Short abstract
Rising temperatures and extreme weather are setting the stage for increases in fungal diseases. As new pathogenic fungi emerge and known threats spread and evolve, scientists and decision makers are responding.
Justin Remais began his career studying schistosomiasis, a disease caused by a parasitic worm common in tropical settings. But when Remais moved his lab to the University of California, Berkeley (UCB), in 2016, he set his sights on another poorly understood pathogen that he found—literally—in his own backyard, Coccidioides immitis. The fungus lives in dust and soil in parts of the western United States, Mexico, and South America.1 Recent years had seen a rise in cases of coccidioidomycosis, or Valley fever—the pneumonia-like respiratory infection caused by the fungus.2,3
Because of the limited environmental and public health surveillance at the time, it was not clear how people were being exposed to Coccidioides and what factors determined who got sick, says Remais, a professor and chair of the Environmental Health Sciences division at UCB School of Public Health. Emerging evidence suggests climate change may be playing a role. However, he says, “There are major gaps in our understanding of the environmental biology of the pathogen and the epidemiology of Valley fever, which makes it difficult to project future risk or develop effective strategies to respond.” The same holds true for other emerging fungal diseases and how climate change may be affecting—and even contributing to emergence of—the environmental pathogens that cause them.

Jace White acquired Valley fever while farming near Fresno, California. Coccidioidomycosis—a pneumonia-like respiratory infection—is named for California’s San Joaquin Valley, where the pathogen was first identified.53 Image: © Carolyn Van Houten/The Washington Post via Getty Images.
Researchers are now working to understand the factors, including climate change, driving the rise in invasive (i.e., systemic) fungal diseases in people worldwide—and how to reduce the threat. Invasive fungal infections are becoming more common, as is the microbes’ resistance to the current small arsenal of antifungal treatments.4 Recognizing the growing global public health problem, in 2022 the World Health Organization (WHO) released its first-ever list of fungal “priority pathogens,”4 calling for policy improvements and research in such areas as fungal disease distribution, patterns of antifungal resistance, and who is most at risk of exposure and disease.
Toward that end, Remais and colleagues have launched a major research project on the environmental epidemiology of fungal diseases in the United States, including Valley fever. They will study the climate sensitivity of key pathogenic fungi and examine how social inequalities may increase disparities in fungal disease incidence and severity across vulnerable populations as they experience climate warming. “Rising temperatures and extreme events such as hurricanes can have very different effects on fungal disease epidemiology across neighborhoods,” says Remais.
Side Effects of Modern Medicine?
About a dozen fungal species cause the majority of human infections,5 and most, like athlete’s foot and ringworm, are superficial or subcutaneous.6 Very few thrive inside the human body under normal conditions for two reasons, explains Arturo Casadevall, a microbiologist and immunologist at Johns Hopkins Bloomberg School of Public Health. First, most fungal species do not grow well at mammalian body temperatures. Second, a healthy human immune system can readily dispense of those that do.7 Yet, “body temperature alone is not enough to protect us,” says Casadevall. A warm body in combination with an intact immune system appears necessary to create a strong defense against pathogenic fungi.

Image: © Ghiglione Claudio/Shutterstock.com.
Most experts acknowledge that fungal disease is on the rise, although the global incidence and prevalence of most fungal infections remain unknown. The 2022 WHO report estimates that more than 300 million people worldwide may be affected by serious fungal infections each year, potentially causing more than 1.5 million deaths annually.8 Yet most fungal diseases are not subject to public health reporting requirements.9 Many clinicians do not initially suspect fungal disease in patients, and even when they do, access to quality diagnostic tests may be limited due to cost, distribution, or technical issues.4
Recognition of fungal diseases grew during the 1950s as advances in cancer care, transplantation, neonatal medicine, and autoimmune disease management led to a larger pool of immunocompromised patients.10 “Fungal infections have emerged as a side effect of the modern medical system,” says Rebecca Drummond, a fungal immunologist at the University of Birmingham in the United Kingdom.
For example, corticosteroids and broad-spectrum antibiotics alter both the immune system and the gut microbiome in ways that increase our susceptibility to fungal disease.10 Fungi are part of the gut microbiome,11 and the use of antimicrobials can alter the balance, leading to an overgrowth of pathogenic species in the gut, explains John Perfect, chief of the Division of Infectious Diseases at the Duke University School of Medicine and coauthor of the 2022 WHO report. Research12 also suggests that “antibiotics may directly disturb how the immune system fights fungal infections,” says Drummond.
Pandemics and Immunity
Most recently, fungal diseases have emerged as a complication of such pandemics as HIV/AIDS10 and COVID-19.13 During the COVID-19 pandemic, outbreaks of antifungal-resistant Candida auris bloodstream infections occurred in crowded intensive care units around the world.13 Such invasive fungal infections among those sick with the virus were not limited to the severely immunocompromised with underlying disease but also struck previously healthy people, says Martin Hoenigl, an associate professor of translational mycology at the Medical University of Graz in Austria. “COVID-19 changed the way we think about fungal disease,” he says.
