Abstract
Introduction:
Dexmedetomidine improves intrapulmonary shunt in thoracic surgery and minimizes inflammatory response during one-lung ventilation (OLV). However, it is unclear whether such benefits translate into less postoperative pulmonary complications (PPCs). Our objective was to determine the impact of dexmedetomidine on the incidence of PPCs after thoracic surgery.
Methods:
Major databases were used to identify randomized trials that compared dexmedetomidine versus placebo during thoracic surgery in terms of PPCs. Our primary outcome was atelectasis within 7 days after surgery. Other specific PPCs included hypoxemia, pneumonia, and acute respiratory distress syndrome (ARDS). Secondary outcome included intraoperative respiratory mechanics (respiratory compliance [Cdyn]) and postoperative lung function (forced expiratory volume [FEV1]). Random effects models were used to estimate odds ratios (OR).
Results:
Twelve randomized trials, including 365 patients in the dexmedetomidine group and 359 in the placebo group, were analyzed in this meta-analysis. Patients in the dexmedetomidine group were less likely to develop postoperative atelectasis (2.3% vs 6.8%, OR 0.42, 95%CI 0.18–0.95, P = 0.04; low certainty) and hypoxemia (3.4% vs 11.7%, OR 0.26, 95%CI 0.10–0.68, P = 0.01; moderate certainty) compared to the placebo group. The incidence of postoperative pneumonia (3.2% vs 5.8%, OR 0.57, 95%CI 0.25–1.26, P = 0.17; moderate certainty) or ARDS (0.9% vs 3.5%, OR 0.39, 95%CI 0.07–2.08, P = 0.27; moderate certainty) was comparable between groups. Both intraoperative Cdyn and postoperative FEV1 were higher among patients that received dexmedetomidine with a mean difference of 4.42 mL/cmH2O (95%CI 3.13–5.72) and 0.27 L (95%CI 0.12–0.41), respectively.
Conclusion:
Dexmedetomidine administration during thoracic surgery may potentially reduce the risk of postoperative atelectasis and hypoxemia. However, current evidence is insufficient to demonstrate an effect on pneumonia or ARDS.
Keywords: Dexmedetomidine, Thoracic surgery, Pulmonary atelectasis, Pneumonia, Hypoxemia, Lung compliance
1. Introduction
One-lung ventilation (OLV) is commonly used in thoracic surgery to facilitate access to the operative site. The non-physiologic collapse of the operative lung and overdistention of the contralateral lung may result in postoperative lung injury due to alveolar strain, oxidative stress, ischemia-reperfusion, and damage of the endothelial glycocalyx [1]. It is therefore necessary to implement evidence-based interventions targeting protective lung ventilation strategy including low tidal volume (i.e., low tidal volume TV [< 6 mL/kg] and use of positive end-expiratory pressure [PEEP]) [2,3], parenchymal-sparing lung resections [4], judicious perioperative fluid administration and selective use of anesthetics shown to have minimal effect on adaptive physiologic processes such as respiratory drive and hypoxic pulmonary vasoconstriction (HPV) [5].
Dexmedetomidine is an alpha-2 (α2) agonist with analgesic and sedation properties that has increasingly been used to reduce postoperative opioid consumption [6]. In experimental models under OLV, dexmedetomidine has been shown to minimize mechanical ventilation-induced lung injury through the inhibition of inflammatory pathways [7], thus enhancing pulmonary function recovery and improving respiratory mechanics [7–9]. There is also clinical evidence to suggest dexmedetomidine may improve respiratory mechanics as well as to prevent non-cardiopulmonary complications in adult cardiac and noncardiac surgical patients [10–12], Despite growing evidence of the physiological benefits of dexmedetomidine, the statistical power of individual studies has not been sufficient to draw definitive conclusions. We hypothesized that dexmedetomidine may improve pulmonary outcomes after thoracic surgery under OLV. The primary objective of this study was to investigate the effects of dexmedetomidine on postoperative pulmonary complications (PPCs) after thoracic surgery through the pooled analysis of randomized trials available in the literature.
2. Methods
Design.
We conducted a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane guidelines. Study protocol was pre-registered in PROSPERO (CRD42022383244), which was amended to include the additional secondary outcomes: intraoperative respiratory mechanics (dynamic respiratory compliance [Cdyn]), peak inspiratory pressure, plateau pressure, and postoperative lung function (forced expiratory volume [FEV1]).
Inclusion and exclusion criteria.
