, protect them from immune rejection, and improve cell engraftment and survival during transplantation of injured brain lesions (Sultan et al., 2021; Figure 9).
Table 2.
Applications of stem cells combined with biomaterials in therapy
| Biomaterial | Stem cell | Animal model | Biological function | Reference |
|---|---|---|---|---|
| Gelatin hydrogel dual-enzymatically cross-linked by horseradish peroxidase and glucose oxidase | BMSC | Weight-drop Mice | - Promoted cellular viability, neural differentiation, and neurotrophins secretion. | Li et al., 2021 |
| BdECM bioink | NSC | Corticectomized Rats | - Inhibited prolonged neuroinflammation that could induce apoptosis and necrosis; - Supported survival and differentiation of NSC; - Suppressed chronic neuroinflammation. |
Bae et al., 2021 |
| SA/CoI/SDF-1 hydrogel | BMSC | Weight-drop Mice | - Promoted transplanted cells to survive, migrate, and differentiate; - Alleviated brain damage, neuronal death, and neuroinflammation. |
Ma et al., 2021 |
| Thermosensitive hydrogel based on CS-HEC-HA/GP | hUC-MSC | Weight-drop Mice | - Enhanced transplanted stem cells to retain, survive, and migrate; - Promoted secretion of neurotrophic factors, and survival and proliferation of neurons; - Inhibited neuron apoptosis; - Accelerated reconstruction of brain and recovery of neural function. |
Yao et al., 2019a |
| Hybrid peptide hydrogel laden with myoglobin | NSCs | Craniotomy Mice | - Induced tissue repair and reconstruction with metabolic requirements while providing functionally appropriate therapies and oxygen to stem cells; - Maintained a beneficial oxygen supply for stem cell grafts, positively affected their lifespan and synthesis, and regulated stem-cell specification; - Improved oxygen release during neural circuit remodeling to guarantee functional synaptic connection. |
Wang et al., 2023 |
BdECM: Brain-derived decellularized extracellular matrix; BMSC: bone marrow-derived mesenchymal stem cell; CS-HEC-HA/GP: chitosan, hydroxyethyl cellulose, HA, and β-glycerophosphate; HA: hyaluronic acid; hUC: human umbilical cord; NSC: neural stem cell; SA/CoI/SDF-1: sodium alginate/collagen type I/stromal cell-derived factor-1; TBI: traumatic brain injury.
Figure 9.
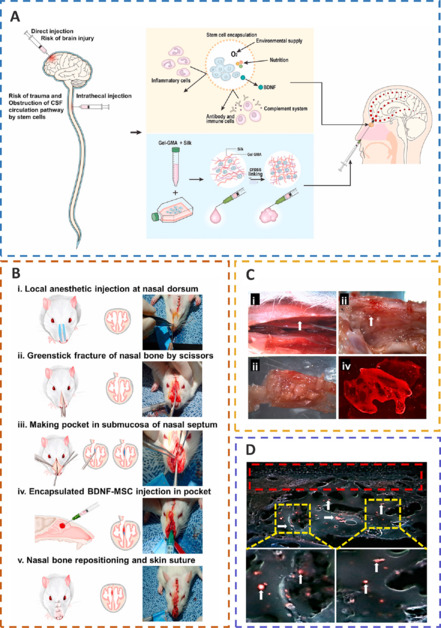
The encapsulation of BDNF-MSCs in biomaterials can protect transplanted cells from immune rejection, offer a favorable milieu, and improve the survival rate of transplanted cells at the injured site.
(A) The biomaterial-encapsulated neural stem cell transplantation platform. (B) Injecting BDNF-MSCs after traumatic brain injury. (C) Localization of cells after transplantation and confocal images of labelled BDNF-MSCs. (D) Fluorescence microscopy imaging of labelled BDNF-hMSCs after transplantation. Reprinted from Sultan et al. (2021) with permission from Elsevier. BDNF: Brain-derived neurotrophic factor; CSF: cerebrospinal fluid; Gel-GMA: glycidyl-methacrylated gelatin; hMSCs: human MSCs; MSCs: mesenchymal stem cells.
In the study by Ma et al. (2021), the longevity, attraction, and proliferation of stem cells were all regulated by stromal cell-derived factor-1 (SDF-1) integrating with its receptor (chemokine C-X-C motif receptor 4, CXCR4). As a result, they created a hydrogel composed of sodium alginate (SA), collagen type I (CoI), and SDF-1, which was infused with bone marrow-derived mesenchymal stem cells (BMSCs; Addington et al., 2015). The SA/CoI/SDF-1 scaffold could maintain a milieu with biological compatibility and degradability for BMSCs to survive, migrate, and differentiate in vitro, while also being able to steadily release SDF-1 (Guo et al., 2020; Liu et al., 2023a). The synthetic scaffold could significantly alleviate motor and cognitive impairment and lessen tension and depressive-like behaviors when it was infused with BMSCs (Hu et al., 2020). Additionally, the BMSC/SA/CoI/SDF-1 scaffold could facilitate BMSC migration in lesions and partially boost neurogenesis by triggering the FAK/PI3K/AKT pathway (Wang et al., 2020a), which was regulated by SDF-1/CXCR4 and could contribute to the reduction in brain damage, neuronal death, and neural inflammation (Ma et al., 2021). In addition, the hydrogel cross-linked by 0.1 U/mL glucose oxidase and 0.5 U/mL horseradish peroxidase exerted superior cytocompatibility and a low immune response, which could further promote the therapeutic effect of GH/BMSC scaffolds (Li et al., 2021). Under 3D culture conditions, neuronal differentiation and neurotrophin secretion might be significantly improved by a dual-enzymatically cross-linked injectable gelatin hydrogel laden with BMSCs (Yao et al., 2019b). The GH/BMSC hydrogel might control the inflammatory response, restrain apoptosis, encourage neurogenesis, and increase the plasticity of neurological function (Cozene et al., 2021; Yuan et al., 2021).
To improve the rate of cell engraftment and survival, some scholars developed a new thermosensitive hydrogel based on chitosan, hydroxyethyl cellulose, HA, and β-glycerophosphate (CS-HEC-HA/GP), which possessed better biocompatibility with human umbilical cord mesenchymal stem cells (hUC-MSCs) and a faster gelation process than traditional hydrogel (Yao et al., 2019a; Yea et al., 2020). In comparison to BMSCs, hUC-MSCs offered the benefits of abundant sources, simple accessibility, few negative outcomes for the donor, low requirement for consistency in human leukocyte antigen matching, and less danger of viral infection following transplantation (Chen et al., 2020a; Barretto et al., 2021). CS-HEC-HA/GP hydrogel-laden hUC-MSCs had the ability to enhance the reservation, survival, and transfer of encapsulated hUC-MSCs (Sultan et al., 2021). Moreover, this synthesized hydrogel could possibly secrete neurotrophic factors and suppress apoptosis to promote the subsistence and multiplication of endogenous neurons (Torres-Ortega et al., 2022; Zhang et al., 2023), and expedite the rebuilding of brain tissue and recovery of neural function in TBI patients (Liu et al., 2020a, 2023b; Bamshad et al., 2023). Jiang et al. (2021) reported that compared with the simple stem cell (SC) or collagen/SF (CS) scaffold, the implantation of CS combined with the SC (CB) scaffold showed better cortical integrity and motor function and more regeneration of nerve fibers (Figure 10).
Figure 10.
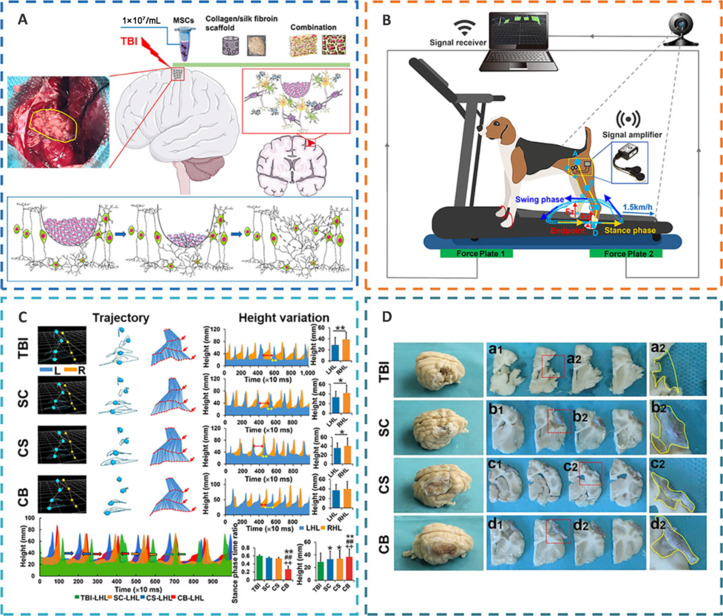
The implantation of regenerative complexes could strengthen the reconstruction of neural networks and the recovery of motor function after TBI.
(A) Complex implantation and nerve repair. (B) Gait detection with motion capture, sEMG and vGRF system. (C) Quantification of motor function recovery after complex implantation in different treatment groups. (D) Gross view of lesion filling and cerebral cortex repair in different treatment groups. Reprinted from Jiang et al. (2021) under CC BY license. CB: CS combined with the SC; CS: collagen/silk fibroin; LHL: left hindlimb; RHL: right hindlimb; SC: stem cell; sEMG: surface electromyography; TBI: traumatic brain injury; vGRF: vertical ground reaction force.
However, typically, the effectiveness of existing stem cell treatment is limited in its ability to address problems including poor implanted stem cell survival and ineffective differentiation into targeted cells (Alvarado-Velez et al., 2021). Bae et al. (2021) developed a brain-derived decellularized extracellular matrix (BdECM) bioink. With the use of BdECM, a manufactured defect-fit stem cell carrier might improve NSC differentiation capacity in vitro and the ability of implanted NSCs to survive in vivo (Bae et al., 2021). Additionally, by extending cell behavior on tissue-specific materials into the brain, the BdECM bioink could increase grafted NSC survival and development into mature neurons while maintaining grafted NSCs on the site without volumetric loss (Hu et al., 2023). Moreover, the BdECM bioink had the ability to perform an anti-neuroinflammation function for a longer period of time, which could cause cell death and necrosis at a secondary damage region from TBI, promote the long-term viability and neuronal differentiation of implanted cells, and dampen persistent neuroinflammation (Damian et al., 2021).
