Abstract
Purpose of Review
Behaviors and practices associated with substance use contribute to lack of HIV virologic suppression and onward transmission. In the USA, many recent HIV outbreaks have been connected with substance use. Evidence-based strategies for integrating care of those at risk for and living with HIV and who use substances continue to evolve. This review, based on scientific and medical literature through March 2023, provides an overview and evaluation of initiatives for integrated care aimed to serve patients at risk for and with HIV and a substance use disorder.
Recent Findings
Integrated care services can improve health outcomes for patients at risk for and with HIV and a substance use disorder; for instance, treatment for an opioid use disorder can help improve HIV viral suppression. Brick-and-mortar facilities can provide successful care integration with appropriate clinic leadership to support multidisciplinary care teams, up-to-date provider training, and sufficient pharmacy stock for substance use treatment. Delivering healthcare services to communities (e.g., mobile healthcare clinics and pharmacies, telehealth) may prove to be an effective way to provide integrated services for those with or at risk of HIV and substance use disorders. Incorporating technology (e.g., mobile phone applications) may facilitate integrated care. Other venues, including harm reduction programs and carceral settings, should be targets for integrated services.
Summary
Venues providing healthcare should invest in integrated care and support legislation that increases access to services related to HIV and substance use.
Keywords: HIV, Substance use disorder, Integrated care models, Harm reduction, Mobile health clinics, Medication treatment of opioid use disorders
Introduction
Human Immunodeficiency Virus
Successful antiretroviral therapy (ART) can suppress HIV viral load, not only improving the health of individuals living with HIV but also preventing transmission to those who are uninfected (i.e., Undetectable = Untransmittable, or U=U) [1••, 2]. Clinicians can now offer long-acting injectable antiretroviral therapy to facilitate HIV medication adherence and reduce treatment burden. Further, HIV pre-exposure prophylaxis (PrEP) is a highly effective biomedical prevention intervention and is now available in two forms—as a daily oral pill or a long-acting injectable medication received as a shot every 2 months. However, it can be difficult to successfully (a) initiate patients on ART or PrEP, (b) support patient adherence to medications, and (c) ensure patients are able to be retained in care. Despite having highly effective tools to combat the HIV epidemic in the USA, in 2020, there were more than 30,000 new HIV diagnoses among those aged 13 and older reported in the USA [3]. Further, it is estimated that 13% of people living with HIV (PWH) in the USA are unaware of their status [3]. Recent (2019) estimates suggest that there are approximately 1.2 million prevalent HIV cases among those aged 13 and over in the USA [3].
Substance Use Disorder
There are many substances that people use, including natural and synthetic opioids, cocaine, methamphetamine, prescription medications, cannabis, and alcohol [4]. While it is possible to use substances without developing problematic use, many are highly addictive and lead to harmful use or polysubstance use that could be diagnosed as a substance use disorder (SUD). As defined by the fifth edition of the Diagnostics and Statistical Manual of Mental Disorders (DSM-5), SUDs encompass eleven key criteria divided into four main themes: impaired control of substance use (e.g., cravings), social impairment (e.g., interference with work obligations), risky use (e.g., using substances in unsafe ways), and pharmacological factors (i.e., tolerance) [5]. SUDs are chronic conditions that impact the brain and can result in significant risk of relapse [6, 7••]. Based on the 2019 National Survey on Drug Use and Health, 20.4 million people aged 12 or over in the USA were diagnosed with a SUD in the prior year, yet less than 11% of those with a SUD received treatment, even though SUDs can be treated effectively using medication and chronic-care model standards [8]. With many serious adverse health effects related to substance use, including both fatal and non-fatal overdose, the response to SUDs requires increased attention.
There are three effective medications for opioid use disorders (MOUD): methadone, buprenorphine, and extended-release naltrexone (XR-NTX). Methadone, a schedule II drug, is a long-acting, full opioid agonist. Currently, methadone can only be prescribed as treatment of OUD through a Drug Enforcement Agency (DEA) registered Opioid Treatment Clinic, thus limiting its widespread access. Buprenorphine is a partial μ-receptor agonist and is ideally administered to patients who are experiencing mild to moderate withdrawal from active opioid use in order to decrease the risk of precipitated withdrawal. It can be used as maintenance treatment for DSM-5 moderate to severe OUD to prevent relapse and death. Buprenorphine comes in many formulations, including daily sublingual forms, weekly and monthly injections, and a subdermal implant that lasts 6 months. Buprenorphine prescribing is no longer limited to those who have received DATA 2020 X-waiver training, but can now be prescribed by any clinician, including HIV providers who have a controlled substance license [9–11]. Lastly, XR-NTX is a competitive μ-receptor antagonist which is administered intramuscularly every 28 days after a required 7-day period of opioid abstinence to prevent precipitated withdrawal.
Managing patients with stimulant use disorders can be difficult, as there are currently no US Food and Drug Administration (FDA)-approved medications to treat this disorder. Thus, contingency management, which includes involvement of multidisciplinary teams (e.g., behavioral therapists and clinical psychologists), is the most successful form of treatment for stimulant use disorders. Currently, there are three FDA-approved treatments for AUD, including disulfiram, acamprosate, and oral and extended-release (XR) naltrexone, with the naltrexone forms being most effective [12, 13••]. Several rigorous research evaluations including large randomized controlled trials have demonstrated the benefits of these medication-based treatments, predominantly NTX forms, alone or in combination with behavioral forms of treatment. These benefits include higher rates of abstinence and less risk of relapse to heavy drinking, with associated improvements in medical and mental health and in quality of life [14].
Intersection of Substance Use and HIV
There is ample evidence that substance use, especially use of opioids and stimulants, contributes to increasing HIV incidence across the USA [15••, 16, 17]. Sharing syringes or other equipment for drug injection (i.e., injection drug use “works”) can increase the risk of HIV acquisition. Moreover, people may engage in transactional sex in exchange or to pay for drug use or engage in risky sexual behaviors (e.g., condomless sexual intercourse) while using drugs or alcohol. PWH, who are at risk of becoming immunocompromised without proper treatment, face additional health challenges with co-morbid SUD (e.g., liver toxicity, immunosuppression, interactions with HIV medication) [18]. Those with untreated SUDs also often have difficulty adhering to medication such as ART (or PrEP), resulting in worse treatment outcomes, higher mortality, and onward transmission [19••].
