Abstract
Obesity leads to many diseases including hypercholesterolemia, type-2 diabetes, hypertension, cardiovascular disease, and cancer. It is the fastest-growing lethal disease in the Western and developing countries. The link between obesity and cancer is relatively underappreciated among the general population. Obesity represents the number one risk factor for type-2 diabetes and a considerable body of epidemiological studies supports the relationship between type-2 diabetes and many cancers. In this review, we examine the obesity-type-2-diabetes–cancer relationships from a mechanistic perspective, and where appropriate, we highlight potential pharmaceutical and dietary interventions.
Keywords: obesity, diabetes, cancer, inflammation, adiponectin, leptin, NSAIDs, statins, metformin, life style
I. INTRODUCTION
The notion that diet affects health has been known for more than 100 years. In 1825, Jean Anthelme Brillat-Savarin wrote, in Physiologie du Gout, ou Meditations de Gastronomie Transcendante: “Dis-moi ce que tu manges, je te dirai ce que tu es,” or “Tell me what you eat and I will tell you what you are.”1 The German philosopher and anthropologist Ludwig Andreas von Feuerbach, in an essay titled Concerning Spiritualism and Materialism, wrote: “Der Mensch ist, was er iβt”: man is what he eats.2,3 These phrases promote the notion that to be fit and healthy, you need to have a good diet.
According to the World Health Organization (WHO), prevalence of obesity across the globe has approximately doubled since 1980. In the United States, approximately one-third of the adult population is obese and an additional one-third is overweight.4 Obesity is the fastest growing lethal disease in the Western and developing countries. People do not die from obesity itself but from its complications, which shorten the lifespan.5,6 Obesity leads to many other diseases including hypercholesterolemia, type-2 diabetes (T2D) and its complication, hypertension, cardiovascular disease, and cancer. The link between obesity and cancer is relatively underappreciated among the general population. As many as 20% of all cancers are due to obesity.7,8 In the United States in 2014, overweight and obesity were estimated to cause 40% of all cancers,9 including cancers of the esophagus, colon, liver, pancreas, gallbladder, breast (postmenopausal), ovary, thyroid, prostate, multiple myeloma, kidney, and many others.10 It is very unlikely that a single mechanism would be at the forefront of all obesity-related cancers. Although the mechanisms linking increased adiposity to malignancy remain incompletely understood, growing evidence suggests that complex interactions between multiple pathways regulating steroid hormone synthesis, insulin resistance, insulin-like growth factor, adipokine and cytokine production, the immune responses, the gastrointestinal (GI) microbiota, and chronic local and systemic inflammation may collectively explain the link between overweight/obesity and carcinogenesis.5,11 In this review, we examine the obesity-type-2-diabetes-cancer relationships from a mechanistic perspective and where appropriate we will highlight potential pharmaceutical and dietary interventions.
II. BODY MASS INDEX AND WHAT DOES IT MEAN?
Currently, the distinction between being overweight and obese is determined by the body mass index (BMI), which is calculated by weight in kilograms divided by height in meters squared.12 BMI is used in assessing obesity, which could potentially be due to its ease of calculation and minimal cost. BMI is divided into four different categories: underweight, normal, overweight, and obese, with BMIs of < 18.5, 18.5–24.9, 25.0–29.9, and ≤ 30, respectively.13 An individual with an ‘obese’ BMI (≤ 30 kg/m2) is at a higher risk of developing cancer than an individual with a BMI of < 25 kg/m2.12 On a global scale, 1.4 billion adults meet the requirement for being overweight and nearly 500 million adults meet the requirement for being obese.14
BMI as a means in determining obesity in adults is extremely limiting and flawed.15 A better way to assess obesity and fat deposits in adults would be to use visceral adipose tissue (VAT) and computed tomography (CT).16 Another suggestion is to use waist circumference ratios to determine obesity, such as that in the waist-to-hip ratio and waist-to-height ratio.12 Visceral fat is more strongly associated with adenocarcinoma in men than when using BMI alone.13 The location of the fat in the body of the individual plays a definitive role in the link between cancer mortality and obesity.17 A positive association was found between cancer mortality and obesity, specifically in the abdominal region.16 Colon, premenopausal breast, endometrium, and esophageal adenocarcinoma cancers have been associated with abdominal adiposity rather than with BMI alone.17
III. ADIPOSE TISSUE INFLAMMATION AND WAT VS. BAT
Adipose tissue inflammation is paramount in the promotion of cancer microenvironment and tumor progression.15 The inflammation sites closely resemble the microenvironment of a healing wound, promoting the production of proinflammatory mediators.15,18 There are two main types of adipose tissue, visceral and subcutaneous, with visceral adipocytes being more metabolically active.19
White adipose tissue (WAT) is a type of subcutaneous adipose tissue that stores energy and excess fat.20 In healthy individuals, WAT can comprise approximately a quarter of body mass21,22 and is a major physiological fuel source. In normal-weight individuals, the adipose tissue microenvironment (ATME) is anti-inflammatory in nature23 with resident macrophages that tend to be M2-polarized.24 These macrophages are triggered by Th2 cytokines and are associated with tissue remodeling and immunosuppression. On the other hand, it has been suggested that M1-polarized macrophages, which are pro-inflammatory, are active in the inflamed obese adipose tissue.25 These macrophages secrete proinflammatory mediators such as PGE2, IL-1β, IL-6, and TNF-α.24
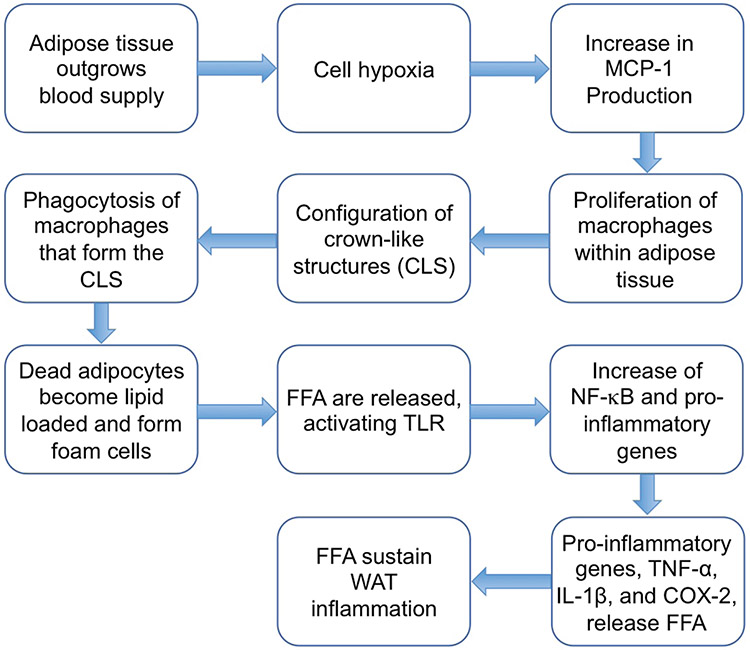
All available data strongly suggest that assessing WAT inflammation status is important in determining whether a patient may be at risk of developing cancer.15 Thus, developing new interventions that reduce WAT has given rise to a new strategy to prevent and treat cancer in obese patients.15 Figure 1 visually depicts the flow of WAT inflammation as discussed by Iyengar et al.15 Adipose tissue outgrows its blood vessels, which leads to hypoxia, adipocyte stress, and death may occur.26 This increases levels of monocyte chemoattractant protein-1 (MCP-1/CCL2), which is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages.27 MCP-1 stimulates proliferation of macrophages within adipose tissue,28 which then leads to formation crown-like structures (CLS), which are essentially dead or dying adipocytes.29 The macrophages that form CLS engage in phagocytosis of the dead or dying adipocyte and become lipid loaded, forming foam cells.30 Free fatty acids (FFAs) are then released from the adipocyte and from other sources, which can activate toll-like receptor (TLR) 4 on the macrophage plasma membrane, leading to increased NF-κB-dependent expression of proinflammatory genes, including TNF-α, IL-1β, and COX-2.31 Lipolysis and release of FFAs are further stimulated by TNF-α and other cytokines, thereby sustaining WAT inflammation.15 This mechanism is consistent with elevated levels of proinflammatory mediators that are found within CLS in visceral fat.15
FIG. 1:
Process of white adipose tissue (WAT) inflammation as described in Iyengar et al.15
Brown adipose tissue (BAT) is a form of adipose tissue located predominantly in the cervical area that contains cells equipped with large numbers of mitochondria and the unique protein, uncoupling protein1 (UCP1).20,32 BAT in newborn humans helps regulate energy expenditure by thermogenesis, but BAT in adult humans has been considered to have no physiologic relevance.33 Research has shown that BAT of adult and geriatric humans is inversely correlated with BMI, suggesting that BAT may potentially play a role in the adult human metabolic systems.33 Notably, WAT can convert to metabolically active fat (BAT) through the process of browning.22 However, the association between BAT activation with cancer progression is not well established.
