Abstract
Background:
Use of highly effective contraception among women living with HIV is critical to prevent unintended pregnancy and subsequent risk of maternal complications and perinatal HIV transmission. However, it is not known whether use of intrauterine devices (IUDs) among women with advanced HIV disease poses an increased risk of pelvic infection or HIV progression and transmission.
Objectives:
To identify evidence regarding the risk of pelvic infection, HIV disease progression or HIV transmission among women with HIV using IUDs and whether this risk differs by severity of HIV disease.
Methods:
We searched the PubMed database for all articles published from database inception through January 2016. For the outcome of pelvic infection, we included studies that examined women using IUDs and reported risk of pelvic inflammatory disease (PID) or pelvic infections among women with varying levels of HIV severity or among women with HIV compared with women without HIV. For the outcomes of HIV disease progression and HIV transmission to noninfected male partners, we included studies of women with HIV using IUDs compared with other contraceptive methods or no method.
Results:
The review identified eight articles from six study populations which addressed pelvic infections or other IUD-related complications and found mixed results. One study that directly compared women with varying levels of HIV disease severity found no differences in complication rates between those with severe or mild disease after short- and longer-term follow-up. The remaining studies generally found low or no incidence of PID among IUD users. Among eight articles from seven study populations that reported on HIV disease progression, there were generally no differences between women using IUDs compared with other contraceptives, nor were there changes between baseline and follow-up. One article that reported directly on HIV disease transmission to noninfected male partners found no difference in HIV disease transmission, and five articles found no differences in genital viral shedding among women using IUDs. No direct evidence addresses potential differences in HIV disease progression or transmission by HIV disease severity.
Conclusion:
Limited evidence of fair to poor quality found no differences in infectious complications when comparing IUD complication rates among women with varying levels of HIV disease severity. One study found that IUD use was not associated with HIV transmission, and studies generally found no differences in genital viral shedding or disease progression; however, there was little direct evidence to address potential differences related to HIV severity.
Keywords: HIV, Pelvic inflammatory disease, Intrauterine device, Systematic review
1. Introduction
Approximately 8300 women in the United States were diagnosed with HIV in 2014, and over 220,000 women are estimated to be living with HIV [1]. Use of highly effective contraception by women living with HIV is critical in order to prevent unintended pregnancy and potential risks of maternal complications and perinatal HIV transmission. According to the US Medical Eligibility Criteria for Contraceptive Use (US MEC), HIV/AIDS is a condition associated with increased risk for adverse health events as a result of unintended pregnancy, and highly effective contraceptive methods may be the best choice [2]. Intrauterine devices (IUDs) are long acting and highly effective with typical use. Women with more advanced HIV disease are likely to be at highest risk for complications of pregnancy and transmission to the infant and would likely benefit most from use of IUDs. However, there are theoretical concerns about use of IUDs among women with HIV, related to the potential for ascending genital tract infection, worsening of HIV disease and transmission to noninfected sexual partners. This systematic review was conducted to identify evidence on risks related to IUD use among women with HIV, with particular focus on women with advanced disease, as part of the process of revising the US MEC. Previous reviews on contraception and HIV have not focused on risks associated with IUD use [3-5].
2. Materials and methods
We conducted this systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [6].
2.1. Key questions
We identified three key questions of interest:
Among IUD users, do women with advanced HIV disease have increased risk of pelvic infection compared with women with less advanced HIV disease or noninfected women?
Among women with advanced HIV disease, does IUD use increase the risk of HIV disease progression compared with nonuse of IUDs or any hormonal contraceptives?
Among women with advanced HIV disease, does IUD use increase the risk of HIV transmission to noninfected partners compared with nonuse of IUDs or any hormonal contraceptives?
2.2. Literature search
We searched the PubMed database for all relevant articles published from database inception through January 2016 using the following search strategy:
(((“Intrauterine Devices”[Mesh] OR “Intrauterine Devices, Copper”[Mesh] OR “Intrauterine Devices, Medicated”[Mesh] OR ((intrauterine OR intra-uterine) AND (device OR system OR contracept*)) OR IUD OR IUCD OR IUS OR mirena OR Skyla OR paragard OR “Copper T380” OR CuT380 OR “Copper T380a” OR “Cu T380a”)) AND (HIV [Mesh] OR “HIV Infections” [Mesh] OR HIV or “human immunodeficiency” OR “Acquired Immunodeficiency Syndrome” [Mesh] OR “acquired immunodeficiency”)).
We searched for all primary research articles published in any language. We also searched reference lists of identified articles and relevant review articles for additional citations of interest. We did not consider unpublished studies, abstracts of conference presentations or dissertations.