Five major databases (PubMed, Embase, Web of Science, Google Scholar, and the China National Knowledge Infrastructure) were used to identify randomized trials that assessed the impact of intraoperative intravenous dexmedetomidine infusion versus placebo on PPCs in thoracic surgery. Search strategy included the following MeSH terms: “thoracic surgery”, “dexmedetomidine”, “pulmonary complications”, “thoracoscopy”, “one-lung ventilation”, “lobectomy”, “segmentectomy”, from inception to January 5th, 2023. We applied no language restrictions. Exclusion criteria were observational studies, use of additional anesthetic adjuncts (e.g., ketamine, nitric oxide), lack of pulmonary outcomes, inhaled administration of study drugs, and cardiac surgeries. Literature search was conducted by two researchers independently. References of studies were also searched manually for relevant trials. Once the abstract was analyzed by the searching reviewer and deemed appropriate, we further studied the full text of the article. In the event of a disagreement, it was resolved through discussion. A third author (M.C.G.) was consulted when there were disagreements in the inclusion of articles. Data were abstracted into a standardized format in Microsoft Excel 2016 (Microsoft Corp, Redmond, Washington, USA). Extracted data pertained to the demographic profile of the patients, country of the trial, disease, procedure performed, surgical approach, anesthetic technique, sample size, incidence of pulmonary complications, infusion rate of dexmedetomidine, use of loading dose. We searched cross-references of relevant articles to ensure enrollment of all eligible studies.
Outcomes.
Our primary outcome was postoperative atelectasis within 7 days after surgery. We also assessed other specific PPCs, such as pneumonia, hypoxemia, and acute respiratory distress syndrome (ARDS) within 7 days after surgery. For this meta-analysis, we did not restrict our analysis to a specific outcome definition. Secondary outcome included intraoperative respiratory mechanics (respiratory compliance [Cdyn]) measured 30 min after OLV and postoperative lung function (forced expiratory volume [FEV1]) measured on postoperative day (POD) 1 and 2. Data extraction was performed by two independent researchers.
Methodological quality assessment.
We used the Cochrane risk of bias assessment tool to assess risk of bias. Each randomized trial was assessed based on seven domains of potential bias (random sequence generation, allocation concealment, blinding of intervention, blinding of outcome assessment, incomplete outcome data, selection reporting and other bias). The overall risk of bias of individual studies was classified as high risk if at least two domains were determined at high risk or if there were more than two domains of unclear risk, moderate risk if at least two domains were determined at unclear risk, and low risk if all the domains were determined at low risk.
Certainty of the evidence.
The GRADE (grading of recommendations, assessment, development, and evaluation) system was used to assess the quality of evidence as high, moderate, low, or very low.
Statistical analysis.
For dichotomous outcomes, pooled odds ratios (OR) were used to estimate the effect size of dexmedetomidine. We used random-effects modeling (DerSimonian and Laird) to perform this metaanalysis. The 95% confidence intervals (CI) were calculated using the Mantel-Haenszel method. For continuous outcomes (e.g., FEV1 and Cdyn), mean difference was computed at the study level and standardized mean difference (SMD) was calculated to pool the results across all studies. The extent of heterogeneity between the trials was quantified using the I2 statistic. Publication bias was estimated using the Begg and Egger’s test, although the value of such testing was only applied for meta-analysis of >10 studies. We planned to conduct subgroup analyses by type of anesthesia (total intravenous anesthesia [TIVA] versus inhaled), lung protective ventilation settings (TV < 6 mL/kg), and surgical approach (open vs minimally invasive). A p-value <0.05 was considered statistically significant. Statistical analysis was performed using Stata 13.0 (Stata Corp, College Station, TX). We also used trial sequential analysis (TSA) to estimate and correct for the effect of smaller trials which are often overruled when results from adequately powered studies emerged. We used Trial Sequential Analysis software 0.9.
3. Results
Study characteristics.
Of the initial 747 articles initially obtained from the literature search, 90 trials were filtered after excluding duplicates (n = 260) and screening title and abstracts (n = 397). We excluded 49 articles due to their retrospective design, 27 due to the lack of pulmonary outcomes, 1 trial that used a bundle of other interventions, and 1 trial that used inhaled dexmedetomidine. In total twelve randomized trials [13–24], comprising 365 patients in the dexmedetomidine group and 359 in the placebo group, were included and analyzed in this meta-analysis (Table 1). In all trials the intervention group consisted of a loading dose (1μg/kg) followed by an infusion of dexmedetomidine (0.3–0.5μg/kg/h), except for 2 trials that only used continuous infusion without loading dose. Eight trials were conducted in China, and 4 from other Asian countries (i.e., 1 from India, 2 from Korea, and 1 from Taiwan). Six trials used lung protective ventilation strategies (TV ≤ 6 mL/kg), 2 trials did not use protective ventilation, and 3 did not report ventilator parameters. Fig. 1 illustrates the flowchart for the section of articles in this meta-analysis.
Table 1.
Summary of trials evaluating the clinical effect dexmedetomidine in one-lung ventilation.
