Abstract
Gamma interferon-deficient (IFN-γ−/−) mice with a C57BL/6 background were infected intraperitoneally with mouse hepatitis virus strain JHM (JHMV). In contrast to IFN-γ-+/− and IFN-γ+/+ mice, JHMV persisted in IFN-γ−/− mice and induced death during the subacute phase of the infection. Unexpectedly, infected IFN-γ−/− mice showed severe peritonitis accompanying the accumulation of a viscous fluid in the abdominal and thoracic cavities in the subacute phase. Destructive changes of hepatocytes were not observed. Administration of recombinant IFN-γ protracted the survival time of IFN-γ−/− mice after JHMV infection. These results demonstrate that IFN-γ plays a critical role in viral clearance in JHMV infection. They also show that a resultant persistent JHMV infection induces another form of disease in IFN-γ−/− mice, which bears a resemblance to feline infectious peritonitis in cats.
Murine coronaviruses, specifically mouse hepatitis viruses, induce a variety of diseases, including hepatitis, enteritis, and encephalitis, in mice. The pathogenic effects vary with virus strain, route of infection, and the strain, age, and immune status of the host (5, 10, 15, 21). For example, if C57BL/6 (B6) mice are infected intracerebrally or intranasally with murine coronavirus strain JHM (JHMV), they will die from acute encephalitis within a week (32). In contrast, B6 mice infected intraperitoneally (i.p.) with the same virus develop an acute mild form of hepatitis but not fatal encephalitis (14, 30). Thus, JHMV is a neurovirulent strain, but if the infection is a systemic one, the virus grows in other tissues including the liver, spleen, and lymph nodes. Although both cell-mediated and humoral immune responses are induced in B6 mice infected i.p. with JHMV, it has been shown that T cells play a key role in protecting against infection (13, 14). Injecting JHMV i.p. into B6 mice that had been selectively depleted of CD4+ T cells induced an infection resembling that of normal B6 mice. If B6 mice were depleted of CD8+ T cells, however, a more severe hepatitis with more extensive viral growth developed than in normal mice (14). These data indicate that CD8+ T cells play a more important role in JHMV clearance than CD4+ T cells.
It has been demonstrated that cloned CD8+ cytotoxic T lymphocytes (CTL) that are JHMV specific inhibit viral growth by inducing apoptosis of virus-infected cells (29). However, CD8+ T cells not only induce direct cytolysis of infected cells by a death receptor-mediated or granule exocytosis mechanism but also secrete a variety of cytokines and chemokines (9, 31). In particular, gamma interferon (IFN-γ) is known to be an antiviral cytokine and is produced by CD8+ CTL, CD4+ Th1 helper T cells, NK cells, and NKT cells (3, 11, 26). In fact, this cytokine has been detected during JHMV infection in mice (22, 23). Since the role of IFN-γ in limiting viral infections in vivo is uncertain (1, 27), we attempted to evaluate its role in i.p. induced JHMV infection of IFN-γ-deficient (IFN-γ−/−) B6 mice.
Production of IFN-γ−/− mice was described previously (33). A 129/SvJ mouse with a disrupted IFN-γ gene was backcrossed with B6 mice five or six times. The genotypes of the mice were determined by PCR as described previously (33), and 8- to 12-week-old female mice were used. Breeding mice were maintained in a laminar flow rack in an environmentally controlled area and were routinely confirmed serologically to be free of mouse hepatitis virus and other pathogenic agents. The mice were infected i.p. with 106 PFU of the DL variant of JHMV and were kept in a safety cabinet in a different area. The experiments were conducted according to institutional ethical guidelines for animal experiments and according to safety guidelines for gene manipulation experiments. For viral isolation and titration, 10% tissue homogenates of samples were serially diluted and plaque assayed on DBT cells (13). Serum-neutralizing antibodies were assayed as described elsewhere (17). Anti-JHMV antibodies were also measured by enzyme-linked immunosorbent assay using a commercial kit (Denka Seiken, Tokyo, Japan). Alanine aminotransferase (ALT) activity in serum was determined by an enzymatic rate method (18) using a commercial kit according to the manufacturer’s instructions (Iatron Laboratories, Tokyo, Japan). Tissue samples were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Viral antigen was detected by immunohistochemical methods with monoclonal antibody J3.3, which is specific for the viral nucleocapsid protein as described elsewhere (22). Statistical analysis was done by Student’s t test or log rank test. Differences were considered statistically significant at a P value of <0.05.