2.3. Selection criteria
Articles were included in this review if they were primary research articles on adverse outcomes among women with HIV using IUDs. The population of interest was women with HIV, particularly those with advanced disease as defined by study authors. The IUDs of interest included copper, levonorgestrel (LNG) or unspecified types. For key Question 1, the reference group of interest was women with mild HIV disease as defined by study authors to allow for assessment of differences in IUD complications related to disease severity. Because studies employed varying definitions for levels of disease severity [e.g., CD4 counts, World Health Organization (WHO) staging or antiretroviral therapy (ART) use], we included all articles in which comparisons were reported between women with advanced and mild disease. Given the limited evidence on this topic, we also included studies comparing women with HIV to women without HIV and studies that did not have a comparison group. Outcomes of interest included pelvic inflammatory disease (PID), pelvic infection, sepsis or any infectious complication. For key Questions 2 and 3, the reference group of interest was women with HIV not using IUDs or any hormonal contraceptives, in order to allow for assessment of the impact of IUDs on disease outcomes. Given the limited evidence, we also included studies that did not have a comparison group. Outcomes of interest for disease progression included changes in CD4 count, plasma RNA, ART initiation, progression to AIDS and death. Outcomes of interest for HIV transmission included direct assessment of seroconversion among noninfected male partners and indirect measures of infectivity such as genital viral shedding.
2.4. Study quality assessment and data synthesis
Two authors (NT and KC) summarized and systematically assessed the evidence. We considered several study features that could impact study quality and potential biases. Related to the study population, we assessed factors such as whether the study included adequate sample size, sufficient length of follow up and appropriate comparison groups. Related to HIV disease, we assessed whether studies reported HIV disease severity at baseline, included women with advanced disease as reported by study authors and reported or controlled for ART use. We assessed whether studies specified IUD type and timing of IUD insertion and whether studies clearly defined measurement of outcomes. We also assessed whether studies controlled for important confounding factors such as condom use and sexually transmitted infections (STIs). We assessed the quality of each individual piece of evidence using the system developed by the United States Preventive Services Task Force [7]. Summary measures were not calculated due to heterogeneity of study designs and outcomes measured.
3. Results
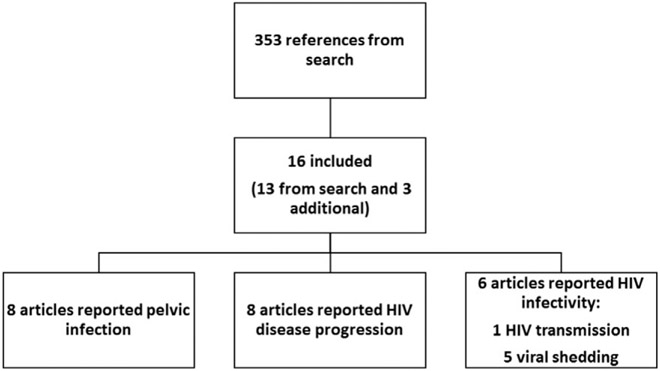
The search identified 353 articles, of which 13 met inclusion criteria (Fig. 1) [8-19]. Three additional articles were identified from one of the previous reviews [20-22]. Therefore, a total of 16 articles were included in this review, which included three articles describing two randomized controlled trials (RCTs) [13,14,16], four articles describing cohort studies [10,12,17,20], two articles describing cross-sectional studies [21,22] and seven articles describing follow-up studies without comparison groups [8,9,11,15,18,19,23]. Eight articles examined copper IUDs [10-14,16,18,23], five articles examined LNG-IUDs [8,9,15,17,23] and four articles did not specify IUD type [19-22].
Fig. 1.
Flow chart of systematic review.
3.1. Key question 1: pelvic infections
Eight articles addressed infectious complications of IUD use, including PID or pelvic infections (Table 1) [9,10,12,13,15,17,19,23].
Table 1.
Key question 1: pelvic infections among women with HIV using IUDs
| Author, year, location, support |
Study design |
Population | IUD type | HIV disease severity at baseline |
Outcomes | Results | Strengths | Limitations | Quality grading |
|---|---|---|---|---|---|---|---|---|---|
| Sinei [12], 1998 Kenya USAID |
Prospective cohort | 156 women with HIV initiating IUDs 493 women without HIV initiating IUDs Follow up for 4 months | Copper IUD (copper T 380 A) | No information on HIV severity | PID (pelvic tenderness and 1 of following: gonorrhea, chlamydia, fever, leucocytosis, pelvic abscess or inflammatory complex) Infection-related complications (pelvic tenderness or removal of IUD for infection or pain) |
PID at 4 months: 1.4% among women with HIV, 0.2% among women without HIV (p-value not reported) Infection-related complications: 6.9% among women with HIV, 5.7% among women without HIV, aOR 1.02 (95% CI=0.46–2.27) Among women with HIV: rates stated to be similar among severely (0%), moderately (8%), and mildly (8%) immunocompromised women (p-value not reported) |
Timing of IUD insertion known Physicians performing exams were blinded to woman’s HIV status Infection diagnostic criteria clearly stated |
No information on HIV severity; levels of severity not further defined No information on ART use during study Lacked power to detect differences in PID rates |
II-2, fair |
| Morrison [10], 2001 (follow up to Sinei [12], 1998) Kenya USAID |
Prospective cohort | 150 women with HIV initiating IUDs 486 women without HIV initiating IUDs Follow up for 24 months |
Copper IUD (copper T 380 A) | No information on HIV severity | PID (pelvic tenderness and 1 of following: gonorrhea, chlamydia, fever, leucocytosis, pelvic abscess, or inflammatory complex) Infection-related complications (pelvic tenderness or removal of IUD for infection or pain) |
PID: 2.0% in women with HIV, 0.4% in women without HIV (p=.09) Infection related complications: 10.7% in women with HIV, 8.8% in women without HIV (p=.5) Among women with HIV: rates similar among severely (9.1%), moderately (14.3%), and mildly (11.1%) immunocompromised women |