| Study (sample size) | Country | Surgery | Dexmedetomidine dose |
Type of anesthesia | Ventilatory settings |
Lung resection | |||
|---|---|---|---|---|---|---|---|---|---|
| Loading dose | Infusion rate | TV | PEEP | FiO2 | |||||
| Shi et al. (n = 120) [19] | China | Open | None | 0.5 μg/kg/h | TIVA | 7 mL/kg | N/A | N/A | N/A |
| Xie et al. (n = 116) [21] | China | VATS | 1 μg/kg | 0.3 μg/kg/h | TIVA | 6 mL/kg | 3 cmH2O | 100% | Lobectomy |
| Meng et al. (n = 40) [18] | China | VATS | 1 μg/kg | 0.5 μg/kg/h | TIVA | 6–8 mL/kg | 5 cmH2O | 100% | Lobectomy |
| Zhang et al. (n = 56) [22] | China | N/A | 1 μg/kg | 0.5 μg/kg/h | Inhaled | 6 mL/kg | 5 cmH2O | N/A | N/A |
| Wu et al. (n = 60) [20] | Taiwan | VATS | None | 0.5 μg/kg/h | Inhaled | 5 mL/kg | 5 cmH2O | 50% | Lobectomy |
| Jannu et al. (n = 80) [15] | India | VATS | 1 μg/kg | 0.5 μg/kg/h | Inhaled | N/A | N/A | N/A | Lob, Segm, Wedge |
| Lee et al. (n = 50) [16] | Korea | VATS | 1 μg/kg | 0.5 μg/kg/h | Inhaled | 6 mL/kg | 3 cmH2O | 60% | Lobectomy |
| Zhu et al. (n = 67) [24] | China | VATS | 1 μg/kg | 0.5 μg/kg/h | TIVA | 6 mL/kg | N/A | 80% | Lob, Segm, Wedge |
| Lee et al. (n = 100) [17] | Korea | VATS | None | 1 μg/kg | Inhaled | N/A | N/A | N/A | Lob, Segm, Wedge |
| Zhou et al. (n = 112) [23] | China | N/A | 1 μg/kg | 0.3 μg/kg/h | N/A | N/A | N/A | N/A | N/A |
| Guo et al. (n = 124) [14] | China | N/A | 1 μg/kg | 0.4 μg/kg/h | TIVA | N/A | N/A | N/A | N/A |
| Fan et al. (n = 50) [13] | China | VATS | 1 μg/kg | 0.5 μg/kg/h | Inhaled | 6 mL/kg | N/A | 60% | Lobectomy |
Fig. 1.
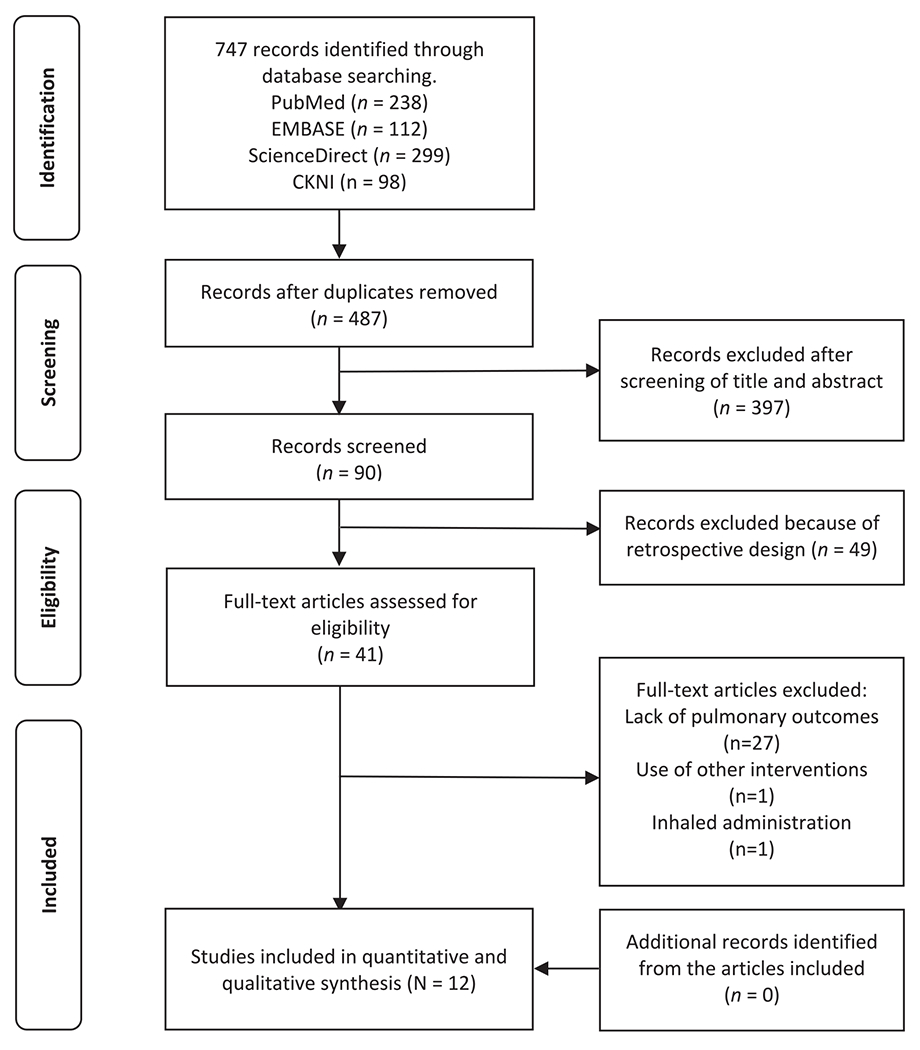
PRISMA flowchart of trial selection.