Bioactive Effects of Biomaterial Scaffolds Carried with Exosomes
In recent studies, stem cell-derived exosomes have been considered an alternative to exogenous stem cells for a wide range of tissue regeneration owing to their advantages such as immunity from T-cell and NK cell attack and their roles as paracrine messengers that regulated the microenvironment to trigger tissue regeneration and promote angiogenesis (Ghosh et al., 2020; Williams et al., 2020; Bambakidis et al., 2022; Zhuang et al., 2022). Liu et al. (2023c) reported a new therapeutic strategy for integrating bone marrow mesenchymal stem cell-derived exosomes (BME) into HA collagen hydrogels (DHC-BME). BME could combine the advantages of stem cells and avoid the risk of immune rejection, but persistent delivery or difficulty focusing at the lesion site were limitations (Zhang et al., 2021f). HA could promote neurodevelopment and inhibit glial scars, thereby constructing an ideal biomimetic neural microenvironment for cell adhesion, growth, and differentiation, but a single collagen hydrogel had the tendency to shrink in vivo, destabilizing the ecological niche of cell growth (Zinger et al., 2021b). The synergy of BME and DHC could enhance the biological functions of both and exert a stable function, such as promoting the differentiation of NSCs into neurons and oligodendrocytes, while inhibiting astrocyte differentiation. DHC-BME could therefore stimulate vascular and neuronal development, from endogenous NSC attraction to neuronal differentiation and vascularization (Mu et al., 2022), collaboratively foster axon regeneration, myelin renewal, synaptic formation, and even neural structure restoration, and could ultimately encourage the rebuilding of nerve function following TBI (Xu et al., 2020b; Figure 11).
Figure 11.
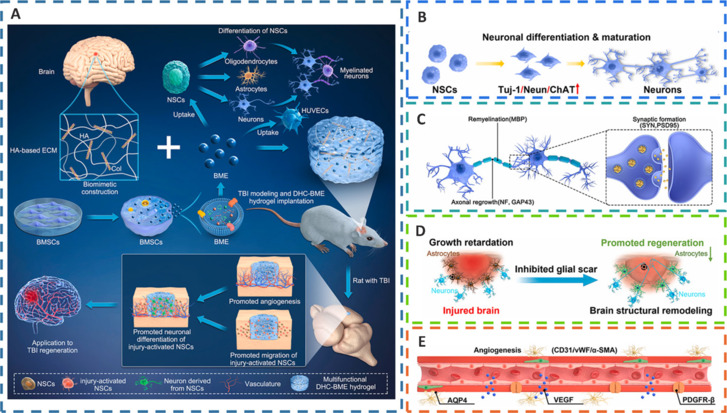
DHC-BME synergistically promotes the recovery of neural structure and function and angiogenesis after TBI.
(A) The biomimetic construction of the BME-incorporated HA-Col hybrid hydrogel, along with the coeffect on the post-implantation. (B) Endogenous neural stem cells (NSCs) differentiating into neurons and then maturing. (C) The effects of the DHC-BME hydrogel on improving axon regeneration, myelination, and synapse formation. (D) The DHC-BME hydrogel promoting brain structural remodeling by inhibiting glial scar formation. (E) The promotion of angiogenesis. Reprinted from Liu et al. (2023c) with permission from Elsevier. AQP4: Aquaporin-4; BMSCs: bone marrow-derived mesenchymal stem cells; ChAT: choline acetyltransferase; DHC-BME: HA collagen hydrogels; ECM: extracellular matrix; HA: hyaluronic acid; HA-Col: hyaluronic acid- collagen; NeuN: neuronal nuclei; PDGFR: platelet-derived growth factor receptor; PSD95: post-synaptic density protein 95; SMA: smooth muscle actin; SYN: synapsin; TBI: traumatic brain injury; Tuj-1: β-III tubulin; VEGF: vascular endothelial growth factor; vWF: von Willebrand factor.
Microglia and astrocytes actively participate in a number of disease events in the CNS. Numerous regulatory substances are produced by activated astrocytes that affect CNS immunity and microglia (Chen et al., 2020c). Microglia have a dual role: pro-inflammatory (M1-like) and anti-inflammatory (M2-like) phenotypes (Yin et al., 2020b). Exosomes and extracellular vesicles, play a key role in initiating, transmitting, and monitoring immune reactions to nearby cells through cargo proteins, RNAs, and miRNAs (Sun et al., 2020; Tonarelli and Quinn, 2020). Astrocyte-derived exosomes (AS-Exos) promote the transformation of the microglial M2 phenotype, improving neuronal flexibility, immune reactions, and neuronal lifespan in a variety of pathological conditions. AS-Exos are rich in numerous miRNAs, of which miR-873a-5p is a highly expressed major component in TBI (Gayen et al., 2020; Wang et al., 2020b), which can significantly inhibit LPS-induced microglial M1 phenotypic transformation and exert anti-inflammatory effects by reducing extracellular regulated protein kinases (ERK) and nuclear factor-κB (NF-κB) p65 phosphorylation (Long et al., 2020; Xin et al., 2023). Thus, activated astrocyte-derived miR-873a-5p can attenuate microglia-mediated nerve inflammation and neural deficits post TBI by inhibiting the nuclear factor-κB signaling pathway. Additionally, it has been demonstrated that AS-Exos drastically lowered the levels of oxidative stress and mitochondrial H2O2 in TBI patients’ hippocampal neurons by increasing the expression of antioxidant enzymes such as superoxide dismutase and catalase (Nourbakhsh et al., 2020; Zhang et al., 2021e). Furthermore, AS-Exos could reduce oxidative stress and neuronal death by triggering Nrf2/HO-1 signaling in TBI patients’ hippocampi (Salman et al., 2020; Zhao et al., 2022).
3D-printed collagen/chitosan scaffolds based on BDNF-stimulated hUC-MSC-derived exosomes (3D-CC-BMExos) were proposed as a viable treatment method for TBI (Xu et al., 2020b; Yuan et al., 2020; Liu et al., 2023b). Collagen and chitosan have the advantages of outstanding biocompatibility and physical properties that enable crucial cell-cell and cell-microenvironment interactions to support the healing of injured tissue (Zhang et al., 2021b; Hajinejad et al., 2023). For in vivo transplantation, the use of 3D printing might further modify the compound scaffold dimensions, form, porosity ratio, and morphology (Liu et al., 2022a). Moreover, the water absorption ratio of 3D-printed scaffolds was suitable, preventing the loss of nutrients and bodily fluids as well as promoting cell proliferation and tissue regeneration (Zhou et al., 2020; Tsui et al., 2021; Zhang et al., 2021c). However, traditional 3D printing techniques might impair the biological activity of exosomes; thus, when printed at a low temperature, the composite scaffolds might very well regulate exosome release (Liu et al., 2023d). In addition, the exosomes generated by 3D-CC-BMExos tended to be more persistent, and their overall release volume was greater than that of CC-BMExos owing to the 3D printing technology’s ability to equally blend and attach the exosomes to the scaffolds (Liu et al., 2020b; Zhang et al., 2020a). Furthermore, 3D-printed collagen/chitosan scaffolds that were based on NSC-derived exosomes and pretreated with insulin-like growth factor 1 (IGF-1-Exos, 3D-CC-INExos) at a low temperature could also promote neural repair by inhibiting inflammation and apoptosis, and accelerating neurogenesis and angiogenesis (Liu et al., 2023d; Figure 12).
Figure 12.
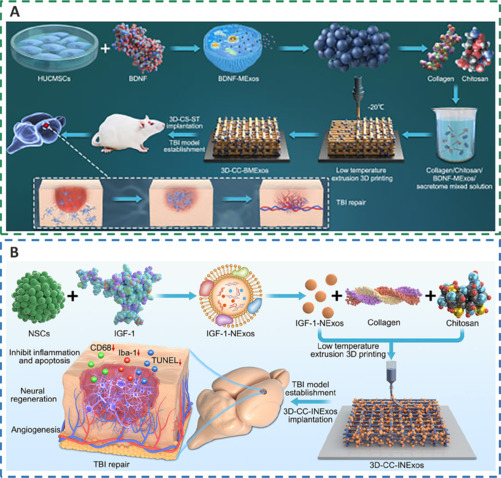
3D-printed collagen/chitosan scaffolds loaded with growth factors promote neural repair and angiogenesis at a low temperature.
(A) The preparation process of 3D-CC-BMExos for TBI treatment. Reprinted from Liu et al. (2023b) under CC BY license. (B) The preparation process and mechanism of 3D-CC-INExos. Reprinted from Liu et al. (2023d) under CC BY license. 3D-CC-BMExos: 3D-printed collagen/chitosan scaffolds based on mesenchymal stem cells-derived exosomes pretreated with BDNF; 3D-CC-INExos: 3D-printed collagen/chitosan scaffolds based on NSC-derived exosomes pretreated with IGF; 3D-CS-ST: 3D-printed collagen/silk fibroin/secretome; BDNF: brain-derived neurotrophic factor; BDNF-MExos: brain-derived neurotrophic factor-stimulated mesenchymal stem cells-derived exosomes; Iba-1: ionized calcium-binding adapter molecule 1; IGF: insulin-like growth factor; IGF-1-NExos: NSC-derived exosomes pretreated with IGF-1; NSCs: neural stem cells; TBI: traumatic brain injury; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Limitations
This review has some limitations. First, little is known about the variety of biological roles performed by NSC-derived exosomes, and it is still unclear how these exosomes connect with recipient cells to modulate target cells. However, this undoubtedly points to a new research focus for cell-to-cell communication (Yuan et al., 2020). Besides, exosome release can be better controlled by 3D-printed composite scaffolds at low temperatures, but it is vital to determine at what temperature exosomes can be stably stored for an extended period while maintaining their integrity (Liu et al., 2023d). Additionally, it is challenging to translate exosome research into clinical applications for the treatment of diseases, as there are yet no reliable, efficient, and safe procedures for the manufacture and purification of exosomes (Zhang et al., 2021e). In summary, creating an NSC exosome amplification system without heterologous components, a unified standard for purification, and storage means of NSC exosome formation should be emphasized, and a method for single-exosome NSC analysis should be defined to better understand the characteristics and mechanisms of exosome-based therapeutics and promote the clinical translation of exosome therapy.
Conclusion and Perspective
Traditional treatments such as drugs and surgery are limited to slowing the progression of disease and relieving pain. The rise and progression of TE and biomaterials have led to novel methods for neural regeneration that can effectively promote the proliferation and differentiation of neural cells, surpassing the limitations of traditional treatments. However, the challenges in translating these therapies into clinical applications may be the complex and unclear key cellular and molecular processes and healing mechanisms, as well as the physical structure and pathophysiological features of the CNS. In addition, the BBB presents a significant obstacle, as it is a highly selective semipermeable membrane that limits the number of potential biomolecules for therapeutic use.
