Abstract
NF-κB is a key regulator of the innate antiviral immune response, due in part to its transcriptional activation of cytokines and adhesion molecules, which, in turn, function in chemotaxis and activation of inflammatory cells. We reported earlier that viral gene expression in hepatocytes transduced with first-generation (E1-deleted) adenoviruses induced NF-κB activation, elevation of serum cytokines, and hepatocellular apoptosis during the first days postinfusion. These events did not occur in mice infused with an adenovirus vector deleted for E1, E2, E3, and late gene expression. In the present study, we used an adenovirus expressing an IκBα supersuppressor (Ad.IκBM) and bcl-2 transgenic mice to unravel the role of virus-induced NF-κB activation and apoptosis in the clearance of recombinant adenovirus vectors from the liver. The combined action of IκBM and Bcl-2 allowed for vector persistence in livers of C57BL/6 × C3H mice. In the absence of Bcl-2, IκBM expression in mouse livers significantly reduced NF-κB activation, cytokine expression, leukocyte infiltration, and the humoral immune response against the transgene product; however, this was not sufficient to prevent the decline of vector DNA in transduced cells. Infusion of Ad.IκBM caused extended apoptosis predominantly in periportal liver regions, indicating that NF-κB activation may protect transduced hepatocytes from apoptosis induced by adenovirus gene products. To confer vector persistence, bcl-2 transgene expression was required to block virus-induced apoptosis if NF-κB protection was inactivated by IκBM. Expression of gene products involved in early stages of apoptotic pathways was up-regulated in response to virus infusion in bcl-2 transgenic mice, which may represent a compensatory effect. Our study supports the idea that the suppression of innate defense mechanisms improves vector persistence.
First-generation recombinant adenoviruses (rAd) deleted for all E1a and E1b genes are widely used for gene transfer in vitro and in vivo (for a review, see reference 17). Systemic application of rAd in mice by tail vein infusion results in predominant transduction of the liver (60). Several studies reported expression of early and late adenovirus proteins in transduced hepatocytes mediated by cellular proteins that can substitute for E1a in its function as transactivator for viral genes (for a review, see reference 28). Expressed viral proteins contribute to toxic effects and elicit an innate and specific immune response directed against the virus or transduced cells (for reviews, see references 7 and 29). In most mouse strains, transgene expression is lost within several weeks after rAd infusion (3, 46). In cases where the decline in transgene expression correlated with the loss of hepatic vector DNA, the etiology of vector clearance was attributed to cytotoxic T-lymphocyte (CTL)-mediated cytolysis of transduced cells involving the Fas (1, 15) and/or perforin/granzyme (65) pathways. In other reports, the humoral immune response against viral and/or transgene products was thought to lead to lysis if transduced cells and/or to interference with the detection of secreted transgene products (33, 45, 46, 57). Alternative factors causing vector clearance include intracellular degradation of vector genomes by innate antiviral mechanisms (initiated, for example, by cytokines such as tumor necrosis factor alpha [TNF-α] and interferons [IFNs] without cell loss or apoptosis induced directly by viral proteins expressed in transduced cells (11, 18, 27, 29, 50).
There have been promising attempts to modulate the antigen-specific host immune response in mice by specific inhibition of costimulatory signals required for B- and T-cell activation (20, 21, 43, 63, 64). Furthermore, newer generations of adenovirus vectors deleted for all viral genes (so-called gutless vectors) that lead to persistent expression of the human α1-antitrypsin (hAAT) gene at high levels in C57BL/6 mice have been developed (44). Nonetheless, first-generation vectors remain an attractive vehicle for gene therapy of tumors and viral infections due, in part, to relatively easy production of purified virus at high titers and the ability to transduce a variety of cell types in vivo. Studies by Ilan et al. indicate that problems of vector-host interaction may also be addressed by overexpression of adenovirus E3 genes that counteract host defenses (18). In this context, the goal of our study was to understand the role of NF-κB activation and of apoptotic pathways that can be blocked by Bcl-2 in adenovirus clearance from mouse liver.
The NF-κB proteins with transactivating function represent a heterodimer of p65 (or c-Rel or RelB) with p50 or p52 (for reviews, see references 12 and 52). NF-κB is sequestered in the cytoplasm by tightly bound inhibitory proteins called IκBs, masking the nuclear localization signal of NF-κB. The most important members of this family include IκBα, -β, and -ɛ, which inhibit different members of the NF-κB family. Phosphorylation of IκB by IκB kinases results in ubiquitination of IκB, which in turn leads to proteasome-mediated degradation of the inhibitor, allowing NF-κB to enter the nucleus and to function as a transcriptional transactivator for a large variety of genes. IκB kinases can be activated through a number of pathways, including the TNF–TNF-α receptor 1 (TNFR1)–TNFR1-associated death domain-containing protein (TRADD)–TRAF2 pathway (12). A variety of viruses, including human immunodeficiency virus, herpes simplex virus, cytomegalovirus, papovaviruses (for reviews, see references 2 and 34), and rAd (8, 29, 31), can activate NF-κB. Viruses have evolved mechanisms to utilize various properties of NF-κB to facilitate their gene expression, replication, and evasion of immune responses. In adenoviruses, NF-κB, for example, transactivates the promoter for the E3 region, which encodes a number of proteins that block TNF-α activity (9). On the other hand, it is known that NF-κB is a key regulator in the antiviral immune response. This is achieved in part by its potential to coordinately transactivate transcription of genes for inflammatory cytokines, or adhesion molecules, which in turn may induce activation or chemotaxis of immune cells (for a review, see reference 12).
NF-κB also regulates apoptotic pathways (for a review, see reference 51). Depending on the specific cell type, differentiation stage, and duration of NF-κB activation, both a proapoptotic role of NF-κB (14, 30) and a role in protection against apoptosis (4, 5, 59, 62) have been observed.
Bcl-2 and related members of the Bcl-2 family are antiapoptotic proteins (for a review, see reference 24). Bcl-2 is localized in mitochondrial, endoplasmic reticulum, and nuclear membranes. In general, the ratio of death antagonists such as Bcl-2 to agonists (e.g., Bax and Bak) determines whether apoptosis will be suppressed or promoted. Hepatocytes normally lack detectable Bcl-2 expression but produce high levels of Bax and Bak, which sensitizes them to Fas-induced apoptosis (22, 23). Hepatic expression of Bcl-2 in Bcl-2 transgenic mice protected them completely from apoptosis induced by synergistic anti-Fas antibody injection (25, 39). The mechanisms behind the antiapoptotic action of Bcl-2 remain enigmatic. In relation to virus infection, Bcl-2 overexpression in vivo inhibited Semliki Forest virus transcription and early replication and delayed virus-induced apoptosis (42). NF-κB-mediated activation of apoptosis after Sindbis virus infection can be inhibited in specific cell lines by Bcl-2 (30).
Activation of cytokines is a central element of the innate immune response against viruses (34). Cytokines are released or produced after systemic application of rAd in a titer-dependent manner (13). Cytokines can directly affect viral replication and gene expression, can induce apoptosis of infected cells, and/or can stimulate production of leukotrienes or adhesion molecules which are involved in chemotaxis and activation of immune cells (35). Among the cytokines activated after rAd infusion is TNF-α (for a review, see reference 37). In the liver, TNF-α is produced primarily by activated Kupffer cells as a biologically active membrane-bound precursor that is cleaved to produce the mature cytokine released in the serum with systemic effects. Released TNF-α binds to two different receptors on a variety of target cell types, including Kupffer cells and hepatocytes. The two TNFRs p55 and p75 have different intracellular domains involved in activation of different signal transduction pathways leading to cell cycle progression, apoptosis, or differentiation. It is thought that among the inflammatory cytokines, TNF-α plays a dominant role in rAd clearance (11, 29). This conclusion is supported by the observation that TNF-α knockout mice or Kupffer cell-depleted mice demonstrated less leukocyte infiltration early after infection and a reduced humoral immune response to the virus. The importance of TNF-α in defense against adenoviruses is also suggested by the fact that four of the ∼25 early adenovirus proteins (E1b-19K, E3-10.4K, and E3-10.4K/14.5K) prevent early TNF-α activity (13). The corresponding genes are removed in E1/E3-deleted first-generation vectors in order to make space for cloning larger inserts.