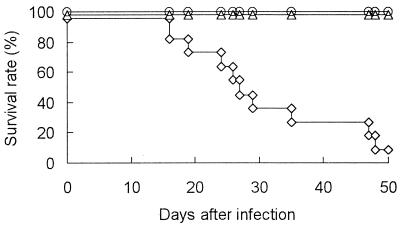
To examine the protective role of IFN-γ in JHMV infection in mice, IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+ B6 mice were infected i.p. with JHMV and monitored for up to 50 days postinfection (p.i.). All the IFN-γ+/+ and IFN-γ+/− mice survived and appeared healthy throughout the experiment (Fig. 1). IFN-γ−/− mice also appeared healthy for the first 10 days p.i. However, some of them died at 16 days p.i. and their survival rate gradually decreased thereafter, so that approximately 90% of IFN-γ−/− mice had died by 50 days p.i. Clinical manifestation of the disease in the mice varied. Most of the infected IFN-γ−/− mice gained weight due to abdominal distension before death. A few mice lost body weight and were inert before their death. A small number of mice showed signs of central nervous system disease such as an inability to turn over rapidly.
FIG. 1.
Mortality of IFN-γ−/− B6 mice after i.p. JHMV infection. Nine IFN-γ−/− (◊), 10 IFN-γ+/− (▵), and 10 IFN-γ+/+ (○) mice were infected i.p. with 106 PFU of JHMV and monitored for 50 days.
It has been suggested that T-cell-mediated immunity plays a key role in viral clearance in the acute phase of JHMV infection in mice (12–14, 34). To assess the importance of IFN-γ in viral clearance in B6 mice infected i.p. with JHMV, viral titers in the liver and IFN-γ levels in serum in IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+ mice were determined. The viral titers in IFN-γ−/− mice were significantly higher than those in IFN-γ+/+ mice at 3 and 5 days p.i., respectively (Table 1). IFN-γ+/− mice showed intermediate values. On the other hand, whereas the serum IFN-γ level in IFN-γ+/+ mice was 5 and 11 pg/ml at 3 and 5 days p.i., respectively, that of IFN-γ−/− mice was almost undetectable. The level in IFN-γ+/− mice was also intermediate (data not shown). These data are compatible with the idea that these two parameters are inversely correlated. In addition, although JHMV was cleared from the liver within 7 days after infection in IFN-γ+/− and IFN-γ+/+ mice, it gradually decreased but persisted in IFN-γ−/− mice during the experimental period (Table 1). These results suggest that IFN-γ plays a critical role in viral clearance during i.p. JHMV infection in B6 mice. They are also consistent with recent reports that clearance of OBLV60, a neuroattenuated variant of JHMV, in IFN-γ−/− mice with a BALB/c background is delayed (14) and that MHV-A59, another strain of murine coronavirus, replicates better in IFN-γ receptor-deficient mice than in wild-type mice (28). Histopathology showed a number of small lesions in the livers of IFN-γ+/+ and IFN-γ+/− mice 5 days after infection (Fig. 2A and B). The liver lesions in IFN-γ−/− mice were larger than those in IFN-γ+/− or IFN-γ+/+ mice and were infiltrated with abundant neutrophils (Fig. 2C). Although a modest increase in serum ALT activity was observed at 5 days p.i. in all groups, its level after 7 days p.i. was normal, even in IFN-γ−/− mice in which JHMV persisted at low levels (Table 1).
TABLE 1.