As Hoenigl and colleagues wrote in a 2022 review, “SARS-CoV-2 infection alters the immune and metabolic responses in patients, which together produce an inflammatory environment that is highly permissive to fungal infection.”13 Accordingly, COVID-19–associated pulmonary aspergillosis, an invasive mold disease, was reported among patients receiving mechanical ventilation and immunosuppressive treatment for respiratory failure.14 A COVID-19–associated mucormycosis outbreak in India garnered global headlines in 2021 with more than 47,500 reported cases.15,16
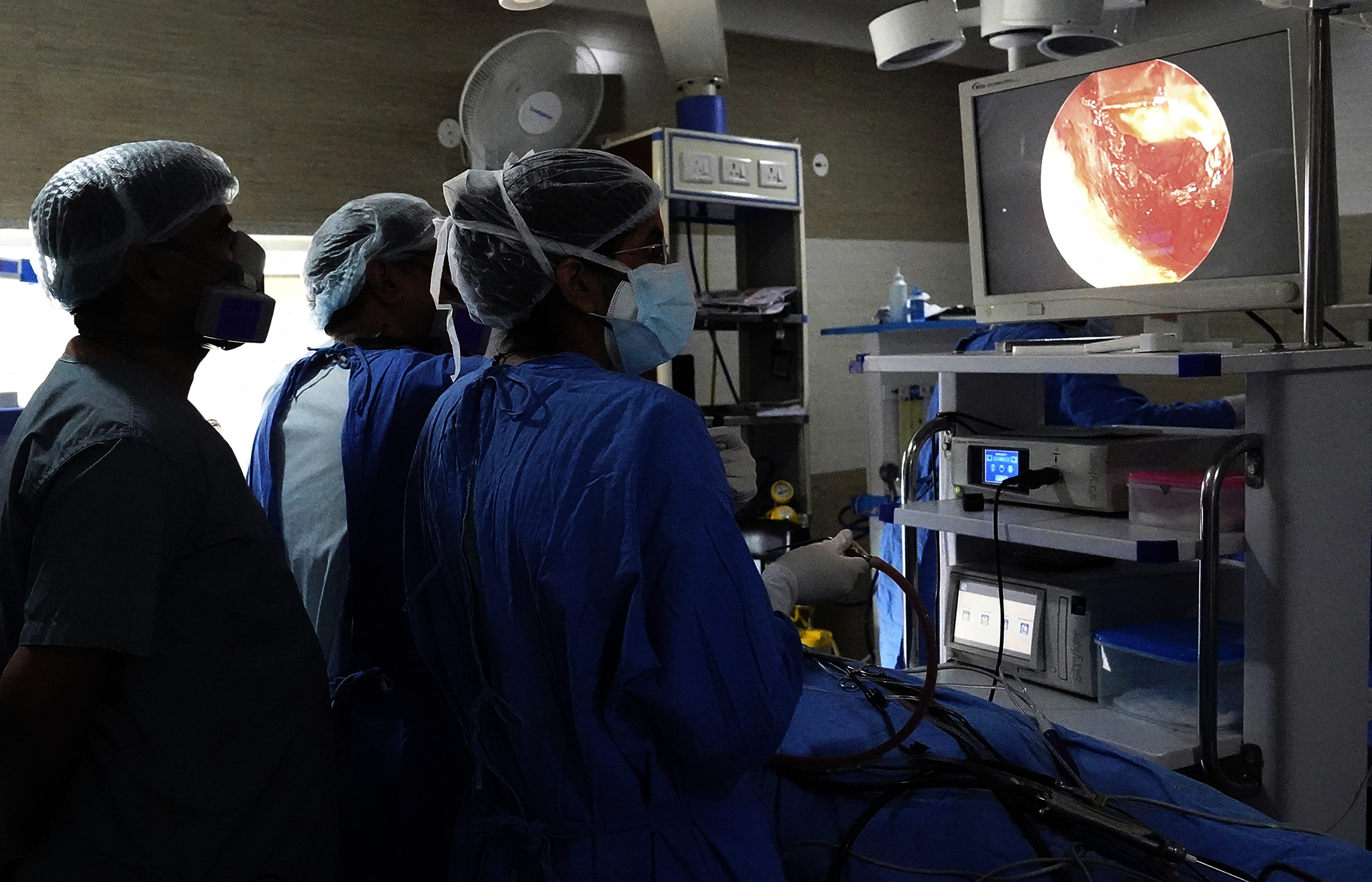
A doctor performs endoscopic sinus surgery on a person with mucormycosis in Ajmer, Rajasthan, India, during the 2021 outbreak. Image: © Himanshu Sharma/Sipa USA via AP.
Fungal Pathogens and Climate Change
But how are people being exposed to pathogenic fungi in the first place? Climate change may drive increased fungal disease risk because warming temperatures, shifting precipitation patterns, and ecological disturbances may help the pathogens spread and evolve.17 “It’s creating more exposure for susceptible individuals,” says Drummond.
Some fungal pathogens have increased in geographic range.17,18 For example, one projection suggests that alternating periods of higher-than-normal precipitation and higher temperatures and drought across the US West could more than double the endemic range of Valley fever by the year 2100, raising the number of affected states from 12 to 17 and causing a 50% increase in cases.18 Multiyear cycles of dry followed by wet winters have been projected to lead to more people getting sick.19 Indeed, record numbers of cases in California followed the drought-ending rainy winter of 2022–2023,20 with its intense atmospheric rivers.21 “Our research19 indicates that the [2023–2024] wet winter in California is likely to lead to further increases in incidence in the summer and fall of 2024,” Remais says.