The duration of transgene expression after gene transfer with first-generation adenovirus varies between different mouse strains (3, 46). In the mouse strain used in this study, reporter gene expression declined to zero by 5 weeks after rAd infusion. The initial goal of this study was to investigate the role of cytokine activation and apoptosis occurring early after rAd infusion in the loss of transgene expression in this mouse strain. Our hypothesis was that virus-induced NF-κB activation was a crucial element in pathways that lead to vector clearance. To dissect the role of NF-κB in activation of cytokines and/or apoptosis, we used an adenovirus vector expressing an IκBα supersuppressor (IκBM). IκBM is not able to respond to activation signals due to mutations in phosphorylation sites and thus remains associated with NF-κB, preventing nuclear translocation. This IκB supersuppressor was used in a number of studies to specifically block NF-κB activation (5, 59, 62). To study the role of Bcl-2-sensitive apoptotic pathways in virus clearance from the mouse liver, we used bcl-2 transgenic mice (C57BL/6 × C3H background). We investigated the effects of individual and combined Bcl-2 and IκBM expression on hepatic NF-κB activation, cytokine gene expression, apoptosis, cellular liver infiltrates, vector genome and transgene persistence, and humoral immune response.
MATERIALS AND METHODS
Adenoviruses.
Ad/RSVhAAT (Ad.hAAT) (19) contains the Rous sarcoma virus long terminal repeat promoter, the hAAT cDNA (1.4 kb), and the bovine growth hormone polyadenylation signal (bPA). Ad.IκBM contains the 1.0-kb cDNA for the dominant negative IκBα (10) fused at the 5′ end to hemagglutinin tag. The IκBM gene was inserted into Ad.PGK between the phosphoglycerokinase (PGK) promoter and the bPA signal (19). The PGK promoter is active in mouse hepatocytes in vivo (19). Ad.Co is a vector that has only the PGK-bPA cassette without any transgene. All adenoviruses were generated by recombination in 293 cells with pJM17 (Microbix, Toronto, Ontario, Canada). pJM17 is an E1-deleted adenovirus type 5 derivative that contains a series of substitutions and deletions that inactivate the expression of the E3-10.4/14.5K and E3-14.7K proteins, which antagonize TNF actions (6). The genes for the E3-11.6K protein and for gp19 are intact in pJM17-derived vectors. Thus, all viruses used in this study are typical first-generation adenoviruses. The plaque titer of all viruses was determined on 293 cells. The presence of replication-competent adenovirus and contamination with endotoxin in virus preparation were excluded by tests described earlier (29). Viruses with a titer of 5 × 1011 PFU/ml were stored at −80°C in 10 mM Tris-Cl (pH 8.0)–1 mM MgCl2–10% glycerol. IκBM expression in mouse livers after Ad.IκBM gene transfer was confirmed by Western blotting with antibodies specific to the hemagglutinin tag or IκBα (C-21; catalog no. sc-371; Santa Cruz Biotechnology, Santa Cruz, Calif.). IκBM expression was detectable beginning 24 h after Ad.IκBM injection.
Animals.
Animal studies were performed in accordance with the institutional guidelines set forth by the University of Washington. All animals were housed in specific-pathogen-free facilities. Human bcl-2 transgenic (bcl-2tg) mice were provided by Stanley Korsmeyer. Transgenic mice had the bcl-2 gene under control of the mouse metallothionein promoter. All experimental animals had the same genetic background (C57BL/6 × C3H inbred offspring); one group was heterozygous for the bcl-2 transgene (bcl-2tg+/−), and the other group was PCR negative for the transgene (bcl-2tg−/−). For breeding, bcl-2tg−/− littermates were mated with bcl-2tg+/− mice because of difficulties in breeding homozygote mice. Mice were screened by PCR with an bcl-2-specific primer (5′CTTTGTGGAACTGTACGGCCCCAGCATGCG), an hGX-specific reverse primer (5′GGAGCAGGGACGTCCGGGAGCC) and 500 ng of genomic DNA obtained from mouse tails (PCR conditions, 40 cycles of 45 s at 95°C, 45 s at 55°C, and 1 min at 72°C). For experiments, 5- to 6-week-old bcl-2tg−/− or bcl-2tg+/− mice were used. For induction of bcl-2 expression, drinking water with 25 mM ZnSO4 was given to the mice 4 days before the experiment. bcl-2 expression after induction was confirmed by Western blotting of liver lysates with Bcl-2-specific antibodies (PharMingen catalog no. 65111A) as described by Pezzella et al. (36). The Bcl-2-specific signal appeared 48 h after provision of the ZnSO4 water and disappeared 5 days after its removal. Adenovirus injection was performed via tail vein infusion with 200 μl of adenovirus diluted in serum-free Dulbecco modified Eagle medium. Blood samples for analysis were obtained by retro-orbital bleeding. Serum samples for hAAT analysis and hAAT antibody analysis were stored at −20°C. Serum hAAT concentrations were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (19). To obtain liver samples, mice were sacrificed by cervical dislocation.
NF-κB electrophoretic mobility shift assay (EMSA).
Nuclear extracts were obtained from mouse livers as described previously (29) and stored at −80°C. Protein concentrations were measured by the Bradford method. The NF-κB binding sequence from the class I major histocompatibility complex enhancer element (H2k) was used as a probe. Double-stranded oligonucleotides were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase. For each reaction, 10 μg of nuclear protein was incubated with 0.2 ng of labeled oligonucleotide probe for 30 min at room temperature and electrophoresed through 5% polyacrylamide Tris-glycine-EDTA gels. For antibody supershift assays, p50- and p65-specific polyclonal antibodies (Santa Cruz Biotechnology) were used. One microgram of the corresponding antibody was added to the samples after 30 min of incubation with the labeled probe. Gels were dried and exposed to Kodak-AR film for 24 h.
Histological analysis.