Pulmonary complications.
Nine trials reported data of one or more postoperative pulmonary complications. Patients in the dexmedetomidine group were less likely to develop atelectasis (2.3% vs 6.8%, OR 0.42, 95%CI 0.18–0.95, P = 0.04; I2 = 0%, P for heterogeneity = 0.71; Fig. 2) and hypoxemia (3.4% vs 11.7%, OR 0.26, 95%CI 0.10–0.68, P = 0.01; I2 = 0%, P for heterogeneity = 0.97; Supplemental Fig. 1) compared to the placebo group. There was small, but not statistically significant difference in the incidence of postoperative pneumonia (3.2% vs 5.8%, OR 0.57, 95%CI 0.25–1.26, P = 0.17; I2 = 0%, P for heterogeneity = 0.94; Fig. 3) and ARDS (OR 0.39, 95%CI 0.07–2.08, P = 0.27; I2 = 0%, P for heterogeneity = 0.97; Supplemental Fig. 2).
Fig. 2.
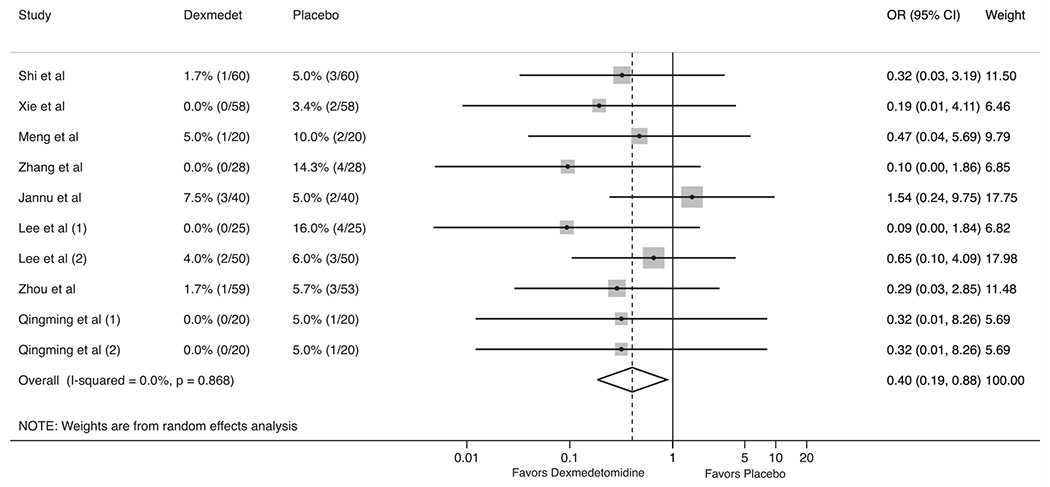
Forest plot for the impact of dexmedetomidine on postoperative atelectasis in thoracic surgery.
Fig. 3.
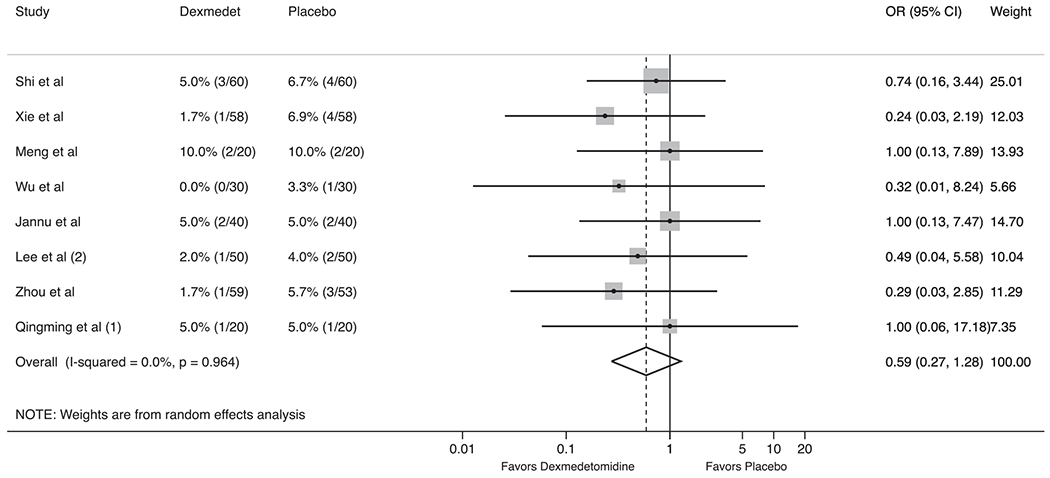
Forest plot for the impact of dexmedetomidine on postoperative pneumonia in thoracic surgery.
For both the primary and secondary outcomes, we examined the sensitivity of the summary estimates to the omission of each study [25]. This sensitivity analysis did not reveal a significant effect for all pulmonary complications after removing trials sequentially (Supplemental Fig. 3). Influence trial analysis showed variable results (Supplemental Fig. 4), most importantly Zhang et al. for atelectasis and Shi et al. for hypoxemia. There was evidence of statistically significant publication bias (Egger’s test P = 0.02) and asymmetry in the funnel plot for atelectasis (Supplemental Fig. 5). For both the primary and secondary outcomes, we examined the sensitivity of the results to the Hartung-Knapp-Sidik-Jonkman modification for random-effects meta-analysis by applying this modification [26,27]. In all cases, the modification does not produce wider confidence intervals.