Long follow up Timing of IUD insertion known Infection diagnostic criteria clearly stated |
Low proportion of women followed up at 24 months and differential between groups (37% women with HIV, 47% women without HIV) No information on HIV severity; levels of severity not further defined No information on ARTuse during study |
II-3, poor |
| Lehtovirta [9], 2007 Finland Helsinki University |
Prospective follow-up without comparison group | 6 women with HIV initiating IUDs Follow up for mean 45 months |
LNG-IUD | Mean CD4 count 0.59×109 cells/L | PID (diagnostic criteria not stated) | No cases of PID | Long follow up Timing of IUD insertion known |
Small sample size No comparison group No information on ART use during study No information on PID diagnostic criteria |
II-3, poor |
| Stringer [13], 2007 Zambia Elizabeth Glaser Pediatric AIDS Foundation, USAID |
RCT | 229 postpartum women with HIV initiating IUDs 206 postpartum women with HIV initiating hormonal method (COCs, POPs, or DMPA) Follow up for at least 24 months |
Copper IUD (copper T 380 A) | IUD group: 10% CD4 count <200 cells/μL Hormonal group: 10% CD4 count <200 cells/μL Excluded women with advanced HIV disease (WHO Stage III or IV) |
PID (pelvic tenderness and 1 of following: fever, pus on culdocentesis, pelvic mass or inflammatory complex, mucopurulent cervicitis) | 1 case of PID in IUD group (rate 0.16 cases per 100 woman–years), 0 cases of PID in hormonal group | Randomization procedure described; baseline characteristics similar between groups Long follow up Infection diagnostic criteria clearly stated |
Comparison group was hormonal users No information on women with severe disease Loss to follow up 23% (IUD group) and 32% (hormonal group) 31% discontinued method; higher among IUD users ART introduced during study duration but analysis did not control for ART use |
I, fair |
| Heikinheimo [17], 2011 Finland Helsinki University |
Prospective follow-up without comparison group | 15 women with HIV using LNG-IUDs Follow up for 5 years |
LNG-IUD | Mean CD4 count 0.54×109/L, 54% using ART | Pelvic infection (diagnostic criteria not stated) | No cases of pelvic infection in IUD users | Long follow up Timing of IUD insertion known |
Small sample size Contraceptive methods among control group not stated No information on women with severe disease No comparison group for question of interest No information on criteria for infection diagnosis |
II-3, poor |
| Coleman [15], 2013 Kenya Puget Sound Partners for Global Health, American College of Obstetricians and Gynecologists, University of Washington |
Prospective follow-up without comparison group | 30 women with HIV initiating LNG-IUDs Follow up for 6 months |
LNG-IUD | CD4 count >250 cells/μL No ART within 90 days of study entry |
PID (pelvic pain plus either adnexal tenderness or cervical motion tenderness) | No cases of PID | 25/30 (83%) included in analysis at 6 months Timing of IUD insertion known |
Small sample size No information on women with severe disease No comparison group No information on ART use during study |
II-3, poor |
| Kakaire [19,23], 2015 Uganda Office of Global AIDS Coordinator, DHHS, HRSA, NIH |
Prospective follow-up without comparison group | 703 women with HIV initiating IUDs Follow up for 12 months |
Copper IUD LNG-IUD |
74% CD4 count >350 cells/μL 88% using ART Excluded women with AIDS who were not on ART |
PID (lower abdominal pain plus cervical or adnexal tenderness, fever, adnexal mass, purulent vaginal discharge) | 2/338 (0.6%) in copper IUD group and 3/334 (0.9%) in LNG-IUD group had PID during 12 months follow up [incidence rate ratio 0.7 (95% CI=0.06–6.04)] | Timing of IUD insertion known Low loss to follow up (4%) |
No information on women with severe disease No comparison group for question of interest |
II-3, poor |
Abbreviations: DHHS, Department of Health and Human Services; HRSA, Health Resources and Services Administration; NIH, National Institutes of Health; USAID, US Agency for International Development.
The only RCT to address pelvic infections was conducted in Zambia, in which postpartum women with HIV were randomized to receive either copper IUDs (n=229) or hormonal contraceptives (self-selection of combined oral contraceptives (COCs), progestin-only pills (POPs) and depot medroxyprogesterone actetate (DMPA)) (n=206) [13]. During 24 months of follow up, there was one case of PID in the IUD group (rate 0.16 cases per 100 women–years) and no cases in the hormonal group.
Two publications reported on a prospective cohort study conducted in Kenya, which examined women who had copper IUDs inserted [10,12]. Sinei reported on 156 women with HIV compared with 493 women without HIV using copper IUDs, and found that the incidence of PID at 4 months was higher among women with HIV (1.4%) than women without HIV (0.2%), although p-values were not reported [12]. There was no statistically significant difference in infection-related complications, defined as pelvic tenderness or removal of IUD for infection or pain, between women with HIV and women without HIV. The authors stated that among women with HIV, rates of infection-related complications were similar among severely (0%), moderately (8%) and mildly (8%) immunocompromised women, although these categories were not defined. A second publication by Morrison found that at 24 months after IUD insertion, 2% of 150 women with HIV and 0.4% of 486 women without HIV were diagnosed with PID; however, this difference was not statistically significant (p=.09) [10]. There was also no statistically significant difference in infection-related complications between women with HIV (11%) and women without HIV (9%). Among women with HIV, rates of pelvic infection were similar when stratified into categories related to CD4 status (severely, moderately and mildly immunocompromised women, not further defined).