Viral growth in the liver and ALT activity in serum in IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+ mice after i.p. JHMV infectiona
| Days after infection | Viral titer (log10 PFU/g)
|
ALT activity (Kunkel units/liter)b
|
||||
|---|---|---|---|---|---|---|
| IFN-γ−/− | IFN-γ+/− | IFN-γ+/+ | IFN-γ−/− | IFN-γ+/− | IFN-γ+/+ | |
| 3 | 4.8 ± 0.5c | 4.2 ± 0.6 | 3.9 ± 0.2 | 68 ± 37 | 33 ± 43 | 39 ± 22 |
| 5 | 4.9 ± 0.3c | 3.7 ± 0.6 | 3.3 ± 0.2 | 154 ± 72 | 93 ± 33 | 113 ± 41 |
| 7 | 4.0 ± 0.8 | <1.7 | <1.7 | 25 ± 8 | 19 ± 4 | 23 ± 3 |
| 14 | 3.2 ± 0.6 | NDd | ND | 37 ± 15 | 31 ± 2 | 29 ± 3 |
| 21 | 2.0 ± 0.2 | ND | ND | 15 ± 4 | 16 ± 4 | 19 ± 7 |
| 30 | 3.0 ± 0.3e | ND | ND | ND | ND | ND |
Samples were collected at the times indicated (n = 3 to 8, except at 30 days p.i., when two mice were used). Data are expressed as the means ± standard deviations.
Levels of ALT activity in uninfected IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+ mice were 24 ± 1, 24 ± 4, and 21 ± 5 Kunkel units/liter (mean ± standard deviation), respectively.
Significant compared to the value for IFN-γ+/+ mice (P < 0.05).
ND, not done.
Infectious virus was detected in the livers of IFN-γ−/− mice at 35, 40, 42, and 48 days p.i.
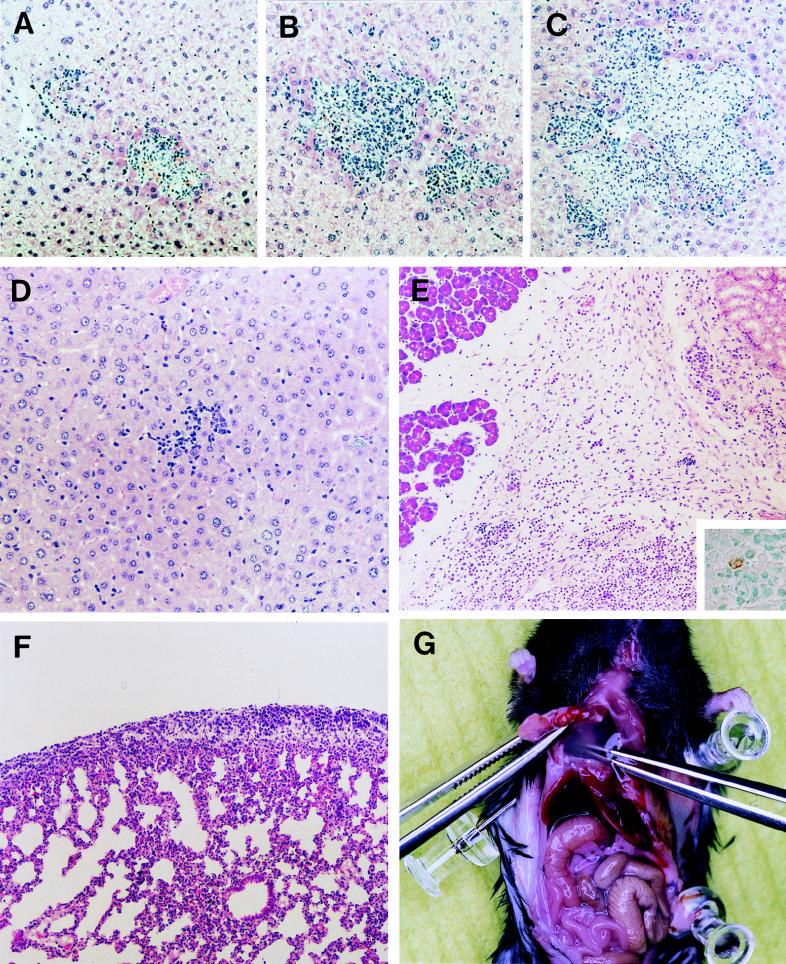
FIG. 2.