Tissue regeneration is hindered by limitations in the interaction between immune cells and biomaterials. Although stem cell therapy has potential for treating diseases, its effectiveness is limited by its low retention rate, viability, and differentiation. To address these issues, biomaterials can act as carriers and regulators to improve the therapeutic effects of stem cells. To ameliorate neural regeneration, effective biomaterials must moderate the tissue microenvironment and cell behavior. Additionally, adaptive biomaterials can respond to undesirable substances in the milieu to boost the lifespan and functionality of host cells, resulting in an adaptable regenerative physiological environment. Last, biomaterial design must also consider the following: 1) Physical hydrogels are easy to manufacture and have minimal toxicity to cells, but they are uncertain and sluggish to react, whereas chemical hydrogels are stable but will have negative effects on the body; 2) Synthetic biomaterials possess stable and excellent properties, but their biological activity and biocompatibility are low; and 3) Natural biomaterials have superior biocompatibility, low cytotoxicity, and high biodegradability, but lack mechanical strength. In conclusion, a promising biomaterial should have optimal biodegradability, stability, biocompatibility, and mechanical quality to support neurite outgrowth and 3D cell culture.
MSCs are a promising treatment for TBI, but they remain unsuccessful because of poor cellular availability, short lifespan of cells, presence of few differentiated neurons, and poor functional integration. Surprisingly, bioactive scaffolds loaded with NSCs might supply a biocompatible and biodegradable milieu that would support NSCs to migrate, survive, and mature while alleviating neuropsychiatric symptoms. However, the current effectiveness of stem cells has certain limitations in solving the issues of transplanted stem cells having a poor survival rate and their ineffectiveness in differentiating into the corresponding cells.
Stem cell exosomes are considered an alternative to exogenous stem cell therapy. Stem cell-derived exosomes combine the advantages of stem cells and avoid the risk of immune rejection. Biomaterials can promote neural development and inhibit glial scar formation, thereby providing a good biomimetic neural microenvironment. The synergistic effect can enhance biological functions and exert a stable function, thereby inducing angiogenesis and neurogenesis and promoting the restoration of brain function in TBI patients. Furthermore, glial cell-derived exosomes can improve neuronal plasticity, immune response, and neuronal survival under various pathological conditions by promoting phenotypic transformation and exerting regulatory effects on biochemical stress and anti-neuroinflammation effects. In addition, bioactive scaffolds combined with exosomes are prospective therapeutic strategies for TBI, as compared to traditional exosome therapy, they possess better biocompatibility and mechanical properties, facilitate cell growth and neural regeneration, release exosomes in a higher total amount, and are more durable.
Funding Statement
Funding: This work was supported by the Sichuan Science and Technology Program, No. 2023YFS0164 (to JC).
Footnotes
Conflicts of interest: There are no actual or potential conflicts of interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials. 2015;72:11–19. doi: 10.1016/j.biomaterials.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Velez M, Enam SF, Mehta N, Lyon JG, LaPlaca MC, Bellamkonda RV. Immuno-suppressive hydrogels enhance allogeneic MSC survival after transplantation in the injured brain. Biomaterials. 2021;266:120419. doi: 10.1016/j.biomaterials.2020.120419. [DOI] [PubMed] [Google Scholar]
- Anderson TN, Hwang J, Munar M, Papa L, Hinson HE, Vaughan A, Rowell SE. Blood-based biomarkers for prediction of intracranial hemorrhage and outcome in patients with moderate or severe traumatic brain injury. J Trauma Acute Care Surg. 2020;89:80–86. doi: 10.1097/TA.0000000000002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari F, Rabiei Faradonbeh D, Malekshahi ZV, Nekounam H, Ghaemi B, Yousefpoor Y, Ghanbari H, Faridi-Majidi R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym. 2022;278:118926. doi: 10.1016/j.carbpol.2021.118926. [DOI] [PubMed] [Google Scholar]
- Ashina H, Iljazi A, Al-Khazali HM, Christensen CE, Amin FM, Ashina M, Schytz HW. Hypersensitivity to calcitonin gene-related peptide in post-traumatic headache. Ann Neurol. 2020a;88:1220–1228. doi: 10.1002/ana.25915. [DOI] [PubMed] [Google Scholar]
- Ashina H, Iljazi A, Al-Khazali HM, Eigenbrodt AK, Larsen EL, Andersen AM, Hansen KJ, Bräuner KB, Mørch-Jessen T, Chaudhry B, Antic S, Christensen CE, Ashina M, Amin FM, Schytz HW. Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: an open-label study. J Headache Pain. 2020b;21:62. doi: 10.1186/s10194-020-01136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Bae M, Hwang DW, Ko MK, Jin Y, Shin WJ, Park W, Chae S, Lee HJ, Jang J, Yi HG, Lee DS, Cho DW. Neural stem cell delivery using brain-derived tissue-specific bioink for recovering from traumatic brain injury. Biofabrication. 2021;13:044110. doi: 10.1088/1758-5090/ac293f. [DOI] [PubMed] [Google Scholar]
- Bailes JE, Borlongan CV. Traumatic brain injury. CNS Neurosci Ther. 2020;26:593–594. doi: 10.1111/cns.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambakidis T, Dekker SE, Williams AM, Biesterveld BE, Bhatti UF, Liu B, Li Y, Pickell Z, Buller B, Alam HB. Early treatment with a single dose of mesenchymal stem cell derived extracellular vesicles modulates the brain transcriptome to create neuroprotective changes in a porcine model of traumatic brain injury and hemorrhagic shock. Shock. 2022;57:281–290. doi: 10.1097/SHK.0000000000001889. [DOI] [PubMed] [Google Scholar]
- Bamshad C, Habibi Roudkenar M, Abedinzade M, Yousefzadeh Chabok S, Pourmohammadi-Bejarpasi Z, Najafi-Ghalehlou N, Sato T, Tomita K, Jahanian-Najafabadi A, Feizkhah A, Mohammadi Roushandeh A. Human umbilical cord-derived mesenchymal stem cells-harvested mitochondrial transplantation improved motor function in TBI models through rescuing neuronal cells from apoptosis and alleviating astrogliosis and microglia activation. Int Immunopharmacol. 2023;118:110106. doi: 10.1016/j.intimp.2023.110106. [DOI] [PubMed] [Google Scholar]
- Barretto TA, Park E, Telliyan T, Liu E, Gallagher D, Librach C, Baker A. Vascular dysfunction after modeled traumatic brain injury is preserved with administration of umbilical cord derived mesenchymal stromal cells and is associated with modulation of the angiogenic response. J Neurotrauma. 2021;38:2747–2762. doi: 10.1089/neu.2021.0158. [DOI] [PubMed] [Google Scholar]
- Bartlett RD, Eleftheriadou D, Evans R, Choi D, Phillips JB. Mechanical properties of the spinal cord and brain: Comparison with clinical-grade biomaterials for tissue engineering and regenerative medicine. Biomaterials. 2020;258:120303. doi: 10.1016/j.biomaterials.2020.120303. [DOI] [PubMed] [Google Scholar]
- Basit RH, Tzerakis N, Jenkins SI, Chari DM. In vitro model of traumatic brain injury to screen neuro-regenerative biomaterials. Mater Sci Eng C Mater Biol Appl. 2021;128:112253. doi: 10.1016/j.msec.2021.112253. [DOI] [PubMed] [Google Scholar]
- Bechinger P, Serrano Sponton L, Grützner V, Musyanovych A, Jussen D, Krenzlin H, Eldahaby D, Riede N, Kempski O, Ringel F, Alessandri B. In-vivo time course of organ uptake and blood-brain-barrier permeation of poly(L-lactide) and poly(perfluorodecyl acrylate) nanoparticles with different surface properties in unharmed and brain-traumatized rats. Front Neurol. 2023;14:994877. doi: 10.3389/fneur.2023.994877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open. 2022;12:e060188. doi: 10.1136/bmjopen-2021-060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Ocampo D, Cawen FA, Álamo-Pindado LA, Negro-Demontel ML, Peluffo H. Safe and neuroprotective vectors for long-term traumatic brain injury gene therapy. Gene Ther. 2020;27:96–103. doi: 10.1038/s41434-019-0073-8. [DOI] [PubMed] [Google Scholar]
- Bonsack B, Corey S, Shear A, Heyck M, Cozene B, Sadanandan N, Zhang H, Gonzales-Portillo B, Sheyner M, Borlongan CV. Mesenchymal stem cell therapy alleviates the neuroinflammation associated with acquired brain injury. CNS Neurosci Ther. 2020;26:603–615. doi: 10.1111/cns.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Pires R, Onggradito H, Ujvari E, Karimi S, Valeo F, Aldhoun J, Edge CJ, Franks NP, Dickinson R. Xenon treatment after severe traumatic brain injury improves locomotor outcome, reduces acute neuronal loss and enhances early beneficial neuroinflammation: a randomized, blinded, controlled animal study. Crit Care. 2020;24:667. doi: 10.1186/s13054-020-03373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, Keller A, Zhang H, Rabinovitch PS, Marcinek DJ, Bruce JE. Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci U S A. 2020;117:15363–15373. doi: 10.1073/pnas.2002250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Shao PL, Li YC, Chiang JY, Sung PH, Chien HW, Shih FY, Lee MS, Chen WF, Yip HK. Human umbilical cord-derived mesenchymal stem cell therapy effectively protected the brain architecture and neurological function in rat after acute traumatic brain injury. Cell Transplant. 2020a;29:963689720929313. doi: 10.1177/0963689720929313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Song Q, Chen Y, Meng S, Zheng M, Huang J, Zhang Q, Jiang J, Feng J, Chen H, Jiang G, Gao X. Tailored reconstituted lipoprotein for site-specific and mitochondria-targeted cyclosporine a delivery to treat traumatic brain injury. ACS Nano. 2020b;14:6636–6648. doi: 10.1021/acsnano.9b09186. [DOI] [PubMed] [Google Scholar]