Five articles also reported pelvic infections among IUD users but did not include a comparison group relevant for this review [9,15,17,19,23]. Three of the studies followed women with HIV using LNG-IUDs (n range, 6–20) and found no cases of pelvic infection or PID during 6 months, 45 months and 5 years of follow up [9,15,17]. One study followed 703 women with HIV using IUDs for 1 year and found that 0.6% of copper IUD users and 0.9% of LNG-IUD users developed PID [19,23].
3.2. Key question 2: HIV disease progression
Eight articles addressed HIV disease progression, including changes in CD4 count, plasma RNA, ART initiation, progression to AIDS and death (Table 2) [8,9,13-18].
Table 2.
Key question 2: HIV disease progression among women with HIV using IUDs
| Author, year, location, support |
Study design | Population | IUD type | HIV disease severity at baseline |
Outcomes | Results | Strengths | Limitations | Quality grading |
|---|---|---|---|---|---|---|---|---|---|
| Heikinheimo [8], 2006 Finland Helsinki University |
Prospective follow-up without comparison group | 12 women with HIV initiating IUDs Follow up for 12 months |
LNG-IUD | CD4 count ≥0.35×109 cells/mL 10 women using ART 2 women not using ART |
CD4 count Plasma HIV RNA |
No significant change in CD4 count (mean 0.63×109 at baseline; mean 0.58×109 at 12 months; p = not significant) Women using ART: 49/50 samples had undetectable plasma HIV RNA Women not using ART: plasma HIV RNA varied between 1350 and 23,600 copies/mL |
100% follow up at 12 months Timing of IUD insertion known |
Small sample size No comparison group No information on pattern of plasma RNA changes in women not using ART |
II-3, poor |
| Lehtovirta [9], 2007 Finland Helsinki University |
Prospective follow-up without comparison group | 6 women with HIV initiating IUDs Follow up for mean 45 months |
LNG-IUD | Mean CD4 count 0.59×109 cells/L | CD4 count Progression to AIDS |
No statistically significant change in CD4 count at 24 months (mean 0.59×109 at baseline; mean 0.47×109 at 24 months; p>.05) One subjected presented with AIDS (length of time after IUD insertion not stated) |
Long follow up Timing of IUD insertion known |
Small sample size No comparison group No information on ART use during study |
II-3, poor |
| Stringer [13], 2007 and Stringer [14], 2009 Zambia Elizabeth Glaser Pediatric AIDS Foundation, USAID |
RCT | 229 postpartum women with HIV initiating IUDs 206 postpartum women with HIV initiating hormonal method (COCs, POPs, or DMPA) Follow up for at least 24 months |
Copper IUD (copper T 380 A) | IUD group: 10% CD4 count <200 cells/μL Hormonal group: 10% CD4 count <200 cells/μL Excluded women with advanced HIV disease (WHO Stage III or IV) |
CD4 count drop below 200 cells/μL Death Initiation of ART |
If initial CD4 count >200 cells/μL: hormonal group more likely to decline to <200 cells/μL than IUD group (hazard ratio 1.6, 95% CI=1.04–2.3) Rate of death not statistically significantly different between 2 groups (rate 2.89/100 patient–years hormonal group; rate 2.01/100 patient–years IUD group; hazard ratio 1.4 [95% CI=0.70–3.0]) Results similar in secondary analysis [14] |
Randomization procedure described; baseline characteristics similar between groups Long follow up |
Comparison group was hormonal users No information on women with severe disease Loss to follow up 23% (IUD group) and 32% (hormonal group) 31% discontinued method; higher among IUD users |
I, fair |
| Heikinheimo [17], 2011 Finland Helsinki University |
Cohort | 15 women with HIV using LNG-IUDs (7 women also included in Heikinheimo 2006 [8]) 25 control women with HIV (methods not specified), matched on age and CD4 count Follow up for 5 years |
LNG-IUD | IUD group: mean CD4 count 0.54×109/L, 54% using ART Control group: mean CD4 count 0.56×109/L, 56% using ART |
Plasma HIV RNA CD4 count Initiation of ART |
Among women not using ART, plasma HIV RNA increased in both groups, not statistically significant from baseline to year 1 in either group (p=.27 in IUD group; p=.25 in control group) No statistically significant difference in CD4 count between groups, when stratified by ART use, during 5 years follow up (p=.59) Among women not using ART at beginning, 45% among LNG-IUD group and 43% among control group initiated ART (p=.91), during 5 years follow up |
Long follow up | Small sample size Timing of IUD insertion not known Contraceptive methods among control group not stated No information on women with severe disease |
II-2, poor |
| Coleman [15], 2013 Kenya Puget Sound Partners for Global Health, American College of Obstetricians and Gynecologists, University of Washington |
Prospective follow-up without comparison group | 30 women with HIV initiating LNG-IUDs Follow up for 6 months |
LNG-IUD | CD4 count >250 cells/μL No ART within 90 days of study entry |
Plasma HIV-1 RNA CD4 count | No statistically significant change in level of plasma HIV-1 RNA (p=.34) Mean CD4 count decreased from 617 cells/μL (enrollment) to 493 cells/μL (6 months) (p<.001) |