Secondary outcomes.
Five trials reported the effect of dexmedetomidine on intraoperative respiratory mechanics, and only two trials reported results of postoperative lung function (i.e., FEV1). After 30 min of OLV, Cdyn was significantly higher among patients that received dexmedetomidine with a mean difference of 4.42 mL/cm2O (95%CI 3.13–5.72, I2 = 0%, P for heterogeneity = 0.80; Supplemental Fig. 3), while plateau pressures were significantly lower with a mean difference of −2.59 (95%CI −4.56 to −0.62, I2 = 85%, P for heterogeneity<0.01; Supplemental Fig. 6). Postoperatively, lung function was slightly better in the dexmedetomidine group at POD 1 (FEV1 0.29 L, 95%CI 0.10–0.49, I2 = 54%, P for heterogeneity = 0.14; Supplemental Fig. 7) and POD 2 (FEV1 0.27 L, 95%CI 0.12–0.41, I2 = 0%, P for heterogeneity = 0.80; Supplemental Fig. 7) compared to the placebo group. Length of hospital stay was reported in 5 trials. Although patients who received dexmedetomidine experienced shorter length of hospital stay compared to placebo (MD −0.85, 95%CI −1.56 to −0.15, P = 0.018, I2 = 18%, P for heterogeneity = 0.29; Supplemental Fig. 8), the results failed to reach statistical significance after the exclusion of Jannu et al as part of the sensitivity analysis (MD −0.58, 95%CI −1.22 to 0.06, P = 0.078, I2 = 0%, P for heterogeneity = 0.96).
Subgroup analysis.
Analysis by type of anesthesia (total intravenous anesthesia [TIVA] versus inhaled anesthesia) showed no evidence of subgroup effects for atelectasis (OR 0.32, 95%CI 0.07–1.37, P = 0.12 among TIVA trials vs OR 0.41, 95%CI 0.16–1.09, P = 0.07 among inhaled anesthetic trials) or pneumonia (OR 0.58, 95%CI 0.20–1.65, P = 0.31 among TIVA trials vs OR 0.63, 95%CI 0.16–2.45, P = 0.50 among inhaled anesthetic trials). The protective effect of dexmedetomidine against postoperative atelectasis remained in the subgroup of trials that used low tidal volume (TV < 6 mL/kg, OR 0.12, 95% 0.02–0.67; Supplemental Fig. 9), but lost statistical significance for those with high tidal volume (TV > 6 mL/kg, OR 0.38, 95%CI 0.07–2.07). There was no effect on the rates of pneumonia in both subgroups (Supplemental Fig. 10). Due to the limited number of studies, we were not able not conduct subgroup analyses by surgical approach as well as for secondary outcomes.
Risk of bias and certainty of evidence.
The trials included in this meta-analysis had low to moderate risk of bias. Supplemental Fig. 11 shows the summary of the risk of bias assessment, which raised important considerations regarding the lack of personnel blinding (6 trials) and allocation concealment (4 trials). The certainty of the evidence was low for atelectasis due to risk of bias and inconsistencies in sensitivity analysis (Table 2). There was moderate certainty of the rest of pulmonary outcomes.
Table 2.
Summary of evidence and GRADE assessment of certainty and quality of the evidence in this meta-analysis.