Moreover, HIV and SUD care cascades can overlap and interact. Achieving HIV virologic suppression can be delayed or difficult to attain among those with comorbid SUD due to lower retention and adherence to HIV prevention or treatment plans. Thus, treatment of concurrent SUD has been found to be an important factor in improving HIV care and prevention outcomes [20••, 21]. All forms of MOUD have been found to reduce HIV risk behaviors, transmission, acquisition of HIV, improve adherence to ART, and promote maintenance of HIV viral suppression [22–27]. Unfortunately, the role of contingency management for stimulant use disorder in HIV care and prevention has not been fully explored, as it is rarely offered in an integrated care model of HIV; this currently remains a major gap in successful HIV prevention for stimulant users [13••, 21, 28–30]. Despite the fact that multiple medications for AUD exist, only XR-NTX for AUD has been demonstrated in a double-blind placebo-controlled trial to show improvement in viral load suppression and reducing alcohol consumption in PWH with AUD who were being released from prisons and jails [31, 32].
Integrated Care Models
Given the concomitant and interconnected epidemics of HIV and substance use, there is a pressing need for integrated care models that can address the unique needs of PWH and PWUD. Medical settings frequently do not provide sufficient resources or provider training to integrate screening, diagnosis, prevention, and treatment for both HIV and SUD. As such, there is a growing body of literature which offer recommendations related to improving outcomes for patients with, or at risk of, HIV as a result of drug use [13••, 19••, 20••, 21, 26, 33, 34]. Included in these recommendations are calls to expand Medicaid in the states that have not done so in order to improve health insurance access, increase funding and access to syringe services programs (SSPs), and to integrate SUD care with treatment and prevention for infectious diseases, including HIV.
Typically, integrated healthcare—including HIV and SUD treatment—has been evaluated using the framework from the Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration (SAMHSA-HRSA), Center for Integrated Health Solutions [35]. This standard consists of 5 levels of care: (1) minimal collaboration; (2) basic collaboration at a distance; (3) basic collaboration onsite; (4) close collaboration in a partly integrated system; and (5) close collaboration in a fully integrated system. Recently released guidelines on how to best integrate OUD and HIV prevention and treatment utilized such a framework and singularly utilize brick-and-mortar clinics to assess success [20••, 21].
There has been an effort to expand the integrated care framework evaluation outside of brick-and-mortar clinics. Resource-limited countries like Zambia and Malawi, which have a much higher burden of HIV than the USA, have developed differentiated service delivery models to reduce burden on clinic staff while simultaneously providing more patient-centered care [36]. This differentiated model includes mobile healthcare service delivery (i.e., vans)—bringing HIV care to patients where they live, using community health workers and peer navigators, operationalizing pharmacy delivery services, and more. This is the proposed model that we recommend in order integrate substance use and SUD prevention, assessment, and treatment alongside HIV prevention, testing, and treatment. Subsequently, this review—which includes the scientific and medical literature through March 2023—expands on existing recommendations and provides an update on current research and initiatives to integrate care for PWH and PWUD (Table 1).
Table 1.
Recommendations for integrating care of HIV and SUD
| Healthcare provider level |
| All clinicians should be trained to screen, diagnose, and treat SUDs and integrate with HIV testing and provision of PrEP/antiretrovirals based on testing results |
| Train CHWs to screen for SUDs and perform rapid HIV testing |
| Healthcare systems level |
| Increase access to telehealth providers, CHWs, peer-support staff, and mobile health clinics for PWUD and PWH |
| Create stigma-free environments in all healthcare programs (brick-and-mortar and mobile) |
| Pharmacies should keep appropriate stock of medications for SUDs, HIV, and PrEP |
| Hospitals and clinics should develop screening procedures for SUD and HIV with integrated treatment and assertive discharge planning to ensure retention on SUD and HIV prevention and treatment |
| Criminal justice settings should offer routine screening and treatment and prevention for SUDs and HIV including MOUD, naloxone, and PrEP with assertive reentry linkage upon release to have uninterrupted medication prescription |
| Policy level |
| Pass state legislation to permit mobile pharmacies that can meet people where they are |
| DEA must remove restriction on mobile pharmacy dispensation of buprenorphine |
| Pass state legislation to eliminate 1 for 1 syringe exchange |
| Introduce and strengthen harm reduction programming and MOUD in prisons and jails |
| Expand Medicaid in all states to improve health insurance access |
| Increase federal and state funding for harm reduction, SSPs, and overdose prevention sites |
| Increase federal and state funding for evidence-based SUD treatment programs and MOUD |
CHW, community health workers; DEA, Drug Enforcement Administration; HIV, human immunodeficiency virus; MOUD, medication for treatment of opioid use disorder; PrEP, pre-exposure prophylaxis; PWH, people with HIV; PWUD, people who use drugs; SSP, syringe services program; SUD, substance use disorder
Ending the HIV Epidemic
In 2019, agencies across the US Department of Health and Human Services (HHS) collaboratively developed and announced an initiative called Ending the HIV Epidemic in the U.S. (EHE). The EHE initiative is guided by four main pillars: diagnose, treat, prevent, respond to the HIV epidemic. The EHE’s primary goal, backed by substantial federal investment, is to reduce the number of incident HIV infections by 75% by 2025 and 90% by 2030 [1••]. As such, the primary indicators for this initiative include quantifying new HIV diagnoses, linking for those with HIV to medical care within a month of their diagnosis, achieving and maintaining HIV viral suppression, and prescribing PrEP for those at risk of HIV infection [37].