IV. OBESITY AND CANCER
Excess weight is associated with an increased risk of multiple types of cancer. Almost 50% of cancers in postmenopausal women can be attributed to obesity.17 Obesity and overweight may increase the likelihood of dying from cancer.34 Abdominal obesity has been shown to increase the risk of cancer mortality by 24%.35 Here, we briefly highlight some of the cancers that are directly linked to obesity.
A. Breast Cancer
Obesity, defined by a BMI of ≥ 30 kg/m2, has been associated with an increased risk of estrogen-dependent breast cancer, specifically in postmenopausal women.36 Alternatively, obesity is associated with reduced breast cancer incidence in premenopausal women and is linked to the recurrence of breast cancer in both post- and premenopausal women.16 Obesity may be a risk factor for bala-like breast cancer regardless of menopausal status.16 In particular, it has been examined that the abundance of abdominal fat increases the risk for postmenopausal breast cancer.8 One study exemplified the link between obesity, inflammation, and aromatase in the mammary gland and visceral fat of mice.37 Necrotic adipocytes were observed in the mammary gland and visceral fat of mice, which formed crown-like structures.37 These crown-like structures are associated with proinflammatory mediators, such as TNF-α, IL-1β, COX-2, and were paralleled by elevated levels of aromatase expression and activity.15,37 Inflammation of white adipose tissue detected by the presence of CLS may be a biomarker for increased risk of cancer.37
B. Colorectal Cancer
Colorectal cancer (CRC) is one of the most common gastrointestinal malignant tumors in the world,12 and it has the highest incidence and mortality among gasterointestinal cancers. This particular cancer also has one of the highest rates of mortality and morbidity worldwide. CRC has been associated with the consumption of red and processed meat.12 After a CRC diagnosis, the consumption of carbohydrates in excess and high-sugar content beverages may increase the risk of recurrence and mortality.12 Excess weight in teenagers is linked with a 200% increase of colon cancer in adulthood.34 In the European Union, keeping a healthy weight was shown to reduce the annual incidence of CRC by up to 21,000 and the annual incidence of breast cancer by up to 13,000 in individuals.17 An increased risk of 60% for CRC has also been reported in men whose weight increased by more than 21 kg after reaching adulthood compared to those whose weight increased by only 1–5 kg.17 The correlation between relative risk of CRC in association with BMI is different in men and women. For men, the correlation is very good; however, in women, the relationship between BMI and the risk of CRC is complicated by the difference in fat distribution.5,7 A better yardstick would be to use waist circumference and waste–hip ratios.38 The mechanisms involved between obesity and CRC are likely to involve insulin and IGF-1 signaling, adipokines, and inflammation, all of which are discussed below.
C. Prostate Cancer
A large number of meta-analyses have indicated that obesity is associated with prostate cancer incidence.7,39-41 Prostate cancer and obesity also have a geographic component.7 Prostate-specific antigen (PSA) levels are lower in obese men42; thus, there is a lower likelihood of a PSA-driven biopsy. This is an important issue when comparing data from US studies, where PSA screening is prevalent, to data from other parts of the world, where PSA screening is less common.7 Notably, in the United States, prostate biopsies are guided by PSA screenings and because obese men have lower PSA levels, there is a reduced chance of undergoing biopsy compared to normal-weight men, lowering the detection rate of prostate cancer in obese men.16
Cytokines, such as IL-6, that are generated in inflamed WAT are known to activate the androgen receptor and promote cancer cell survival and proliferation in the prostate.15 Although only limited data are available, the increase in prevalence of prostate cancer is thought to be connected to lifestyle changes such as physical inactivity and higher intake of dietary fat and meat.43 An increased risk of high-grade, aggressive prostate cancer is also thought to be associated with high BMIs.44
D. Pancreatic Cancer
Obesity and pancreatic cancer (PC) are strongly associated. For example, there is a higher relative risk for PC for individuals whose BMI falls within the obese range (≥ 30), compared to those that are within of a healthy zone (< 25 )44; this applies to both men and women.45,46 In the US where there are a significant number of overweight adults, there is a two-fold increased risk of developing PC and mortality.47,48 The underlying cause appears to be due to inflammation that is caused by excess triglycerides leading to an increase in adipocyte number and size, which leads to hypoxia as a result of devascularization, and macrophage infiltration.20 This leads to local secretion of adiponectin, leptin, TNF-α, interleukins, and monocyte chemoattractant proteins47,49; all of which are discussed further in other sections below.
E. Esophageal Cancer
In the United States, the incidence of esophageal adenocarcinoma has significantly increased over the past few decades, which may in part be due to the increasing weight trends.13 Association between esophageal adenocarcinoma and individuals with a BMI ≥ 30 kg/m2 has been reported, and the risk increases as BMI increases.50,51 Many studies have identified obesity as a risk factor for esophageal squamous cell carcinoma and adenocarcinoma (EAC) in men and women.52 Obese patients have a higher prevalence of gastroesophageal reflux disease, which can lead to Barrett’s esophagus (BE) and intestinal metaplasia, which are precursors to EAC.5,53,54 A good correlation has been shown between abdominal fat/obesity and BE.55 Notably, obesity early in life increases the risk of EAC.5,56
F. Liver Cancer
Obesity has been recognized as a major independent risk factor for liver cancer, which has a 5-year survival rate of just 4%–8%.57 As fat accumulates, leading to liver dysfunction, the liver synthesizes more triglycerides, but it does not transport them out, which leads to the development of fatty liver disease, defined as triglyceride content > 5% of the organ’s weight.57,58 Fatty liver disease leads to the dysfunction of adipose tissue and creates a proinflammatory milieu and production of cytokines,58 which makes the liver more susceptible to tumor formation and tumor growth.
V. OBESITY AND MOLECULAR TARGETS IN CANCER
Many factors appear to be important in linking obesity and cancer. However, three main factors are linked to endocrine and paracrine dysregulation of adipose tissue in obesity, which are considered to be the hallmarks: the insulin–IGF-1 axis, steroid hormones, and adipocyte-derived cytokines.59,60
A. Insulin and IGF-1 Signaling
Insulin is an anabolic hormone secreted by the pancreatic β-cells; it is intimately involved in regulating carbohydrate, fat, and protein metabolisms. Its anabolic actions in the muscle, adipose tissue, and liver increase tissue mass, regulate glucose uptake, and synthesize nutrients. These anabolic effects are not directly related to carcinogenesis.5 In fact, in alloxan-induced diabetes, rats treated with the mammary tumor inducing carcinogen, 7,12-dimethylbenz(a)anthracene were completely free of tumors.5,61 Moreover, induction of alloxan diabetes in tumor-bearing rats produced the rapid regression of 90% of the tumors.61 These data show that tumors that are insulin dependent have a stringent requirement for insulin to grow. Generally, obese patients have hyperinsulinemia independently of T2D, potentially as a result of higher energy needs.5 In a study of 695 middle-aged, nondiabetic, and normoinsulinemic men, those with a BMI of ≥ 26.7 kg/m2 had a 6.6-fold increased risk of developing hyperinsulinemia, compared with men with body mass index of < 24.4 kg/m2.62 In hyperglycemia, the availability of nutrients is plentiful, and cancer cells metabolize glucose via the Warburg effect, which is a metabolic shift in ATP generation from oxidative phosphorylation to glycolysis, which means that cancer cells can produce energy through high rates of glycolysis.59
All available epidemiological data indicate a strong connection between T2D and certain cancers. The molecular basis for this association could involve the insulin-like growth factor 1 (IGF-1) signaling which is mitogenic under conditions of hyperglycemia and/or hyperinsulinemia, independently of the metabolic features of T2D.5