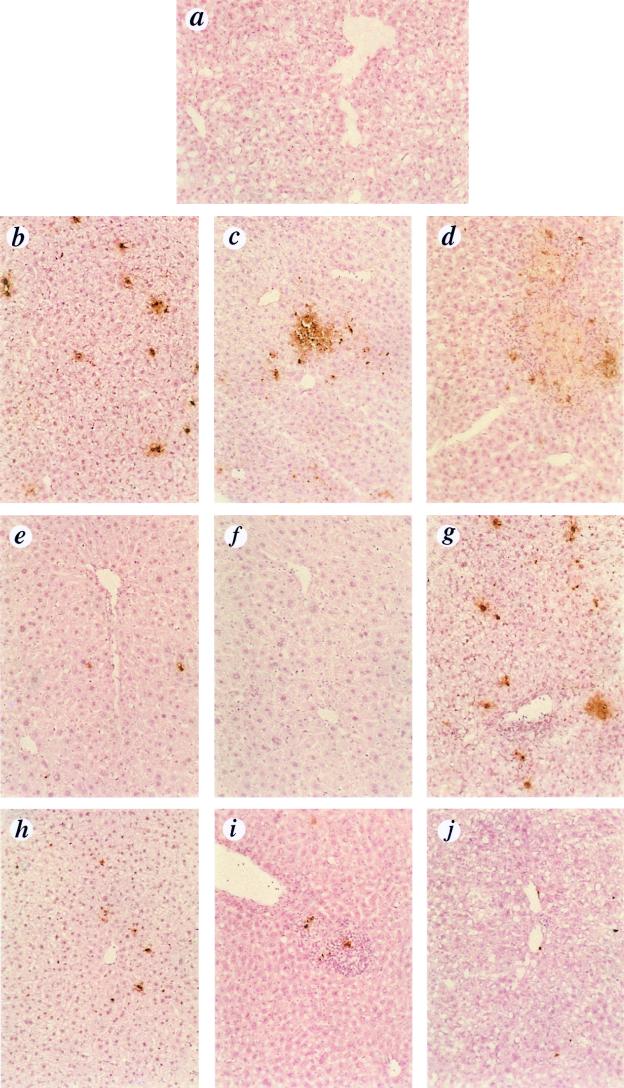
For histological analysis, liver samples from different lobes were used. For terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis, liver tissue was frozen in OCT compound (Miles, Inc., Elkhart, Ind.) and cryosectioned in 10-μm sections. An In Situ Cell Death Detection kit (Boehringer Mannheim) was used to quantify apoptosis in hepatocytes as specified by the manufacturer. Liver sections were counterstained with hematoxylin and eosin and analyzed by light microscopy at a magnification of ×60. A higher magnification (×190) was used to identify the cellular infiltrate as polymorphonuclear leukocytes. Representative samples were photographed with a Nikon VFM camera. Figure 3 was scanned from color film slides, assembled in Adobe Photoshop, and printed on a dye sublimation printer.
FIG. 3.
TUNEL analysis of liver sections. Adenovirus was administered to mice as described for Fig. 1. Liver sections were obtained at days 1, 2, 5, and 40 p.i. and analyzed for apoptotic cell death by the TUNEL technique (counterstaining with hematoxylin and eosin). (a) Naive mouse; (b) bcl-2tg−/−/Ad.Co, day 1; (c) bcl-2tg−/−/Ad.IκBM, day 1; (d) bcl-2tg−/−/Ad.IκBM, day 2; (e) bcl-2tg+/−/Ad.Co, day 1; (f) bcl-2tg+/−/Ad.IκBM, day 1; (g) bcl-2tg−/−/Ad.Co, day 5; (h) bcl-2tg−/−/Ad.IκBM, day 5; (i) bcl-2tg+/−/Ad.Co, day 5; (j) bcl-2tg+/−/Ad.IκBM, day 5. Note the neutrophil infiltration in panels g and i. Magnification, ×60.
RPA.
RNA was isolated from 100 mg of snap-frozen liver tissue and stored in liquid nitrogen, using an RNeasy mini kit (Qiagen). RNA was dissolved on RNase-free water and stored in aliquots at −80°C. The RNase protection assay (RPA) was performed by using PharMingen RPA kits, mAPO-3 Multi-Probe Template Set (45355P) for mouse apoptosis gene expression, and mCK-3b Multi-Probe Template Set for mouse cytokine gene expression according to the protocol provided by the manufacturer. Probes were labeled with [32P]UTP. Quantification of all bands was performed on a model 400S PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). The signals from specific mRNAs were normalized to signals from housekeeping genes (mouse L32 [mL32] and mouse glyceraldehyde 3-phosphate dehydrogenase [mGAPDH]) run on each lane to adjust for loading differences.
hAAT antibodies.
Anti-hAAT antibodies were determined by ELISA as described previously (46). Briefly, ELISA plates coated with anti-hAAT capture monoclonal antibody (MAb) were blocked and then incubated with hAAT protein (calibrator serum 4; Atlantic Antibodies, Stillwater, Minn.) diluted 1:50 in blocking buffer for 2 h at room temperature. For each two sample wells, two additional wells were mock loaded with blocking buffer only to determine whether individual high serum hAAT levels would interfere with the assay. Mouse serum samples, diluted 1:1,000 and 1:10,000, were loaded onto the plate along with a similarly diluted naive serum as a negative control. A murine immunoglobulin G2b (IgG2b) anti-hAAT MAb (178260; Calbiochem, La Jolla, Calif.) serially diluted in blocking buffer from 10−2 to 10−6 was also included on each plate as a positive control. Following another 2 h of incubation at room temperature, the plates were incubated with horseradish peroxidase-labeled sheep anti-mouse IgG whole-molecule antibody (A-6782; Sigma, St. Louis, Mo.) for another 2 h. To avoid interference with high endogenous hAAT levels, the values for the control wells (without hAAT calibrator) were subtracted from the sample values.
Southern analysis.
For genomic DNA preparation, mouse livers were flushed with 5 ml of phosphate-buffered saline via the portal vein. Genomic DNA was extracted from 100 mg of liver tissue as described earlier (28). DNA concentrations were determined spectrophotometrically. Ten micrograms of genomic DNA was digested with BamHI, run on a 0.8% agarose gel, and electrotransferred to a Hybond N+ nylon filter (Amersham). The blots were hybridized in rapid hybridization buffer (Amersham) with a [α-32P]dCTP-labeled hAAT probe, using a random priming kit (Gibco BRL).
RESULTS
Inhibition of NF-κB activation by Ad.IκBM.
Recently, we reported that infusion of a first-generation virus (rAd) or a vector deleted for 25 kb, including E1, E2, E3, and late genes, activated NF-κB in mouse livers within minutes, probably as a result of particle-receptor interaction and/or internalization (29). This early NF-κB activation was followed by a second phase of NF-κB activity beginning at day 3 after infusion of rAd but not the deleted vector, suggesting that viral gene expression was responsible for NF-κB activation. The same pattern of NF-κB activation was observed in mice depleted for Kupffer cells, indicating that NF-κB activation takes place in hepatocytes. A biphasic elevation of serum TNF was observed, with a first peak occurring shortly after rAd infusion as a result of TNF release from Kupffer cells and a second peak that correlated with viral gene expression in hepatocytes (29).
Our initial hypothesis in this study was that cytokines produced in hepatocytes early after rAd transduction are responsible in large part for loss of vector DNA by activation of innate defenses (e.g., apoptosis) and/or by facilitating subsequent antigen-specific clearance mechanisms and that the inhibition of cytokine expression can prolong vector persistence. To study whether virus-induced NF-κB activation is causally linked to expression of cytokine genes, we used IκBM to specifically block the NF-κB activation pathway after rAd infusion. All of the following experiments were performed with four groups of animals. Each bcl-2tg+/− or bcl-2tg−/− mouse was injected with 4 × 109 PFU of Ad.IκBM together with an equal dose of Ad.RSV/hAAT (expressing hAAT as a reporter gene) or 4 × 109 PFU of Ad.Co (rAd without transgene) with 4 × 109 PFU Ad.RSV/hAAT. From earlier experiments, it was known that a dose of 8 × 109 PFU transduces >95% of hepatocytes with 20 to 40 viral genome copies per cell, such that both hAAT and IκBM were coexpressed in host cells (60, 61). Endogenous hepatic Bcl-2 expression was not detectable by Western blotting in bcl-2tg+/− and bcl-2tg−/− mice (data not shown). bcl-2 transgene expression from the metallothionein promoter in bcl-2tg+/− mice can be induced by adding ZnSO4 to the drinking water (see Materials and Methods).