Acute and subacute pathological changes in JHMV-infected IFN-γ−/− B6 mice. Paraffin-embedded sections of the livers from IFN-γ+/+ (A), IFN-γ+/− (B), and IFN-γ−/− (C) mice obtained at 5 days p.i. were stained with hematoxylin and eosin (magnification, ×126). Paraffin-embedded sections of the livers (D) (magnification, ×175), pancreases (E, inset), kidneys (E), and lungs (F) (magnification, ×87.5) of IFN-γ−/− mice obtained at 21 days p.i. were stained with hematoxylin and eosin. Viral antigen was detected in an inflammatory lesion near the pancreas by immunohistochemistry (inset in E) (magnification, ×350). Copious translucent fluid accumulated in the thoracic cavity in IFN-γ−/− mice at 1 month p.i. (G).
Since JHMV-infected IFN-γ−/− mice died in the subacute phase, the mice were sacrificed at 14 days, 21 days, and 1 month p.i. and examined for pathological changes. At 14 days p.i., a pseudomembrane was observed on the surfaces of the livers of some IFN-γ−/− mice. At 21 days p.i., most mice had peritonitis, and organs in their abdominal cavities had adhered to each other and to the peritoneum. Approximately half of the mice also had an accumulation of ascites fluid. The severity of peritonitis varied among the mice but appeared progressive. Interestingly, the adhesion began in the upper left part of the abdominal cavity and then spread through the whole cavity. No bacteria were isolated from the ascites fluid (data not shown), excluding the possibility of a bacterial infection. Although some leukocytes were observed in the livers of IFN-γ−/− mice in the subacute phase, no hepatocyte injury was observed (Fig. 2D). This is consistent with a normal level of ALT activity in serum of the mice (Table 1). Microscopically, the disease was characterized by disseminated granulomatous inflammation and exudative fibrinous serositis with a notable number of plasma cells and eosinophils in the abdominal cavity (Fig. 2E). The same lesions were observed not only in the abdominal cavity but also in the thoracic cavity (Fig. 2F). Accumulation of a translucent fluid in the thoracic cavity was more frequent than ascites in IFN-γ−/− mice in the subacute phase (Fig. 2G). The infectious virus was recovered from various tissues, including the liver, spleen, kidney, pancreas, mesenterium, and lung, of some IFN-γ−/− mice 21 days after infection (Table 2). The highest level of viral growth was observed in the mesenterium, where active inflammation had been occurring, but a few cells were positive for the viral antigen (Fig. 2E). Since some ascites fluid contained infectious viruses, we could not exclude the possibility that they may have contaminated other samples taken from the peritoneal cavity. Viremia was not observed in IFN-γ−/− mice in the subacute phase.
TABLE 2.
Virus present in IFN-γ−/− B6 mice with i.p. subacute JHMV infectionsa
| Sample | No. of positive samples/ no. tested | Viral titerb |
|---|---|---|
| Liver | 3/3 | 50–2,000 |
| Spleen | 1/3 | 1,500 |
| Kidney | 2/3 | 50–500 |
| Pancreas | 3/3 | 1,000–2,000 |
| Mesenterium | 3/3 | 2,600–10,500 |
| Lung | 1/3 | 50 |
| Brain | 1/5 | 8,500 |
| Blood | 0/5 | NAc |
| Ascites fluid | 2/3 | 50–3,500 |
Samples were collected at 21 days p.i. 10% homogenates of each sample were inoculated onto DBT cells, and the viral titer was determined.
Expressed as PFU per milliliter for blood and ascites fluid and as PFU per gram for other tissues.
NA, not applicable.
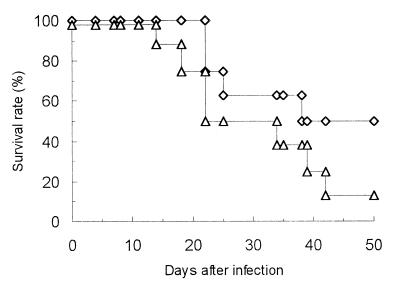
The effect of recombinant IFN-γ administration on JHMV-induced fatal disease in IFN-γ−/− mice was examined. Recombinant IFN-γ was obtained from Genzyme Corp. (Cambridge, Mass.). IFN-γ−/− mice were injected i.p. with 3,000 U of recombinant IFN-γ twice a week during the experiment (Fig. 3). Recombinant IFN-γ administration partially inhibited JHMV-induced fatal disease and protracted the survival time. The log rank test revealed that the survival curves were significantly different (P < 0.05), confirming that the deficiency of IFN-γ contributed to the pathogenesis of JHMV-induced subacute fatal disease in IFN-γ−/− mice.