Coccidioides live in desert soils and proliferate during wet periods.18 Soil disturbances from wind or digging in dry soil can lift the spores into the air, where they can be inhaled. These filamentous fungi act like aerosolized particulates, says Remais. “It’s important to understand it as similar in key ways to particulate matter air pollutants,” he explains, although it differs in how it affects the body. “It has emissions sources and transport characteristics. Just as with particulate matter, the sources and dispersion of Coccidioides can shift with environmental change.” The US Centers for Disease Control and Prevention (CDC) recommends protective measures—such as N95 respirators and staying inside during dust storms—for people at risk for severe illness, although these strategies have not been proven to prevent illness.22
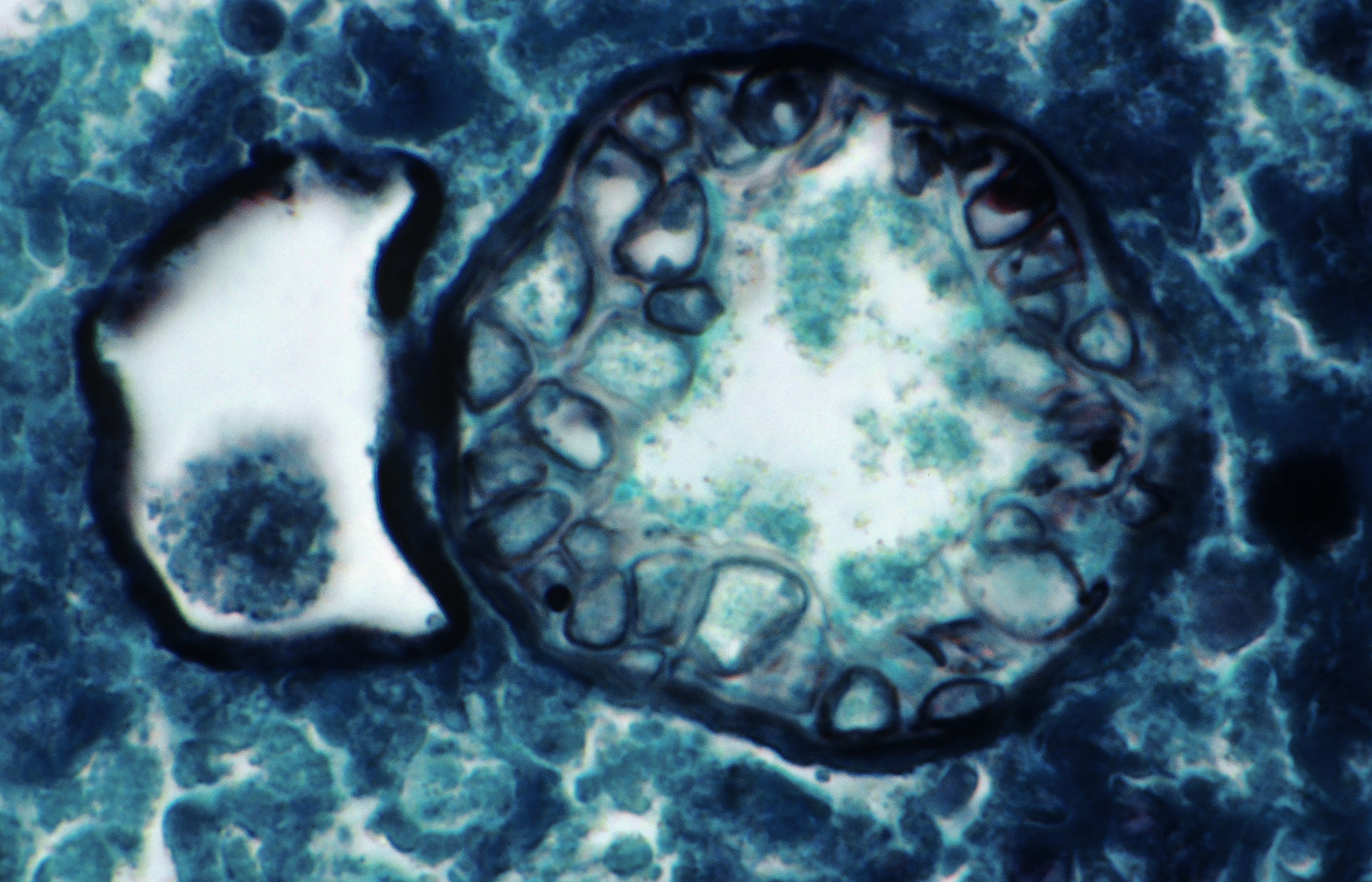
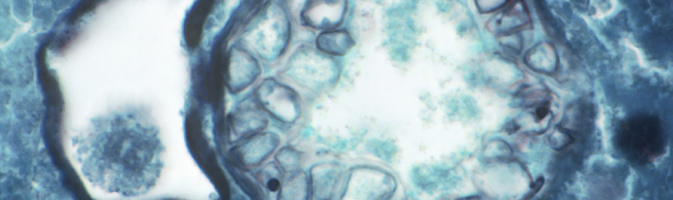
With respect to human health, the pathogen that causes Valley fever behaves like an aerosolized particulate, says Remais. Light micrograph shows a Coccidioides immitis spherule, capable of releasing spores and spreading infection, in a human lung. Image: © Michael Abbey/Science Source.
Changes in temperature and moisture combined with forest disturbances from tree harvesting may have driven outbreaks of cryptococcosis caused by Cryptococcus gattii on Canada’s Vancouver Island starting in the late 1990s.23 Researchers found associations between an increase in logging, which releases fungal spores into the environment, and C. gattii incidence on the island.24 This fungus is common in soil and on certain trees in tropical and subtropical regions; the timing of its first appearance on Vancouver Island remains unclear.25
Other fungi may be adapting to survive at warmer temperatures—closer to human body temperature.17 In other words, fungal pathogens that are not currently a threat to mammals may evolve to survive at higher temperatures and thus become a health threat.
Indeed, it may already have happened at least once. The best candidate for the theory of evolving thermotolerance is the recent emergence of Candida auris, says Casadevall. C. auris is a type of yeast that can cause severe infections—particularly in people who receive invasive medical devices—and spreads easily among patients in health care facilities. Even more concerning, C. auris shows multidrug resistance. First identified in 2009 during treatment of an ear infection in a woman in Japan,26 by 2019, the CDC had classified C. auris as an urgent threat to public health, due to its growing drug resistance.27
C. auris is the first completely new fungal species hypothesized to have emerged due to climate change.17 Casadevall points to the simultaneous, unexplained emergence of three types of C. auris on three continents as circumstantial evidence of a global warming link.28 “You see the rapid emergence of a microbe not previously known to medicine suddenly causing disease in Venezuela, South Africa, and India,” said Casadevall. All of those locations have experienced extensive warming, according to Casadevall.