| No. trials | Risk of bias | Inconsistency | Indirectness | Imprecision | Absolute Effect |
Relative Effect |
Certainty | |
|---|---|---|---|---|---|---|---|---|
| Events / Intervention | Events / Control | OR (95% CI) | ||||||
| Postoperative atelectasis (follow up: 3 days) | 8/340 (2.3%) |
23/334 (6.9%) |
OR 0.42 (95% CI 0.18–0.95) |
⊕⊕ΟΟ | ||||
| 8 | Serious | Serious | Not serious | Not serious | Low | |||
| Postoperative hypoxemia (follow up: 3 days) | 6/177 (3.4%) |
20/171 (11.7%) |
OR 0.26 (95% CI 0.10–0.68) |
⊕⊕⊕Ο | ||||
| 3 | Not serious | Serious | Not serious | Not serious | Moderate | |||
| Postoperative pneumonia (follow up: 3 days) | 10/317 (3.2%) |
18/311 (5.8%) |
OR 0.57 (95% CI 0.25–1.26) |
⊕⊕⊕Ο | ||||
| 7 | Serious | Not serious | Not serious | Not serious | Moderate | |||
| Postoperative ARDS (follow up: 3 days) | 1/113 (0.9%) |
4/113 (3.5%) |
OR 39 (95% CI 0.07–2.08) | ⊕⊕⊕Ο | ||||
| 3 | Not serious | Serious | Not serious | Not serious | Moderate | |||
| Respiratory compliance (follow up: 30 min after OLV) |
MD 4.42 (95% CI 3.13 to 5.72) |
⊕⊕⊕Ο | ||||||
| 3 | Serious | Not serious | Not Serious | Serious | – | – | Moderate | |
| Plateau pressure (follow up: 30 min after OLV) |
MD −2.59 (95% CI −4.56 to −0.62) |
⊕⊕ΟΟ | ||||||
| 3 | Serious | Not serious | Not Serious | Serious | – | – | Low | |
| Peak inspiratory pressure (follow up: 30 min after OLV) | – | – |
MD −3.32 (95% CI −7.82 to 1.19) |
⊕⊕ΟΟ | ||||
| 2 | Serious | Not serious | Not serious | Serious | Low | |||
| Forced expiratory volume FEV1 (follow up: day 1 after surgery) | – | – |
MD 0.29 (95% CI 0.10 to 0.49) |
⊕⊕⊕Ο | ||||
| 2 | Not serious | Not serious | Not serious | Serious | Moderate | |||
| Forced expiratory volume FEV1 (follow up: day 2 after surgery) | – | – |
MD 0.27 (95% CI 0.12 to 0.41) |
⊕⊕⊕Ο | ||||
| 2 | Not serious | Serious | Not serious | Not serious | Moderate | |||
| Length of hospital stay | – | – | ⊕⊕ΟΟ | |||||
| 4 | Not serious | Serious | Serious | Not serious | MD | Low | ||
CI: Confidence interval; MD: Mean difference; OR: Odds ratio; ARDS: acute respiratory distress syndrome.
4. Discussion
This meta-analysis including 724 patients from 12 randomized controlled trials analyzed the impact of intraoperative dexmedetomidine on pulmonary outcomes after thoracic surgery. We found that intraoperative dexmedetomidine may potentially reduce the risk of postoperative atelectasis and hypoxemia. We also observed an improvement in respiratory mechanics (e.g., Cdyn and plateau pressure) during OLV among patients receiving dexmedetomidine. The level of certainty of the current clinical evidence is low to moderate, which highlights the need of further studies with better methodological quality and larger sample size.
Dexmedetomidine is has been investigated for a wide array of potentially beneficial effects in the perioperative setting. It is increasingly utilized as an opioid-sparing anesthetic, largely due to its limited impact on respiratory drive [28]. Recent evidence has shown several benefits of dexmedetomidine in cardiothoracic surgery, including reduced incidence of AKI and postoperative delirium [10]. There is also evidence that dexmedetomidine may be protective against myocardial ischemia [29,30]. Our meta-analysis demonstrated that patients who received intraoperative dexmedetomidine during OLV experienced less postoperative atelectasis and improved oxygenation. Some postulate that dexmedetomidine has a direct anti-inflammatory effect through the inhibition of a cascade of cytokines (i.e., TNF, IL-1, IL-6, IL-8) and lipid peroxidation [8], which are partially responsible for acute lung injury [31]. Other studies have demonstrated lower ventilation/perfusion (V/Q) mismatch after dexmedetomidine administration, which may explain improved postoperative oxygenation. Similarly, histopathological studies have shown less alveolar damage after dexmedetomidine administration, which can be attributed to the attenuation of ischemia-reperfusion due to sympatholytic effects as well as preservation of the glycocalyx [32–34]. Supplemental Table 1 summarizes the postulated mechanisms of lung protection that have been associated with dexmedetomidine.
Optimizing respiratory mechanics during thoracic surgery can be challenging due to ventilation of a single lung and changes in respiratory compliance due to extrinsic forces (i.e., mediastinum shift from gravity and limited diaphragmatic excursion) [31]. In addition to the implementation of lung protective strategies (i.e., low tidal volume ventilation and alveolar recruitment) [35], dexmedetomidine has been studied as a potential pharmacological intervention to minimize lung injury secondary to OLV. It is thought that dexmedetomidine enhances hypoxic pulmonary vasoconstriction by increasing nitric oxide in lung circulation, thereby decreasing shunt fraction and improving oxygenation [36]. Additionally, dexmedetomidine enhances airway smooth muscle relaxation through inhibition of alpha-2 adrenoreceptors [37].
This study has several limitations. First, all included trials were small in sample size and event number, with fewer than 5 events in each treatment group for all trials. As a result, we observed wide confidence intervals for all meta-analyzed effect estimates, including intervals overlapping the null for the outcomes of pneumonia and ARDS. Larger trials are needed to understand whether there is a clinically significant difference in the incidence of these uncommon events between treatment groups. Additionally, the specific definition of the outcomes was not always reported and could vary significantly between the trials. Similarly, there was high heterogeneity in studies examining plateau pressures and pulmonary function tests. Second, trial characteristics varied with respect to dexmedetomidine loading dose and infusion rate, type of anesthesia, ventilatory settings, and resection type. We attempted to mitigate potential heterogeneity through use of a random effects model, which does not assume a fixed treatment effect across studies. It is also unclear whether fluid management strategy, duration of OLV, and need of blood transfusions could affect the protective effect of dexmedetomidine. Additionally, the I2 value was 0% for all assessed effects, suggesting minimal to no intra-study effect heterogeneity. Third, most of the trials included in meta-analysis were performed in Asia, thus limiting the extrapolation of our results to other places Finally, the GRADE assessment of the certainty of the evidence was moderate for the outcomes of hypoxemia, pneumonia, and ARDS, but low for atelectasis. This, combined with likely publication bias for atelectasis, suggests that further studies with larger sample size are needed to understand the impact of dexmedetomidine on this outcome.