FIG. 3.
Effect of recombinant murine IFN-γ treatment on JHMV-induced fatal disease in IFN-γ−/− B6 mice. IFN-γ−/− mice (n = 16) were infected i.p. with JHMV, and 3,000 U of recombinant IFN-γ were injected twice per week (◊). Control IFN-γ−/− mice (n = 16) were infected i.p. with JHMV and injected with the same volume of phosphate-buffered saline. (▵). Both groups of mice were monitored for up to 50 days p.i.
Some immunological features of JHMV-infected IFN-γ−/− mice were characterized. To examine antiviral antibody responses in IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+ mice, sera were obtained from the mice at 21 days after infection and antiviral-antibody titers were determined by enzyme-linked immunosorbent assay. The antibody titers in IFN-γ−/− mice were significantly higher (1:734) than those in IFN-γ+/− (1:165) and IFN-γ+/+ (1:253) mice (P < 0.05). Virus neutralization antibody titers were also determined, and the titers in IFN-γ−/− mice were significantly higher (1:390) than those in IFN-γ+/− (1:94) and IFN-γ+/+ mice (1:109) (P < 0.05). Continuous antigenic stimulation due to persistent JHMV infection might enhance antibody production in IFN-γ−/− mice.
IFN-γ−/− mice that had been depleted of CD8+ T cells through injection of anti-CD8 monoclonal antibody and then infected i.p. with JHMV suffered severe hepatitis and died within 2 weeks (15a). This indicates that CD8+ T cells in IFN-γ−/− mice play a role in protection against JHMV infection. In fact, ex vivo flow-cytometric analysis showed that CD8+ T cells expressing a small amount of CD62L (i.e., activated CD8+ T cells) were induced in IFN-γ−/− mice after JHMV infection. Also, an assay of in vitro-stimulated CTL (2, 4) confirmed that they were cytotoxic against EL-4 cells sensitized with JHMV CD8+ T cell epitope S-510-518 and S-598-605 (15a). Thus, CD8+ T cells in IFN-γ+/+ mice would minimize viral infection in vivo not only by producing IFN-γ, which induces an antiviral state in adjacent uninfected cells, but also by lysing virus-infected cells producing progeny virions (30, 37). The dissociation of these functions in the hosts would be disadvantageous for protection against viral infections. This may be why CD8+ CTL, CD4+ Th1 helper T cells, NK cells, and NKT cells possess killer activity as well as the potential to produce IFN-γ.
Although both cell-mediated and humoral immune responses were activated during JHMV infection in IFN-γ−/− mice, JHMV persisted in IFN-γ−/− mice. This might be explained by the following hypotheses. First, IFN-γ may be a prerequisite for JHMV clearance, especially from the peritoneal cavity. This may result in a persistent infection in the peritoneal cavity. Although we cannot explain why viral clearance is particularly marked in the peritoneal cavity, the peritoneal and pleural cavities are immunologically unique sites where B-1 cells (Ly-1 B cells) are distributed. Since B-1 cells are suspected of being involved in some autoimmune diseases (19), it would be interesting to focus on B-1 cells in JHMV-infected IFN-γ−/− mice. Alternatively, IFN-γ is involved in the commitment and stabilization of CD4+ helper T cells, which are categorized into Th1 and Th2 subsets according to their pattern of cytokine secretion (20). JHMV-induced subacute disease in IFN-γ−/− mice may be explained by a preferential Th2 response. Future studies should aim at determining whether the polarized Th2 response is involved in the pathogenesis.