Clinical strains of C. auris also may be more thermotolerant than strains found in nature. In 2021, researchers isolated C. auris from a salt marsh and a beach with no human activity on India’s Andaman Islands in the Bay of Bengal.29 The fungus had never before been identified outside a hospital setting. The C. auris strain found in the Andaman salt marsh was less thermotolerant than the strains causing outbreaks in hospitals worldwide—it struggled to grow at , human body temperature. The observation that an environmental fungal strain in its natural habitat grew more slowly at human body temperature than did clinical strains is consistent with the hypothesis that the fungus recently adapted to higher temperatures. That adaptation is what may allow C. auris to thrive in a new host—humans—explains Casadevall.
He argues that the heat island effect (urban areas that are several degrees warmer than the surrounding region due to buildings, traffic, and other factors30) means fungi there could face more selective pressures for thermotolerance. Accordingly, in a study available in preprint,31 he and his team, led by postdoctoral fellow Daniel Smith, sampled the fungal population of four sidewalks in Baltimore, Maryland. They found fungi from hotter sidewalks were lighter-colored and absorbed less heat than those isolated from cooler, shadier sidewalks. These findings could inform future studies on “how urban environments may drive stress/thermotolerance in fungi, which could alter fungal interactions with humans and impact human health,” wrote the researchers.31
Climate-Related Disasters
The more than 11,000 extreme weather disasters worldwide between 1970 and 202132 offer another route by which people were exposed to pathogenic fungi.33 For instance, an outbreak of necrotizing mucormycosis occurred among those injured by a 2011 tornado in Joplin, Missouri.34 In a 2012 case–control study of 48 people injured during the tornado, those with penetrating wounds and a greater number of wounds experienced increased risk of fungal infection.34 In California hospitals between 2014 and 2018, researchers found that admissions for coccidioidomycosis increased by 20% in the month following exposure to wildfire smoke, which can disperse fungal spores.35

In addition to altering distribution and characteristics of pathogenic fungi, climate change also leads to increased storm intensity. After the 2011 F5 tornado in Joplin, Missouri, people with more wounds and puncture wounds had increased risk of rare cutaneous mucormycosis.34 Image: © iStock.com/Eyecrave Productions.
Extreme wet-weather events create conditions in which fungal pathogens thrive17 and people get hurt. Wound contamination and exposure to mold in housing are two potential sources of post-disaster fungal infection, according to Hoenigl. Extensive flooding caused by Hurricane Maria in Puerto Rico36 and Hurricane Harvey in Texas,37 both in 2017, was linked to increases in invasive mold infections post-hurricane.36,37
People of any race, gender, or socioeconomic status can be exposed to fungal pathogens as a result of a disaster. However, when Hoenigl and colleagues analyzed existing racial and ethnic disparities in risk for invasive fungal infections, they found that social determinants of health—nonmedical factors that shape an individual’s everyday living circumstances, such as working conditions, housing, and access to high-quality, affordable medical care—largely accounted for their increased risk.38 “Genetic risk may play a small role for some fungal infections, but social determinants of health are the main driver of racial and ethnic disparity,” says Hoenigl.
“We know that climate change impacts health by exacerbating the role of social disadvantage,” says Remais. This suggests that the global poor are likely to bear the brunt of a future increase in fungal infections.38 Measures that could protect the socially vulnerable include the following:
Ensuring access to N95 masks in certain work environments with potentially high exposure to fungal spores, such as agriculture, landscaping, or construction.
Addressing leaks, mold, and poor ventilation in housing.
Increasing access to fungal diagnostics and antifungal treatments in hospitals and clinics, especially in low- and middle-income countries.38
A better understanding of the epidemiology of different fungal infections—and the role of climate change—can further help guide measures to protect the most vulnerable individuals, according to Remais. In their new project on the environmental and social epidemiology of fungal diseases (described above), he and colleagues will apply modeling approaches that join data from millions of electronic medical records together with climate information to project the US distribution of fungal diseases 30–50 years into the future.39 They also will look at neighborhood differences, such as lack of investment in protective infrastructure, that could exacerbate the risk of fungal infections among certain groups.