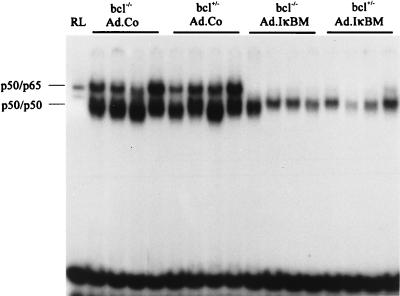
The activation status of NF-κB was analyzed by EMSA, which reflects the DNA binding activity in nuclear extracts from livers. For the analysis of NF-κB activity, we selected day 3 postinfusion (p.i.) because it corresponded to the second phase of NF-κB activation and could potentially be inhibited by IκBM expressed after Ad.IκBM infusion. NF-κB activation at day 3 p.i. was significantly reduced in nuclear extracts from livers transduced with Ad.IκBM, regardless of the bcl-2tg status (Fig. 1). It cannot be excluded that IκBM expression from Ad.IκBM was not high enough in all transduced cells to completely block NF-κB activation.
FIG. 1.
Hepatic NF-κB activity after adenovirus infusion. bcl-2tg−/− and bcl-2tg+/− mice were injected with 4 × 109 PFU of Ad.Co (without transgene) or 4 × 109 PFU Ad.IκBM together with an equal dose of Ad.hAAT, and nuclear extracts were analyzed by EMSA at day 3 p.i. Reticulocyte lysate (RL) was used as the marker to determine the position of the p50-p65 complex. Positions of the NF-κB p50-p65 complex and the p50 homodimers were identified in supershift assays with specific antibodies (30). Each lane represents an individual animal.
Cytokine expression in relation to NF-κB activation.
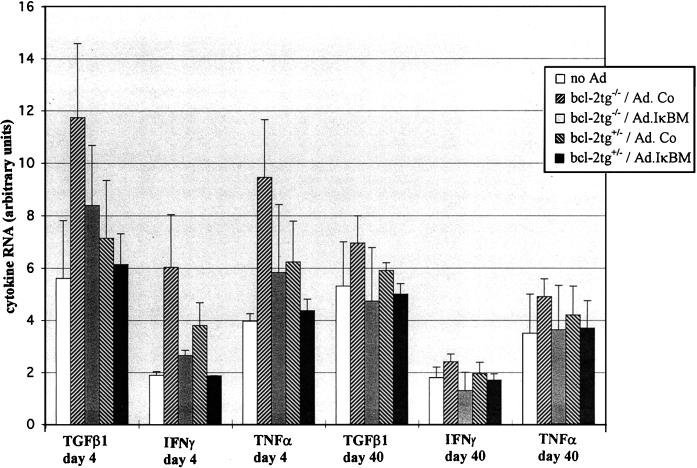
To study the activation of cytokine gene expression in relation to NF-κB status, we decided to quantify cytokine-specific RNA in livers by RPA rather than by measuring serum cytokine levels. The latter cannot identify the organ that produced cytokines and cannot discriminate between de novo expression and cytokine release. We concentrated on the main inflammatory cytokines transforming growth factor β1 (TGF-β1), IFN-γ, TNF-α, and interleukin-6 (IL-6). Total liver RNA was isolated at days 4 and 40 after rAd infusion from the four experimental groups described above and analyzed by RPA with a mouse cytokine template set (PharMingen) (Fig. 2). Detectable IL-6 transcription was not found in liver RNA from any of the animals in all four experimental groups. TGF-β1, IFN-γ, and TNF-α RNA expression was higher by more than a factor of 2 at day 4 after infusion of rAd in the bcl-2tg−/−/Ad.Co group than in naive animals that did not receive adenovirus. Ad.IκBM administration reduced significantly the rAd-induced activation of cytokine gene transcription in bcl-2tg+/− and bcl-2tg−/− mice. At day 40 after rAd infusion, cytokine expression was not significantly higher than in naive mice. The data suggest that NF-κB was one of the factors responsible for activation of cytokine expression in hepatocytes shortly after rAd infusion.
FIG. 2.
Concentrations of cytokine RNAs after adenovirus infusion. Adenovirus was administered to mice as described for Fig. 1. At days 4 and 40 p.i., 2.5 μg of total liver RNA was quantified for RNAs specific for the inflammatory cytokines TGF-β1, IFN-γ IL-6, and TNF-α by RPA using the mCK-3b Multi-Probe Template Set (PharMingen) for mouse cytokine gene expression. Noninfused bcl-2tg−/− mice were used as controls. Cytokine mRNA expression in these mice was the same as in naive bcl-2tg+/− animals. Signals from protected fragments were quantified on a PhosphorImager. Represented are the average mRNA intensities normalized to the signals of the housekeeping genes (mL32 and mGAPDH) and expressed as arbitrary units. Results are means ± standard deviations for at least three animals. (IL-6 mRNA was not detectable by RPA in any of the groups and therefore was not included in the graphic.)
Histological analysis for liver apoptosis and inflammation.
To study the effects of NF-κB and Bcl-2 on hepatic apoptosis and inflammation induced early after rAd transduction, we obtained liver sections from three mice per group at days 1, 2, 5, and 40 p.i. and analyzed them for signs of apoptosis by TUNEL and for neutrophil infiltration. Representative samples from each group obtained at days 1, 2, and 5 p.i. are shown in Fig. 3. Liver histology performed on samples from day 40 p.i. did not differ from those obtained at day 5 p.i. (data not shown). TUNEL signals in about 2 to 3% of hepatocytes were observed as early as 24 h (Fig. 3b) as well as 5 days after Ad.Co infusion into bcl-2tg−/− mice (Fig. 3g). In addition to these sparse signals, intense focal hepatocellular apoptosis appeared 24 h after infusion of Ad.IκBM into bcl-2tg−/− mice, particularly in periportal regions, which theoretically received the highest viral load and probably expressed the highest concentrations of IκBM (Fig. 3c). These focal apoptotic areas were infiltrated with neutrophils by day 2 (Fig. 3d) and resolved by day 5 p.i. (Fig. 3h). Hepatocellular apoptosis was completely absent (Fig. 3f, i, and j) or observed in fewer than 0.1% hepatocytes (Fig. 3e) in bcl-2tg+/− transgenic mice. Isolated TUNEL-positive cells in Fig. 3i and j were from nonparenchymal cells or infiltrated inflammatory cells. Cellular infiltrates observed at day 5 p.i. (Fig. 3g and i) were mostly polymorphonuclear neutrophils. Hepatic infiltrates at day 5 p.i. were significantly reduced in livers transduced with Ad.IκBM. Bcl-2 expression in combination with Ad.IκBM transduction resulted in normal liver morphology at day 5 after rAd infusion. Taken together, these data demonstrate that (i) IκBM expression inhibits hepatic leukocyte infiltration after rAd administration, (ii) efficient inhibition of NF-κB by IκBM may be the reason for hepatocellular apoptosis induced by viral proteins, indicating an antiapoptotic role of NF-κB, and (iii) hepatocellular apoptosis can be blocked by Bcl-2.
Expression of gene products involved in apoptotis.