IGF-1, also called somatomedin C, is a hormone similar to insulin in its molecular structure63 that has an important role in childhood growth and anabolic effects in adults. IGF-1 is transported in blood by insulin-like growth factor-binding protein-1 (IGFBP)-1. Insulin is exclusively produced and secreted by pancreatic β cells, whereas IGF-1 is mainly produced in the liver. Insulin binds to the insulin receptor (IR) and IGF-1 binds to the IGF-1 receptor (IGF-1R). IGF-1 signaling pathway is involved in promotion of cell growth and inhibition of apoptosis. These oncogenic properties are mediated through the signal transduction crosstalk between two IGF-R-activated pathways: Ras/Raf/MEK/ERK/MAPK (Ras pathway) and PI3K/AKT (AKT pathway). The Ras and AKT pathways have been shown to upregulate key cell-cycle checkpoint proteins.64 Obesity can contribute to carcinogenesis by activating the insulin-IGF-1 pathway. Overweight individuals have elevated circulating levels of IGF-1,65 and low serum levels of IGFBP-1, which could confer increased susceptibility to cancer development.63,66,67 Notably, IGFBP has six iso-forms, and IGFBP-3 is the most abundant in humans.5,59 Blocking the IGF-1R can inhibit the cellular actions of IGF-1. The antibody CP-751781 (Figitumumab), which blocks IGF-1R, was tested in a phase 1 dose-escalation study of 6 patients with CRC with acceptable tolerability, expected side effects of hyperglycemia, and preliminary evidence of efficacy.68,69 In another phase 1 clinical trial assessing the safety and tolerability of dacomitinib-figitumumab combination therapy in patients with advanced solid tumors, the combination therapy was tolerable with significant dose reductions of both agents to less than the recommended single-agent phase II dose of each drug with some indications in efficacy.70 However, a number of studies have indicated that IGF-1R monoantibodies had no significant value in cancer treatment.71-74 Three sets of data from the following relatively recent clinical trials (NCT00372996, 2015; NCT00887159, 2015; NCT00684983, 2016) have also indicated the insignificant cancer curative value of anti–IGF-1R agents.75
B. Steroid Hormones
1. Estrogen
Hormonally driven cancers are increasing among postmenopausal women.11 Increasing BMI and obesity are pivotal risk factors for the development of postmenopausal hormone receptor-positive breast cancer and for endometrial cancer.76,77 In premenopausal women, the ovaries are the main organs responsible for estrogen production. However, in postmenopausal women, estrogen is produced locally within the adipose tissues and skin.78 Peripheral conversion of androgens to estrogens is catalyzed by the enzyme cytochrome P450 aromatase, which is encoded by the CYP19 gene.79 The rate of conversion of androgens to estrogens is elevated in postmenopausal women and with increasing BMI.80,81 In obesity, aromatase gene expression can also be regulated by other factors such as cytokines, prostaglandins, and hormones. For example, PGE2 is elevated in obesity, and it promotes the expression of aromatase in breast adipose tissues.82 Furthermore, multiple other interactive signaling pathways involving estrogen, and its receptors, insulin and IGF-1, and adipokines are also implicated in breast carcinogenesis.11
Inhibiting the enzymatic activity of aromatase to reduce estrogen production is an effective therapeutic strategy for management of estrogen receptor-positive breast cancer. A number of these agents are in clinical trials.83-85
2. Androgens
In prostate cancer cell lines, inflammatory cytokine such as IL-6 and IGF-1 have been shown to modulate the androgen receptor (AR) signaling.86 Also, IL-6 and other proinflammatory cytokines generated in inflamed WAT can activate the AR and promote cancer cell survival and proliferation in the prostate.15 Selective androgen receptor modulators (SARMs) have numerous possible clinical applications, with promise for the safe use in the treatment of cachexia, benign prostatic hyperplasia (BPH), hypogonadism, as well as breast and prostate cancers.87
C. Adipocyte-Derived Cytokines
Adipokines are produced mainly by WAT, preadipocytes, and mature adipocytes. Adipokines such as leptin and adiponectin have a significant role in many cancers, including that of breast, colon, pancreas, and HCC. Adipokines are bioactive proteins that are synthesized and secreted from the adipose tissue. They have an important role in lipid metabolism, insulin sensitivity, inflammation, angiogenesis, and cell proliferation.11,88,89 Metabolic homeostasis and the balance between cell proliferation and apoptosis are regulated in part by adipokines.11 In obesity, adipokines are deregulated, which ultimately may lead to cancer progression and metastasis.90
1. Leptin
Leptin is a 16 kD hormone that was first identified in adipocytes in 1994.91,92 It is primarily known for its role in the mammalian central nervous system, where it regulates food intake.59 Factors that alter leptin production and action include insulin, estrogen, and inflammatory mediators such as IL-1β, IL-6, and TNF-α.93 Lipopolysaccharides (LPS) (membrane components of gram-negative bacteria) also increase leptin expression in WAT.94 The actions of leptin in human cancer cell lines can be quite pleotropic. In a human colon-cancer cell line (HCT-116), leptin increased cell proliferation by activating the PI3K–AKT pathway; this effect was blocked by LY294002, a PI3K inhibitor.95 Cell proliferation of colon cancer cells (HT-29) was also shown to be mediated through the Jak2–STAT3-activating capacity of leptin.96 The proliferative and antiapoptotic effects of leptin were reduced by AG490, an inhibitor of JAK2, with LY294002, a phosphatidylinositol 3′-kinase (PI3 kinase) inhibitor, and by SP600125, a c-Jun NH2-terminal kinase (JNK) inhibitor.96 In vivo studies with Lepob/ob mice have shown less tumor formation when challenged with the carcinogen azoxymethane (AOM), suggesting a role for leptin in stimulating initiation of colon cancer.97 The role of leptin in human colon cancer is quite controversial. Studies have shown that men with colorectal adenomas had elevated circulating leptin levels98; other studies have indicated no correlation between leptin levels and increased in CRC when BMI and waist circumference were taken into account.99
Elevated circulating levels of leptin have also been observed in women with breast cancer100-102 and in some cases this has correlated with poorer prognosis.103 Leptin was shown to increase proliferation of breast cancer cell lines by activating the JAK–STAT and PI3K signaling pathways.104,105
Leptin has a role in pancreatic cancer (PC) pathogenesis. It binds to its receptors to mediate downstream signaling.106 Leptin receptor and hypoxia inducible factor-1 (HIF-1) are coexpressed in PC cell lines, and this has been associated with poor prognosis, decreased overall survival, and increased metastasis in PC patients. Silencing of HIF-1 inhibited leptin receptor expression in PC cells, suggesting that a positive feedback loop between HIF-1 and leptin receptor mediates PC progression.20,106 In PC, cell migration and invasion have been shown to involve Janus kinase 2 and signal transducer and activator of transcription 3 (JAK2/STAT3) pathway.20,107
2. Adiponectin
Adiponectin, also known as AdipoQ, is a 30-kilodalton polypeptide with a C-terminal globular domain similar to TNF-α. It has a significant role in insulin sensitization of tissues such as muscle and liver.20,108 It regulates carbohydrate as well as lipid metabolism through the adenosine monophosphate-activated protein kinase (AMPK) pathway, with its expression being decreased in obesity and diabetes. However, relatively recent studies strongly suggest that adiponectin modulates the activity of a ceramidase, which leads to decreased intracellular levels of ceramides, improved insulin sensitivity, and inhibition of apoptosis. These effects are mediated through activation of its cognate receptors AdipoR1 and AdipoR2.109 In a murine model of nonalcoholic steatohepatitis, adiponectin-knockout mice had significantly more hepatic tumors.110 Adiponectin also increased caspase-3 activation and apoptosis in HCC cell lines, via inhibition of serine–threonine-protein kinase mTOR (mTOR), in a c-JNK dependent manner.59,111 In vivo studies with nude mice, overexpression of adiponectin inhibited liver tumor growth and metastasis by suppression of tumor angiogenesis and downregulation of the ROCK/IP10/matrix metalloproteinase 9 pathway.112 Epidemiological studies strongly suggest that obesity in association with low levels of adiponectin is correlated with increased incidence of breast cancer.113
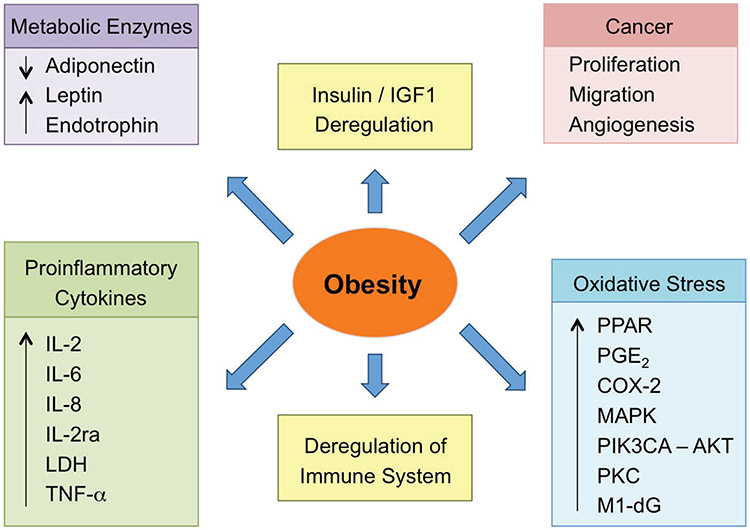
The biochemical features of obesity include IGF-1 deregulation, alterations in metabolic enzymes, some proinflammatory cytokines, deregulation of the immune system, increases in the oxidative stress markers. All of these lead to cancer angiogenesis, migration, and proliferation, as depicted in a schematic diagram in Fig. 2.
FIG. 2:
Schematic diagram of the biochemical features of obesity. Reprinted from Martinez-Useros and Garcia-Foncillas,12 with permission from Springer, Copyright 2016.