While the EHE initiative is an admirable step forward, the effort did not explicitly provide guidance or goals for people who use drugs (PWUD), despite the fact that PWUD continue to experience a high risk of HIV acquisition and contribute to HIV transmission. Ultimately, to reach its goals, the EHE initiative will need to both broaden and revamp its methodology to include the unique needs of PWUD. Focused attention on PWUD is crucial, as PWUD commonly experience substantial gaps to accessing HIV testing, treatment, and prevention from systems-level and social barriers, including housing instability, justice system-related or legal issues, racism, stigma, and lack of transportation as well as concurrent untreated mental health conditions [38–41]. To address this key limitation of EHE effort, the National Institute on Drug Abuse (NIDA) issued a Notice of Special Interest (NOSI) in late 2022 for research to meet these EHE goals that focuses on PWUD who are living with, or are at risk for, HIV [42].
Brick-and-Mortar Clinics
To create and maintain integrated care models in traditional brick-and-mortar settings, patients should be offered routine screening for SUD and AUD, HIV testing, and appropriate prevention (PrEP) or treatment (ART) as needed [13••]. Similarly, PWH who have OUD and AUD should be offered medication treatment integrated with HIV prevention and treatment services [43]. Currently, many uninsured or underinsured PWH have access to direct and many ancillary services provided through the Ryan White HIV/AIDS Program (RWHAP). However, depending on state laws, not all RWHAPs provide access to screening and treatment of SUDs like medication treatment of OUD [34]. These gaps, among others, must be addressed in order to integrate care and better serve PWH and PWUD and meet the EHE goals.
All healthcare providers, and importantly HIV prevention and treatment providers, should be trained to identify, screen, and treat SUDs—even if they are not addiction medicine or psychiatry specialists—as recommended by the IAS-USA guidelines [13••, 33]. In fact, addiction care specialists do not provide the vast majority of SUD treatment in the USA—this is currently accomplished by internal medicine, family medicine, and psychiatry physicians in the face of addiction medicine specialist workforce shortages [7••]. Moreover, infectious disease physicians often see many patients with SUDs, as patients often present with infections related to injection drug use: bacteremia, endocarditis, skin and soft tissue infections, wounds, or skin abscesses [7••]. Thus, it has been previously proposed that infectious disease physicians integrate screening for SUDs, subsequent treatment, and linkage to care after discharge into their current practice [7••, 44••]. Simple referral to SUD treatment is not sufficient—providers should proactively provide care while the patient is present in the clinic [21], and resources and support for linkage beyond their clinical setting is critical. Providing integrated, holistic, and continued care in this format can improve patient outcomes related to opioid withdrawal, pain management, and ultimately improves retention in HIV prevention and care and limits unplanned or incomplete discharges while also simultaneously being cost effective [7••, 44••, 45].
Patients who are diagnosed with a SUD should immediately be assessed for treatment readiness and be provided with treatment or medications that can aid in alleviating withdrawal symptoms [33]. Recent progress has been made to alleviate systems-level barriers for patients seeking prescriptions to buprenorphine and methadone [13••, 20••]. In January 2023, after substantial advocacy and lobbying, the Biden administration eliminated the requirement for providers to receive a prohibitory, specialty waiver from the DEA to prescribe buprenorphine (i.e., X-waiver, removed through the Section 1262 of the Consolidated Appropriations Act, 2023) [19••, 20••, 46]. Separate provisions under the Medication Access and Training Expansion Act (i.e., the MATE Act), which went into effect in June 27, 2023, provide stipulations to reduce the training burden for clinicians—which helps to facilitate buprenorphine prescription [47].
As a result of these previous restrictions, few physicians are properly informed on how to provide safe, effective, and evidence-based treatment for OUD. Moreover, due to substantial stigma around OUD, it can be difficult for patients to get MOUD prescriptions filled due to pharmacy stocking issues. With changes in federal regulations to reduce barriers for buprenorphine prescribing, it is now more critical than ever for brick and mortar pharmacies to keep an appropriate stock of FDA-approved medications for SUDs [33].
A key limitation to brick-and-mortar healthcare settings is that there are many barriers preventing patients from visiting traditional health care clinics, including but not limited to transportation, lack of childcare, medical mistrust, and racism [13••]. These challenges, among others, are commonly experienced by PWUD and PWH. Because these health conditions are highly stigmatized, particularly HIV among justice-involved persons, these patient populations face additional impediments to accessing care in traditional settings. In addition, in settings where care is not integrated, these barriers may be even more pronounced because patients must go to multiple providers for different care needs where providers may not have the appropriate clinical skills or cultural competency to manage these co-morbid conditions. Moreover, being uninsured or underinsured is also a known barrier in limiting access to quality care. For example, low-income patients living in states that elected to not expand Medicaid often struggle to access PrEP [48]. In response to these existing barriers, brick-and-mortar settings should work to reduce stigma around substance use and HIV, develop screening procedures for SUD and HIV with warm referrals when detected, utilize support staff (e.g., task-shifting), train healthcare providers to enhance clinical skills, develop workflows to provide integrated care for PWUD and PWH, implement multidisciplinary teams that can manage patients with comorbid diagnoses (i.e., HIV, SUD) including the use of peers or those with lived experience, and advocate for state and federal policies like the Infectious Disease Society of America (IDSA) and American Society of Addiction Medicine (ASAM) and other groups that promote integrated care models [7••, 13••].
Mobile Health Clinic Interventions
Mobile health clinics (MHCs) are designed to provide a dynamic model of healthcare delivery that can facilitate improved access to healthcare among marginalized or vulnerable populations. MHCs can help overcome healthcare acquisition barriers that these populations may face related to transportation, housing instability, health insurance, documentation, stigma, and comfort around the healthcare setting [49–51]. Importantly, MHCs can move around and across communities, allowing them to offer services across places and times that typically do not offer healthcare. This is a powerful tool to overcome important structural barriers to health care provision for under-resourced populations, which are often impacted by both SUD and HIV. The MHC has the ability to meet people “where they are,” both physically and psychologically. As such, there have been increasing efforts to provide mobile healthcare services to both PWUD and PWH with and without justice involvement to facilitate engagement in care [4, 52–54].