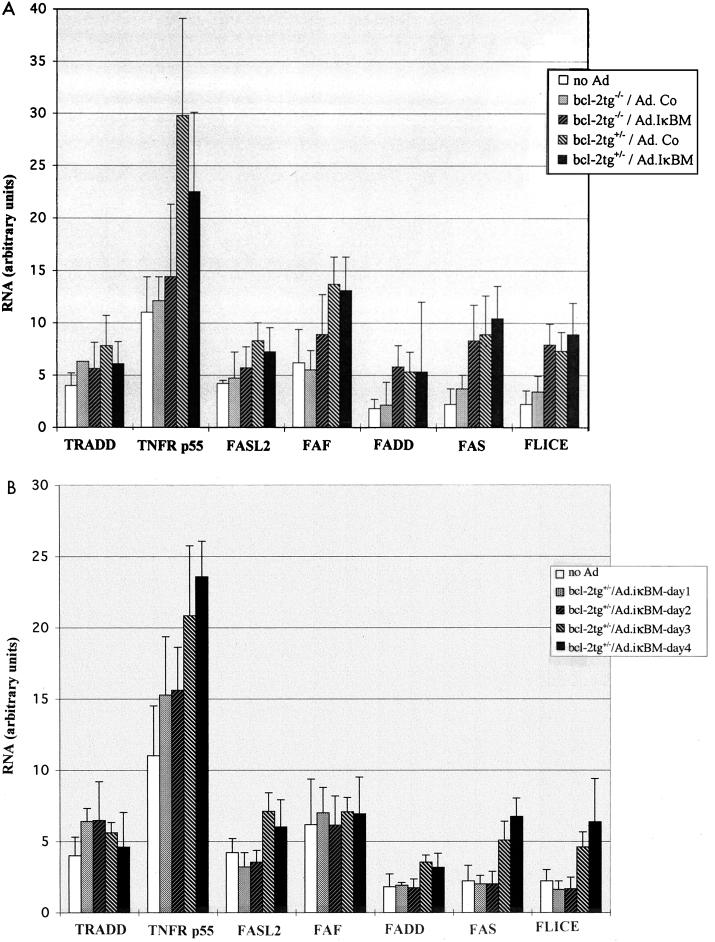
Certain chronic apoptotic stimuli may up-regulate expression of death proteins. Moreover, a compensatory reaction in the form of induction of apoptotic gene expression is thought to take place if specific pathways or the balance between death proteins are affected (24, 53). In an attempt to begin to unravel the mechanisms involved in rAd-induced apoptosis, we studied the expression of major components of the cell death pathways triggered by TNF or Fas ligand (FasL). At day 4 after rAd infusion, total liver RNA was quantified by RPA for RNAs specific for murine FasL2 (also called TRAIL), Fas, FLICE (caspase-81), FAF, and Fas-associated death domain-containing protein (FADD) (for the Fas pathway) and TNFR p55 and TRADD (Fig. 4). Transcription of the genes for TNFR p55, FAF, Fas, and FLICE was increased significantly in both groups of bcl-2tg+/− transgenic mice, with the most pronounced activation (threefold) for TNFR p55 expression. To a lesser extent, FAS and FLICE expression was stimulated in bcl-2tg−/− mice that received Ad.IκBM, while TRADD, FasL2, and FADD expression was not significantly increased (Fig. 4A). Induction of gene expression in bcl-2tg+/− mice occurred gradually during the first 4 days after rAd infusion (Fig. 4B). The data suggest that rAd (Ad.Co) infusion did not significantly increase expression of apoptotic genes in bcl-2tg−/− mice, whereas in bcl-2tg+/− mice, expression of certain cell death proteins involved in the early stages of apoptotic pathways was up-regulated.
FIG. 4.
Expression of proapoptotic genes after adenovirus infusion. Adenovirus was administered to mice as described for Fig. 1. (A) At days 4 and 40 p.i., total liver RNA from all four experimental groups was quantified by RPA for RNAs specific for gene products involved in apoptotic pathways. (B) Analysis of gene expression in bcl-2tg+/− mice at days 1, 2, 3, and 4 after infusion of Ad.IκBM. Signals from protected bands were quantified on a PhosphorImager. The signals from specific mRNAs were normalized to signals from housekeeping genes (mL32 and mGAPDH) run on each lane to adjust for loading differences and expressed as arbitrary units. Apoptotic gene expression in the experimental groups is represented in comparison to that of normal animals that did not receive rAd. Results are means ± SD for at least three animals.
Effects of bcl-2 and IκBM on transgene expression and vector DNA persistence.
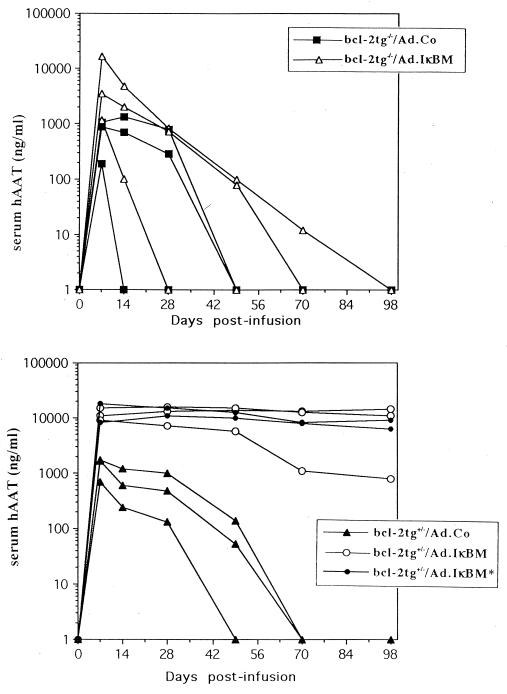
Based on our hypothesis that inflammation and apoptosis activated by cytokines and/or viral proteins affect vector persistence, we monitored hAAT transgene expression after Ad.RSV/hAAT infusion in relation to Bcl-2 expression and NF-κB inhibition by Ad.IκBM, which can block the above-mentioned mechanisms (Fig. 5). hAAT expression declined to undetectable levels by weeks 5 to 6 in bcl-2tg−/− and bcl-2tg+/− mice infused with Ad.hAAT in combination with a control virus. Two of three bcl-2tg−/− mice that received Ad.IκBM and Ad.hAAT expressed hAAT for 1 or 70 days. Coinfusion of Ad.IκBM with Ad.hAAT into bcl-2tg+/− mice resulted in persistent hAAT expression over the analyzed time period of 100 days. Transient induction of Bcl-2 expression throughout the first week after adenovirus infusion was sufficient to confer stable transgene expression.
FIG. 5.
Serum hAAT levels after Ad.hAAT infusion in relation to bcl-2 expression and NF-κB inhibition by IκBM. A dose of 4 × 109 PFU of Ad.Co or 4 × 109 PFU of Ad.IκBM together with an equal dose of Ad.hAAT (first-generation vector) was injected via tail vein into bcl-2tg−/− and bcl-2tg+/− mice. In one group of bcl-2tg+/− animals that received Ad.IκBM (bcl-2tg+/−/Ad.IκBM∗), bcl-2 expression was induced only during the first 7 days after adenovirus infusion by ZnSO4. All the other animals were under ZnSO4 for the full duration of the experiment. Serum was analyzed periodically for hAAT by ELISA. Each line represents an individual animal.
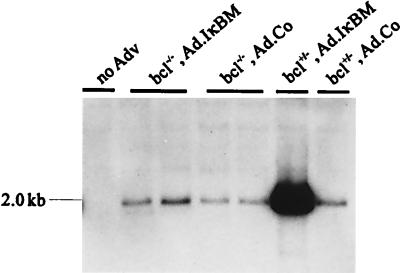
To understand the reasons for this expression pattern, vector DNA in genomic liver DNA was analyzed by Southern blotting. The analysis was performed on the animals used for hAAT studies represented in Fig. 6 at day 100 p.i. At this time point, hAAT expression was present only in Ad.IκBM-infused bcl-2tg+/− mice. At day 100 p.i., a high concentration of vector (Ad.hAAT) DNA was detected only in bcl-2tg+/− mice that received Ad.IκBM. Vector DNA in all other groups was significantly reduced but still detectable. Liver DNA from bcl-2tg−/− mice that received Ad.IκBM had a slightly higher amount of vector DNA than did liver DNA from bcl-2tg−/− animals injected with Ad.Co. The data indicate that inhibition of NF-κB prolonged transgene expression, but the block of apoptosis by coexpression of Bcl-2 was necessary to achieve persistence.