VI. DIABETES AND CANCER
The prevalence of diabetes is increasing, and in addition to 415 million adults who have diabetes, 318 million also have impaired glucose tolerance. Approximately 642 million people worldwide will have diabetes by the year 2040.114,115 T2D which used to be referred to adult-onset or non–insulin-dependent diabetes, accounts for > 90%–95% of all diabetes. T2D is a complex metabolic disorder essentially characterized by alterations in lipid metabolism, insulin resistance, and pancreatic β-cell dysfunction.116 Obesity is the number one risk factor for T2D; it may lead to elevated serum triglycerides, hypertension, and insulin resistance.117
Compared to women of normal weight, those with a BMI of 30 kg/m2 and 35 kg/m2 have 28 times and 93 times greater risk of developing diabetes, respectively.34,118 In normal-weight men, T2D occurred a rate of 1.6 per 1000 person years, whereas in obese men this number was 11.4,119 and obese men developed T2D 7 times more often than normal-weight men. The rate at which T2D is increasing in the youth is alarming. Between 2001 to 2016, the prevalence of T2D increased by ~ 35% among youth aged 10–19 years.120
Patients with diabetes experience a roughly 20%–25% higher cancer incidence than individuals without diabetes, and they also have a higher mortality rate.34,121 Both men and women with T2D were shown to have a two-fold increased risk of developing hepatocellular carcinomas (HCC), compared with nondiabetics.122 Furthermore, this risk appears to be independent of age, obesity, and sex.123 Also, obesity and hepatic steatosis have been shown to be independent predictors of HCC, suggesting that metabolic derangements and diabetes work synergistically to increase HCC risk.123 A considerable body of evidence supports an association between diabetes and an increased risk of breast cancer.124,125
A considerable number of epidemiological studies support the relationship between T2D and colorectal cancer. Patients with T2D were shown to have a 27% increased risk of colorectal cancer compared to nondiabetics.126 The evidence is even more overwhelming when it comes to pancreatic cancer. Based on data from 23 cohort and 13 case-controlled studies,5 the World Cancer Research Fund panel concluded that there is a “convincing increased risk” of pancreatic adenocarcinoma related to body adiposity and a “probable increased risk” with abdominal adiposity.127 A comprehensive meta-analysis showed that individuals with T2D for < 4 years had a 50% increased risk of pancreatic cancer than did patients with diabetes for ≥ 5 years.128 Although the connection between T2D and PC is convincing, with T2D patients having a two-fold increased risk in developing PC, the literature strongly suggests that insulin resistance, one of the hallmarks of T2D, and diabetes may in some cases be a consequence of PC.128-130
VII. PHARMACEUTICAL INTERVENTIONS
A. Metformin
Metformin is a biguanide that is widely used as an insulin-sparing agent to treat diabetes. Some studies have indicated that those who used this drug, as a cohort had lower total cancer incidence.131,132 However, there are also other studies that do not support these general observations.133 A meta-analysis reported that metformin use was associated with reduced breast cancer incidence and mortality,134 but other studies failed to demonstrate a protective effect of metformin on breast cancer incidence133,135,136 or mortality137 or a difference in the frequency of estrogen receptor (ER)-positive breast cancer.138 In a tumor murine model of ER-positive breast cancer, metformin treatment slowed down tumor growth and this was shown to be through suppression of obesity associated adipokine levels and reduced Akt/mTOR pathway activation.139 In a randomized clinical trial where patients were administered metformin for 1 month prior to breast cancer resection, the metformin-treated patients had reduced Ki67 expression, a cellular marker for proliferation, in HER2-positive resected tumors.140 In preclinical studies, metformin suppressed overexpression of the HER2 protein by inhibiting the Akt/mTOR pathway141 and delayed the onset of adenocarcinoma in a transgenic murine model of HER2-positive breast cancer.142
In diabetic men, a considerable body of data shows an inverse association between metformin use and prostate cancer risk143-145 and prostate cancer-specific mortality.146,147 Following primary therapy for prostate cancer, those receiving metformin had reduced risk of recurrence.145
At the cellular level, metformin inhibited proliferation of the human prostate cancer cell lines, LNCaP, PC3, and DU145, causing G0/G1 or S phase cell cycle arrest via inhibition of cyclinD1, while having no effect on normal epithelial prostate cells. Also, in a xenograft mouse model of prostate cancer, metformin slowed tumor growth.148
Metformin has been shown to prevent carcinogen-induced pancreatic cancer induction in hamsters maintained on a high-fat diet.149 Notably, the growth of the pancreatic cell lines’ PANC1 and MIAPaCa-2 tumor xenografts were significantly reduced after daily intraperitoneal treatment with metformin.150
Metformin use has also been associated with reductions in GI malignancies including gastric and CRC.135,151,152 Interestingly, the use of metformin has been shown to improve traditional chemotherapeutic response rates.153 The underlying anti-cancer action of metformin includes the inhibition of the mammalian target of rapamycin complex 1 (mTORC1) pathway, which plays an important role in metabolism, growth, and proliferation of cancer cells.154 The mTOR pathway is activated through IGF-1 and insulin ligand binding to their respective receptors, which causes the insulin receptor substrate (IRS) signal to transmit to phosphoinositide 3-kinase (PI3K), and Akt/protein kinase B (PKB), which indirectly activate mTORC1.34,155
B. Thiazolidinediones
Thiazolidinediones (also called glitazones, TZDs) are a class of orally available hypoglycemic medications that may be used for the treatment of T2D. They act by increasing insulin sensitivity through the peroxisome proliferator-activated receptor gamma, PPAR-γ. Attachment of the ligand to the PPAR-γ receptor activates a series of genes that are involved in glucose and fatty acid metabolism; the overall effect is an increase in insulin effect.156 In a recent study using Umuc-3 and 5637 bladder cancer cells, rosiglitazone and pioglitazone markedly induced cell cycle G2 arrest and apoptosis, which resulted in inhibition of cell proliferation in vitro and suppression of tumor growth in vivo. The underlying mechanism involved marked inhibition of PI3K-Akt pathway.157
Efatutazone is a novel third-generation thiazolidinedione that is at least 50 times more potent than rosiglitazone and 500 times more potent than troglitazone for PPAR-γ response element activation and the inhibition of cancer cell growth.158 It has shown good activity in cell culture, inhibiting proliferation of human pancreatic and anaplastic thyroid tumor cell.159
A recent meta-analysis indicated a protective association between TZDs use and CRC risk in patients with T2D.160 PPAR-γ agonists suppress CRC cell growth in vitro and in vivo by inhibiting the mTOR and LKB-1 pathways.161-163
C. Statins
Cholesterol comes from the diet and is also endogenously synthesized. It is an essential component of cellular plasma membrane in animals and has a crucial role in maintaining membrane fluidity.164 Cholesterol is also a precursor of endogenous sex steroid hormones; thus, it has a potential role in breast and prostate cancers, two hormone-dependent tumors. Cholesterol that is derived from the diet can increase the risk of ER-positive breast cancer development,165,166 and some data strongly suggest an increased risk of breast cancer with increasing serum cholesterol levels.167 There is good correlation between high dietary cholesterol intake and increased risk of ER-positive, but not ER-negative, breast cancer.167
Cholesterol content of normal prostate epithelial cells is relatively high, and this increases further during progression to cancer, suggesting a role in prostate cancer progression.164 As reviewed in Allott and Hursting,16 elevated cholesterol levels may be associated with increased risk of aggressive prostate cancer168-171 but not total prostate cancer,171,172 although other studies have reported no association between cholesterol or its subfractions and prostate cancer risk.173,174
In prostate cancer cell lines and in murine xenograft models, cholesterol has promoted cell growth.175 Conversely, cholesterol reduction slowed tumor growth in xenograft murine models of human prostate cancer by lowering intratumoral androgen levels.176
Statins are cholesterol-lowering drugs that are widely used in both men and women. As a class, they competitively and reversibly inhibit the enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, which is the rate-limiting enzyme for cholesterol biosynthesis in the liver. This leads to decreases in serum low-density lipoprotein cholesterol (LDL-C), triglyceride, and cholesterol levels.163
Statin use has been associated with reduced total cancer risk177 and lower cancer-specific mortality.178 However, there does not seem to be any association between statin use and total breast cancer risk,179,180 although the UK Cancer Registry suggests a protective effect of statin use on breast cancer-specific mortality.181 One study reported lower rates of ER-negative breast cancer among statin users,182 but other researchers found no such association.183 However, a strong inverse association between statin uses and risk of breast cancer recurrence and mortality has been reported.184,185
In prostate cancer, there does not seem to be an association between statin use and total prostate cancer risk186-189: however, an inverse association between statin use and risk of aggressive disease has been detected.186,187 Postdiagnosis statin use has been associated with reduced risk of recurrence.190
D. Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
Obesity is associated with subclinical chronic inflammation88,191 and elevated levels of COX-2 expression.192 The anti-inflammatory properties of NSAIDs occur primarily through the inhibition of the COX pathway, which lowers PG levels.193 Considerable evidence suggests that the long-term use of NSAIDs is associated with a significant reduction in many forms of cancer: colon,194-197 breast,198-200 pancreas,201 bladder,202,203 head and neck,204 esophageal,205 ovarian,206,207 prostate,208 hepatocellular,209 and skin.210-212 Of these, cellular and molecular mechanisms of CRC, which in many ways represent the prototypical case for cancer prevention, have been studied most extensively.