In conclusion, dexmedetomidine administration (infused at a rate of 0.3–0.5μg/kg/h with or without loading dose) during thoracic surgery may potentially reduce the risk of postoperative atelectasis and hypoxemia. Dexmedetomidine may also improve respiratory mechanics during OLV.
Supplementary Material
HIGHLIGHTS.
Translational research has demonstrated that dexmedetomidine minimizes inflammatory response during one-lung ventilation.
Our meta-analysis aimed to determine whether dexmedetomidine reduces pulmonary complications after thoracic surgery.
Dexmedetomidine administration reduces atelectasis and improves oxygenation in the postoperative period.
The current evidence is not sufficient to demonstrate any effect of dexmedetomidine on postoperative pneumonia.
A large, randomized trial is warranted to further clarify the potential benefit of dexmedetomidine on pulmonary outcomes
KeyPoints.
Question:
Does dexmedetomidine reduce the risk of postoperative pulmonary complications and improve intraoperative respiratory mechanics in thoracic surgery?
Findings:
Dexmedetomidine reduces the odds of postoperative atelectasis and hypoxemia by 46% and 57%, respectively, but there is insufficient evidence to determine whether there is an effect pneumonia or respiratory distress.
Interpretation:
Dexmedetomidine may have beneficial effects to protect against postoperative atelectasis and hypoxemia.
Central message.
Dexmedetomidine administration compared to placebo during thoracic surgery may potentially reduce atelectasis and improve oxygenation in the postoperative period. The current evidence is not sufficient to demonstrate any effect of dexmedetomidine on postoperative pneumonia.
Perspective.
A large, randomized trial is warranted to further clarify the potential benefit of dexmedetomidine on pulmonary outcomes after thoracic surgery.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinane.2023.111345.
References
- [1].Lohser J, Slinger P. Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg 2015;121(2):302–18. [DOI] [PubMed] [Google Scholar]
- [2].Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth 2019;123(6):898–913. [DOI] [PubMed] [Google Scholar]
- [3].Gao S, Zhang Z, Brunelli A, et al. The Society for Translational Medicine: clinical practice guidelines for mechanical ventilation management for patients undergoing lobectomy. J Thorac Dis 2017;9(9):3246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mori S, Shibazaki T, Noda Y, et al. Recovery of pulmonary function after lung wedge resection. J Thorac Dis 2019;11(9):3738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schorer R, Dombret AL, Hagerman A, Bedat B, Putzu A. Impact of pharmacological interventions on intrapulmonary shunt during one-lung ventilation in adult thoracic surgery: a systematic review and component network meta-analysis. Br J Anaesth 2023;130(1):e92–105. [DOI] [PubMed] [Google Scholar]
- [6].Beloeil H, Garot M, Lebuffe G, et al. Balanced opioid-free anesthesia with Dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology 2021;134(4):541–51. [DOI] [PubMed] [Google Scholar]
- [7].Zhu CH, Yu J, Wang BQ, Nie Y, Wang L, Shan SQ. Dexmedetomidine reduces ventilator-induced lung injury via ERK1/2 pathway activation. Mol Med Rep 2020;22(6):5378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Yi X, Jiang L, et al. Protective effects of dexmedetomidine on lung in rats with one-lung ventilation. Exp Ther Med 2019;17(1):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu B, Gao H, Li D, Hu C, Yang J. Nebulized dexmedetomidine improves pulmonary shunt and lung mechanics during one-lung ventilation: a randomized clinical controlled trial. PeerJ 2020;8:e9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Peng K, Li D, Applegate RL 2nd, Lubarsky DA, Ji FH, Liu H. Effect of Dexmedetomidine on cardiac surgery-associated acute kidney injury: a Meta-analysis with trial sequential analysis of randomized controlled trials. J Cardiothorac Vase Anesth 2020;34(3):603–13. [DOI] [PubMed] [Google Scholar]
- [11].Sanders RD, Wehrman J, Irons J, Dieleman J, Scott D, Shehabi Y. Meta-analysis of randomised controlled trials of perioperative dexmedetomidine to reduce delirium and mortality after cardiac surgery. Br J Anaesth 2021;127(5):e168–70. [DOI] [PubMed] [Google Scholar]
- [12].Qin C, Jiang Y, Lin C, Li A, Liu J. Perioperative dexmedetomidine administration to prevent delirium in adults after non-cardiac surgery: a systematic review and meta-analysis. J Clin Anesth 2021;73:110308. [DOI] [PubMed] [Google Scholar]
- [13].Fan Z, Tian J, Chen Y, Jiang H. Protective effects of Ulinastatin combined with Dexmedetomidine on patients undergoing one lung ventilation. Indian J Pharm Sci 2021;83(1):219–24. [Google Scholar]