25/30 (83%) included in analysis at 6 months Timing of IUD insertion known |
Small sample size Short follow up for changes in disease status No information on women with severe disease No comparison group No information on ART use during study |
II-3, poor |
| Haddad [16], 2013 Malawi Anonymous foundation, Emory University |
RCT | 99 women with HIV initiating copper IUD 101 women with HIV initiating DMPA Follow up for 48 weeks |
Copper IUD | IUD group: mean CD4 count 430 cells/μL DMPA group: mean CD4 count 550 cells/μL |
CD4 count Change in ART regimen |
Mean CD4 count unchanged among both groups (p=.56) No significant difference in number of women who changed ART regimen (p=.40) |
Randomization described Small loss to follow-up (2 from each group) Timing of IUD insertion known |
Comparison group hormonal users Short follow up for changes in disease status No information on women with severe disease |
I, fair |
| Landolt [18], 2013 Thailand Chulalongkorn University |
Prospective follow-up without comparison group | 29 women with HIV initiating copper IUD Follow up for 6 months |
Copper IUD (Multiload Cu 375) | CD4 ≥350 cells/μL (ART naïve) or ART for 6 months or undetectable viral load (ART experienced) 28/29 women using ART at baseline |
CD4 count HIV RNA | No significant changes in CD4 count or HIV RNA at 6 months (p values not reported) | Timing of IUD insertion known | Small sample size Short follow up for changes in disease status No information on women with severe disease No comparison group No information on change in ART use during study |
II-3, poor |
Three publications from two RCTs addressed HIV disease progression [13,14,16]. Two publications from the RCT conducted by Stringer reported changes in disease progression between women using copper IUDs and hormonal contraceptives [13,14]. Among women with initial CD4 count >200 cells/μL, the hormonal group was more likely to experience decline to <200 cells/μL than the IUD group [hazard ratio: 1.6, 95% confidence interval (CI)= 1.04–2.3]. The rate of death was not statistically significantly different between the two groups. A secondary analysis of data from this trial found similar results, when examining a composite outcome of death, declining CD4 count below 200 cells/μL or initiation of ART [14]. The hazard ratio for the composite outcome was statistically significantly higher among women using oral contraceptive pills (OCPs) or DMPA than among women using IUDs. The rate of death was not statistically significantly different between women using IUDs, OCPs or DMPA. Haddad conducted an RCT in Malawi that randomized women with HIV to receive copper IUDs (n=99) or DMPA (n=101) [16]. During 48 weeks of follow up, the mean CD4 count was unchanged among both groups, and there was no significant difference between groups in the number of women who changed ART regimen.
One cohort study addressed HIV disease progression [17]. Heikinheimo compared 15 women with HIV using LNG-IUDs to 25 women with HIV using unspecified contraceptive methods and found that among women not using ART, plasma HIV RNA increased in both groups; however, the change was not statistically significant from baseline to year 1 in either group. During 5 years of follow up, there were no statistically significant differences between the IUD and control groups in CD4 count, when stratified by ART use, or proportion of women initiating ART.
Four follow-up studies without comparison groups reported HIV disease progression among IUD users [8,9,15,18]. Heikinheimo found that among 12 women using LNG-IUDs, there was no significant change in CD4 count during 12 months of follow up [8]. Among women using ART, 49/50 samples had undetectable plasma HIV RNA. Among women not using ART, plasma HIV RNA varied between 1350 and 23,600 copies/mL; however, no further information was given. Lehtovirta found that among six women using LNG-IUDs, there was no statistically significant change in CD4 count during 24 months of follow up [9]. One subject presented with AIDS, although the length of time after IUD insertion was not stated. Coleman found that among 30 women with HIV using LNG-IUDs, during 6 months of follow up, the mean CD4 count decreased from 617 cells/μL at enrollment to 493 cells/μL at 6 months (p<.001) [15]. There was no statistically significant change in plasma HIV-1 RNA during the 6 months of follow up. Landolt found that among 29 women with HIV using copper IUDs, there were no significant changes in CD4 count or HIV RNA at 6 months of follow up [18].
3.3. Key question 3: HIV transmission
Six articles addressed HIV transmission, including one article that provided direct evidence on transmission from women with HIV to noninfected male partners [20] and five articles that provided indirect evidence on surrogate markers of infectivity such as genital viral shedding (Table 3) [8,11,15,21,22]. Plasma viral load may also be a surrogate marker of infectivity; however, changes in viral load are discussed above as part of key Question 2 on HIV disease progression.
Table 3.