Some data support the upregulation of the aromatase pathway by COX-2-mediated PGE2213 and therefore support the notion that NSAIDs should be effective in the management of ER-positive breast cancer. NSAID use has been shown to reduce serum estradiol levels in women with breast cancer.214 NSAIDs are also protective against breast cancer incidence and mortality.215,216 COXIBs or COX-2 specific NSAIDs, such as celecoxib, may have a role in preventing HER2-positive breast cancer217; however, the general impact of NSAIDs in this subtype of breast cancer needs to be investigated further.
In prostate cancer, COX-2 expression is also high relative to the normal adjacent tissue.218 A meta-analysis reported an inverse association between NSAID use and aggressive prostate cancer risk.219 However, regular NSAIDs use can lower PSA levels.220
VIII. OTHER MODES OF TACKLING OBESITY
A. Lifestyle Interventions: Diet
Research suggests that weight reduction aids in restoring pathways that were once deregulated due to obesity.15,16 Restricting caloric intake for 28 days in a proof-of-principle study resulted in individuals having improved inflammatory gene expression signatures in the subcutaneous WAT.16 Caloric excess and diets that are rich in calories, overabundant in alcohol and animal fats, and/or lacking in plant products lead to increased cancer incidence.16,17 There is an association between low-cancer risk and higher intake of fruits and vegetables,17 but more research should be conducted because this may only be relative to those eating high-protein, high-fat diets.17 Low levels of vitamin D in individuals may be responsible for 20% of the cancer risk association with an increased BMI.17 Dieting by restricting caloric intake may lead to WAT inflammation improvement and, in turn, to reduced incidence of cancer related to a high BMI.15
B. Physical Activity
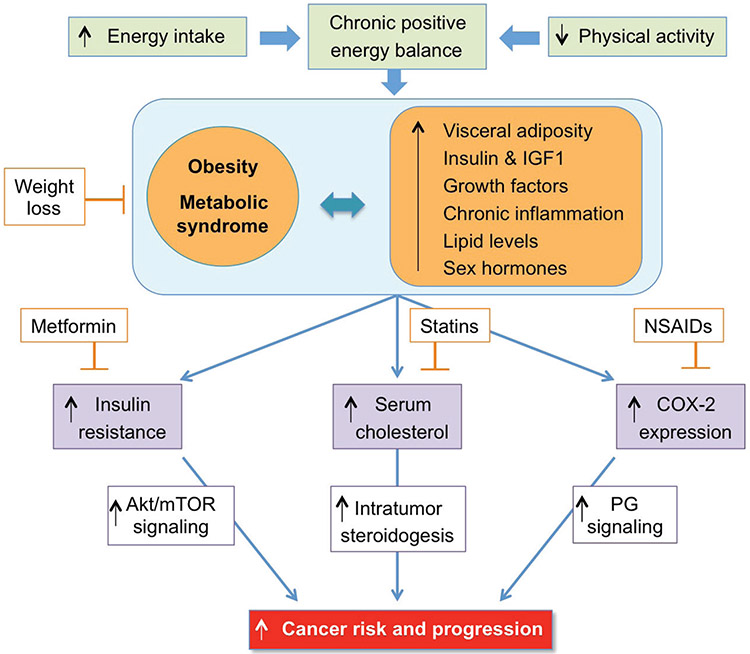
To prevent the onset of chronic diseases, individuals should partake in daily exercise. Physical exercise can lower the risk of breast cancer and has been correlated to decreases in the risks of colon and breast cancer development.17 Men and women who are physically active have a 30%–40% decreased risk in developing colon cancer compared to those who are less active, with the risk declining at higher levels of physical activity.221 Physically active women have a 20%–30% reduction in risk of developing breast cancer compared to women who are physically inactive.221 Physical activity decreases the risk of pancreatic cancer in individuals who are overweight.46 Figure 3 represents a schematic diagram linking obesity with cancer and the rationale for the use of various pharmacological agents.
FIG. 3:
Putative mechanisms linking obesity with cancer risk and progression: lessons from studies of chemopreventive agents. Reprinted from Allott and Hursting,16 with permission from Bioscientifica Limited.
IX. CONCLUSIONS AND FUTURE DIRECTIONS
Because the prevalence of obesity and T2D is on the rise, we need to achieve greater insight into the mechanisms that relate the obesity-T2D-cancer axis. Certainly there are links between obesity and T2D, between obesity and cancer, and between T2D and cancer. Collectively, these relationships are of public health interest; hence, more clinical studies are needed to decipher them. Patients that are clinically obese may require pharmacological interventions as well as lifestyle changes. To that end, more studies are needed to access the utility of agents such as metformin, statins, NSAIDs, and TZDs in the management of such patients. It would help if BMI measurements or at least another yardstick became routinely available in general medical practice. Considering that 1.6 million deaths occur internationally each year that are essentially obesity related, targeting this problem may ultimately decrease cancer-related deaths and also heart disease, stroke, and diabetes.
ACKNOWLEDGMENT
This study was supported in part by the National Institutes of Health (grant nos. R24 DA018055 and R01GM123508).
ABBREVIATIONS
- T2D
type-2 diabetes
- JAK2
Janus kinase 2
- STAT3
signal transducer and activator of transcription 3
- AMPK
adenosine monophosphate-activated protein kinase
- MCP-1/CCL2
monocyte chemoattractant protein-1
- FFA
free fatty acid
- WAT
white adipose tissue
- BAT
brown adipose tissue
- CLS
crown-like structures
- BMI
body mass index
- CRC
colorectal cancer
- PC
pancreatic cancer
- HCC
hepatocellular carcinoma
- IGFBP-1
insulin-like growth factor-binding protein-1
- PI3 kinase
phosphatidylinositol 3′-kinase
- PSA
prostate-specific antigen
- HIF-1
hypoxia inducible factor-1
- NSAIDs
nonsteroidal anti-inflammatory drugs
- ATME
adipose tissue microenvironment
- TNF-α
tumor necrosis factor-α
- IL-1β
interleukin 1β
- IL-6
interleukin-6
- PG
prostaglandin
- COX-2
cyclooxygenase-2
- ER
estrogen receptor
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- mTORC1
mammalian target of rapamycin complex 1
REFERENCES
- 1.Brillat-Savarin JA. Physiologie du Goût, ou Méditations de Gastronomie Transcendante; ouvrage théorique, historique et á l’ordre du jour, dédié aux Gastronomes parisiens, par un Professeur, membre de plusieurs sociétés littéraires et savantes. France; 1825. (in French). [Google Scholar]
- 2.Abbt I. [Is man what he eats?]. Schweizerische Rundschau fur Medizin Praxis = Revue Suisse de medecine Praxis. 1993;82(38):1029–32. [PubMed] [Google Scholar]
- 3.Cherno M. Feuerbach’s “Man is what he eats”: a rectification. J Hist Ideas. 1963;24(3):397–406. [Google Scholar]
- 4.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–93. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, UK). 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 8.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, Puckett M, Richardson LC. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morbid Mortal Wkly Rep. 2017;66(39):1052–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gucalp A, Iyengar NM, Hudis CA, Dannenberg AJ. Targeting obesity-related adipose tissue dysfunction to prevent cancer development and progression. Semin Oncol. 2016;43(1):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Zhuang H, Liu Y. The association between obesity factor and esophageal caner. J Gastrointest Oncol. 2012;3(3):226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strong AL, Burow ME, Gimble JM, Bunnell BA. Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells (Dayton, OH). 2015;33(2):318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. 2015;22(6):R365–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obesity. 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agurs-Collins T, Ross SA, Dunn BK. The many faces of obesity and its influence on breast cancer risk. Frontiers Oncol. 2019;9:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothuraju R, Rachagani S, Junker WM, Chaudhary S, Saraswathi V, Kaur S, Batra SK. Pancreatic cancer associated with obesity and diabetes: an alternative approach for its targeting. J Exp Clin Cancer Res. 2018;37(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrud LG, Flegal KM, Looker AC, Everhart JE, Harris TB, Shepherd JA. Body composition data for individuals 8 years of age and older: US population, 1999–2004. Vital Health Stat 11. 2010(250):1–87. [PMC free article] [PubMed] [Google Scholar]
- 22.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nature Rev Endocrinol. 2019;15(3):139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical Cancer Res. 2013;19(22):6074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investigation. 2007;117(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1-2):20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflammation. 2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metabol. 2014;19(1):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, Chen A, Bluher M, Shai I, Rudich A. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metabol. 2013;98(3):1173–81. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Bio Chem. 2001;276(20):16683–89. [DOI] [PubMed] [Google Scholar]
- 32.Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes. 2009;58(7):1482–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashamalla M, Youssef I, Yacoub M, Jayarangaiah A, Gupta N, Ray J, Iqbal S, Miller R, Singh J, McFarlane SI. Obesity, diabetes and gastrointestinal malignancy: the role of metformin and other anti-diabetic therapy. Glob J Obes Diabet Metab Syndr. 2018;5(2):008–14. [Google Scholar]
- 35.Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, Hand GA, Blair SN. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009;45(10):1831–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahid H, Subbaramaiah K, Iyengar NM, Zhou XK, Chen IC, Bhardwaj P, Gucalp A, Morrow M, Hudis CA, Dannenberg AJ, Brown KA. Leptin regulation of the p53-HIF1α/PKM2-aromatase axis in breast adipose stromal cells: a novel mechanism for the obesity-breast cancer link. Int J Obes (Lond). 2018;42(4):711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila). 2011;4(3):329–46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556–65. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91(3):421–30. [DOI] [PubMed] [Google Scholar]