The subacute disease in IFN-γ−/− mice may involve immune escape variants that emerge under the reduced antiviral immunity caused by the deficiency of IFN-γ. Recent studies have clearly demonstrated that CTL-resistant variants arise soon after JHMV infection in B6 mice. Such results suggest that these mutated viruses may contribute to viral persistence and JHMV-induced demyelinating disease in the central nervous system (24, 25). In addition to CTL-resistant variants, antibody-resistant variants of JHMV are known to show different pathogenicities in the central nervous system in mice (6, 8). Characterization of viruses that persisted in IFN-γ−/− mice would reveal whether this is the case. Even if escape variants do emerge, the finding that i.p. JHMV infection induced subacute peritonitis but not hepatitis in IFN-γ−/− mice is still an unexpected one.
A similar disease was reported earlier in ICR-nude mice at a lower rate after MHV-NuU infection (35, 36). These mice also showed signs of peritonitis with abundant ascites fluid in the abdominal cavity and died at 25 to 52 days p.i. Nevertheless, these two diseases are not identical; whereas MHV-NuU-infected ICR-nude mice died of progressive hepatitis, hepatitis was almost resolved in JHMV-infected IFN-γ−/− mice in the subacute phase. The cause of death of JHMV-infected IFN-γ−/− mice remains obscure because of the variation in clinical manifestations. However, the accumulation of a fluid in their thoracic cavities might have induced compressive atelectasis in most cases. Feline infectious peritonitis is known to be a progressive lethal disease caused by a group of feline coronaviruses (7). There is neither a good remedy nor an effective vaccine for the disease. Common pathological changes such as exudative fibrinous serositis in the abdominal and thoracic cavities associated with feline infectious peritonitis and experimental JHMV infection in IFN-γ−/− mice suggest that this experimental model may provide a unique opportunity to address the pathogenesis of virus-induced peritonitis. Although detailed comparison of the diseases may help to clarify their natures, an optimistic speculation is that IFN-γ may be effective against feline infectious peritonitis because treatment with recombinant IFN-γ protracted the survival time of JHMV-infected IFN-γ−/− mice.
Acknowledgments
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann C C, Yao Q, Lin M, Stohlman S A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted CTL response. J Gen Virol. 1996;77:315–325. doi: 10.1099/0022-1317-77-2-315. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Castro R F, Perlman S. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J Virol. 1995;69:8127–8131. doi: 10.1128/jvi.69.12.8127-8131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compton S R, Barthold S W, Smith A L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993;43:15–28. [PubMed] [Google Scholar]
- 6.Dalziel R G, Lampert P W, Talbot P J, Buchmeier M J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986;59:463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groot R J, Horzinek M C. Feline infectious peritonitis. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 293–315. [Google Scholar]
- 8.Fleming J O, Trousdale M D, El-Zaatari F A K, Stohlman S A, Weiner L P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 10.Homberger F R. Enterotropic mouse hepatitis virus. Lab Anim. 1996;31:97–115. doi: 10.1258/002367797780600189. [DOI] [PubMed] [Google Scholar]
- 11.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 12.Kyuwa S, Yamaguchi K, Toyoda Y, Fujiwara K. Effect of sensitized T cell transfer on mouse hepatitis virus type 4 infection in athymic nude mice. Jpn J Vet Sci. 1989;51:219–221. doi: 10.1292/jvms1939.51.219. [DOI] [PubMed] [Google Scholar]
- 13.Kyuwa S, Machii K, Okumura A, Toyoda Y. Characterization of T cells expanded in vivo during primary mouse hepatitis virus infection in mice. J Vet Med Sci. 1995;58:431–437. doi: 10.1292/jvms.58.431. [DOI] [PubMed] [Google Scholar]
- 14.Kyuwa S, Machii K, Shibata S. Role of CD4+ and CD8+ T cells in mouse hepatitis virus infection in mice. Exp Anim. 1996;45:81–83. doi: 10.1538/expanim.45.81. [DOI] [PubMed] [Google Scholar]
- 15.Kyuwa S, Stohlman S A. Pathogenesis of a neurotropic murine coronavirus, strain JHM in the central nervous system of mice. Semin Virol. 1990;1:273–280. [Google Scholar]
- 15a.Kyuwa, S. Unpublished data.