Key question 3: HIV disease transmission to noninfected male partners or markers of infectivity among women with HIV using IUDs
| Author, year, location, support | Study design | Population | IUD type | HIV disease severity at baseline | Outcomes | Results | Strengths | Limitations | Quality grading |
|---|---|---|---|---|---|---|---|---|---|
| European Study Group [20], 1992 9 European countries Commission of the European Communities | Cohort | 10 women with HIV using IUDs 86 women with HIV using no contraceptive | IUD (type not specified) | Most stage II or III with CD4 count >200×106/L 36 women stage IV or CD4 count <200×106/L | Seroconversion of male partner | Male partners of IUD users: 10% (n=1/10) seroconverted Male partners ofwomen using no method: 12% (n=10/86) seroconverted Not significant (exact p value not reported but not identified as significant) | Multiple countries | Small sample size for comparison of interest Index cases primarily drug users (may limit generalizability) Length of follow up not stated Response rates and follow-up rates not reported IUD type not specified Unclear if reported IUD use was at baseline or at time of transmission Analysis not adjusted | II-2, poor |
| Mostad [22], 1997 Kenya NIH, Clinical Nutrition Research Unit | Cross-sectional | 6 women with HIV using IUDs 182 women with HIV using no contraceptive | IUD (type not specified) | Mean CD4 count 452; 17% < 200; no information for IUD users | Cervical HIV-1 DNA (swab) Vaginal HIV-1 DNA (swab) | Odds of cervical HIV-1 DNA among IUD users versus no method: OR 0.2 (95% CI=0.0–2.2) No vaginal HIV-1 DNA in IUD users | Small sample size of IUD users IUD type not specified Large percentage of sex workers (may limit generalizability) No information on ART use | II-3, Poor | |
| Richardson [11], 1999 (follow up to Sinei [12], 1998) Kenya USAID | Prospective follow-up without comparison group | 98 women with HIV initiating IUDs Follow up for 4 months | Copper IUD (copper T 380 A) | At 1 month follow-up, 7% had CD4 counts <200 | Cervical HIV-1 DNA (endocervical swab) | No significant difference in prevalence of cervical shedding at 4 months (43%) compared with baseline (50%): adjusted OR 0.6 (95% CI=0.3–1.1) | Physicians performing exams were blinded to woman's HIV status Timing of IUD insertion known Cervical shedding analysis controlled for confounders 76% follow-up at 4 months Study powered to detect clinically relevant change in cervical HIV-1 DNA | Small sample size Short follow up for changes in disease status No comparison group No information on ART use during study | II-3, fair |
| Kovacs [21], 2001 United States NIH, Agency for Health Care Policy and Research, CDC | Cross-sectional | Women with HIV using IUDs (N not stated) | IUD (type not specified) | 32% CD4 count >500 cells/mm3 50% CD4 count 200–499 cells/mm3 18% CD4 count <200 cells/mm3 All on stable ART for 1 month | Genital HIV shedding (endocervical swab and cervicovaginal lavage) | No association of IUD use with genital HIV shedding (data not shown) | Included women with severe disease | Sample size using IUDs not stated IUD types not stated OR for comparison of IUD versus non-IUD users not shown | II-3, poor |
| Heikinheimo [8], 2006 Finland Helsinki University | Prospective follow-up without comparison group | 12 women with HIV initiating IUDs Follow up for 12 months | LNG-IUD | CD4 count ≥0.35×109 cells/mL 10 women using ART 2 women not using ART | Genital HIV RNA (cervicovaginal lavage) | Women usingART (N=10): Nodifference in genitalHIV RNA (6 women undetectable) Women not using ART (N=2): genital HIV RNA present in half of samples, level decreased in 1 woman and increased in 1 woman after IUD initiation | 100% follow up at 12 months Timing of IUD insertion known | Small sample size No comparison group No information on women with severe disease | II-3, poor |
| Coleman [15], 2013 Kenya Puget Sound Partners for Global Health, American College of Obstetricians and Gynecologists, University of Washington | Prospective follow-up without comparison group | 30 women with HIV initiating LNG-IUDs Follow up for 6 months | LNG-IUD | CD4 count >250 cells/μL No ART within 90 days of study entry | Genital HIV-1 RNA (endocervical secretions and cervical vaginal lavage) | No statistically significant change in genital HIV-1 RNA (p=.55) | 25/30 (83%)with evaluable specimens at 6 months Timing of IUD insertion known | Small sample size Short follow up for changes in disease status No information on women with severe disease No comparison group No information on ART use during study | II-3, poor |
The European Study Group provided the only direct evidence on disease transmission from women with HIV to noninfected male partners [20]. In this cohort study, unadjusted analysis revealed no significant difference in rate of seroconversion of males whose HIV-infected female partners used IUDs (10%; n=1/10) or no contraceptive (12%; n=10/86).
The remaining studies were cross-sectional or follow-up studies without comparison groups. Mostad conducted a cross-sectional study in Kenya that found that rate of detection of cervical HIV-1 DNA did not differ between IUD users (n=6) compared with no contraceptive method (n=182) [odds ratio (OR) 0.2; 95% CI=0.0–2.2] [22]. In addition, there were no IUD users with vaginal HIV-1 DNA detected. Richardson found that, among 98 copper IUD users, there were no statistically significant differences in prevalence of cervical HIV-1 DNA shedding at 4 months (43%) compared with baseline (50%) [11]. In a cross-sectional study by Kovacs, there was no association of IUD use with genital shedding (data not shown) [21]. Heikinheimo found that among 12 women using LNG-IUDs and who were using ART at baseline, there was no difference in genital HIV RNA at 12 months of follow up [8]. Among two women not using ART, genital HIV RNA was present in half of samples. RNA levels increased in one woman and decreased in one woman after IUD initiation. Coleman found that there was no statistically significant change in levels of genital HIV-1 RNA during the 6 months of follow up [15].