- [14].Guo YB, Xu JD, Ji XX, Zhang JX, Liang JX, Zhou GB. Protective effect of dexmedetomidine against perioperative inflammation and on pulmonary function in patients undergoing radical resection of lung cancer. Nan Fang Yi Ke Da Xue Xue Bao 2017;37(12):1673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jannu V, Dhorigol MG. Effect of intraoperative Dexmedetomidine on postoperative pain and pulmonary function following video-assisted Thoracoscopic surgery. Anesth Essays Res 2020;14(1):68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: a randomised double-blinded trial. Eur J Anaesthesiol 2016;33(4):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee SH, Lee CY, Lee JG, Kim N, Lee HM, Oh YJ. Intraoperative Dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted Thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine (Baltimore) 2016;95(7):e2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meng J, Lv Q, Yao J, Wang S, Yang K. Effect of Dexmedetomidine on postoperative lung injury during one-lung ventilation in Thoracoscopic surgery. Biomed Res Int 2020;2020:4976205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Shi Z, Mi W. Application of dexmedetomidine for lung injury in elderly patients undergoing one-lung ventilation. Arch Med Sci 2023;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu CY, Lu YF, Wang ML, et al. Effects of Dexmedetomidine infusion on inflammatory responses and injury of lung tidal volume changes during one-lung ventilation in Thoracoscopic surgery: a randomized controlled trial. Mediators Inflamm 2018;2018:2575910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xie Y, Jiang W, Zhao L, Wu Y, Xie H. Effect of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing radical resection of lung cancer. Int J Clin Exp Pathol 2020;13(10):2544–53. [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang J, Dong N, Qian H, Yu W. Dexmedetomidine improves function of lung oxygenation in patients with moderate chronic obstructive pulmonary disease underwent lung cancer surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017;42(3):271–6. [DOI] [PubMed] [Google Scholar]
- [23].Zhou Y, Dong X, Zhang L. Dexmedetomidine can reduce the level of oxidative stress and serum miR-10a in patients with lung cancer after surgery. Thorac Cardiovasc Surg 2022;71(3):197–205. [DOI] [PubMed] [Google Scholar]
- [24].Zhu L, Zhang Y, Zhang Z, Ding X, Gong C, Qian Y. Activation of PI3K/Akt/HIF-1 alpha signaling is involved in lung protection of dexmedetomidine in patients undergoing video-assisted Thoracoscopic surgery: a pilot study. Drug Des Devel Ther 2020;14:5155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36(3):1–48. [Google Scholar]
- [26].Hartung J. An alternative method for meta-analysis. Biom J 1999;41:901–16. [Google Scholar]
- [27].Sidik KJJ. A simple confidence interval for meta-analysis. Stat Med 2002;21:3153–9. [DOI] [PubMed] [Google Scholar]
- [28].Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care 2000;4(5):302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013;127(15):1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ibacache M, Sanchez G, Pedrozo Z, et al. Dexmedetomidine preconditioning activates pro-survival kinases and attenuates regional ischemia/reperfusion injury in rat heart. Biochim Biophys Acta 2012;1822(4):537–45. [DOI] [PubMed] [Google Scholar]
- [31].Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19(1):5–10. [DOI] [PubMed] [Google Scholar]
- [32].Zhang W, Zhang JQ, Meng FM, Xue FS. Dexmedetomidine protects against lung ischemia-reperfusion injury by the PI3K/Akt/HIF-1alpha signaling pathway. J Anesth 2016;30(5):826–33. [DOI] [PubMed] [Google Scholar]
- [33].Kucukebe OB, Ozzeybek D, Abdullayev R, Ustaoglu A, Tekmen I, Kume T. Effect of dexmedetomidine on acute lung injury in experimental ischemia-reperfusion model. Braz J Anesthesiol 2017;67(2):139–46. [DOI] [PubMed] [Google Scholar]
- [34].Dong W, Yang H, Cheng M, et al. Dexmedetomidine alleviates pulmonary ischemia-reperfusion injury through modulating the miR-21-5p/Nr4a1 signaling pathway. Acta Biochim Pol 2020;67(4):521–9. [DOI] [PubMed] [Google Scholar]
- [35].Peel JK, Funk DJ, Slinger P, Srinathan S, Kidane B. Tidal volume during 1-lung ventilation: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2022;163(4):1573–85 e1. [DOI] [PubMed] [Google Scholar]
- [36].Xia R, Xu J, Yin H, et al. Intravenous infusion of dexmedetomidine combined isoflurane inhalation reduces oxidative stress and potentiates hypoxia pulmonary vasoconstriction during one-lung ventilation in patients. Mediators Inflamm 2015;2015:238041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mikami M, Zhang Y, Kim B, Worgall TS, Groeben H, Emala CW. Dexmedetomidine’s inhibitory effects on acetylcholine release from cholinergic nerves in guinea pig trachea: a mechanism that accounts for its clinical benefit during airway irritation. BMC Anesthesiol 2017;17(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