- 40.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. [DOI] [PubMed] [Google Scholar]
- 41.Hu MB, Liu SH, Jiang HW, Bai PD, Ding Q. Obesity affects file biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: a dose-response meta-analysis of 29,464 patients. PLoS One. 2014;9(9):e106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC, Moul JW, Freedland SJ. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275–80. [DOI] [PubMed] [Google Scholar]
- 43.Hsing AW, Sakoda LC, Chua S. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86(3):s843–57. [DOI] [PubMed] [Google Scholar]
- 44.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132(6):2208–25. [DOI] [PubMed] [Google Scholar]
- 45.Gumbs AA. Obesity, pancreatitis, and pancreatic cancer. Obesity Surg. 2008;18(9):1183–87. [DOI] [PubMed] [Google Scholar]
- 46.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MT, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–29. [DOI] [PubMed] [Google Scholar]
- 47.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6(8):852–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11 (12):886–95. [DOI] [PubMed] [Google Scholar]
- 50.Doyle SL, Donohoe CL, Finn SP, Howard JM, Lithander FE, Reynolds JV, Pidgeon GP, Lysaght J. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107(2):196–204. [DOI] [PubMed] [Google Scholar]
- 51.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC, Ahsan H, West AB, Rotterdam H, Niwa S, Fraumeni JF. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90(2):150–55. [DOI] [PubMed] [Google Scholar]
- 52.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarker Prevent. 2006;15(5):872–78. [DOI] [PubMed] [Google Scholar]
- 53.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53(8):1070–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122(6):1569–91. [DOI] [PubMed] [Google Scholar]
- 55.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133(2):403–11. [DOI] [PubMed] [Google Scholar]
- 56.de Jonge PJ, Steyerberg EW, Kuipers EJ, Honkoop P, Wolters LM, Kerkhof M, van Dekken H, Siersema PD. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2006;101(7):1421–29. [DOI] [PubMed] [Google Scholar]
- 57.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56(3):704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Divella R, Mazzocca A, Daniele A, Sabba C, Paradiso A. Obesity, nonalcoholic fatty liver disease and adipocytokines network in promotion of cancer. Int J Bio Sci. 2019;15(3):610–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocrine Rev. 2011;32(4):550–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heuson JC, Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972;32(2):226–32. [PubMed] [Google Scholar]
- 62.Lakka HM, Salonen JT, Tuomilehto J, Kaplan GA, Lakka TA. Obesity and weight gain are associated with increased incidence of hyperinsulinemia in non-diabetic men. Hormone Metabol Res = Hormon- und Stoffwechselforschung = Hormones Metabolisme. 2002;34(9):492–98. [DOI] [PubMed] [Google Scholar]
- 63.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28. [DOI] [PubMed] [Google Scholar]
- 64.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials–early lessons. J Mamm Gland Biol Neoplasia. 2008;13(4):471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Gronbaek H, Tjonneland A, Halkjaer J, Dossus L, Boeing H, Kroger J, Trichopoulou A, Zylis D, Trichopoulos D, Boutron-Ruault MC, de Lauzon-Guillain B, Clavel-Chapelon F, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Gils CH, Peeters PH, Gram IT, Rodriguez L, Jakszyn P, Molina-Montes E, Navarro C, Barricarte A, Larranaga N, Khaw KT, Rodwell S, Rinaldi S, Slimani N, Norat T, Gallo V, Riboli E, Kaaks R. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Human Biol. 2011;38(2):194–202. [DOI] [PubMed] [Google Scholar]
- 66.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nature Rev Endocrinol. 2011;7(1):11–24. [DOI] [PubMed] [Google Scholar]
- 67.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metabol. 2006;17(8):328–36. [DOI] [PubMed] [Google Scholar]
- 68.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5834–40. [DOI] [PubMed] [Google Scholar]
- 69.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calvo E, Soria JC, Ma WW, Wang T, Bahleda R, Tolcher AW, Gernhardt D, O’Connell J, Millham R, Giri N, Wick MJ, Adjei AA, Hidalgo M. A Phase I clinical trial and independent patient-derived xenograft study of combined targeted treatment with dacomitinib and figitumumab in advanced solid tumors. Clin Cancer Res. 2017;23(5):1177–85. [DOI] [PubMed] [Google Scholar]
- 71.Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, Benner RJ, Scranton JR, Olszanski AJ, Jassem J. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2014;32(19):2059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moran T, Felip E, Keedy V, Borghaei H, Shepherd FA, Insa A, Brown H, Fitzgerald T, Sathyanarayanan S, Reilly JF, Mauro D, Hsu K, Yan L, Johnson DH. Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with non-small-cell lung cancer: a phase I/II randomized trial. Exper Hematol Oncol. 2014;3(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK, Blanke CD. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase 1b and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120(19):2980–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Cutsem E, Eng C, Nowara E, Swieboda-Sadlej A, Tebbutt NC, Mitchell E, Davidenko I, Stephenson J, Elez E, Prenen H, Deng H, Tang R, McCaffery I, Oliner KS, Chen L, Gansert J, Loh E, Smethurst D, Tabernero J. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res. 2014;20(16):4240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu X, Wu Z, Dong W, Zhang T, Wang L, Pang Z, Ma W, Du J. Update of IGF-1 receptor inhibitor (ganitumab, dalotuzmnab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy. Oncotarget. 2017;8(17):29501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ (Clin Res). 2007;335(7630):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Rev. 1994;15(3):342–55. [DOI] [PubMed] [Google Scholar]
- 79.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocrine Rev. 2009;30(4):343–75. [DOI] [PubMed] [Google Scholar]
- 80.Chen J. Multiple signal pathways in obesity-associated cancer. Obesity Rev. 2011;12(12):1063–70. [DOI] [PubMed] [Google Scholar]
- 81.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metabol. 2012;23(2):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE. Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res. 2007;67(18):8914–22. [DOI] [PubMed] [Google Scholar]
- 83.Bahrami N, Sauer T, Engebretsen S, Aljabri B, Bemanian V, Lindstrom J, Luders T, Kristensen V, Lorentzen A, Loeng M, Odegard HP, Kvaloy JO, Vestol IB, Geisler SB, Gravdehaug B, Gundersen JM, Geisler J. The NEOLETEXE trial: a neoadjuvant cross-over study exploring the lack of cross resistance between aromatase inhibitors. Future Oncol (London, UK). 2019;15(32):3675–82. [DOI] [PubMed] [Google Scholar]
- 84.Tang M, O’Connell RL, Amant F, Beale P, McNally O, Sjoquist KM, Grant P, Davis A, Sykes P, Mileshkin L, Moujaber T, Kennedy CJ, deFazio A, Tan K, Antill Y, Goh J, Bonaventura T, Scurry J, Friedlander M. PARAGON: A Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol. 2019;154(3):531–38. [DOI] [PubMed] [Google Scholar]
- 85.Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, Hazra A, Kammler R, Thurlimann B, Colleoni M, Viale G, Brown M, Tamimi RM. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1-98. Breast Cancer Res. 2019;21(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinogen. 2011;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective androgen receptor modulators: current knowledge and clinical applications. Sex Med Rev. 2019;7(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, Kakarala M, Brodie A, Berger NA. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila). 2012;5(11):1260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83. [DOI] [PubMed] [Google Scholar]
- 90.Housa D, Housova J, Vemerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55(3):233–44. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. [DOI] [PubMed] [Google Scholar]
- 92.Friedman J. The long road to leptin. J Clin Investig. 2016;126(12):4727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obesity Rel Metabol Disord. 2002;26(11):1407–33. [DOI] [PubMed] [Google Scholar]
- 94.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Investig. 1996;97(9):2152–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei M, Wang L, Zhong M. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37(1):91–101. [DOI] [PubMed] [Google Scholar]
- 96.Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colored Dis. 2007;22(4):401–9. [DOI] [PubMed] [Google Scholar]
- 97.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60(10):1363–71. [DOI] [PubMed] [Google Scholar]
- 98.Chia VM, Newcomb PA, Lampe JW, White E, Mandelson MT, McTiernan A, Potter JD. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol Biomarker Prevent. 2007;16(12):2697–703. [DOI] [PubMed] [Google Scholar]