Many populations may benefit from MHC integrated care models for HIV and SUDs, including rural communities, transgender peoples, women, unhoused, and/or uninsured populations [41]. Furthermore, justice-involved people also face numerous barriers to health care acquisition and retention, namely, from disruptions to the care continuum resulting from periods of incarceration [19••]. By providing additional accessibility and flexibility, the MHC model can help those reentering their communities and other populations struggling with social determinants of health to achieve improved health outcomes.
In order to meet some of the unique health needs of people who inject drugs, the National Institutes of Health is currently supporting an HIV Prevention Trials Network (HPTN) study called HPTN 094 or INTEGRA (A Vanguard Study of Health Service Delivery in a Mobile Health Delivery Unit to Link Persons who Inject Drugs to Integrated Care and Prevention for Addiction, HIV, HCV, and Primary Care). This is a randomized, open label, two-arm trial aimed at examining the efficacy of MHCs which provide integrated, “one stop” health services for PWID with OUD and at risk or living with HIV compared to peer navigation to brick and mortar clinics providing these services [55]. The MHC is staffed with a clinical team that is cross trained in both HIV prevention and care as well as OUD management, so study participants can receive integrated services for MOUD and HIV PrEP and/or ART from the same clinical staff. The MHC parks in community locations convenient to participants to help overcome transportation and stigma barriers to care. The primary study outcomes include engagement in MOUD and HIV PrEP for those at risk for HIV or HIV care for those living with HIV.
Additionally, the ACTION Study (Addressing risk through Community Treatment for Infectious disease and Opioid use disorder Now), a 5-year NIDA funded hybrid type 1 effectiveness-implementation randomized control trial, aims to compare the effectiveness of a patient navigators (PN) intervention to a MHC service delivery for those who have used opioids and/or stimulants and were recently from a custody setting ( i.e., prison, jail) or those who have been justice-involved (e.g., on probation or parole) in the last 6 months in two sites in Connecticut and two sites in Texas [4••]. The primary outcome of interest is the length of time to initiation of PrEP or ART, depending on current HIV status. The MHC arm of the study provides integrated HIV and substance use disorder testing, prevention, diagnosis, treatment, and care on a MHC. Additionally, a community health worker (CHW) on the MHC assists the participants with accessing housing, transportation, insurance, and cell phones if needed.
While MHCs present a considerable opportunity to meet the needs of PWUD, these MHCs alone are not a solution without adequate support and knowledge about the community one serves. For instance, MHCs that circulate at specific scheduled locations may not reach all the vulnerable populations, especially PWUD. MHCs need to be truly mobile to meet PWUD where they are. In order to serve PWUD effectively, MHC teams need to know the geographic area well and have an ongoing understanding of where PWUD or those at risk of overdosing are located and provide integrated services in stigma-free environments where PWUD feel comfortable receiving care. This requires timely and dynamic data from multiple sources, including public health agencies such as health departments and fire and emergency management systems that track HIV infection and emergency responses to overdoses in the community, community-based organizations that serve these populations, and other constituents with lived experience.
A NIDA-funded Director’s Pioneer Award (DP1), also known as an Avant Garde Award, was awarded to author S. Springer to facilitate an integrated mobile healthcare clinic service model that is positioned to overcome the barriers described above. The study, InMOTION (Integrated Mobile Opioid Treatment and Infectious disease cOordinated care in your Neighborhood), is designed to establish and test a novel new mobile hub-and-spokes model that integrates mobile retail pharmacy services for PWUD at risk and/or living with HIV (Fig. 1) [54]. Starting first in Connecticut, patients will be identified by community healthcare workers (CHWs), who connect patients to (a) an on-demand medical provider via telehealth and (b) ancillary services they may need (e.g., housing, transportation, insurance, cell phones). The CHWs, who may share a lived experience of substance use, HIV, incarceration, and/or being unhoused, will serve as a key asset to programmatic success, as they are the primary contact with community members, and can bypass stigma. The CHWs will be trained to provide support to OUD/SUD diagnoses and perform rapid point of care HIV testing [56]. In this novel model, CHWs will help facilitate real-time linkage between patients and clinicians using telehealth [57••]. The significance of a telehealth component in this study should not be overlooked, as on-demand providers can both diagnose patients and subsequently prescribe the medications delineated in the EHE (i.e., PrEP, ART) as well as MOUD or contingency management for stimulants. The electronic prescription generated from the clinician will then be sent to a newly developed mobile retail pharmacy, the next key component to this novel model. This DP1 study will evaluate the efficacy of the provision of a mobile pharmacy to patients in facilitating engagement in these important medical interventions. Importantly, the mobile pharmacy will have a pharmacist to (a) fill prescriptions like PrEP/ART, HCV treatment, or MOUD, (b) dispense medications directly to the patient where they live, and (c) work with the CHW to return to the patient to assist with refills and evaluations for any potential side effects.
Fig. 1.