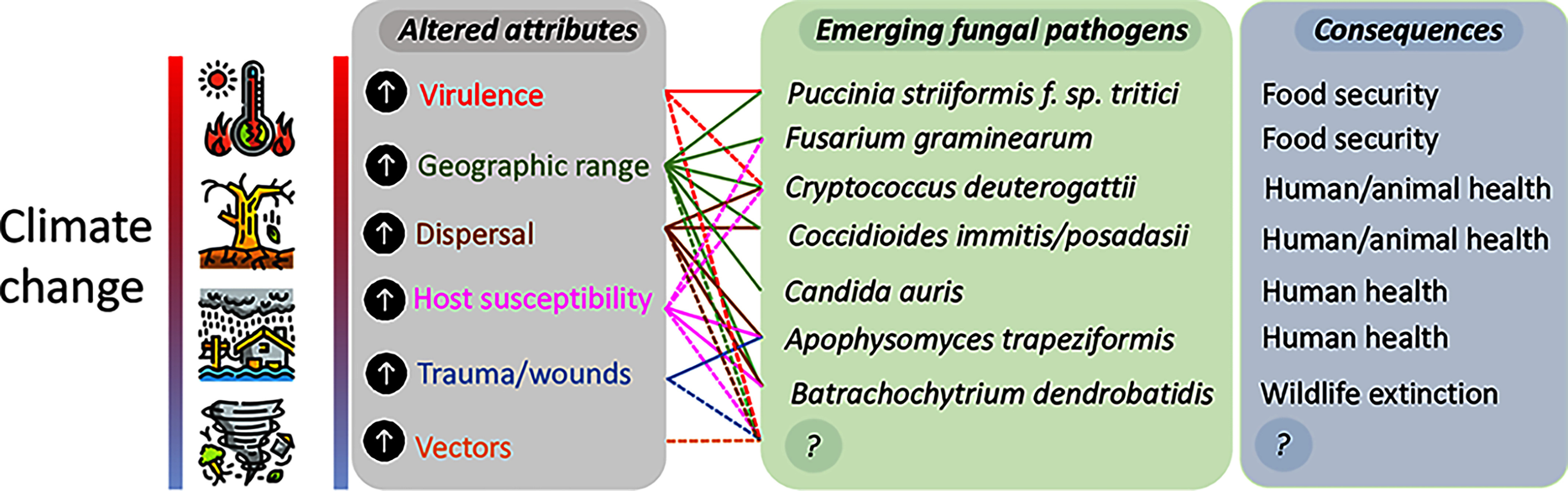
“Attributes of the fungus, environment, and host, altered by climate change, can drive emergence of novel, uncommon, or adapted species, with consequences for health, biodiversity, and food security,” investigators wrote in a 2021 article.17 In this graphic, solid lines show links supported by published evidence; dashed lines show probable but unproven links. “?” represents as-yet unknown fungal species and consequences. Image: © Nnadi and Carter (2021).17 Used under Creative Commons license CC-BY 4.0 DEED [https://creativecommons.org/licenses/by/4.0/].
Environmental Influences on Antifungal Resistance
In addition to raising the risk of infection, climate change may play an indirect role in driving antifungal resistance.17 As one example, researchers have linked more aggressive, thermotolerant strains of stripe rust (a major disease of wheat crops) to a warming climate.17 More virulent strains of plant pathogens and broader geographic range could lead to greater usage of broad-spectrum antifungals in agriculture, according to Hoenigl.
Fungal crop pathogens overwintering more successfully in milder climates also could lead to increased use of antifungals on crops, says Robin May, a professor of infectious diseases at the University of Birmingham in England and chief scientific adviser to the UK Food Standards Agency. “We know that agricultural antifungal use can select for resistance in human fungal pathogens,” says May. Mounting evidence suggests infection from such antifungal-resistant strains may be acquired directly from the environment.40
The best example may be drug-resistant Aspergillus fumigatus infections, according to May. A. fumigatus is a mold that can cause invasive aspergillosis and other health conditions, particularly among people who are immunocompromised.40 Resistance to triazole antifungal agents—used in cereal crops such as wheat and barley—has emerged in the last three decades as a global problem in Aspergillus infections41; some researchers hypothesize resistance may have spread via azole-treated tulip bulbs from the Netherlands.42 Inhaling spores of resistant strains—which have been isolated from decaying plant bulb waste, garden waste, and wood chips, for example43—can lead to infection.44
Researchers are trying to better understand how fungi develop resistance to antifungal agents. It is a big task. In contrast with bacteria, where small, circular pieces of DNA called plasmids are the main vehicle for antimicrobial-resistant gene transfer,45 fungi appear to employ a host of mechanisms, says Joanna Rhodes, a microbiologist at Imperial College London. “With fungi, resistance tends to be inherited, and fungi can reproduce in many ways,” she says.
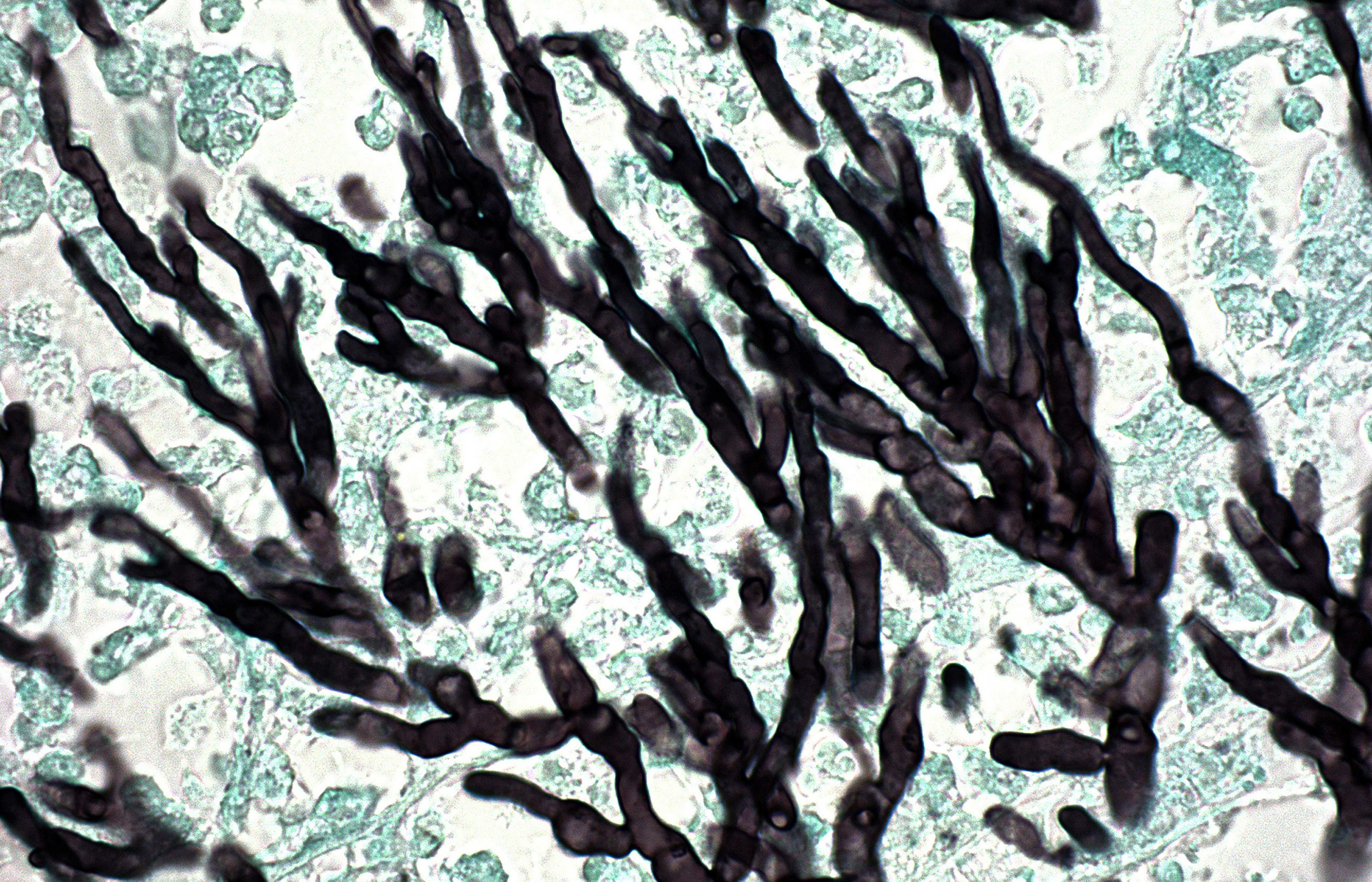
Strains of Aspergillus fumigatus are developing resistance to triazole antifungal agents.41 This microscopic section showing the microbe’s branching hyphae—a form of vegetative growth—is from an immunosuppressed individual. Image: © Ralph C. Eagle, Jr./Science Source.
Of special concern, says Rhodes, are fungal infections that develop resistance very quickly—in some cases over a few weeks—through a process called hypermutation. In bacteria, hypermutation may result from exposure to mutagens, including ultraviolet radiation, she explains. Is this also the case with fungi? “We don’t know. We don’t yet know what’s causing it or what the genetic basis for it is,” says Rhodes, who, with colleagues, in 2017 published the first evidence of hypermutation in the pathogenic fungus Cryptococcus neoformans.46 Some researchers have speculated that heat stress from rising global temperatures may favor fungal hypermutations that allow increased thermotolerance.47