FIG. 6.
Southern blot analysis of adenovirus vector (Adv) DNA in transduced mouse livers. A dose of 4 × 109 PFU of Ad.Co or 4 × 109 PFU of Ad.IκBM together with an equal dose of Ad.hAAT (first-generation vector) was injected into bcl-2tg−/− and bcl-2tg+/− mice. Mice were sacrificed at day 100 p.i. Ten micrograms of BamHI-digested genomic liver DNA was loaded on each lane. Blots were hybridized with a labeled hAAT probe; Exposure was for 4 days. The fragment specific for Ad.hAAT is 2.0 kb. The analysis was performed with animals used for hAAT studies represented in Fig. 5.
Effects of Bcl-2 and IκBM on anti-hAAT antibody production.
The absence of hAAT expression at a time point when vector DNA was still detectable may be related to a block of transgene expression at the level of transcription, translation, or posttranslational processing. Tsui et al. reported that CTL-derived cytokines can induce posttranscriptional clearance of hepatitis B virus RNA in infected hepatocytes (58). Furthermore, certain cytokines can inhibit transcription from the Rous sarcoma virus promoter used for hAAT expression (38). We did not investigate these possibilities but instead concentrated on an observation recently made by our group (45, 46) and others (33), that antibodies to the expressed transgene product (hAAT) reduce the level of detectable serum hAAT in C3H mice. To evaluate this possibility, we determined whether antibodies to hAAT were present in the serum at day 4, 7, 14, and 30 p.i. (Table 1). Anti-hAAT IgG antibody levels in serum samples of bcl-2tg−/− and bcl-2tg+/− mice infused with Ad.IκBM were at least 100 times lower than in those of mice that received the control virus. In most of the Ad.IκBM-infused bcl-2tg+/− mice, anti-hAAT antibodies were undetectable. This indicates (i) that anti-hAAT antibodies interfered with hAAT detection by ELISA and (ii) that inhibition of NF-κB effectively prevented the humoral immune response against the transgene product hAAT.
TABLE 1.
Anti-hAAT antibody levels in mouse serum after adenovirus infusiona
| Mouse/virus | Antibody level
|
|||
|---|---|---|---|---|
| Day 4 p.i. | Day 7 p.i. | Day 14 p.i. | Day 30 p.i. | |
| bcl-2tg−/−/Ad.Co | +/+/+ | +++/++/++ | +++/+++/+++ | +++/+++/+++ |
| bcl-2tg−/−/Ad.IκBM | −/−/− | +/−/− | +/+/− | +/+/− |
| bcl-2tg+/−/Ad.Co | +/+/+ | ++/++/++ | +++/+++/+++ | +++/+++/+++ |
| bcl-2tg+/−/Ad.IκBM | −/−/− | +/−/− | +/−/− | +/−/− |
Virus infusion was done as described in the legend to Fig. 6. Serum obtained at days 4, 7, 14, and 30 p.i. was analyzed for anti-hAAT IgG by a semiquantitative ELISA in comparison to a standard curve of a commercial anti-hAAT MAb. Anti-hAAT antibody levels are expressed for individual animals as not detected (−) or detection similar to that of the standard MAb diluted 1:1,000 (+++), 1:100 (++), or 1:10 (+). Results of three separate determinations are shown.
DISCUSSION
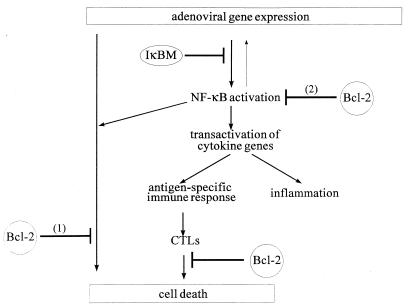
This study addressed the hypothesis that apoptosis of transduced cells is a key mechanism for rAd vector clearance from the mouse liver. We postulated that viral proteins expressed in transduced hepatocytes induce apoptosis by a combination of two major mechanisms: (i) a direct proapoptotic activity inherent to specific adenovirus early or late proteins and (ii) activation of cytokines (e.g., TNF-α) that cause apoptosis through direct action or by recruitment of CTLs or NK cells, which trigger apoptotic cell death (Fig. 7). We hypothesized that cytokine induction involves the activation of NF-κB. These hypotheses were based on an earlier study where we demonstrated that viral gene expression in hepatocytes transduced with first-generation adenoviruses induced an innate response characterized by NF-κB activation, elevation of serum cytokines, and hepatocellular apoptosis during the first 3 days p.i. These reactions were absent in mice infused with an adenovirus vector deleted for 25 kb, including the E1, E2, E3, and late genes (29).
FIG. 7.
Hypothetical mechanisms for the roles of NF-κB and Bcl-2 in vector persistence. Expressed adenovirus proteins can induce early apoptosis. (1) NF-κB has an antiapoptotic role. Bcl-2 blocks virus-induced apoptosis if NF-κB protection is inactivated by IκBM. (2) NF-κB transactivates cytokine expression, which is a key element in recruitment and activation of immune cells. Hence, NF-κB promotes cytokine/CTL-mediated cell death. Bcl-2 is required to block residual NF-κB activity, which was not inhibited by IκBM.
In an attempt to delineate the role of virus-induced NF-κB activation and apoptosis in clearance of rAd vectors from the liver, we used IκBM and bcl-2 transgene expression to block the corresponding mechanisms. Strikingly, the combined action of IκBM and Bcl-2 led to vector persistence in livers of the mouse strain used in this study.
Role of hepatocellular cytokine expression.
The down-regulation of cytokine expression by blocking the NF-κB transactivation mechanisms inhibited infiltration and probably activation of immune cells. This is reflected in part by the fact that in mice infused with Ad.IκBM, leukocyte infiltrates in transduced livers were absent and the levels of anti-hAAT antibodies were markedly reduced. Reduced levels of cytokine transcripts detected in total liver RNA at day 4 after infusion of Ad.IκBM may be the result of inhibited cytokine expression in hepatocytes and of subsequently suppressed infiltration of inflammatory cells, which can potentially produce cytokines (20, 64). Our hypothesis that the initial IκBM-mediated block of cytokine activation occurred primarily in hepatocytes and not in nonparenchymal liver cells is supported by the following observations. (i) A number of reports demonstrated the expression of TNF-α, TGF-β1, and IFN-γ genes in isolated primary hepatocytes or hepatocyte lines after adequate activation (40, 41, 47, 54). (ii) The same pattern of NF-κB activation occurs in mice depleted for Kupffer cells, suggesting that rAd-induced NF-κB activation takes place in hepatocytes (29). (iii) Inhibition of cytokine expression correlates with the blockage of NF-κB activation by IκBM in hepatocytes. Hepatocytes are the cell type that is predominantly transduced after intravenous rAd infusion in mice. There are no reported data showing that rAd-mediated transgene expression can occur in Kupffer cells or immune cells.
Role of apoptosis induced by viral proteins expressed in transduced hepatocytes.