- Chen X, Huang X, Liu C, Li S, Yang Z, Zhang F, Chen X, Shan H, Tao L, Zhang M. Surface-fill H(2)S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 2022;154:259–274. doi: 10.1016/j.actbio.2022.11.021. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li J, Ma B, Li N, Wang S, Sun Z, Xue C, Han Q, Wei J, Zhao RC. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging (Albany NY) 2020c;12:18274–18296. doi: 10.18632/aging.103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang N, Ma X, Wang P, Dong W, Chen Z, Wu M, Wang Z, Wang L, Guan D, Zhao R. Spatial-temporal changes of iron deposition and iron metabolism after traumatic brain injury in mice. Front Mol Neurosci. 2022;15:949573. doi: 10.3389/fnmol.2022.949573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Gao Y, Li J, Rui T, Li Q, Chen H, Jia B, Song Y, Gu Z, Wang T, Gao C, Wang Y, Wang Z, Wang F, Tao L, Luo C. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic Biol Med. 2023;194:184–198. doi: 10.1016/j.freeradbiomed.2022.12.002. [DOI] [PubMed] [Google Scholar]
- Cozene B, Sadanandan N, Farooq J, Kingsbury C, Park YJ, Wang ZJ, Moscatello A, Saft M, Cho J, Gonzales-Portillo B, Borlongan CV. Mesenchymal stem cell-induced anti-neuroinflammation against traumatic brain injury. Cell Transplant. 2021;30:9636897211035715. doi: 10.1177/09636897211035715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeiter E, Amrein K, Gravesteijn BY, Lecky F, Menon DK, Mondello S, Newcombe VFJ, Richter S, Steyerberg EW, Vyvere TV, Verheyden J, Xu H, Yang Z, Maas AIR, Wang KKW, Büki A. Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine. 2020;56:102785. doi: 10.1016/j.ebiom.2020.102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian C, Ghuman H, Mauney C, Azar R, Reinartz J, Badylak SF, Modo M. Post-stroke timing of ECM hydrogel implantation affects biodegradation and tissue restoration. Int J Mol Sci. 2021;22:11372. doi: 10.3390/ijms222111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Deng L, Yao S, Wu W, Cao J, Sun L, Bai Y, Li H, Weng X, Ren H, Ren W. Protective effects of curcumin against thyroid hormone imbalance after gas explosion-induced traumatic brain injury via activation of the hypothalamic-pituitary-thyroid axis in male rats. Environ Sci Pollut Res Int. 2022;29:74619–74631. doi: 10.1007/s11356-022-20943-2. [DOI] [PubMed] [Google Scholar]
- Du M, Wu C, Yu R, Cheng Y, Tang Z, Wu B, Fu J, Tan W, Zhou Q, Zhu Z, Balawi E, Huang X, Ma J, Liao ZB. A novel circular RNA, circIgfbp2, links neural plasticity and anxiety through targeting mitochondrial dysfunction and oxidative stress-induced synapse dysfunction after traumatic brain injury. Mol Psychiatry. 2022;27:4575–4589. doi: 10.1038/s41380-022-01711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Edelbrock AN, Àlvarez Z, Simkin D, Fyrner T, Chin SM, Sato K, Kiskinis E, Stupp SI. Supramolecular nanostructure activates TrkB receptor signaling of neuronal cells by mimicking brain-derived neurotrophic factor. Nano Lett. 2018;18:6237–6247. doi: 10.1021/acs.nanolett.8b02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Yang W, Zhang L, Cai H, Zhuang Y, Chen Y, Zhao Y, Dai J. Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Biomaterials. 2022;288:121689. doi: 10.1016/j.biomaterials.2022.121689. [DOI] [PubMed] [Google Scholar]
- Fang J, Yuan Q, Du Z, Zhang Q, Yang L, Wang M, Yang W, Yuan C, Yu J, Wu G, Hu J. Overexpression of GPX4 attenuates cognitive dysfunction through inhibiting hippocampus ferroptosis and neuroinflammation after traumatic brain injury. Free Radic Biol Med. 2023;204:68–81. doi: 10.1016/j.freeradbiomed.2023.04.014. [DOI] [PubMed] [Google Scholar]
- Finbloom JA, Sousa F, Stevens MM, Desai TA. Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv Drug Deliv Rev. 2020;167:89–108. doi: 10.1016/j.addr.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JL, Dill LK, Wood RJ, Wang S, Robertson K, Murray SS, Zamani A, Semple BD. Acute treatment with TrkB agonist LM22A-4 confers neuroprotection and preserves myelin integrity in a mouse model of pediatric traumatic brain injury. Exp Neurol. 2021;339:113652. doi: 10.1016/j.expneurol.2021.113652. [DOI] [PubMed] [Google Scholar]
- Fure SCR, Howe EI, Andelic N, Brunborg C, Sveen U, Røe C, Rike PO, Olsen A, Spjelkavik Ø, Ugelstad H, Lu J, Ponsford J, Twamley EW, Hellstrøm T, Løvstad M. Cognitive and vocational rehabilitation after mild-to-moderate traumatic brain injury: A randomised controlled trial. Ann Phys Rehabil Med. 2021;64:101538. doi: 10.1016/j.rehab.2021.101538. [DOI] [PubMed] [Google Scholar]
- Fyfe I. Nanoparticles improve outcomes of traumatic brain injury in mice. Nat Rev Neurol. 2020;16:129. doi: 10.1038/s41582-020-0325-7. [DOI] [PubMed] [Google Scholar]
- Galarza S, Crosby AJ, Pak C, Peyton SR. Control of astrocyte quiescence and activation in a synthetic brain hydrogel. Adv Healthc Mater. 2020;9:e1901419. doi: 10.1002/adhm.201901419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen M, Bhomia M, Balakathiresan N, Knollmann-Ritschel B. Exosomal microRNAs released by activated astrocytes as potential neuroinflammatory biomarkers. Int J Mol Sci. 2020;21:2312. doi: 10.3390/ijms21072312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Xue X, Xian J, Yuan L, Wang L, Zou Y, Zhong J, Jiang Z, Shi J, Chen T, Su H, Feng H, Hu S. Ferrostatin-1 alleviates white matter injury via decreasing ferroptosis following spinal cord injury. Mol Neurobiol. 2022;59:161–176. doi: 10.1007/s12035-021-02571-y. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Garg S, Ghosh S. Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem Neurosci. 2020;11:2045–2047. doi: 10.1021/acschemneuro.0c00368. [DOI] [PubMed] [Google Scholar]
- Grebenik EA, Surin AM, Bardakova KN, Demina TS, Minaev NV, Veryasova NN, Artyukhova MA, Krasilnikova IA, Bakaeva ZV, Sorokina EG, Boyarkin DP, Akopova TA, Pinelis VG, Timashev PS. Chitosan-g-oligo(L,L-lactide) copolymer hydrogel for nervous tissue regeneration in glutamate excitotoxicity: in vitro feasibility evaluation. Biomed Mater. 2020;15:015011. doi: 10.1088/1748-605X/ab6228. [DOI] [PubMed] [Google Scholar]
- Guo K, Yao X, Wu W, Yu Z, Li Z, Ma Z, Liu D. HIF-1α/SDF-1/CXCR4 axis reduces neuronal apoptosis via enhancing the bone marrow-derived mesenchymal stromal cell migration in rats with traumatic brain injury. Exp Mol Pathol. 2020;114:104416. doi: 10.1016/j.yexmp.2020.104416. [DOI] [PubMed] [Google Scholar]
- Hajinejad M, Ebrahimzadeh MH, Ebrahimzadeh-Bideskan A, Rajabian A, Gorji A, Sahab Negah S. Exosomes and nano-SDF scaffold as a cell-free-based treatment strategy improve traumatic brain injury mechanisms by decreasing oxidative stress, neuroinflammation, and increasing neurogenesis. Stem Cell Rev Rep. 2023;19:1001–1018. doi: 10.1007/s12015-022-10483-0. [DOI] [PubMed] [Google Scholar]
- Han Z, Han Y, Huang X, Ma H, Zhang X, Song J, Dong J, Li S, Yu R, Liu H. A novel targeted nanoparticle for traumatic brain injury treatment: combined effect of ROS depletion and calcium overload inhibition. Adv Healthc Mater. 2022;11:e2102256. doi: 10.1002/adhm.202102256. [DOI] [PubMed] [Google Scholar]
- Hasanzadeh E, Seifalian A, Mellati A, Saremi J, Asadpour S, Enderami SE, Nekounam H, Mahmoodi N. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater Today Bio. 2023;20:100614. doi: 10.1016/j.mtbio.2023.100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Shi X, Wang J, Wang X, Wang Q, Yu D, Ge B, Zhang X, Huang F. Reactive oxygen species-induced aggregation of nanozymes for neuron injury. ACS Appl Mater Interfaces. 2020;12:209–216. doi: 10.1021/acsami.9b17509. [DOI] [PubMed] [Google Scholar]
- Hong JY, Seo Y, Davaa G, Kim HW, Kim SH, Hyun JK. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020;101:357–371. doi: 10.1016/j.actbio.2019.11.012. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jia Y, Wang S, Ma Y, Huang G, Ding T, Feng D, Genin GM, Wei Z, Xu F. An ECM-mimicking, injectable, viscoelastic hydrogel for treatment of brain lesions. Adv Healthc Mater. 2023;12:e2201594. doi: 10.1002/adhm.202201594. [DOI] [PubMed] [Google Scholar]
- Hu Z, Gajavelli S, Spurlock MS, Mahavadi A, Quesada LS, Gajavelli GR, Andreoni CB, Di L, Janecki J, Lee SW, Rivera KN, Shear DA, Bullock RM. Human neural stem cell transplant location-dependent neuroprotection and motor deficit amelioration in rats with penetrating traumatic brain injury. J Trauma Acute Care Surg. 2020;88:477–485. doi: 10.1097/TA.0000000000002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ye Y, Zhang J, Zhang X, Ma H, Zhang Y, Fu X, Tang J, Jiang N, Han Y, Liu H, Chen H. Reactive oxygen species scavenging functional hydrogel delivers procyanidins for the treatment of traumatic brain injury in mice. ACS Appl Mater Interfaces. 2022;14:33756–33767. doi: 10.1021/acsami.2c04930. [DOI] [PubMed] [Google Scholar]
- Jeong DU, Bae S, Macks C, Whitaker J, Lynn M, Webb K, Lee JS. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed Mater. 2021;16:035002. doi: 10.1088/1748-605X/abc7f1. [DOI] [PubMed] [Google Scholar]