“Thus far, we have not seen the same level of antimicrobial resistance in human fungal pathogens that we see in some bacteria,” says May. But he points to the emergence of C. auris, which is resistant to many antifungals, as evidence for how problematic such strains can be, even if rare.17 “Since we have far fewer drugs against fungi than we do against bacteria, the antifungal arsenal is very limited, and resistance to only one or two drugs can render a fungus essentially untreatable,” he says.
Looking Ahead
No new classes of antifungals have reached the market in the last 20 years,48,49 but Perfect, Hoenigl, and others are optimistic about a number of new classes of antifungals in late-stage clinical development. “The antifungal pipeline is pretty robust,” says Perfect.
Drummond sees potential for immune-based therapies, too.50 “Different groups of patients have different susceptibilities,” she says. In patients with AIDS, for instance, T cells are depleted, she explains. “What are the T cells producing that drives protection against fungal infection [in healthy individuals]? Could replacing that molecule boost immunity? There’s a lot on the immunology side to catch up on.”
Mapping fungal diversity could help identify potential threats in the environment by giving clues about which fungal species may be most likely to adapt to human body temperatures under global warming,51 according to Casadevall. “We know very little about fungi in the natural world beyond the fungi that interfere with us. Most fungal species are not characterized,” he says.
Remais stresses the need for transdisciplinary science to tackle the changing epidemiology of fungal infections. Drawing on expertise from epidemiology, ecology, and environmental microbiology, his lab team recently reported indications that small burrowing mammals, including mice and rats, may shape the distribution of Coccidioides in soil.52 That study adds to evidence that the pathogen has a natural reservoir among wildlife and provides a piece of a much bigger puzzle that could one day lead to better protection against fungal diseases acquired from soils associated with animal colonies. “If we approach the problem purely through an epidemiology lens,” Remais says, “we will miss opportunities to predict and prevent these infections from spreading.”
Biography
Lindsey Konkel Neabore is a New Jersey–based journalist who reports on science, health, and the environment.
References
- 1.CDC (Centers for Disease Control and Prevention). 2021. Fungal Diseases: Maps. [Website.] https://www.cdc.gov/fungal/diseases/coccidioidomycosis/maps.html [accessed 15 March 2024].
- 2.Benedict K, McCotter OZ, Brady S, Komatsu K, Sondermeyer Cooksey GL, Nguyen A, et al. . 2019. Surveillance for coccidioidomycosis—United States, 2011–2017. MMWR Surveill Summ 68(7):1–15, PMID: 31538631, 10.15585/mmwr.ss6807a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd-Kundeti G, Sondermeyer Cooksey GL, Jain S, Vugia DJ. 2020. Valley fever (coccidioidomycosis) awareness—California, 2016–2017. MMWR Morb Mortal Wkly Rep 69(42):1512–1516, PMID: 33090980, 10.15585/mmwr.mm6942a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (World Health Organization). 2022. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. https://iris.who.int/bitstream/handle/10665/363682/9789240060241-eng.pdf [accessed 15 March 2024].