In the absence of cytokine-mediated immune cell recruitment or activation, apoptosis induced by expressed viral proteins may be the central mechanism responsible for the decline in vector DNA in transduced hepatocytes. In general, lytic viruses regulate cell viability, inhibiting apoptosis during the early stages of replication and then promoting apoptosis late in infection so that progeny virions can be released from the cell (for a review, see reference 55). In adenovirus, these processes are regulated mainly by E1a and the antiapoptotic proteins E1B-55K and E1B-19K, which are deleted in rAd vectors. Apoptotic signals observed in mouse livers after infusion of rAd may be causally linked to other adenovirus proapoptotic proteins such as the E4 open reading frame 4 (50), E3-11.6K (56), and pre terminal (pTP) E2 (26) proteins or specific late proteins like penton or fiber (48). Two observations that we made earlier support the etiological role of expressed viral proteins in hepatocellular apoptosis. A small percentage of apoptotic hepatocytes was detected in mouse liver only with adenovirus vectors that expressed early and late viral proteins, not with vectors deleted for viral genes (29). Furthermore, a considerable amount of vector genome is lost during the first 36 h p.i. in both immunocompetent and immunodeficient mice, which may be attributed, in part, to death of infected cells due to virus-induced apoptosis (28). Although apoptosis was observed in only 2 to 3% of all hepatocytes at each time point, it cannot be excluded that an asynchronous turnover of the majority of hepatocytes takes place in the first day(s) after rAd infusion. This hypothesis would be consistent with the DNA replication in ∼70% of hepatocytes occurring at day 4 p.i. as a result of liver damage and cytokine activation (28, 29, 44).
NF-κB has a protective role against virus-induced apoptosis in hepatocytes.
Infusion of Ad.IκBM caused extended apoptosis in periportal liver regions that probably received the highest viral load and therefore express IκBM at levels sufficient for complete NF-κB inhibition. This finding indicates that NF-κB activation may protect transduced cells from apoptosis induced by adenovirus gene products. This may be why only in combination with Bcl-2 expression did IκBM prevent vector clearance. In general, an antiapoptotic role of NF-κB is supported by the fact that relA knockout mice were found to display embryonic lethality due to apoptosis in the liver (4) and by in vitro studies where inhibition of NF-κB activation promoted cell death (5, 59, 62) whereas ectopic NF-κB overexpression protected against apoptosis (51).
Inhibition of apoptosis by Bcl-2.
Endogenous Bcl-2 is not expressed at detectable levels in the liver (23, 24). In our studies, bcl-2 transgene expression in mouse livers prevented virus-induced apoptosis. Bcl-2 protection is most pronounced in the absence of antiapoptotic NF-κB mechanisms (Fig. 7, hypothesis 1). We hypothesize that Bcl-2 can override the virus-induced death signals activated upon the loss of NF-κB activity due to IκBM overexpression. The mechanisms behind the antiapoptotic action of Bcl-2 are still unclear; however, our observation that cytokine expression is slightly reduced in bcl-2 transgenic mice indicates that Bcl-2 may inhibit the transactivation activity of p65 as suggested previously (14) and neutralize the residual hepatic NF-κB activity left after Ad.IκBM infusion (Fig. 7, hypothesis 2).
Hepatic expression of the bcl-2 transgene was associated with up-regulated transcription of proapoptotic genes. It appears that all induced gene products are involved in Fas/TNF-mediated pathways at or before a step that is blocked by Bcl-2, suggesting that this up-regulation represents a compensatory reaction to a Bcl-2-mediated block (24, 53). More refined analysis with specific inhibitors for Fas, TRAIL (FasL2), caspases, or other components are required to support this hypothesis.
Mechanism for extinction of transgene expression.
We demonstrated that IκBM expression alone was sufficient to inhibit the humoral immune response against the transgene and the infiltration of inflammatory cells into the liver; however, the additional expression of Bcl-2 was required to counteract vector loss in transduced cells. This finding suggests that at least in this mouse strain, a humoral immune response, while affecting the detection of hAAT, was not primarily responsible for vector clearance. Also, loss of vector DNA was a result of at least two mechanisms: (i) the cell-mediated immune response, which is triggered by cytokines and cannot be blocked by Bcl-2 expression alone (Fig. 6, bcl-2tg+/−/Ad.Co) and (ii) early apoptosis induced by viral proteins, which can be prevented by Bcl-2. This conclusion implies that other strategies that can block cytokine activation or action may prolong vector persistence. Notably, a rAd vector that overexpressed all adenovirus E3 proteins suppressed the production of neutralizing antibodies and resulted in transgene persistence (18). The adenovirus E3 region encodes proteins that protect against TNF- and Fas-mediated apoptosis (49). Interestingly, the E3 promoter is transactivated by NF-κB (9), which may be one of the mechanisms for how NF-κB exerts its antiapoptotic function after rAd transduction.
An argument against our hypothesis that early apoptosis induced by cytokines, CTLs, and/or viral proteins, which is associated with loss of transduced cells, represents a central mechanism for vector clearance is that destruction of >90% of transduced hepatocytes over a short time should be fatal. However, this argument does not consider that the liver can regenerate quickly in response to damage and cytokine release, particularly if apoptosis is asynchronous. Hepatocellular proliferation in response to rAd was reported by a number of groups (28, 29, 44, 64).
Importance for gene therapy.
Our data suggest that transient expression of Bcl-2 at early time points after rAd infusion results in persistent gene expression if the vector is coadministered with Ad.IκBM. If, in addition to transient Bcl-2 expression, a transient, inducible expression of IκBM during a critical period after rAd infusion were sufficient to confer vector persistence, then our study could be the basis for a practicable approach to achieve persistent gene expression for therapeutic purposes. On the contrary, a permanent inactivation of NF-κB will probably have consequences including tumor development and lost protection against infectious agents (for a review, see reference 32).
With respect to permanent Bcl-2 expression, it is notable that an aberrant overexpression of Bcl-2 is often observed in tumors and may be responsible for resistance of tumor cells to apoptotic stimuli such as radiotherapy and chemotherapy (for a review, see reference 16). Nonetheless, our study contributes to improving the understanding of early host antiviral host mechanisms. Further efforts in this direction may include the use of specific compounds for NF-κB inhibition (e.g., oligonucleotide decoys) and blockage of specific apoptotic pathways (e.g., by caspase inhibitors).
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1 DK51807 (M.A.K.) and the Cystic Fibrosis Foundation (A.L.).