4. Discussion
This systematic review identified eight articles that addressed pelvic infections and found an overall low incidence of PID among women with HIV using IUDs. Two analyses from the same study population compared women with varying levels of HIV disease severity and found no differences in complication rates between those with severe or mild disease [10,12]. These analyses found that the incidence of pelvic infection was higher among HIV-infected compared with uninfected women, although differences were not statistically significant. One RCT found only one case of PID among IUD users, and the follow-up studies without comparison groups found low (0.7%) or no incidence of PID among IUD users. Among the eight articles that reported on HIV disease progression, there were generally no differences between women using IUDs compared with other contraceptives, nor were there changes between baseline (e.g., study recruitment, IUD initiation or some time point after IUD initiation) and follow-up. Among the one article that reported on risk of HIV transmission and five articles that reported on markers of HIV infectivity, there was no difference in transmission or genital viral shedding among women using IUDs. There was no direct evidence to address potential differences in HIV disease progression or transmission by HIV disease severity.
This body of evidence is of fair to poor quality and is subject to several limitations. Related to the study population, we assessed sample size, generalizability of study population, follow-up and use of appropriate comparison groups. Most of the studies included small sample sizes or lacked power to detect differences in outcomes [8,9,11,12,15,17,18,20-22]. Several studies included women with specific high-risk characteristics, such as drug use or sex workers [20,22], or included only postpartum women [13,14] which may limit generalizability of results. Several of the studies reporting HIV disease outcomes had relatively short follow-up times (less than 1 year), which may have prevented the observation of slowly progressive effects [11,15,16,18]. Several studies had low rates of follow up, which may introduce bias if loss to follow up is related to disease severity [10,13,14]. Studies that included hormonal contraceptive users as the comparison group may have failed to find differences if both groups experienced adverse effects and studies that did not include a comparison group provide limited information regarding potential effects of IUDs [8-11,13-19,23].
Related to HIV disease severity, we assessed whether studies reported HIV disease severity at baseline, included women with advanced disease and reported or controlled for ART use. Several studies did not describe HIV disease severity of the study population or included only women with mild HIV disease [8,10,12-19,23]. In addition, studies used varying definitions to define HIV disease severity, which limits interpretation of results. The only study which directly compared women by disease severity did not define those levels [10,12]. Two studies excluded women with advanced disease, which was defined differently in each study [13,14,19,23]. Several studies included only women with CD4 counts above a certain level [8,15,18], and several studies included all women with HIV but reported mean CD4 count or proportion of women with high or low CD4 counts [9,11,16,17,20-22]. Use of ART is an important potential confounder, and several studies did not provide information on ART use during the study or control for ART use in analyses [9-12,15,18,20,22].
We assessed several features of the studies related to the measurement and reporting of the exposure, outcomes and analyses. Related to IUD use, we assessed whether studies specified IUD type and timing of IUD insertion. Four studies did not specify IUD types, and one of these additionally did not clarify timing of IUD insertion related to outcome measurement [19-22]. Related to the outcome of genital viral shedding, there is no clear consensus on what or how to measure and studies reported a variety of viral markers collected in different ways, making comparisons between studies difficult. With regard to the outcome of PID or pelvic infection, several studies used only physical examination findings without any objective criteria or did not describe what diagnostic criteria were used to make the diagnoses [9,15,17]. We also assessed whether studies controlled for important confounding factors, such as condom use or STIs. The one study examining HIV transmission among IUD users reported unadjusted analyses and failed to account for condom use or other important confounders [20]. STI infection status at the time of IUD insertion was not known in most studies; most studies either did not perform STI screening or did not report whether STI screening was performed at the time of IUD insertion.
The primary theoretical concern related to IUD use by women with HIV is risk of ascending infection and PID, as women with advanced HIV disease may be at higher risk for infectious complications due to higher viral load burden and decreased immune status. Because of the limited evidence on this question, we considered analogous situations, such as similar procedures among women with HIV or IUD insertions among women with other immunocompromised conditions. Studies examining complications among women with HIV who undergo cervical and uterine procedures such as loop electrosurgical excision procedure (LEEP) or dilation and curettage have generally not found statistical differences in infectious complications compared with noninfected women [24-27]. Evidence on infectious complications related to IUD use among other immunocompromised women is limited to case reports or severely underpowered studies [28-30].
Additional theoretical concerns related to IUD use by women with HIV include worsening of HIV disease and transmission to noninfected male partners, via possible mechanisms such as alterations in genital immune cell populations or genital viral shedding which could theoretically be affected by presence of an IUD. One study found that neither copper nor LNG-IUDs affected genital tract immune cells in HIV noninfected women [31]. Studies have found conflicting results on whether cervical HIV RNA levels increase following cervical procedures (including cryosurgery, LEEP and cold-knife cone procedures) [24,32,33]; however, it is not known whether these results can be extrapolated to IUD insertion.
The limited body of evidence on safety of IUDs among women with HIV highlights the need for further study on the issue. Consistency is needed in definitions of HIV disease severity used by researchers. WHO classifies HIV into four stages, based on clinical symptoms [34]. The US Centers for Disease Control and Prevention (CDC) classifies HIV infection into three stages, based on CD4 count or opportunistic illnesses; however, these definitions are for surveillance purposes and may not be appropriate for patient care or research [35]. Future studies should also clearly define comparison groups, IUD type and timing of initiation and outcomes, and should account for important confounders such as ART, condom use and STIs.