- 5.Denham ST, Wambaugh MA, Brown JCS. 2019. How environmental fungi cause a range of clinical outcomes in susceptible hosts. J Mol Biol 431(16):2982–3009, PMID: 31078554, 10.1016/j.jmb.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Society for Microbiology. 2019. One Health: Fungal Pathogens of Humans, Animals, and Plants. Colloquium Report. Washington, DC: American Society for Microbiology. [PubMed] [Google Scholar]
- 7.Köhler JR, Casadevall A, Perfect J. 2015. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med 5(1):a019273, PMID: 25367975, 10.1101/cshperspect.a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher MC, Denning DW. 2023. The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol 21(4):211–212, PMID: 36747091, 10.1038/s41579-023-00861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DJ, Gold JAW, Benedict K, Wu K, Lyman M, Jordan A, et al. . 2023. Public health research priorities for fungal diseases: a multidisciplinary approach to save lives. J Fungi (Basel) 9(8):820, PMID: 37623591, 10.3390/jof9080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall A. 2022. Immunity to invasive fungal diseases. Annu Rev Immunol 40:121–141, PMID: 35007128, 10.1146/annurev-immunol-101220-034306. [DOI] [PubMed] [Google Scholar]
- 11.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. . 2017. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5(1):153, PMID: 29178920, 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond RA, Desai JV, Ricotta EE, Swamydas M, Deming C, Conlan S, et al. . 2022. Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host Microbe 30(7):1020–1033.e6, PMID: 35568028, 10.1016/j.chom.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, et al. . 2022. COVID-19-associated fungal infections. Nat Microbiol 7(8):1127–1140, PMID: 35918423, 10.1038/s41564-022-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feys S, Lagrou K, Lauwers HM, Haenen K, Jacobs C, Brusselmans M, et al. . 2024. High burden of COVID-19 associated pulmonary aspergillosis in severely immunocompromised patients requiring mechanical ventilation. Clin Infect Dis 78(2):361–370, PMID: 37691392, 10.1093/cid/ciad546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahalaxmi I, Jayaramayya K, Venkatesan D, Subramaniam MD, Renu K, Vijayakumar P, et al. . 2021. Mucormycosis: an opportunistic pathogen during COVID-19. Environ Res 201:111643, PMID: 34237335, 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raut A, Huy NT. 2021. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? Lancet Respir Med 9(8):e77, PMID: 34090607, 10.1016/S2213-2600(21)00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nnadi NE, Carter DA. 2021. Climate change and the emergence of fungal pathogens. PLoS Pathog 17(4):e1009503, PMID: 33914854, 10.1371/journal.ppat.1009503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorris ME, Treseder KK, Zender CS, Randerson JT. 2019. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. Geohealth 3(10):308–327, PMID: 32159021, 10.1029/2019GH000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head JR, Sondermeyer-Cooksey G, Heaney AK, Yu AT, Jones I, Bhattachan A, et al. . 2022. Effects of precipitation, heat, and drought on incidence and expansion of coccidioidomycosis in western USA: a longitudinal surveillance study. Lancet Planet Health 6(10):e793–e803, PMID: 36208642, 10.1016/S2542-5196(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.California Department of Public Health. 2024. Health Advisory. Substantial Rise in Coccidioidomycosis in California: recommendations for California Healthcare Providers. [Website.] 18 January 2024. https://www.cdph.ca.gov/Programs/OPA/Pages/CAHAN/Substantial-Rise-in-Coccidioidomycosis-in-California-Recommendations-for-California-Healthcare-Providers.aspx [accessed 16 February 2024].
- 21.National Environmental Satellite Data and Information Service. 2023. Atmospheric rivers hit West Coast. 25 January 2023. https://www.nesdis.noaa.gov/news/atmospheric-rivers-hit-west-coast [accessed 14 March 2024].
- 22.CDC. 2022. Fungal Diseases: Risk & Prevention. [Webpage]. Last reviewed 11 July 2022. https://www.cdc.gov/fungal/diseases/coccidioidomycosis/risk-prevention.html [accessed 25 March 2024].
- 23.Osuolale O. 2023. Stirring up trouble? Forest disturbance and the spread of a fungal disease. Environ Health Perspect 131(10):104003, PMID: 37878795, 10.1289/EHP13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acheson ES, Otterstatter M, Galanis E. 2023. Forest disturbance and disease: exploring the effects of tree harvesting area on Cryptococcus gattii sensu lato infection risk, Vancouver Island, Canada, 1998–2014. Environ Health Perspect 131(7):077009, PMID: 37466219, 10.1289/EHP12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. . 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA 101(49):17258–17263, PMID: 15572442, 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auras sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53(1):41–44, PMID: 19161556, 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 27.CDC. 2019. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC. [Google Scholar]
- 28.Casadevall A, Kontoyiannis DP, Robert V. 2021. Environmental Candida auris and the global warming emergence hypothesis. mBio 12(2):e00360-21, PMID: 33727350, 10.1128/mBio.00360-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora P, Singh P, Wang Y, Yadav A, Pawar K, Singh A, et al. . 2021. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio 12(2):e03181-20, PMID: 33727354, 10.1128/mBio.03181-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US EPA (US Environmental Protection Agency). 2024. Heat island effect. https://www.epa.gov/heatislands [accessed 25 March 2024].
- 31.Smith DFQ, Bencomo A, Faiez TS, Casadevall A. 2023. Thermal and pigment characterization of environmental fungi in the urban heat island of Baltimore City. bioRxiv. Preprint posted online 10 November 2023, PMID: 37986923, 10.1101/2023.11.10.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Meteorological Organization. 2021. WMO Atlas of Mortality and Economic Losses from Weather, Climate and Water Extremes (1970–2019). WMO-No. 1267. https://library.wmo.int/records/item/57564-wmo-atlas-of-mortality-and-economic-losses-from-weather-climate-and-water-extremes-1970-2019 [accessed 15 March 2024].