REFERENCES
- 1.Ashany D, Song X, Lacy E, Nikolic-Zugic J, Friedman S M, Elkon K B. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 pathway. Proc Natl Acad Sci USA. 1995;92:11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M. Strain related variations in adenoviral mediated transgene expression from mouse hepatocytes in vivo: comparison between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–156. [PubMed] [Google Scholar]
- 4.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the relA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 5.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Bett A J, Krougliak V, Graham F L. DNA sequence of the deletion/insertion in early region 3 of Ad5 dl 309. Virus Res. 1995;39:75–82. [PubMed] [Google Scholar]
- 7.Boucher R C. Current status of CF gene therapy. Trends Genet. 1996;12:81–84. doi: 10.1016/0168-9525(96)81410-7. [DOI] [PubMed] [Google Scholar]
- 8.Clesham G J, Adam P J, Proudfoot D, Flynn P D, Efstathiou S, Weissberg P L. High adenoviral loads stimulate NF-κB-dependent gene expression in human vascular smooth muscle cells. Gene Ther. 1998;5:174–180. doi: 10.1038/sj.gt.3300576. [DOI] [PubMed] [Google Scholar]
- 9.Deryckere F, Burger H-G. Tumor necrosis factor alpha induces the adenovirus early promoter by activation of NF-κB. J Biol Chem. 1996;47:30249–30255. doi: 10.1074/jbc.271.47.30249. [DOI] [PubMed] [Google Scholar]
- 10.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkon K B, Liu C-C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Song X, Zhou J L, Old L J, Crystal R G, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore T D. The Rel/NF-κB signal transduction pathway. Cancer Biol. 1997;8:61–62. doi: 10.1006/scbi.1997.0056. [DOI] [PubMed] [Google Scholar]
- 13.Gooding L R. Regulation of TNF-mediated cell death and inflammation by human adenoviruses. Infect Agents Dis. 1994;3:106–115. [PubMed] [Google Scholar]
- 14.Grimm S, Bauer M K A, Baeuerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henkart P. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 16.Hickman J A. Apoptosis and chemotherapy resistance. Eur J Cancer. 1996;32A:921–926. doi: 10.1016/0959-8049(96)00080-9. [DOI] [PubMed] [Google Scholar]
- 17.Hitt M M, Addison C L, Graham F L. Human adenoviral vectors for gene transfer into mammalian cells. Adv Pharmacol. 1997;40:137–205. doi: 10.1016/s1054-3589(08)60140-4. [DOI] [PubMed] [Google Scholar]
- 18.Ilan Y, Droguett G, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay M A, Graham F, Leland F, Woo S L. Therapeutic serum concentrations of human alpha1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 20.Kay M A, Holterman A-X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 21.Kay M A, Meuse L, Gown A M, Linsley P, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiefer M C, Brauer M J, Powers V C, Wu J J, Umansky S R, Tomei L D, Barr P J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 23.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang H G, Reed J C. Immunohistochemical determination of in vivo distribution of bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145:1323–1326. [PMC free article] [PubMed] [Google Scholar]
- 24.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;6:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 25.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 26.Langer S J, Schaak J. 293 cell lines that inducibly express high level adenovirus type 5 precurser terminal protein. Virology. 1996;221:172–179. doi: 10.1006/viro.1996.0363. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Kang J, Horwitz M S. Interaction of an adenovirus 14.7-kilodalton protein inhibitor of tumor necrosis factor alpha cytolysis with a new member of the GTPase superfamily of signal transducers. J Virol. 1997;71:1576–1582. doi: 10.1128/jvi.71.2.1576-1582.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieber A, He C-Y, Kirillova I, Kay M A. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieber A, He C-Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K, Lee S-H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeser P, Jennings G S, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus promoter in the mouse liver: involvement of NF-κB. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luque I, Gelinas C. Rel/NF-κB and IκB factors in oncogenesis. Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 33.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wildtype and E2a deleted vectors. Hum Gene Ther. 1997;8:1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 34.Mosialos G. The role of rel/NF-κB proteins in viral oncogenesis and the regulation of viral transcription. Cancer Biol. 1997;8:121–129. doi: 10.1006/scbi.1997.0063. [DOI] [PubMed] [Google Scholar]
- 35.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak T W, Holzmann B, Kronke M. Crucial role of 55-kDa TNF receptor in TNF induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 36.Pezzella F, Tse A G, Cordell J L, Pulford K A, Gatter K C, Mason D Y. Expression of the Bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- 37.Pfizenmaier K, Wajant H, Grell M. Tumor necrosis factor in 1996. Cytokine Growth Factor Rev. 1996;3:271–277. doi: 10.1016/s1359-6101(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 38.Quin L H, Ding Y Z, Pahud D R, Chang E, Imperiale M J, Bromberg J S. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez I, Matsuura K, Khatib K, Reed J C, Nagata S, Vassalli P. A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J Exp Med. 1996;183:1031–1036. doi: 10.1084/jem.183.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth S, Schurek J, Gressner A M. Expression and release of the latent transforming growth factor beta binding protein by hepatocytes from rat liver. Hepatology. 1997;25:1398–1405. doi: 10.1002/hep.510250616. [DOI] [PubMed] [Google Scholar]
- 41.Rowell D L, Eckmann L, Dwinell M B, Carpenter S P, Raucy J L, Yang S K, Kagnoff M F. Human hepatocytes express an array of proinflammatory cytokines after agonist stimulation or bacterial invasion. Am J Physiol. 1997;273:G322–G332. doi: 10.1152/ajpgi.1997.273.2.G322. [DOI] [PubMed] [Google Scholar]
- 42.Scallan M, Allsopp T E, Fazakerley J K. Bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaria A, St-George J A, Gregory R J, Noelle R J, Wadsworth S C, Smith A E, Kaplan J M. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- 44.Schiedner G, Morral N, Parks R J, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 45.Schowalter D B, Meuse L, Wilson C B, Linsley P S, Kay M A. Constitutive expression of murine CTLA4Ig from a recombinant adenovirus vector results in prolonged transgene expression. Gene Ther. 1997;4:853–860. doi: 10.1038/sj.gt.3300466. [DOI] [PubMed] [Google Scholar]
- 46.Schowalter, D. B., C. L. Himeda, B. L. Winther, C. B. Wilson, and M. A. Kay. 1998. Unpublished data.
- 47.Schroeder R A, Gu J S, Kuo P C. Interleukin 1beta-stimulated production of nitric oxide in rat hepatocytes is mediated through endogenous synthesis of interferon gamma. Hepatology. 1998;27:711–719. doi: 10.1002/hep.510270312. [DOI] [PubMed] [Google Scholar]
- 48.Shenk T. Adenoviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publisher; 1996. pp. 2111–2148. [Google Scholar]
- 49.Shishler J, Yang C, Walter B, Ware C F, Gooding L R. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol. 1997;71:8299–8306. doi: 10.1128/jvi.71.11.8299-8306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shtrichman R, Kleinberger T. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J Virol. 1998;72:2975–2983. doi: 10.1128/jvi.72.4.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonenshein G E. Rel/NF-κB transcription factors and the control of apoptosis. Cancer Biol. 1997;8:113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 52.Stancovski I, Baltimore D. NF-κB activation: the IκB kinase revealed. Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 53.Strasser A, O’Connor L. Fas-ligand—caught between Scylla and Charybdis. Nat Med. 1998;4:21–22. doi: 10.1038/nm0198-021. [DOI] [PubMed] [Google Scholar]
- 54.Sueoka E, Soeoka N, Okabe S, Kozu T, Komori A, Ohta T, Suganuma M, Kim S J, Lim I K, Fujiki H. Expression of the tumor necrosis factor alpha gene and early response genes by nodularin, a liver tumor promoter, in primary cultured rat hepatocytes. J Cancer Res Clin Oncol. 1997;123:413–419. doi: 10.1007/BF01372544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tollefson A E, Scaria A, Hermiston T W, Ryerse J S, Wold L J, Wold W S M. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tripathy S, Black H, Goldwasser E, Leiden •. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 58.Tsui L V, Guidotti L G, Ishikawa T, Chrisari F V. Post-transcriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci USA. 1995;92:12398–12402. doi: 10.1073/pnas.92.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 60.Vrancken Peeters M-J, Lieber A, Perkins J, Kay M A. Method for multiple portal vein infusions in mice: quantification of adenovirus-mediated hepatic gene transfer. BioTechniques. 1996;20:278–285. doi: 10.2144/96202rr05. [DOI] [PubMed] [Google Scholar]
- 61.Vrancken Peeters M-J, Patjin G A, Lieber A, Meuse L, Kay M-A. Adenovirus-mediated hepatic gene transfer in mice: comparison of intravascular and biliary administration. Hum Gene Ther. 1996;7:1693–1699. doi: 10.1089/hum.1996.7.14-1693. [DOI] [PubMed] [Google Scholar]
- 62.Wang C-Y, Mayo M W, Baldwin A S. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 65.Yang X-Z, Ertl H C, Wilson J M. Upregulation of class I MHC antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]