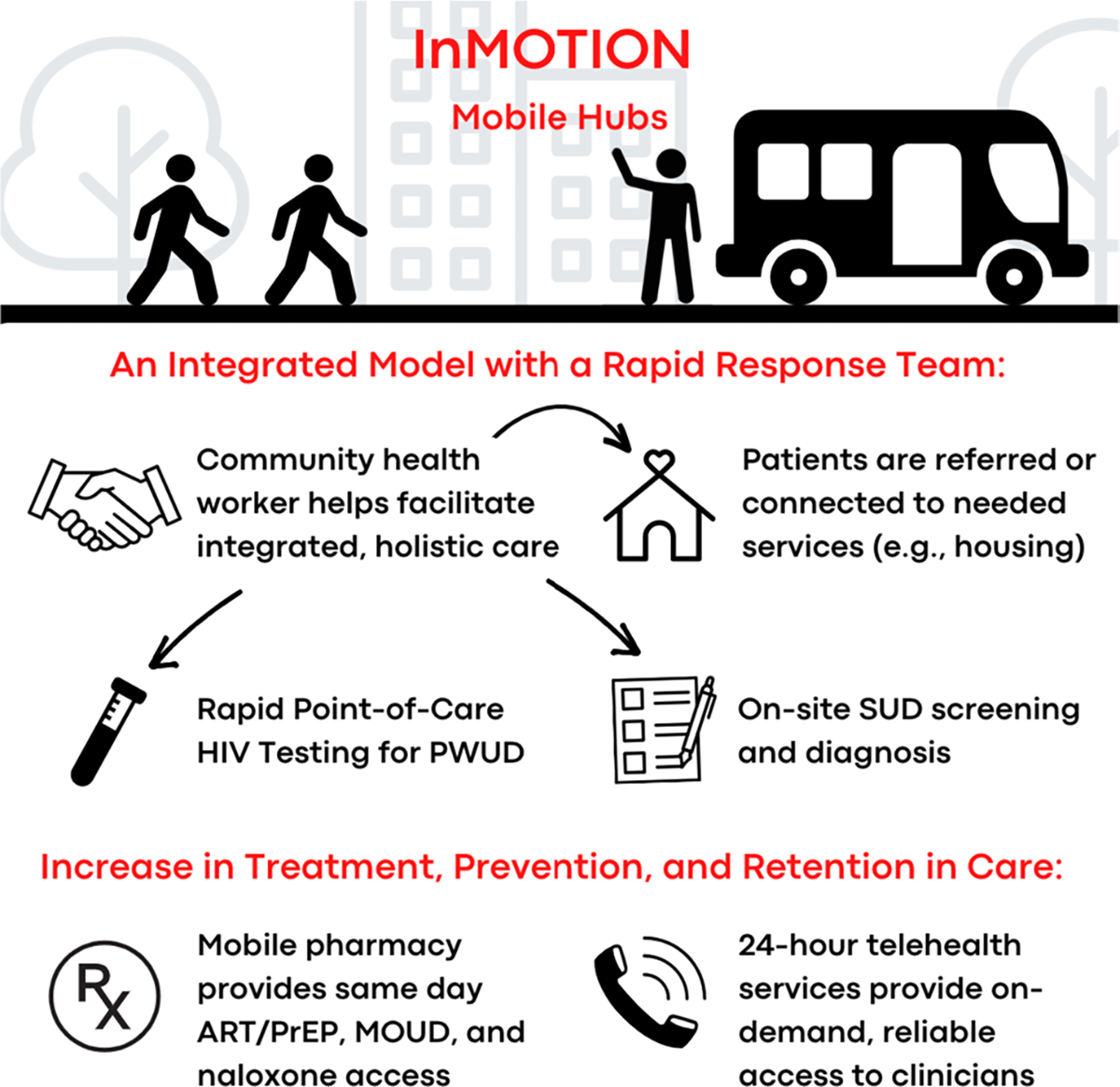
Project design for InMOTION (Integrated Mobile Opioid Treatment and Infectious disease cOordinated care in your Neighborhood)
Patient access to a pharmacy is pivotal. However, mobile retail pharmacies that are able to meet people where they live are currently illegal and/or heavily regulated across most of the USA except California—which recently passed a State Senate Bill 872, Mobile Pharmacy Vans, but only for local municipalities to use [58]. For integrated mobile care models to be successful, each state must pass legislation that allows retail pharmacies to be mobile and carry and dispense medications like PrEP/ART and buprenorphine for PWUD at risk or living with HIV. Accordingly, legislation in Connecticut has now passed, initiated by author S. Springer and the Drug Control Division of the Department of Consumer Protection, entitled An Act Concerning Pharmacies and Pharmacists [59]. These regulatory actions are pivotal, as mobile pharmacies should be able to provide ART and PrEP in line with the EHE that recommends “Rapid” or same day provision of treatment at the time of HIV diagnosis [1••]. Mobile pharmacies should also be able to dispense treatment for OUD without the barriers that currently may make this service impractical for providers (e.g., stringent security regulations due to fear of diversion). Although the DEA has now allowed Opioid Treatment Programs to dispense from mobile units since the COVID-19 pandemic, only those attached to a brick-and-mortar address are allowed to dispense methadone and buprenorphine in the state where the treatment program is located [60]. This will not be sufficient, as many communities do not have an Opioid Treatment Program and/or have not integrated SUD care with HIV treatment and prevention. The DEA must allow buprenorphine to be dispensed from mobile retail pharmacies as they become available, regardless of linkage with an Opioid Treatment Program. Knowing that ART, PrEP, and MOUD retention are key for patient success and ending the HIV and OUD epidemics, MHCs and mobile pharmacies will play an important role in integrating care for the hardest to reach PWUD and PWH—who are often at highest risk [54].
Other Settings
Mobile Devices
Technology in the form mobile device health (mHealth) provides substantial opportunities for improving care for patients with HIV and/or SUD, including navigating health services, providing education, and social support. For instance, a pilot mHealth educational intervention application provided to those with OUD was able to significantly increase patients’ knowledge about HIV transmission and risk [61]. Additionally, the PositiveLinks application, with knowledge resources and anonymous virtual support group, has demonstrated improvement in HIV virologic suppression [62]. Another mobile application, called DynamiCare Health, was recently granted a Breakthrough Device Designation by the FDA for their digital therapeutic for contingency management [63–66]. The CARE+ Corrections study, a cellphone-based mHealth intervention for PWH recently released from carceral settings, demonstrated a higher rate of viral suppression among those in the intervention arm, although this finding was not statistically significant [67]. In sum, harnessing technology can provide holistic, person-centered, integrated care for PWUD and PWH.
Harm Reduction, Syringe Services Programs, and Safe Injection Sites
Organizations providing harm reduction services and supplies (e.g., sterile syringes, naloxone, fentanyl test strips, wound care supplies) are uniquely situated to provide integrated care for PWUD and PWH. They can be the front line where HIV testing and SUD/OUD screening can occur and thus be a critical first “integrated care” touchpoint. Consequently, community-based harm reduction programs should be supported at the federal and state levels [19••]. Legislation that impedes successful harm reduction programming (e.g., requirements of one for one syringe exchange) must be reversed to ensure PWUD and PWH are able to receive quality, evidence-based integrated care and harm reduction services [19••, 20••].