- Jha KA, Gentry J, Del Mar NA, Reiner A, Sohl N, Gangaraju R. Adipose tissue-derived mesenchymal stem cell concentrated conditioned medium alters the expression pattern of glutamate regulatory proteins and aquaporin-4 in the retina after mild traumatic brain injury. J Neurotrauma. 2021;38:1702–1716. doi: 10.1089/neu.2020.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Liu X, Cao Y, Niu H, Lan Z, Li R, Li F, Sun D, Shi M, Wa L, Liu X, Yang G, Chen F, Zhang S, Zhang J. Deferoxamine ameliorates neurological dysfunction by inhibiting ferroptosis and neuroinflammation after traumatic brain injury. Brain Res. 2023;1812:148383. doi: 10.1016/j.brainres.2023.148383. [DOI] [PubMed] [Google Scholar]
- Jiang J, Liu X, Chen H, Dai C, Niu X, Dai L, Chen X, Zhang S. 3D printing collagen/heparin sulfate scaffolds boost neural network reconstruction and motor function recovery after traumatic brain injury in canine. Biomater Sci. 2020;8:6362–6374. doi: 10.1039/d0bm01116a. [DOI] [PubMed] [Google Scholar]
- Jiang J, Dai C, Liu X, Dai L, Li R, Ma K, Xu H, Zhao F, Zhang Z, He T, Niu X, Chen X, Zhang S. Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery. Theranostics. 2021;11:768–788. doi: 10.7150/thno.50540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowski MP, Bettio L, E KW, Patten A, Yau SY, Gil-Mohapel J. Beyond the Hippocampus and the SVZ: Adult neurogenesis throughout the brain. Front Cell Neurosci. 2020;14:576444. doi: 10.3389/fncel.2020.576444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZM, Wilts E, Vlaisavljevich E, Long TE, Verbridge SS. Electroresponsive hydrogels for therapeutic applications in the brain. Macromol Biosci. 2022;22:e2100355. doi: 10.1002/mabi.202100355. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. 2020;134:104679. doi: 10.1016/j.nbd.2019.104679. [DOI] [PubMed] [Google Scholar]
- Kolias AG, Adams H, Timofeev IS, Corteen EA, Hossain I, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Posti JP, Servadei F, et al. Evaluation of outcomes among patients with traumatic intracranial hypertension treated with decompressive craniectomy vs standard medical care at 24 months: a secondary analysis of the rescueicp randomized clinical trial. JAMA Neurol. 2022;79:664–671. doi: 10.1001/jamaneurol.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien JM. The pathogenesis of neurotrauma indicates targets for neuroprotective therapies. Curr Neuropharmacol. 2021;19:1191–1201. doi: 10.2174/1570159X19666210125153308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé A, Brot S, Gaillard A. Beneficial effects of hyaluronan-based hydrogel implantation after cortical traumatic injury. Cells. 2022;11:3831. doi: 10.3390/cells11233831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassarén P, Lindblad C, Frostell A, Carpenter KLH, Guilfoyle MR, Hutchinson PJA, Helmy A, Thelin EP. Systemic inflammation alters the neuroinflammatory response: a prospective clinical trial in traumatic brain injury. J Neuroinflammation. 2021;18:221. doi: 10.1186/s12974-021-02264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ducker M, Sun B, Szele FG, Czernuszka JT. Interpenetrating polymer networks of collagen, hyaluronic acid, and chondroitin sulfate as scaffolds for brain tissue engineering. Acta Biomater. 2020a;112:122–135. doi: 10.1016/j.actbio.2020.05.042. [DOI] [PubMed] [Google Scholar]
- Li H, Jin K, Luo M, Wang X, Zhu X, Liu X, Jiang T, Zhang Q, Wang S, Pang Z. Size dependency of circulation and biodistribution of biomimetic nanoparticles: red blood cell membrane-coated nanoparticles. Cells. 2019;8:881. doi: 10.3390/cells8080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang D, Guo S, Zhao C, Wang L, Ma S, Guan F, Yao M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int J Biol Macromol. 2021;187:200–213. doi: 10.1016/j.ijbiomac.2021.07.111. [DOI] [PubMed] [Google Scholar]
- Li QS, Jia YJ. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen Res. 2023;18:506–512. doi: 10.4103/1673-5374.350187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang C, Haggerty AE, Yan J, Lan M, Seu M, Yang M, Marlow MM, Maldonado-Lasunción I, Cho B, Zhou Z, Chen L, Martin R, Nitobe Y, Yamane K, You H, Reddy S, Quan DP, Oudega M, Mao HQ. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials. 2020b;245:119978. doi: 10.1016/j.biomaterials.2020.119978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Shen Z, Sun X, Yu D, Liu K, Mugo SM, Chen W, Wang D, Zhang Q. Electron conductive and transparent hydrogels for recording brain neural signals and neuromodulation. Adv Mater. 2023;35:e2211159. doi: 10.1002/adma.202211159. [DOI] [PubMed] [Google Scholar]
- Liao J, Li X, He W, Guo Q, Fan Y. A biomimetic triple-layered biocomposite with effective multifunction for dura repair. Acta Biomater. 2021;130:248–267. doi: 10.1016/j.actbio.2021.06.003. [DOI] [PubMed] [Google Scholar]
- Lim TC, Mandeville E, Weng D, Wang LS, Kurisawa M, Leite-Morris K, Selim MH, Lo EH, Spector M. Hydrogel-based therapy for brain repair after intracerebral hemorrhage. Transl Stroke Res. 2020;11:412–417. doi: 10.1007/s12975-019-00721-y. [DOI] [PubMed] [Google Scholar]
- Liu H, Feng Y, Che S, Guan L, Yang X, Zhao Y, Fang L, Zvyagin AV, Lin Q. An electroconductive hydrogel scaffold with injectability and biodegradability to manipulate neural stem cells for enhancing spinal cord injury repair. Biomacromolecules. 2023a;24:86–97. doi: 10.1021/acs.biomac.2c00920. [DOI] [PubMed] [Google Scholar]
- Liu X, Song S, Chen Z, Gao C, Li Y, Luo Y, Huang J, Zhang Z. Release of O-GlcNAc transferase inhibitor promotes neuronal differentiation of neural stem cells in 3D bioprinted supramolecular hydrogel scaffold for spinal cord injury repair. Acta Biomater. 2022a;151:148–162. doi: 10.1016/j.actbio.2022.08.031. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang J, Cheng X, Liu P, Feng Q, Wang S, Li Y, Gu H, Zhong L, Chen M, Zhou L. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen Biomater. 2023b;10:rbac085. doi: 10.1093/rb/rbac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu C, Zhang Y, Chen S, Ding J, Chen Z, Wu K, Wu X, Zhou T, Zeng M, Wei D, Sun J, Fan H, Zhou L. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr Polym. 2023c;306:120578. doi: 10.1016/j.carbpol.2023.120578. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chang ZH, Chen C, Liang J, Shi JX, Fan X, Shao Q, Meng WW, Wang JJ, Li XH. 3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats. Stem Cell Res Ther. 2022b;13:525. doi: 10.1186/s13287-022-03208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Feng YH, Feng QB, Zhang JY, Zhong L, Liu P, Wang S, Huang YR, Chen XY, Zhou LX. Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury. Neural Regen Res. 2023d;18:1990–1998. doi: 10.4103/1673-5374.366497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wei MG, Liang J, Xu HH, Wang JJ, Wang J, Yang XP, Lv FF, Wang KQ, Duan JH, Tu Y, Zhang S, Chen C, Li XH. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J Neurochem. 2020a;153:230–251. doi: 10.1111/jnc.14859. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hsu YH, Huang AP, Hsu SH. Semi-interpenetrating polymer network of hyaluronan and chitosan self-healing hydrogels for central nervous system repair. ACS Appl Mater Interfaces. 2020b;12:40108–40120. doi: 10.1021/acsami.0c11433. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Z, Zhang Y, Luo B, Liu X, Cao Y, Pei R. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomater. 2023e;158:178–189. doi: 10.1016/j.actbio.2022.12.048. [DOI] [PubMed] [Google Scholar]
- Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, Zhao K, Zhang H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation. 2020;17:89. doi: 10.1186/s12974-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Preza FI, Huerta de la Cruz S, Santiago-Castañeda C, Silva-Velasco DL, Beltran-Ornelas JH, Tapia-Martínez J, Sánchez-López A, Rocha L, Centurión D. Hydrogen sulfide prevents the vascular dysfunction induced by severe traumatic brain injury in rats by reducing reactive oxygen species and modulating eNOS and H(2)S-synthesizing enzyme expression. Life Sci. 2023;312:121218. doi: 10.1016/j.lfs.2022.121218. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhou X, Cheng J, Ma Q. Early intensified rehabilitation training with hyperbaric oxygen therapy improves functional disorders and prognosis of patients with traumatic brain injury. Adv Wound Care (New Rochelle) 2021;10:663–670. doi: 10.1089/wound.2018.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb Perspect Biol. 2015;7:a019034. doi: 10.1101/cshperspect.a019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zhao Y, Huang L, Xiao Z, Chen B, Shi Y, Shen H, Dai J. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020a;237:119830. doi: 10.1016/j.biomaterials.2020.119830. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhou J, Huang T, Zhang Z, Xing Q, Zhou X, Zhang K, Yao M, Cheng T, Wang X, Wen X, Guan F. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021;131:185–197. doi: 10.1016/j.actbio.2021.06.038. [DOI] [PubMed] [Google Scholar]