- 99.Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, Rinaldi S, Fedirko V, Romieu I, Riboli E, Gunter MJ, Westphal S, Overvad K, Tjonneland A, Halkjaer J, Racine A, Boutron-Ruault MC, Clavel-Chapelon F, Kaaks R, Lukanova A, Trichopoulou A, Lagiou P, Trichopoulos D, Mattiello A, Pala V, Palli D, Tumino R, Vineis P, Buckland G, Sanchez MJ, Amiano P, Huerta JM, Barricarte A, Menendez V, Peeters PH, Soderberg S, Palmqvist R, Allen NE, Crowe FL, Khaw KT, Wareham N, Pischon T. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012;72(20):5328–37. [DOI] [PubMed] [Google Scholar]
- 100.Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The Association between leptin level and breast cancer: a meta-analysis. PLoS One. 2013;8(6):e67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao JQ, Zhang QK, Zhou YX, Chen LH, Wu PF. Association between circulating leptin concentration and G-2548A gene polymorphism in patients with breast cancer: a meta-analysis. Arch Med Sci. 2019;15(2)275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barone I, Giordano C, Bonofiglio D, Ando S, Catalano S. The weight of obesity in breast cancer progression and metastasis: clinical and molecular perspectives. Semin Cancer Biol. 2019; in press. [DOI] [PubMed] [Google Scholar]
- 103.Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118(6):1414–19. [DOI] [PubMed] [Google Scholar]
- 104.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105(4):956–64. [DOI] [PubMed] [Google Scholar]
- 105.Thiagarajan PS, Zheng Q, Bhagrath M, Mulkearns-Hubert EE, Myers MG, Lathia JD, Reizes O. STAT3 activation by leptin receptor is essential for TNBC stem cell maintenance. Endocr Relat Cancer. 2017;24(8):415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren H, Jia L, Zhao T, Zhang H, Chen J, Yang S, Liu J, Yu M, Hao J. Hypoxia inducible factor (HIF)-1alpha directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014;354(1):172–80. [DOI] [PubMed] [Google Scholar]
- 107.Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao F, Yao M, Gu J, Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6(18):16120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Bio Chem. 1996;271(18):10697–703. [DOI] [PubMed] [Google Scholar]
- 109.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47(4):556–64. [DOI] [PubMed] [Google Scholar]
- 111.Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139(5):1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZX, Cheung JS, Wu EX, Sun CK, Poon RT, Fan ST. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res. 2010;16(3):967–77. [DOI] [PubMed] [Google Scholar]
- 113.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metabol. 2007;92(4):1510–16. [DOI] [PubMed] [Google Scholar]
- 114.International Diabetes Federation. IDF Diabetes Atlas, 7th edition. Belgium; 2015. [Google Scholar]
- 115.Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed Pharmacother = Biomedecine Pharmacotherapie. 2017;95:605–13. [DOI] [PubMed] [Google Scholar]
- 116.Podell BK, Ackart DF, Richardson MA, DiLisio JE, Pulford B, Basaraba RJ. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis Model Mechan. 2017;10(2):151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gheibi S, Bakhtiarzadeh F, Jeddi S, Farrokhfall K, Zardooz H, Ghasemi A. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide Bio Chem. 2017;64:39–51. [DOI] [PubMed] [Google Scholar]
- 118.Barnes AS. The epidemic of obesity and diabetes: trends and treatments. Texas Heart Inst J. 2011;38(2):142–44. [PMC free article] [PubMed] [Google Scholar]
- 119.Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assessment (Winchester, UK). 2004;8(21):iii–iv, 1–182. [DOI] [PubMed] [Google Scholar]
- 120.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scappaticcio L, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine. 2017;56(2):231–39. [DOI] [PubMed] [Google Scholar]
- 122.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–48. [DOI] [PubMed] [Google Scholar]
- 123.Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B, Cheung R, Wong RJ. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Digest Dis Sci. 2016;61(2):636–45. [DOI] [PubMed] [Google Scholar]
- 124.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabet Care. 2010;33(7):1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ (Clin Res). 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 126.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26(11):863–76. [DOI] [PubMed] [Google Scholar]
- 127.Thompson R. Preventing cancer: the role of food, nutrition and physical activity. J Fam Health Care. 2010;20(3):100–2. [PubMed] [Google Scholar]
- 128.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Brit J Cancer. 2005;92(11):2076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129(2):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogawa Y, Tanaka M, Inoue K, Yamaguchi K, Chijiiwa K, Mizumoto K, Tsutsu N, Nakamura Y. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94(9):2344–49. [DOI] [PubMed] [Google Scholar]
- 131.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clin Res). 2005;330(7503):1304–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3(11):1451–61. [DOI] [PubMed] [Google Scholar]
- 133.Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, Ioannidis JP. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabet Care. 2014;37(9):2522–32. [DOI] [PubMed] [Google Scholar]
- 134.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–18. [DOI] [PubMed] [Google Scholar]
- 135.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Safety. 2015;24(8):865–74. [DOI] [PubMed] [Google Scholar]
- 137.Lega IC, Shah PS, Margel D, Beyene J, Rochon PA, Lipscombe LL. The effect of metformin on mortality following cancer among patients with diabetes. Cancer Epidemiol Biomarker Prevent. 2014;23(10):1974–84. [DOI] [PubMed] [Google Scholar]
- 138.Besic N, Satej N, Ratosa I, Horvat AG, Marinko T, Gazic B, Petric R. Long-term use of metformin and the molecular subtype in invasive breast carcinoma patients—a retrospective study of clinical and tumor characteristics. BMC Cancer. 2014;14:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fuentes-Mattei E, Velazquez-Torres G, Phan L, Zhang F, Chou PC, Shin JH, Choi HH, Chen JS, Zhao R, Chen J, Gully C, Carlock C, Qi Y, Zhang Y, Wu Y, Esteva FJ, Luo Y, McKeehan WL, Ensor J, Hortobagyi GN, Pusztai L, Fraser Symmans W, Lee MH, Yeung SC. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014;106(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, Pruneri G, Serrano D, Schwab M, Hofmann U, Mora S, Aristarco V, Macis D, Bassi F, Luini A, Lazzeroni M, Bonanni B, Pollak MN. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat. 2014;148(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle (Georgetown, TX). 2009;8(1):88–96. [DOI] [PubMed] [Google Scholar]
- 142.Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle (Georgetown, TX). 2010;9(1):188–97. [DOI] [PubMed] [Google Scholar]
- 143.Clyne M. Prostate cancer: metformin–the new wonder drug? Nat Rev Urol. 2014;11(7):366. [DOI] [PubMed] [Google Scholar]
- 144.Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, Mucci LA, Adami HO, Sorensen HT. Metformin use and prostate cancer risk. Eur Urol. 2014;66(6):1012–20. [DOI] [PubMed] [Google Scholar]
- 145.Yu H, Yin L, Jiang X, Sun X, Wu J, Tian H, Gao X, He X. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One. 2014;9(12):e116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31(25):3069–75. [DOI] [PubMed] [Google Scholar]
- 147.Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol Biomarker Prevent. 2014;23(10):2111–18. [DOI] [PubMed] [Google Scholar]
- 148.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576–86. [DOI] [PubMed] [Google Scholar]
- 149.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120(5):1263–70. [DOI] [PubMed] [Google Scholar]
- 150.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69(16):6539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tseng CH. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging. 2016;8(8):1636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Skinner HD, McCurdy MR, Echeverria AE, Lin SH, Welsh JW, O’Reilly MS, Hofstetter WL, Ajani JA, Komaki R, Cox JD, Sandulache VC, Myers JN, Guerrero TM. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol (Stockholm, Sweden). 2013;52(5):1002–9. [DOI] [PubMed] [Google Scholar]
- 154.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13(10):433–42. [DOI] [PubMed] [Google Scholar]
- 155.Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Trans Med. 2014;2(6):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spencer CM, Markham A. Troglitazone. Drugs. 1997;54(1):89–101; discussion 2. [DOI] [PubMed] [Google Scholar]
- 157.Lv S, Wang W, Wang H, Zhu Y, Lei C. PPARgamma activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer. 2019;19(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Copland JA, Marlow LA, Kurakata S, Fujiwara K, Wong AK, Kreinest PA, Williams SF, Haugen BR, Klopper JP, Smallridge RC. Novel high-affinity PPARgamma agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25(16):2304–17. [DOI] [PubMed] [Google Scholar]