- 16.Lane T E, Paoletti A D, Buchmeier M J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin M T, Stohlman S A, Hinton D R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi U, Guidi G. A new colorimetric ultramicromethod for serum glutamic-oxalacetic and glutamic-pyruvic transaminase determination. Clin Chim Acta. 1970;28:431–437. doi: 10.1016/0009-8981(70)90069-0. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Honjo T. Involvement of B-1 cells in mucosal immunity and autoimmunity. Immunol Today. 1995;16:534–539. doi: 10.1016/0167-5699(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Lee R K, Nam S Y, Podack E R, Bottomly K, Flavell R A. Roles of IL-4 and IFN-γ in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–2653. [PubMed] [Google Scholar]
- 21.Ohtsuka N, Taguchi F. Mouse susceptibility to mouse hepatitis virus infection is linked to viral receptor genotype. J Virol. 1997;71:8860–8863. doi: 10.1128/jvi.71.11.8860-8863.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra B, Hinton D R, Lin M T, Cua D J, Stohlman S A. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology. 1997;233:260–270. doi: 10.1006/viro.1997.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce B D, Hobbs M V, McGraw T S, Buchmeier M J. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J Virol. 1994;68:5483–5495. doi: 10.1128/jvi.68.9.5483-5495.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pewe L, Wu G, Barnett E M, Castro R, Perlman S. Cytotoxic T cell-resistant variants are selected in a virus-induced demyelinating disease. Immunity. 1996;5:253–262. doi: 10.1016/s1074-7613(00)80320-9. [DOI] [PubMed] [Google Scholar]
- 25.Pewe L, Xue S, Perlman S. Cytotoxic T-cell-resistant variants arise at early times after infection in C57BL/6 but not in SCID mice infected with a neurotropic coronavirus. J Virol. 1997;71:7640–7647. doi: 10.1128/jvi.71.10.7640-7647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramshaw I, Ruby J, Ramsay A, Ada G, Karupiah G. Expression of cytokines by recombinant vaccinia viruses: a model for studying cytokines in virus infection in vivo. Immunol Rev. 1992;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Hamilton-Easton A-M, Mo X Y, Doherty P C. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–3921. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schijns V E, Wierda C M, van Hoeij M, Horzinek M C. Exacerbated viral hepatitis in IFN-γ receptor-deficient mice is not suppressed by IL-12. J Immunol. 1996;157:815–821. [PubMed] [Google Scholar]
- 29.Shibata S, Kyuwa S, Lee S-K, Toyoda Y, Goto N. Apoptosis induced in mouse hepatitis virus-infected cells by a virus-specific CD8+ cytotoxic T-lymphocyte clone. J Virol. 1994;68:7540–7545. doi: 10.1128/jvi.68.11.7540-7545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A L, Barthold S W, de Souza M S, Bottomly K. The role of gamma interferon in infection of susceptible mice with murine coronavirus, MHV-JHM. Arch Virol. 1991;121:89–100. doi: 10.1007/BF01316746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth M J, Trapani J A. The relative role of lymphocyte granule exocytosis versus death receptor-mediated cytotoxicity in viral pathophysiology. J Virol. 1998;72:1–9. doi: 10.1128/jvi.72.1.1-9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stohlman S A, Frelinger J A. Resistance to fatal nervous system disease by mouse hepatitis virus, strain JHM. 1. Genetic analysis. Immunogenetics. 1978;6:277–281. [Google Scholar]
- 33.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 34.Williamson J S P, Stohlman S A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanagisawa T, Nakanaga K, Kyuwa S, Fujiwara K. Ascitic disease in nude mice infected with mouse hepatitis virus. Jpn J Vet Sci. 1985;47:171–174. doi: 10.1292/jvms1939.47.171. [DOI] [PubMed] [Google Scholar]
- 36.Yanagisawa T, Nakanaga K, Kyuwa S, Fujiwara K. Ascitic disease in ICR-nude mice due to mouse hepatitis virus. Jpn J Vet Sci. 1986;48:7–14. doi: 10.1292/jvms1939.48.7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Hinton D R, Cua D J, Stohlman S A, Lai M M C. Expression of interferon-γ by a coronavirus defective-interfering RNA vector and its effect on viral replication, spread, and pathogenicity. Virology. 1997;233:327–338. doi: 10.1006/viro.1997.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]