- Ma X, Agas A, Siddiqui Z, Kim K, Iglesias-Montoro P, Kalluru J, Kumar V, Haorah J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact Mater. 2020b;5:124–132. doi: 10.1016/j.bioactmat.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Manley GT, Abrams M, Åkerlund C, Andelic N, Aries M, Bashford T, Bell MJ, Bodien YG, Brett BL, Büki A, Chesnut RM, Citerio G, Clark D, Clasby B, Cooper DJ, Czeiter E, Czosnyka M, Dams-O’Connor K, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21:1004–1060. doi: 10.1016/S1474-4422(22)00309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macks C, Jeong D, Bae S, Webb K, Lee JS. Dexamethasone-loaded hydrogels improve motor and cognitive functions in a rat mild traumatic brain injury model. Int J Mol Sci. 2022;23:11153. doi: 10.3390/ijms231911153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, DeGutis J, Levin H, Newsome MR, Bell MD, Grills C, French LM, Sullivan KW, Kim SJ, Rose A, Stasio C, Merzenich MM. A randomized clinical trial of plasticity-based cognitive training in mild traumatic brain injury. Brain. 2021;144:1994–2008. doi: 10.1093/brain/awab202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man W, Yang S, Cao Z, Lu J, Kong X, Sun X, Zhao L, Guo Y, Yao S, Wang G, Wang X. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials. 2021;276:120971. doi: 10.1016/j.biomaterials.2021.120971. [DOI] [PubMed] [Google Scholar]
- Mason H, Rai G, Kozyr A, De Jonge N, Gliniewicz E, Berg LJ, Wald G, Dorrier C, Henderson MJ, Zakharov A, Dyson T, Audley J, Pettinato AM, Padilha EC, Shah P, Xu X, Leto TL, Simeonov A, Zarember KA, McGavern DB, et al. Development of an improved and specific inhibitor of NADPH oxidase 2 to treat traumatic brain injury. Redox Biol. 2023;60:102611. doi: 10.1016/j.redox.2023.102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary DD, Resick PA, Penzien DB, McGeary CA, Houle TT, Eapen BC, Jaramillo CA, Nabity PS, Reed DE, 2nd, Moring JC, Bira LM, Hansen HR, Young-McCaughan S, Cobos BA, Mintz J, Keane TM, Peterson AL. Cognitive behavioral therapy for veterans with comorbid posttraumatic headache and posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Neurol. 2022;79:746–757. doi: 10.1001/jamaneurol.2022.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishchenko TA, Klimenko MO, Kuznetsova AI, Yarkov RS, Savelyev AG, Sochilina AV, Mariyanats AO, Popov VK, Khaydukov EV, Zvyagin AV, Vedunova MV. 3D-printed hyaluronic acid hydrogel scaffolds impregnated with neurotrophic factors (BDNF, GDNF) for post-traumatic brain tissue reconstruction. Front Bioeng Biotechnol. 2022;10:895406. doi: 10.3389/fbioe.2022.895406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J, Ma T, Chen J, Zhang C, Zhang X, Lu T, Kong X, Sun J, Gao J. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10:1803–1811. doi: 10.1039/d1bm01722e. [DOI] [PubMed] [Google Scholar]
- Mu X, He H, Wang J, Long W, Li Q, Liu H, Gao Y, Ouyang L, Ren Q, Sun S, Wang J, Yang J, Liu Q, Sun Y, Liu C, Zhang XD, Hu W. Carbogenic nanozyme with ultrahigh reactive nitrogen species selectivity for traumatic brain injury. Nano Lett. 2019;19:4527–4534. doi: 10.1021/acs.nanolett.9b01333. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nam J, Lim HK, Kim NH, Park JK, Kang ES, Kim YT, Heo C, Lee OS, Kim SG, Yun WS, Suh M, Kim YH. Supramolecular peptide hydrogel-based soft neural interface augments brain signals through a three-dimensional electrical network. ACS Nano. 2020;14:664–675. doi: 10.1021/acsnano.9b07396. [DOI] [PubMed] [Google Scholar]
- Nemkova SA. Modern approaches to the diagnostics and treatment of the consequences of traumatic brain injury in children and adolescents. Zh Nevrol Psikhiatr Im S I’m Korsakova. 2022;122:20–29. doi: 10.17116/jnevro202212206120. [DOI] [PubMed] [Google Scholar]
- Ngo TB, Spearman BS, Hlavac N, Schmidt CE. Three-dimensional bioprinted hyaluronic acid hydrogel test beds for assessing neural cell responses to competitive growth stimuli. ACS Biomater Sci Eng. 2020;6:6819–6830. doi: 10.1021/acsbiomaterials.0c00940. [DOI] [PubMed] [Google Scholar]
- Nguyen LTB, Hsu CC, Ye H, Cui Z. Development of an in situ injectable hydrogel containing hyaluronic acid for neural regeneration. Biomed Mater. 2020;15:055005. doi: 10.1088/1748-605X/ab8c43. [DOI] [PubMed] [Google Scholar]
- Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater. 2018;17:642–651. doi: 10.1038/s41563-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson MKL, Johansson B, Carlsson ML, Schuit RC, Rönnbäck L. Effect of the monoaminergic stabiliser (-)-OSU6162 on mental fatigue following stroke or traumatic brain injury. Acta Neuropsychiatr. 2020;32:303–312. doi: 10.1017/neu.2020.22. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh M, Zarrintaj P, Jafari SH, Hosseini SM, Aliakbari S, Pourbadie HG, Naderi N, Zibaii MI, Gholizadeh SS, Ramsey JD, Thomas S, Farokhi M, Saeb MR. Fabricating an electroactive injectable hydrogel based on pluronic-chitosan/aniline-pentamer containing angiogenic factor for functional repair of the hippocampus ischemia rat model. Mater Sci Eng C Mater Biol Appl. 2020;117:111328. doi: 10.1016/j.msec.2020.111328. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Mu X, Wang J, Li Q, Gao Y, Liu H, Sun S, Ren Q, Yan R, Wang J, Liu Q, Sun Y, Liu C, He H, Long W, Zhang XD. Carbon dot targeting to nitrogen signaling molecules for inhibiting neuronal death. J Mater Chem B. 2020;8:2321–2330. doi: 10.1039/c9tb02447f. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Musyaju S, Modi HR, Cao Y, Flerlage WJ, Huynh L, Kociuba B, Visavadiya NP, Kobeissy F, Wang K, Gilsdorf JS, Scultetus AH, Shear DA. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free Radic Biol Med. 2023;198:44–58. doi: 10.1016/j.freeradbiomed.2023.02.001. [DOI] [PubMed] [Google Scholar]
- Park HH, Kim YM, Anh Hong LT, Kim HS, Kim SH, Jin X, Hwang DH, Kwon MJ, Song SC, Kim BG. Dual-functional hydrogel system for spinal cord regeneration with sustained release of arylsulfatase B alleviates fibrotic microenvironment and promotes axonal regeneration. Biomaterials. 2022;284:121526. doi: 10.1016/j.biomaterials.2022.121526. [DOI] [PubMed] [Google Scholar]
- Park S, Yuk H, Zhao R, Yim YS, Woldeghebriel EW, Kang J, Canales A, Fink Y, Choi GB, Zhao X, Anikeeva P. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun. 2021;12:3435. doi: 10.1038/s41467-021-23802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischiutta F, Caruso E, Lugo A, Cavaleiro H, Stocchetti N, Citerio G, Salgado A, Gallus S, Zanier ER. Systematic review and meta-analysis of preclinical studies testing mesenchymal stromal cells for traumatic brain injury. NPJ Regen Med. 2021;6:71. doi: 10.1038/s41536-021-00182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Han Y, Han Z, Zhang D, Zhang L, Zhao G, Li S, Jin G, Yu R, Liu H. In Situ implantable, post-trauma microenvironment-responsive, ROS depletion hydrogels for the treatment of traumatic brain injury. Biomaterials. 2021;270:120675. doi: 10.1016/j.biomaterials.2021.120675. [DOI] [PubMed] [Google Scholar]
- Roh EJ, Kim DS, Kim JH, Lim CS, Choi H, Kwon SY, Park SY, Kim JY, Kim HM, Hwang DY, Han DK, Han I. Multimodal therapy strategy based on a bioactive hydrogel for repair of spinal cord injury. Biomaterials. 2023;299:122160. doi: 10.1016/j.biomaterials.2023.122160. [DOI] [PubMed] [Google Scholar]
- Roquilly A, Moyer JD, Huet O, Lasocki S, Cohen B, Dahyot-Fizelier C, Chalard K, Seguin P, Jeantrelle C, Vermeersch V, Gaillard T, Cinotti R, Demeure Dit Latte D, Mahe PJ, Vourc’h M, Martin FP, Chopin A, Lerebourg C, Flet L, Chiffoleau A, et al. Effect of continuous infusion of hypertonic saline vs standard care on 6-month neurological outcomes in patients with traumatic brain injury: The COBI randomized clinical trial. JAMA. 2021;325:2056–2066. doi: 10.1001/jama.2021.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau N, Bonzanni M, Erndt-Marino JD, Sievert K, Ramirez CG, Rusk W, Levin M, Kaplan DL. A 3D tissue model of traumatic brain injury with excitotoxicity that is inhibited by chronic exposure to gabapentinoids. Biomolecules. 2020;10:1196. doi: 10.3390/biom10081196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, Bulger EM, Idris AH, Christenson J, Morrison LJ, Frascone RJ, Bosarge PL, Colella MR, Johannigman J, Cotton BA, Callum J, McMullan J, Dries DJ, Tibbs B, Richmond NJ, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324:961–974. doi: 10.1001/jama.2020.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui T, Wang H, Li Q, Cheng Y, Gao Y, Fang X, Ma X, Chen G, Gao C, Gu Z, Song S, Zhang J, Wang C, Wang Z, Wang T, Zhang M, Min J, Chen X, Tao L, Wang F, et al. Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. J Pineal Res. 2021;70:e12704. doi: 10.1111/jpi.12704. [DOI] [PubMed] [Google Scholar]
- Ruppert KA, Prabhakara KS, Toledano-Furman NE, Udtha S, Arceneaux AQ, Park H, Dao A, Cox CS, Olson SD. Human adipose-derived mesenchymal stem cells for acute and sub-acute TBI. PLoS One. 2020;15:e0233263. doi: 10.1371/journal.pone.0233263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S, May RM. Oxford: Oxford University Press; 1928. Degeneration and regeneration of the nervous system. [Google Scholar]
- Salman M, Tabassum H, Parvez S. Nrf2/HO-1 mediates the neuroprotective effects of pramipexole by attenuating oxidative damage and mitochondrial perturbation after traumatic brain injury in rats. Dis Model Mech. 2020;13:dmm045021. doi: 10.1242/dmm.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish L, Santra S, Tsurkan MV, Werner C, Jana M, Sahoo H. Conformational changes of GDNF-derived peptide induced by heparin, heparan sulfate, and sulfated hyaluronic acid - Analysis by circular dichroism spectroscopy and molecular dynamics simulation. Int J Biol Macromol. 2021;182:2144–2150. doi: 10.1016/j.ijbiomac.2021.05.194. [DOI] [PubMed] [Google Scholar]