Prisons and Jails
Addiction is not cured through punishment [21]. However, it can be managed with evidence-based approaches. Incarcerated individuals have higher rates of HIV and SUDs than the general population, yet most carceral settings in the USA do not provide sufficient care for SUD and HIV prevention or treatment within their facilities. Incarcerated men and women often struggle to gain access to testing, quality treatment, or preventive care for such conditions. As such, prisons and jails can and should be considered key locations to strengthen integrated ART, PrEP, MOUD, and even harm reduction services including provision of sterile syringes [19••, 20••, 68]. Even more important, post-release linkage to SUD and HIV care is critical in maintaining any health care gains that may have been achieved during incarceration.
Conclusion
PWUD, and key issues related to HIV diagnosis, treatment, and prevention for this specific population, were overlooked in the federally funded, collaborative EHE initiative. Yet, in order to end both the HIV epidemic and highly fatal substance use epidemic in the USA, provision of quality care that integrates HIV and SUD services is of paramount importance (Table 2). New models of integrated mobile HIV and SUD care are urgently needed and are currently being tested. Existing traditional brick-and-mortar clinics could strengthen their services by incorporating integrated HIV and SUD screening, treatment, and prevention services. However, to meet the EHE goals equitably, we must reach the most vulnerable, particularly those with SUD and justice involvement, by going beyond traditional brick and mortar clinics through expanding options for MHC units and mHealth interventions to improve how we meet patients where they are in trusting and non-stigmatizing environments. The optimal scopes and synergies between PNs, CHWs, telehealth platforms, and mobile pharmacies must be developed and measured to ensure integrated care models are able to meet complex community needs and that successful models can be scaled. The recent regulatory changes in buprenorphine prescribing practices which expands who can prescribe buprenorphine and reduces the once onerous training requirements are an important first step that can facilitate the development of integrated care models, but more expansion is needed. Furthermore, to achieve the idealized model of quality integrated care, legislative change at the state level is needed to allow dispensing methadone and buprenorphine in mobile pharmacies without linkage to brick-and-mortar Opioid Treatment Programs. People need integrated care to meet all of their major health care needs, with access to PrEP, ART, and MOUD in the moment they receive care and in the best setting in which to receive that care. Because PWUD and PWID still remain at high risk for HIV infection and transmission, important structural changes to care integration for HIV and SUD should be a priority for medical and public health professionals alike to better serve this population and meet the ambitious EHE goals.
Table 2.
Integrated care locations and models
| Ability to provide integrated HIV and SUD care | Meets patients where they are | Utilizes community health workers | Incorporates telehealth technologies | Includes access to pharmacy services | |
|---|---|---|---|---|---|
|
| |||||
| Brick-and-mortar clinics | X | X | |||
| Mobile health clinics | X | X | X | ||
| InMOTION | X | X | X | X | X |
| mHealth interventions | X | X | |||
| Harm reduction services | X | X | X | ||
| Prisons and jails | X | ||||
HIV, human immunodeficiency virus; InMOTION, Integrated Mobile Opioid Treatment and Infectious disease cOordinated care in your Neighborhood; SUD, substance use disorder
Acknowledgements
Additionally, we would like to thank Alysse Schultheis, Cynthia Frank, PhD, and the ACTION and DP1 research teams at Yale for their input and feedback.
Funding
This work was funded by the National Institutes on Drug Abuse (NIDA) (DP1DA056106, Springer and U01DA047982, Springer). The funders were not involved in the research design, analysis, or interpretation of the data or the decision to publish the manuscript.
Footnotes
Conflict of Interest Author S. Springer has received paid scientific consultation honoraria from Alkermes Inc and has received in-kind study drug donations from Alkermes Inc and Indivior Pharmaceutical Company for NIH-funded research. S. Shenoi’s spouse worked for Merck Pharmaceuticals 1997–2007 and retains company stock in his retirement account. There is no conflict of interest, but it is included in the interest of full disclosure. Authors K. Hill, I. Kuo, and M. Desruisseaux have nothing to declare.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. Jama. 2019(1538–3598 (Electronic)); This paper delineated the Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce the number of incident HIV infections by 75% by 2025 and 90% by 2030.
- 2.Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. The Lancet. 2023; 10.1016/S0140-6736(23)00877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Estimated HIV incidence and prevalence in the United States, 2015. –2019, 2021. [Google Scholar]
- 4.••. Springer SA, Nijhawan AE, Knight K, Kuo I, Di Paola A, Schlossberg E, et al. Study protocol of a randomized controlled trial comparing two linkage models for HIV prevention and treatment in justice-involved persons. BMC Infect Dis. 2022;22(1):380. 10.1186/s12879-022-07354-x. This manuscript provides a protocol for a 5-year hybrid type 1 effectiveness-implementation randomized controlled trial which aims to inform delivery of HIV, HCV, STI, and SUD prevention, testing, and treatment for justice-involved individuals in the community.
- 5.Hasin DS, Cp OB, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am, J Psychiatry. 2013; 1535–7228 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samet PCF-,HJ, Samet JH. In the clinic. Substance use disorders. Ann Intern, Med. 2016(1539–3704 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 7.••. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis. 2020;70(5):968–72. 10.1093/cid/ciz804. This work explored the need for infectious disease/addiction medicine specialists to both improve patient outcomes among those with serious infections caused by drug use.
- 8.SAMHSA. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2020. [Google Scholar]
- 9.Medication Access and Training Expansion Act 2021. [Google Scholar]
- 10.Mainstreaming Addiction Treatment Act. 2021. [Google Scholar]
- 11.Restoring Hope for Mental Health and Well-Being Act. 2022. [Google Scholar]
- 12.Fairbanks J, Umbreit A, Kolla BP, Karpyak VM, Schneekloth TD, Loukianova LL, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin, Proc. 2020; 1942–5546 (Electronic) [DOI] [PubMed] [Google Scholar]
- 13.••. Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA. 2023(1538–3598 (Electronic); This manuscript, written by expert physician scientists, provides up-to-date recommendations for HIV treatment and prevention, including the need for integrated, evidence-based approaches for those with HIV and substance use disorder.