- 159.Shimazaki N, Togashi N, Hanai M, Isoyama T, Wada K, Fujita T, Fujiwara K, Kurakata S. Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor gamma agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer. 2008;44(12):1734–43. [DOI] [PubMed] [Google Scholar]
- 160.Liu Y, Jin PP, Sun XC, Hu TT. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: a meta-analysis. Saudi J Gastroenterol. 2018;24(2):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burton JD, Goldenberg DM, Blumenthal RD. Potential of peroxisome proliferator-activated receptor gamma antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008;2008:494161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Okumura T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J Gastroenterol. 2010;45(11):1097–102. [DOI] [PubMed] [Google Scholar]
- 163.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–24. [DOI] [PubMed] [Google Scholar]
- 164.Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013;1835(2):219–29. [DOI] [PubMed] [Google Scholar]
- 165.Fagherazzi G, Fabre A, Boutron-Ruault MC, Clavel-Chapelon F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: results from the prospective E3N cohort. Eur J Cancer Prevent. 2010;19(2):120–25. [DOI] [PubMed] [Google Scholar]
- 166.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Annals Oncol. 2012;23(2):491–500. [DOI] [PubMed] [Google Scholar]
- 167.Jakovljevic J, Touillaud MS, Bondy ML, Singletary SE, Pillow PC, Chang S. Dietary intake of selected fatty acids, cholesterol and carotenoids and estrogen receptor status in premenopausal breast cancer patients. Breast Cancer Res Treat. 2002;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 168.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer. 2012;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22(11):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103(11):885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123(7):1693–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20(7):1181–92. [DOI] [PubMed] [Google Scholar]
- 174.Jacobs EJ, Stevens VL, Newton CC, Gapstur SM. Plasma total, LDL, and HDL cholesterol and risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control. 2012;23(8):1289–96. [DOI] [PubMed] [Google Scholar]
- 175.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–31. [PubMed] [Google Scholar]
- 176.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7(1):e30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100(2):134–39. [DOI] [PubMed] [Google Scholar]
- 178.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–802. [DOI] [PubMed] [Google Scholar]
- 179.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treatment. 2012;135(1):261–69. [DOI] [PubMed] [Google Scholar]
- 180.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23(34):8606–12. [DOI] [PubMed] [Google Scholar]
- 181.Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology (Cambridge, MA). 2015;26(l):68–78. [DOI] [PubMed] [Google Scholar]
- 182.Kumar AS, Benz CC, Shim V, Minami CA, Moore DH, Esserman LJ. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarker Prevent. 2008;17(5):1028–33. [DOI] [PubMed] [Google Scholar]
- 183.Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, Jay A, Bock C, Cote M, Petrucelli N, Rosenberg CA, Peters U, Agalliu I, Budrys N, Abdul-Hussein M, Lane D, Luo J, Park HL, Thomas F, Wactawski-Wende J, Simon MS. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol Biomarker Prevent. 2013;22(10):1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109(3):573–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123(4):899–904. [DOI] [PubMed] [Google Scholar]
- 187.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120(4):833–43. [DOI] [PubMed] [Google Scholar]
- 189.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295(1):74–80. [DOI] [PubMed] [Google Scholar]
- 190.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2014;114(5):661–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2(4):356–65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 193.Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv Pharmacol (San Diego, CA). 2009;57:31–89. [DOI] [PubMed] [Google Scholar]
- 194.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet (London). 2012;379(9826):1602–12. [DOI] [PubMed] [Google Scholar]
- 195.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–99. [DOI] [PubMed] [Google Scholar]
- 196.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–90. [DOI] [PubMed] [Google Scholar]
- 197.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, Ford LG, Jacobs EJ, Jankowski JA, La Vecchia C, Law M, Meyskens F, Rothwell PM, Senn HJ, Umar A. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.de Pedro M, Baeza S, Escudero MT, Dierssen-Sotos T, Gomez-Acebo I, Pollan M, Llorca J. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2015;149(2):525–36. [DOI] [PubMed] [Google Scholar]
- 199.Yiannakopoulou EC. Aspirin and NSAIDs for breast cancer chemoprevention. Eur J Cancer Prevent. 2014;24(5):416–21. [DOI] [PubMed] [Google Scholar]
- 200.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100(20):1439–47. [DOI] [PubMed] [Google Scholar]
- 201.Cui XJ, He Q, Zhang JM, Fan HJ, Wen ZF, Qin YR. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43(1):135–40. [DOI] [PubMed] [Google Scholar]
- 202.Nicastro HL, Grubbs CJ, Margaret Juliana M, Bode AM, Kim MS, Lu Y, You M, Milne GL, Boring D, Steele VE, Lubet RA. Preventive effects of NSAIDs, NO-NSAIDs, and NSAIDs plus difluoromethylornithine in a chemically induced urinary bladder cancer model. Cancer Prev Res (Phila). 2014;7(2):246–54. [DOI] [PubMed] [Google Scholar]
- 203.Daugherty SE, Pfeiffer RM, Sigurdson AJ, Hayes RB, Leitzmann M, Schatzkin A, Hollenbeck AR, Silverman DT. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am J Epidemiol. 2011;173(7):721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Becker C, Wilson JC, Jick SS, Meier CR. Non-steroidal anti-inflammatory drugs and the risk of head and neck cancer: a case-control analysis. Int J Cancer. 2015;137(10):2424–31. [DOI] [PubMed] [Google Scholar]
- 205.Sun L, Yu S. Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma. Dis Esophagus. 2011;24(8):544–49. [DOI] [PubMed] [Google Scholar]
- 206.Baandrup L, Kjaer SK, Olsen JH, Dehlendorff C, Friis S. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann Oncol. 2015;26(4):787–92. [DOI] [PubMed] [Google Scholar]
- 207.Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, Brinton LA, Webb PM, Nagle CM, Jordan SJ, Risch HA, Rossing MA, Doherty JA, Goodman MT, Lurie G, Kjaer SK, Hogdall E, Jensen A, Cramer DW, Terry KL, Vitonis A, Bandera EV, Olson S, King MG, Chandran U, Anton-Culver H, Ziogas A, Menon U, Gayther SA, Ramus SJ, Gentry-Maharaj A, Wu AH, Pearce CL, Pike MC, Berchuck A, Schildkraut JM, Wentzensen N. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106(2):djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, Grubb R, Church TR, Chia D, Zhou C, Chu LW, Huang WY, Peters U, Kirsh VA, Chatterjee N, Leitzmann MF, Hayes RB, Hsing AW. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Brit J Cancer. 2012;107(1):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, Hollenbeck AR, Freedman ND, McGlynn KA. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Clouser MC, Roe DJ, Foote JA, Harris RB. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol Drug Safety. 2009;18(4):276–83. [DOI] [PubMed] [Google Scholar]
- 211.Elmets CA, Viner JL, Portland AP, Cantrell W, Lin HY, Bailey H, Kang S, Linden KG, Heffernan M, Duvic M, Richmond E, Elewski BE, Umar A, Bell W, Gordon GB. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102(24):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goodman JR, Grossman D. Aspirin and other NSAIDs as chemoprevention agents in melanoma. Cancer Prev Res (Phila). 2014;7(6):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137(12):5739–42. [DOI] [PubMed] [Google Scholar]
- 214.Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, Vogel VG, Weissfeld JL. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarker Prevent. 2008;17(3):680–87. [DOI] [PubMed] [Google Scholar]
- 215.Allott EH, Tse CK, Olshan AF, Carey LA, Moorman PG, Troester MA. Non-steroidal anti-inflammatory drug use, hormone receptor status, and breast cancer-specific mortality in the Carolina Breast Cancer Study. Breast Cancer Res Treat. 2014;147(2):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291(20):2433–40. [DOI] [PubMed] [Google Scholar]
- 217.Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, Thaler HT, Muller WJ, Du B, Brown AM, Dannenberg AJ. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62(19):5405–7. [PubMed] [Google Scholar]
- 218.Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89(3):589–96. [PubMed] [Google Scholar]
- 219.Liu Y, Chen JQ, Xie L, Wang J, Li T, He Y, Gao Y, Qin X, Li S. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. 2014;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chang SL, Harshman LC, Presti JC Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28(25):3951–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee IM. Physical activity and cancer prevention–xdata from epidemiologic studies. Med Sci Sports Exerc. 2003;35(11):1823–27. [DOI] [PubMed] [Google Scholar]