- Schirmer L, Hoornaert C, Le Blon D, Eigel D, Neto C, Gumbleton M, Welzel PB, Rosser AE, Werner C, Ponsaerts P, Newland B. Heparin-based, injectable microcarriers for controlled delivery of interleukin-13 to the brain. Biomater Sci. 2020;8:4997–5004. doi: 10.1039/d0bm01249a. [DOI] [PubMed] [Google Scholar]
- Selvakumar GP, Ahmed ME, Iyer SS, Thangavel R, Kempuraj D, Raikwar SP, Bazley K, Wu K, Khan A, Kukulka K, Bussinger B, Zaheer S, Burton C, James D, Zaheer A. Absence of glia maturation factor protects from axonal injury and motor behavioral impairments after traumatic brain injury. Exp Neurobiol. 2020;29:230–248. doi: 10.5607/en20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma GP, Frei AC, Narayanan J, Gasperetti T, Veley D, Amjad A, Albano K, Fish BL, Himburg HA. Brain-derived neurotrophic factor promotes immune reconstitution following radiation injury via activation of bone marrow mesenchymal stem cells. PLoS One. 2021;16:e0259042. doi: 10.1371/journal.pone.0259042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Ifergan I, Kurz JE, Linsenmeier RA, Xu D, Cooper JG, Miller SD, Kessler JA. Intravenous immunomodulatory nanoparticle treatment for traumatic brain injury. Ann Neurol. 2020;87:442–455. doi: 10.1002/ana.25675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Xu B, Yang C, Xue W, You Z, Wu X, Ma D, Shao D, Leong K, Dai J. A DAMP-scavenging, IL–10-releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials. 2022;280:121279. doi: 10.1016/j.biomaterials.2021.121279. [DOI] [PubMed] [Google Scholar]
- Sultan MT, Choi BY, Ajiteru O, Hong DK, Lee SM, Kim HJ, Ryu JS, Lee JS, Hong H, Lee YJ, Lee H, Suh YJ, Lee OJ, Kim SH, Suh SW, Park CH. Reinforced-hydrogel encapsulated hMSCs towards brain injury treatment by trans-septal approach. Biomaterials. 2021;266:120413. doi: 10.1016/j.biomaterials.2020.120413. [DOI] [PubMed] [Google Scholar]
- Sun D, Liu K, Li Y, Xie T, Zhang M, Liu Y, Tong H, Guo Y, Zhang Q, Liu H, Fang J, Chen X. Intrinsically bioactive manganese-eumelanin nanocomposites mediated antioxidation and anti-neuroinflammation for targeted theranostics of traumatic brain injury. Adv Healthc Mater. 2022a;11:e2200517. doi: 10.1002/adhm.202200517. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu J, Gao C, Zheng J, Zhang J, Ding Y, Gong W, Yang M, Li Z, Wang Y, Yang Y, Gao C. Targeted delivery of PARP inhibitors to neuronal mitochondria via biomimetic engineered nanosystems in a mouse model of traumatic brain injury. Acta Biomater. 2022b;140:573–585. doi: 10.1016/j.actbio.2021.12.023. [DOI] [PubMed] [Google Scholar]
- Sun MK, Passaro AP, Latchoumane CF, Spellicy SE, Bowler M, Goeden M, Martin WJ, Holmes PV, Stice SL, Karumbaiah L. Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. J Neurotrauma. 2020;37:1358–1369. doi: 10.1089/neu.2019.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Marushima A, Nagasaki Y, Hirayama A, Muroi A, Puentes S, Mujagic A, Ishikawa E, Matsumura A. Novel neuroprotection using antioxidant nanoparticles in a mouse model of head trauma. J Trauma Acute Care Surg. 2020;88:677–685. doi: 10.1097/TA.0000000000002617. [DOI] [PubMed] [Google Scholar]
- Tarudji AW, Gee CC, Romereim SM, Convertine AJ, Kievit FM. Antioxidant thioether core-crosslinked nanoparticles prevent the bilateral spread of secondary injury to protect spatial learning and memory in a controlled cortical impact mouse model of traumatic brain injury. Biomaterials. 2021;272:120766. doi: 10.1016/j.biomaterials.2021.120766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarudji AW, Miller HA, Curtis ET, Porter CL, Madsen GL, Kievit FM. Sex-based differences of antioxidant enzyme nanoparticle effects following traumatic brain injury. J Control Release. 2023;355:149–159. doi: 10.1016/j.jconrel.2023.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Louca I, Bolan F, Sava OR, Allan SM, Lawrence CB, Pinteaux E. Regenerative potential of hydrogels for intracerebral hemorrhage: lessons from ischemic stroke and traumatic brain injury research. Adv Healthc Mater. 2021;10:e2100455. doi: 10.1002/adhm.202100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonarelli SB, Quinn DK. Comment: Biomarkers for mild traumatic brain injury: Role of the exosomes. Neurology. 2020;94:1017. doi: 10.1212/WNL.0000000000009574. [DOI] [PubMed] [Google Scholar]
- Torres-Ortega PV, Del Campo-Montoya R, Plano D, Paredes J, Aldazabal J, Luquin MR, Santamaría E, Sanmartín C, Blanco-Prieto MJ, Garbayo E. Encapsulation of MSCs and GDNF in an injectable nanoreinforced supramolecular hydrogel for brain tissue engineering. Biomacromolecules. 2022;23:4629–4644. doi: 10.1021/acs.biomac.2c00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JM, Sali G, Homburg HB, Cassidy NT, Sanders ME, Fung KM, Andrews BT, Nudo RJ, Bohnstedt BN, Detamore MS. Thiolated bone and tendon tissue particles covalently bound in hydrogels for in vivo calvarial bone regeneration. Acta Biomater. 2020;104:66–75. doi: 10.1016/j.actbio.2019.12.035. [DOI] [PubMed] [Google Scholar]
- Tsui CT, MacGillivray SR, Weber SM, McAllister L, Churchward MA, Dennison CR, Todd KG. Applying a novel 3D hydrogel cell culture to investigate activation of microglia due to rotational kinematics associated with mild traumatic brain injury. J Mech Behav Biomed Mater. 2021;114:104176. doi: 10.1016/j.jmbbm.2020.104176. [DOI] [PubMed] [Google Scholar]
- Tuladhar A, Obermeyer JM, Payne SL, Siu RCW, Zand S, Morshead CM, Shoichet MS. Injectable hydrogel enables local and sustained co-delivery to the brain: Two clinically approved biomolecules, cyclosporine and erythropoietin, accelerate functional recovery in rat model of stroke. Biomaterials. 2020;235:119794. doi: 10.1016/j.biomaterials.2020.119794. [DOI] [PubMed] [Google Scholar]
- Verma M, Lizama BN, Chu CT. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl Neurodegener. 2022;11:3. doi: 10.1186/s40035-021-00278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viet QHN, Nguyen VQ, Le Hoang DM, Thi THP, Tran HP, Thi CHC. Ability to regulate immunity of mesenchymal stem cells in the treatment of traumatic brain injury. Neurol Sci. 2022;43:2157–2164. doi: 10.1007/s10072-021-05529-z. [DOI] [PubMed] [Google Scholar]
- Waheed TO, Hahn O, Sridharan K, Mörke C, Kamp G, Peters K. Oxidative stress response in adipose tissue-derived mesenchymal stem/stromal cells. Int J Mol Sci. 2022;23:13435. doi: 10.3390/ijms232113435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang S, Ge X, Yin Z, Li M, Guo M, Hu T, Han Z, Kong X, Li D, Zhao J, Wang L, Liu Q, Chen F, Lei P. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J Neuroinflammation. 2022a;19:185. doi: 10.1186/s12974-022-02550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yin Z, Wang F, Han Z, Wang Y, Huang S, Hu T, Guo M, Lei P. Hydrogen exerts neuroprotection by activation of the miR-21/PI3K/AKT/GSK-3β pathway in an in vitro model of traumatic brain injury. J Cell Mol Med. 2020a;24:4061–4071. doi: 10.1111/jcmm.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Han X, Zha W, Wang X, Liu L, Li Z, Shi Y, Kan X, Wang G, Gao D, Zhang B. GDNF promotes astrocyte abnormal proliferation and migration through the GFRα1/RET/MAPK/pCREB/LOXL2 signaling axis. Mol Neurobiol. 2022b;59:6321–6340. doi: 10.1007/s12035-022-02978-1. [DOI] [PubMed] [Google Scholar]
- Wang P, Ma H, Zhang Y, Zeng R, Yu J, Liu R, Jin X, Zhao Y. Plasma exosome-derived micrornas as novel biomarkers of traumatic brain injury in rats. Int J Med Sci. 2020b;17:437–448. doi: 10.7150/ijms.39667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zoneff ER, Thomas JW, Hong N, Tan LL, McGillivray DJ, Perriman AW, Law KCL, Thompson LH, Moriarty N, Parish CL, Williams RJ, Jackson CJ, Nisbet DR. Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands. Nat Commun. 2023;14:457. doi: 10.1038/s41467-023-36133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehn AC, Khalin I, Duering M, Hellal F, Culmsee C, Vandenabeele P, Plesnila N, Terpolilli NA. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol Commun. 2021;9:138. doi: 10.1186/s40478-021-01236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Higgins GA, Bhatti UF, Biesterveld BE, Dekker SE, Kathawate RG, Tian Y, Wu Z, Kemp MT, Wakam GK, Liu B, Li Y, Buller B, Alam HB. Early treatment with exosomes following traumatic brain injury and hemorrhagic shock in a swine model promotes transcriptional changes associated with neuroprotection. J Trauma Acute Care Surg. 2020;89:536–543. doi: 10.1097/TA.0000000000002815. [DOI] [PubMed] [Google Scholar]
- Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, Shao A, Shi L, Lu J, Mei S, Wang X, Guo X, Wang Y, Zhao Z, Zhang J. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18:2. doi: 10.1186/s12974-020-02041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Sun X, Zhou C, Yan J, Cheng C. Neuroblasts migration under control of reactive astrocyte-derived BDNF: a promising therapy in late neurogenesis after traumatic brain injury. Stem Cell Res Ther. 2023a;14:2. doi: 10.1186/s13287-022-03232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Jia S, Xu H, Gao Z, Wang Z, Lu B, Ai Y, Liu Y, Liu R, Yang T, Luo R, Hu C, Kong L, Huang D, Yan L, Yang Z, Zhu L, Hao D. Supramolecular hydrogel microspheres of platelet-derived growth factor mimetic peptide promote recovery from spinal cord injury. ACS Nano. 2023b;17:3818–3837. doi: 10.1021/acsnano.2c12017. [DOI] [PubMed] [Google Scholar]
- Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther. 2019;25:465–475. doi: 10.1111/cns.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Baokun Z, Zhiheng C, Qiang S, Erzhu Y, Jianguang X, Xiaofeng L. Biodegradable bilayer hydrogel membranes loaded with bazedoxifene attenuate blood-spinal cord barrier disruption via the NF-κB pathway after acute spinal cord injury. Acta Biomater. 2023;159:140–155. doi: 10.1016/j.actbio.2023.01.056. [DOI] [PubMed] [Google Scholar]
- Xu C, Diao YF, Wang J, Liang J, Xu HH, Zhao ML, Zheng B, Luan Z, Wang JJ, Yang XP, Wei MG, Duan JH, Wang KQ, Chen C, Chen F, Ming D, Zhang S, Sun HT, Li XH. Intravenously infusing the secretome of adipose-derived mesenchymal stem cells ameliorates neuroinflammation and neurological functioning after traumatic brain injury. Stem Cells Dev. 2020a;29:222–234. doi: 10.1089/scd.2019.0173. [DOI] [PubMed] [Google Scholar]
- Xu D, Bhattacharyya S, Wang W, Ifergan I, Wong MAC, Procissi D, Yeldandi A, Bale S, Marangoni RG, Horbinski C, Miller SD, Varga J. PLG nanoparticles target fibroblasts and MARCO+ monocytes to reverse multiorgan fibrosis. JCI Insight. 2022;7:e151037. doi: 10.1172/jci.insight.151037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jia Z, Ma K, Zhang J, Dai C, Yao Z, Deng W, Su J, Wang R, Chen X. Protective effect of BMSCs-derived exosomes mediated by BDNF on TBI via miR-216a-5p. Med Sci Monit. 2020b;26:e920855. doi: 10.12659/MSM.920855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, Wan XD, Chen AD, Yan ZQ, Lu YF, Liu JC, Wang YZ, Wang J, Zhao Y, Wu SX, Cai GH. Characteristics of traumatic brain injury models: from macroscopic blood flow changes to microscopic mitochondrial changes. Neural Regen Res. 2023a;18:2268–2277. doi: 10.4103/1673-5374.369125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hsu CC, Cao TT, Ye H, Chen J, Li YQ. A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats. Neural Regen Res. 2023b;18:657–663. doi: 10.4103/1673-5374.350212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Chen Y, Zhang J, Gao F, Ma S, Guan F. Chitosan-based thermosensitive composite hydrogel enhances the therapeutic efficacy of human umbilical cord MSC in TBI rat model. Mater Today Chem. 2019a;14:100192. [Google Scholar]
- Yao M, Gao F, Xu R, Zhang J, Chen Y, Guan F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater Sci. 2019b;7:4088–4098. doi: 10.1039/c9bm00749k. [DOI] [PubMed] [Google Scholar]
- Ye J, Jin S, Cai W, Chen X, Zheng H, Zhang T, Lu W, Li X, Liang C, Chen Q, Wang Y, Gu X, Yu B, Chen Z, Wang X. Rationally designed, self-assembling, multifunctional hydrogel depot repairs severe spinal cord injury. Adv Healthc Mater. 2021;10:e2100242. doi: 10.1002/adhm.202100242. [DOI] [PubMed] [Google Scholar]
- Yea JH, Bae TS, Kim BJ, Cho YW, Jo CH. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020;114:104–116. doi: 10.1016/j.actbio.2020.07.020. [DOI] [PubMed] [Google Scholar]
- Yin R, Zhao S, Qiu C. Brain-derived neurotrophic factor fused with a collagen-binding domain inhibits neuroinflammation and promotes neurological recovery of traumatic brain injury mice via TrkB signalling. J Pharm Pharmacol. 2020a;72:539–550. doi: 10.1111/jphp.13233. [DOI] [PubMed] [Google Scholar]
- Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, Wang L, Yu J, Li W, Wang Y, Li D, Zhao J, Wang Y, Zuo Y, Li Y, Kong X, Chen F, Lei P. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun. 2020b;83:270–282. doi: 10.1016/j.bbi.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Yuan J, Botchway BOA, Zhang Y, Wang X, Liu X. Combined bioscaffold with stem cells and exosomes can improve traumatic brain injury. Stem Cell Rev Rep. 2020;16:323–334. doi: 10.1007/s12015-019-09927-x. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yuan W, Ding L, Shi M, Luo L, Wan Y, Oh J, Zhou Y, Bian L, Deng DYB. Cell-adaptable dynamic hydrogel reinforced with stem cells improves the functional repair of spinal cord injury by alleviating neuroinflammation. Biomaterials. 2021;279:121190. doi: 10.1016/j.biomaterials.2021.121190. [DOI] [PubMed] [Google Scholar]
- Zhai K, Duan H, Wang W, Zhao S, Khan GJ, Wang M, Zhang Y, Thakur K, Fang X, Wu C, Xiao J, Wei Z. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B. 2021;11:3493–3507. doi: 10.1016/j.apsb.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu C, Li F, Zheng M, Liu Y, Li L, Yang H, Zhang S, Wang C, Rong H, Guo H, Li Y, Li Y, Fu Y, Zhao Z, Zhang J. Extracellular mitochondria activate microglia and contribute to neuroinflammation in traumatic brain injury. Neurotox Res. 2022a;40:2264–2277. doi: 10.1007/s12640-022-00566-8. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chang R, Ren Y, He Y, Guo S, Guan F, Yao M. Injectable and reactive oxygen species-scavenging gelatin hydrogel promotes neural repair in experimental traumatic brain injury. Int J Biol Macromol. 2022b;219:844–863. doi: 10.1016/j.ijbiomac.2022.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu X, Ma K, Chen M, Xu H, Niu X, Gu H, Wang R, Chen X, Sun H. Collagen/heparin scaffold combined with vascular endothelial growth factor promotes the repair of neurological function in rats with traumatic brain injury. Biomater Sci. 2021a;9:745–764. doi: 10.1039/c9bm01446b. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fan C, Hao W, Zhuang Y, Liu X, Zhao Y, Chen B, Xiao Z, Chen Y, Dai J. NSCs migration promoted and drug delivered exosomes-collagen scaffold via a bio-specific peptide for one-step spinal cord injury repair. Adv Healthc Mater. 2021b;10:e2001896. doi: 10.1002/adhm.202001896. [DOI] [PubMed] [Google Scholar]
- Zhang N, Lin J, Lin VPH, Milbreta U, Chin JS, Chew EGY, Lian MM, Foo JN, Zhang K, Wu W, Chew SY. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv Sci (Weinh) 2021c;8:e2100805. doi: 10.1002/advs.202100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Sun S, Wang J, Li Q, Yan R, Gao Y, Liu H, Liu S, Hao W, Dai H, Liu C, Sun Y, Long W, Mu X, Zhang XD. Catalytic patch with redox Cr/CeO(2) nanozyme of noninvasive intervention for brain trauma. Theranostics. 2021d;11:2806–2821. doi: 10.7150/thno.51912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hong J, Zhang H, Zheng W, Yang Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY) 2021e;13:21642–21658. doi: 10.18632/aging.203508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yu Q, Zhou P, Yang S, Xia J, Deng T, Yu C. Blood-brain barrier penetrating carbon dots with intrinsic anti-inflammatory and drug-loading properties. Biomater Adv. 2022c;139:212995. doi: 10.1016/j.bioadv.2022.212995. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y. Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study. Neurorehabil Neural Repair. 2020a;34:616–626. doi: 10.1177/1545968320926164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Chopp M, Pang H, Zhang ZG, Mahmood A, Xiong Y. MiR-17-92 cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma. 2021f;38:1535–1550. doi: 10.1089/neu.2020.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Chopp M, Pang H, Chen L, Zhang ZG, Mahmood A, Xiong Y. Therapeutic role of microRNAs of small extracellular vesicles from human mesenchymal stromal/stem cells in treatment of experimental traumatic brain injury. J Neurotrauma. 2023;40:758–771. doi: 10.1089/neu.2022.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Liang J, Yan JX, Ye YC, Wang JJ, Chen C, Sun HT, Chen F, Tu Y, Li XH. TBHQ improved neurological recovery after traumatic brain injury by inhibiting the overactivation of astrocytes. Brain Res. 2020b;1739:146818. doi: 10.1016/j.brainres.2020.146818. [DOI] [PubMed] [Google Scholar]
- Zhao J, Qu D, Xi Z, Huan Y, Zhang K, Yu C, Yang D, Kang J, Lin W, Wu S, Wang Y. Mitochondria transplantation protects traumatic brain injury via promoting neuronal survival and astrocytic BDNF. Transl Res. 2021;235:102–114. doi: 10.1016/j.trsl.2021.03.017. [DOI] [PubMed] [Google Scholar]
- Zhou P, Xu P, Guan J, Zhang C, Chang J, Yang F, Xiao H, Sun H, Zhang Z, Wang M, Hu J, Mao Y. Promoting 3D neuronal differentiation in hydrogel for spinal cord regeneration. Colloids Surf B Biointerfaces. 2020;194:111214. doi: 10.1016/j.colsurfb.2020.111214. [DOI] [PubMed] [Google Scholar]
- Zhao P, Wei Y, Sun G, Xu L, Wang T, Tian Y, Chao H, Tu Y, Ji J. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J Neuroinflammation. 2022;19:269. doi: 10.1186/s12974-022-02633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Chen L, Wu Z, Li W, Liu X, Wang Y, Guo M, Ito Y, Wang L, Zhang P, Wang H. Bioorthogonal DOPA-NGF activated tissue engineering microunits for recovery from traumatic brain injury by microenvironment regulation. Acta Biomater. 2022;150:67–82. doi: 10.1016/j.actbio.2022.07.018. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Liu M, Luo J, Zhang X, Dai Z, Zhang B, Chen H, Xue J, He M, Xu H, Liu A. Exosomes derived from bone marrow mesenchymal stem cells attenuate neurological damage in traumatic brain injury by alleviating glutamate-mediated excitotoxicity. Exp Neurol. 2022;357:114182. doi: 10.1016/j.expneurol.2022.114182. [DOI] [PubMed] [Google Scholar]
- Zinger A, Soriano S, Baudo G, De Rosa E, Taraballi F, Villapol S. Biomimetic nanoparticles as a theranostic tool for traumatic brain injury. Adv Funct Mater. 2021a;31:2100722. doi: 10.1002/adfm.202100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger A, Cvetkovic C, Sushnitha M, Naoi T, Baudo G, Anderson M, Shetty A, Basu N, Covello J, Tasciotti E, Amit M, Xie T, Taraballi F, Krencik R. Humanized biomimetic nanovesicles for neuron targeting. Adv Sci (Weinh) 2021b;8:e2101437. doi: 10.1002/advs.202101437. [DOI] [PMC free article] [PubMed] [Google Scholar]