- 33.Benedict K, Park BJ. 2014. Invasive fungal infections after natural disasters. Emerg Infect Dis 20(3):349–355, PMID: 24565446, 10.3201/eid2003.131230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, et al. . 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367(23):2214–2225, PMID: 23215557, 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 35.Mulliken JS, Hampshire KN, Rappold AG, Fung M, Babik JM, Doernberg SB. 2023. Risk of systemic fungal infections after exposure to wildfires: a population-based, retrospective study in California. Lancet Planet Health 7(5):e381–e386, PMID: 37164514, 10.1016/S2542-5196(23)00046-3. [DOI] [PubMed] [Google Scholar]
- 36.Vélez-Torres LN, Bolaños-Rosero B, Godoy-Vitorino F, Rivera-Mariani FE, Maestre JP, Kinney K, et al. . 2022. Hurricane María drives increased indoor proliferation of filamentous fungi in San Juan, Puerto Rico: a two-year culture-based approach. PeerJ 10:e12730, PMID: 35261816, 10.7717/peerj.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toda M, Williams S, Jackson BR, Wurster S, Serpa JA, Nigo M, et al. . 2023. Invasive mold infections following Hurricane Harvey—Houston, Texas. Open Forum Infect Dis 10(3):ofad093, PMID: 36910694, 10.1093/ofid/ofad093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenks JD, Prattes J, Wurster S, Sprute R, Seidel D, Oliverio M, et al. . 2023. Social determinants of health as drivers of fungal disease. EClinicalMedicine 66:102325, PMID: 38053535, 10.1016/j.eclinm.2023.102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.University of California, Berkeley. 2023. Berkeley Public Health launches major study on the effects of climate change and social inequality on deadly fungal infections. [Press release.] 17 August 2023. https://publichealth.berkeley.edu/news-media/research-highlights/bph-launches-major-study-on-deadly-fungal-infections/ [accessed 15 March 2024].
- 40.Rhodes J, Abdolrasouli A, Dunne K, Sewell TR, Zhang Y, Ballard E, et al. . 2022. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat Microbiol 7(5):663–674, PMID: 35469019, 10.1038/s41564-022-01091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verweij PE, Snelders E, Kema GHJ, Mellado E, Melchers WJG. 2009. Azole resistance in Aspergillus fumigatus: a side effect of environmental fungicide use? Lancet Infect Dis 9(12):789–795, PMID: 19926038, 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 42.Dunne K, Hagen F, Pomeroy N, Meis JF, Rogers TR. 2017. Intercountry transfer of triazole-resistant Aspergillus fumigatus on plant bulbs. Clin Infect Dis 65(1):147–149, PMID: 28369271, 10.1093/cid/cix257. [DOI] [PubMed] [Google Scholar]
- 43.Schoustra SE, Debets AJM, Rijs AJMM, Zhang J, Snelders E, Leendertse PC, et al. . 2019. Environmental hotspots for azole resistance selection of Aspegillus fumigatus, the Netherlands. Emerg Infect Dis 25(7):1347–1353, PMID: 31211684, 10.3201/eid2507.181625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verweij PE, van de Sande-Bruisma N, Kema GHJ, Melchers WJG. 2012. Azole resistance in Aspergillus fumigatus in the Netherlands—increase due to environmental fungicides? [in Dutch]. Ned Tijdschr Geneeskd 156(25):A4458, PMID: 22748367. [PubMed] [Google Scholar]
- 45.Haavisto V. 2023. Plasmids and the spread of antibiotic resistance [Website]. https://asm.org/articles/2023/january/plasmids-and-the-spread-of-antibiotic-resistance-g [accessed 15 March 2024].
- 46.Rhodes J, Beale MA, Vanhove M, Jarvis JN, Kannambath S, Simpson JA, et al. . 2017. A population genomics approach to assessing the genetic basis of within-host microevolution underlying recurrent cryptococcal meningitis infection. G3 (Bethesda) 7(4):1165–1176, PMID: 28188180, 10.1534/g3.116.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gusa A, Yadav V, Roth C, Williams JD, Shouse EM, Magwene P, et al. . 2023. Genome-wide analysis of heat stress-stimulated transposon mobility in the human fungal pathogen Cryptococcus deneoformans. Proc Natl Acad Sci USA 120(4):e2209831120, PMID: 36669112, 10.1073/pnas.2209831120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. . 2021. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81(15):1703–1729, PMID: 34626339, 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perfect JR. 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16(9):603–616, PMID: 28496146, 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lionakis MS, Drummond RA, Hohl TM. 2023. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol 23(7):433–452, PMID: 36600071, 10.1038/s41577-022-00826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casadevall A. 2023. Global warming could drive the emergence of new fungal pathogens. Nat Microbiol 8(12):2217–2219, PMID: 38030896, 10.1038/s41564-023-01512-w. [DOI] [PubMed] [Google Scholar]
- 52.Head J, Campo S, Weaver A, Jones I, Montoya L, Lee E, et al. . 2023. Small mammal burrows shape the distribution of Coccidioides in soils: evidence from a long-term ecological experiment in the Carrizo Plain National Monument, California, USA. [Abstract]. In: Proceedings of the 67th Annual Coccidioidomycosis Study Group Meeting. 31 March–1 April 2023. Pittsburgh, PA: Coccidioidomycosis Study Group, 58 Oral. https://vfce.arizona.edu/sites/default/files/proceedings_2023.pdf [accessed 25 March 2024]. [Google Scholar]
- 53.Hirschmann JV. 2007. The early history of coccidioidomycosis: 1892–1945. Clin Infect Dis 44(9):1202–1207, PMID: 17407039, 10.1086/513202. [DOI] [PubMed] [Google Scholar]