- 14.Mason B, Heyser C. Alcohol use disorder: the role of medication in recovery. Alcohol, Res. 2021(2169–4796 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.••. Alpren C, Dawson EL, John B, Cranston K, Panneer N, Fukuda HD, et al. Opioid use fueling HIV transmission in an urban setting: an outbreak of HIV infection among people who inject drugs-Massachusetts, 2015–2018. Am, J Public Health. 2020(1541–0048 (Electronic); This study documented the a recent HIV outbreak in Massachusetts, identified epidemiologically and through molecular surveillance, among those who inject drugs, demonstrating the often interconnected nature of HIV and OUD.
- 16.Schwetz TA, Calder T, Rosenthal E, Kattakuzhy S, Fauci AS. Opioids and infectious diseases: a converging public health crisis. J Infect Dis. 2019(1537–6613 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, et al. Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J Infect Dis. 2017;216(9):1053–62. 10.1093/infdis/jix307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Wilson TE, Adedimeji A, Merenstein D, Milam J, Cohen J, et al. The impact of substance use on adherence to antiretroviral therapy among HIV-infected women in the United States. AIDS Behav. 2018;22(3):896–908. 10.1007/s10461-017-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.••. Springer SA, Barocas JA, Wurcel A, Nijhawan A, Thakarar K, Lynfield R, et al. Federal and state action needed to end the infectious complications of illicit drug use in the United States: IDSA and HIVMA’s advocacy agenda. J Infect Dis. 2020. (1537–6613 (Electronic); This piece, by an IDSA and HIVMA working group, highlights evidence-based practices that have the opportunity to address the overlapping HIV and opioid epidemics in the USA.
- 20.••. Springer SA, Merluzzi AP, Del Rio C. Integrating responses to the opioid use disorder and infectious disease epidemics: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA. 2020;324(1):37–8. 10.1001/jama.2020.2559. This work highlights successful, integrated services for those with infectious diseases and OUD across the USA, as well as barriers to the integration of care.
- 21.National Academies of Sciences E, and Medicine. Integrating responses at the intersection of opioid use disorder and infectious disease epidemics: proceedings of a workshop. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 22.Gowing L, Mf F, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst, Rev. 2011(1469–493X (Electronic)). [DOI] [PubMed] [Google Scholar]
- 23.Springer SA, J Q, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. P LoS One. 2012(1932. –6203 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Springer SA, Di Paola A, Azar MM, Barbour R, Biondi BE, Desabrais M, et al. Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: results of a double-blind, placebo-controlled randomized trial. J Acquir Immune Defic Syndr. 2018. (1944–7884 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altice FL, Rd B, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011(1944–7884 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara KB, B. E., Hernández-Ramírez RU, Taweh N, Grimshaw AA, Springer S. A systematic review and meta-analysis of studies evaluating the effect of medication treatment for opioid use disorder on infectious disease outcomes. Open Forum Infect, Dis. 2021(2328–8957 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taweh N, Schlossberg E, Frank C, Nijhawan A, Kuo I, Knight K, et al. Linking criminal justice-involved individuals to HIV, hepatitis C, and opioid use disorder prevention and treatment services upon release to the community: progress, gaps, and future directions. Int, J Drug Policy. 2021(1873–4758 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown HD, DeFulio A. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alcohol Depend. 2020;216:108307. 10.1016/j.drugalcdep.2020.108307. [DOI] [PubMed] [Google Scholar]
- 29.McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiat. 2013;170(1):94–101. 10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zastepa E, Sun JC, Clune J, Mathew N. Adaptation of contingency management for stimulant use disorder during the COVID-19 pandemic. J Subst Abuse Treatment. 2020;118:108102. 10.1016/j.jsat.2020.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Springer SA, Di Paola A, Barbour R, Azar MM, Altice FL. Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV and alcohol use disorders transitioning to the community: results from a double-blind, placebo-controlled trial. J Acquir Immune Defic Syndr. 2018(1944–7884 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer SA, Di Paola A, Azar MM, Barbour R, Krishnan A, Altice FL. Extended-release naltrexone reduces alcohol consumption among released prisoners with HIV disease as they transition to the community. Drug Alcohol, Depend. 2017(1879–0046 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer SA, Korthuis PT, Del Rio C. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: a call for action after a National Academies of Sciences, Engineering, and Medicine workshop. Ann Intern, Med. 2018(1539–3704 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McManus KA, Davy-Mendez T, Killelea A, Schranz AJ. Access to medications for opioid use disorder for persons with human immunodeficiency virus in the United States: gaps in coverage by state AIDS drug assistance programs. Open Forum Infect, Dis. 2022;9(4):ofac057. 10.1093/ofid/ofac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath B, Wise Romero P, Reynolds K. A review and proposed standard framework for levels of integrated healthcare. Washington DC: SAMHSA-HRSA, Center for Integrated Health Solutions; March 2013. [Google Scholar]
- 36.Huber A, Pascoe S, Nichols B, Long L, Kuchukhidze S, Phiri B, et al. Differentiated service delivery models for HIV treatment in Malawi, South Africa, and Zambia: a landscape analysis. Glob Health Sci, Pract. 2021(2169–575X (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.America’s HIV Epidemic Analysis Dashboard (AHEAD). https://ahead.hiv.gov/ (2021). Accessed. [Google Scholar]
- 38.Tofighi B, Sindhu SS, Chemi C, Lewis CF, Dickson VV, Lee JD. Perspectives on the HIV continuum of care among adult opioid users in New York City: a qualitative study. Harm Reduct J. 2019;16(1):58. 10.1186/s12954-019-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamdan S, Smyth E, Murphy ME, Grussing ED, Wei M, Guardado R, et al. Racial and ethnic disparities in HIV testing in people who use drugs admitted to a tertiary care hospital. Aids Patient Care STDS. 2022(1557–7449 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lancaster KE, Endres-Dighe S, Sucaldito AD, Piscalko H, Madhu A, Kiriazova T, et al. Measuring and addressing stigma within HIV interventions for people who use drugs: a scoping review of recent research. Curr HIV/AIDS Rep. 2022;19(5):301–11. 10.1007/s11904-022-00619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Springer SA, Biondi BE, Frank C, El-Bassel N. A call to action to combat the opioid epidemic among women. J Addict Med. 2020(1935–3227 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varthakavi V, Lee-Win A. Notice of special interest (NOSI): research to address ‘ending the HIV epidemic’ initiative goals relevant to substance using populations at-risk for or living with HIV. National Institute on Drug Abuse; 2022. [Google Scholar]
- 43.Springer SA, Rio CD. Lessons learned from the response to the human immunodeficiency virus epidemic that can inform addressing the opioid epidemic. Infect Dis Clin North, Am. 2020(1557–9824 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.••. Seval N, Eaton E, Springer SA. Beyond antibiotics: a practical guide for the infectious disease physician to treat opioid use disorder in the setting of associated infectious diseases. Open Forum Infect, Dis. 2020(2328–8957 (Print); This paper provides guidance and strategies for infectious disease physicians who see patients with co-occurring OUD, including how to screen for OUD and initiate MOUD.
- 45.Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect, Dis. 2019. (1537–6591 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SAMHSA. Removal of DATA waiver (X-waiver) requirement. 2023. [Google Scholar]
- 47.Medication Access and Training Expansion Act of 2021. H R 2067 1st Session ed 2021. [Google Scholar]
- 48.Karletsos DS C Impact of Medicaid expansion on PrEP utilization in the US: 2012–2018. AIDS Behav. 2021(1573–3254 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 49.Yu SWY, Hill C, Ricks ML, Bennet J, Oriol NE. The scope and impact of mobile health clinics in the United States: a literature review. Int J Equity Health. 2017;16(1):178. 10.1186/s12939-017-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malone NC, Williams MM, Smith Fawzi MC, Bennet J, Hill C, Katz JN, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. 10.1186/s12939-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmack HJ, Bouchelle Z, Rawlins Y, Bennet J, Hill C, Oriol NE. Mobilizing a narrative of generosity: patient experiences on an urban mobile health clinic. Commun Q. 2017;65(4):419–35. 10.1080/01463373.2017.1279677. [DOI] [Google Scholar]
- 52.Robinowitz N, Smith ME, Serio-Chapman C, Chaulk P, Johnson KE. Wounds on wheels: implementing a specialized wound clinic within an established syringe exchange program in Baltimore, Maryland. Am J Public Health. 2014;104(11):2057–9. 10.2105/AJPH.2014.302111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liebman J, M PL, Altice F. Effectiveness of a mobile medical van in providing screening services for STDs and HIV. Public Health, Nurs. 2002(0737–1209 (Print)). [DOI] [PubMed] [Google Scholar]
- 54.Springer S Ending the HIV epidemic for persons who use drugs: the practical challenges of meeting people where they are. J Gen Intern Med. 2023(1525–1497 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoptaw S, El-Bassel N. HPTN 094 protocol. US National Institutes of Health; 2020. [Google Scholar]
- 56.Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS). J Correct Health Care. 2015;21(1):12–26. 10.1177/1078345814557513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.••. Lambdin BH, Kan D, Kral AH. Improving equity and access to buprenorphine treatment through telemedicine at syringe services programs. Subst Abuse Treat Prev, Policy. 2022(1747–597X (Electronic)). This study, which assessed early findings from an intervention providing telemedicine to initiate buprenorphine, found that 87% of participants were able to be inducted on the day of their buprenorphine referral, and 64% returned for their second refill of buprenorphine.
- 58.Hopkins H SB 872 – mobile pharmacy vans. California Senator Bill Dodd; 2022. [Google Scholar]
- 59.An Act Concerning Pharmacies and Pharmacists. January ed2023. [Google Scholar]
- 60.Chan B, Hoffman KA, Bougatsos C, Grusing S, Chou R, McCarty D. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treatment. 2021;129:108483. 10.1016/j.jsat.2021.108483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochalek TA, Heil SH, Higgins ST, Badger GJ, Sigmon SC. A novel mHealth application for improving HIV and hepatitis C knowledge in individuals with opioid use disorder: a pilot study. Drug Alcohol Depend. 2018;190:224–8. 10.1016/j.drugalcdep.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dillingham R, Ingersoll K, Flickinger TE, Waldman AL, Grabowski M, Laurence C, et al. PositiveLinks: a mobile health intervention for retention in HIV care and clinical outcomes with 12-month follow-up. AIDS Patient Care STDs. 2018;32(6):241–50. 10.1089/apc.2017.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammond A, Sweeney M, Chikosi T, Stitzer M. Digital delivery of a contingency management intervention for substance use disorder: a feasibility study with DynamiCare Health. J Subst Abuse Treat. 2021(1873–6483 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeFulio A, Brown H, Davidson R, Regnier S, Kang N, Ehart M. Feasibility, acceptability, and preliminary efficacy of a smartphone-based contingency management intervention for buprenorphine adherence. Behav Anal, Pract. 2023(1998–1929 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen T-A. DynamiCare Health digital therapeutic receives FDA breakthrough device designation for treatment of smoking during pregnancy. PRWEB; 2023. [Google Scholar]
- 66.Gastfriend E DynamiCare Health digital therapeutic receives FDA breakthrough device designation for treatment of alcohol use disorder. PRWEB; 2023. [Google Scholar]
- 67.Kuo I, Liu T, Patrick R, Trezza C, Bazerman L, Uhrig Castonguay BJ, et al. Use of an mHealth intervention to improve engagement in HIV community-based care among persons recently released from a correctional facility in Washington, DC: a pilot study. AIDS Behav. 2019;23(4):1016–31. 10.1007/s10461-018-02389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Springer SA, E P, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004(1537–6591 (Electronic)). [DOI] [PubMed] [Google Scholar]