In conclusion, limited evidence found no differences in infectious complications when comparing IUD complication rates among women with varying levels of HIV disease severity. Studies generally found that IUD use was not associated with HIV transmission, infectivity or disease progression; however, there was little direct evidence to address potential differences related to HIV severity. The US MEC currently recommends that IUDs can generally be used by women with HIV but generally should not be inserted in women with severe disease. However, the evidence identified by this review does not demonstrate additional risk for women with HIV who use IUDs. The information from all but one of the articles [23] was presented at a meeting held by CDC in August 2015 to update the US MEC, and the findings of this systematic review will be incorporated into the forthcoming updated US MEC.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- [1].CDC. HIV surveillance report, volume 26. Available at http://www.cdc.gov/hiv/library/reports/surveillance/index.html [Accessed June 15, 2016]. [Google Scholar]
- [2].CDC. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep 2010;59(RR-4) 1–86. [PubMed] [Google Scholar]
- [3].Curtis KM, Nanda K, Kapp N. Safety of hormonal and intrauterine methods of contraception for women with HIV/AIDS: a systematic review. AIDS 2009;23(Suppl 1):S55–67. [DOI] [PubMed] [Google Scholar]
- [4].Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a systematic review. AIDS 2013; 27:787–94. [DOI] [PubMed] [Google Scholar]
- [5].Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS 2013;27:493–505. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US preventive services task force: a review of the process. Am J Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- [8].Heikinheimo O, Lehtovirta P, Suni J, Paavonen J. The levonorgestrel-releasing intrauterine system (LNG-IUS) in HIV-infected women–effects on bleeding patterns, ovarian function and genital shedding of HIV. Hum Reprod 2006;21:2857–61. [DOI] [PubMed] [Google Scholar]
- [9].Lehtovirta P, Paavonen J, Heikinheimo O. Experience with the levonorgestrel-releasing intrauterine system among HIV-infected women. Contraception 2007;75:37–9. [DOI] [PubMed] [Google Scholar]
- [10].Morrison CS, Sekadde-Kigondu C, Sinei SK, Weiner DH, Kwok C, Kokonya D. Is the intrauterine device appropriate contraception for HIV-1-infected women? Obstet Gynaecol 2001;108:784–90. [DOI] [PubMed] [Google Scholar]
- [11].Richardson BA, Morrison CS, Sekadde-Kigondu C, Sinei SK, Overbaugh J, Panteleeff DD, et al. Effect of intrauterine device use on cervical shedding of HIV-1 DNA. AIDS 1999;13:2091–7. [DOI] [PubMed] [Google Scholar]
- [12].Sinei SK, Morrison CS, Sekadde-Kigondu C, Allen M, Kokonya D. Complications of use of intrauterine devices among HIV-1-infected women. Lancet 1998;351:1238–41. [DOI] [PubMed] [Google Scholar]
- [13].Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am J Obstet Gynecol 2007;197:144.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stringer EM, Levy J, Sinkala M, Chi BH, Matongo I, Chintu N, et al. HIV disease progression by hormonal contraceptive method: secondary analysis of a randomized trial. AIDS 2009;23:1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coleman JS, Mwachari C, Balkus J, Sanguli L, Muliro A, Agnew K, et al. Effect of the levonorgestrel intrauterine device on genital HIV-1 RNA shedding among HIV-1-infected women not taking antiretroviral therapy in Nairobi, Kenya. J Acquir Immune Defic Syndr 2013;63:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haddad LB, Cwiak C, Jamieson DJ, Feldacker C, Tweya H, Hosseinipour M, et al. Contraceptive adherence among HIV-infected women in Malawi: a randomized controlled trial of the copper intrauterine device and depot medroxyprogesterone acetate. Contraception 2013;88:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heikinheimo O, Lehtovirta P, Aho I, Ristola M, Paavonen J. The levonorgestrel-releasing intrauterine system in human immunodeficiency virus-infected women: a 5-year follow-up study. Am J Obstet Gynecol 2011;204:126.e1–4. [DOI] [PubMed] [Google Scholar]
- [18].Landolt NK, Phanuphak N, Teeratakulpisarn N, Kriengsinyot R, Ahluwalia J, Pinyakorn S, et al. Uptake and continuous use of copper intrauterine device in a cohort of HIV-positive women. AIDS Care 2013;25:710–4. [DOI] [PubMed] [Google Scholar]
- [19].Kakaire O, Byamugisha JK, Tumwesigye NM, Gemzell-Danielsson K. Clinical versus laboratory screening for sexually transmitted infections prior to insertion of intrauterine contraception among women living with HIV/AIDS: a randomized controlled trial. Hum Reprod 2015;30:1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Comparison of female to male and male to female transmission of HIV in 563 stable couples. European study group on heterosexual transmission of HIV. BMJ 1992;304:809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kovacs A, Wasserman SS, Burns D, Wright DJ, Cohn J, Landay A, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet 2001;358:1593–601. [DOI] [PubMed] [Google Scholar]
- [22].Mostad SB, Overbaugh J, DeVange DM, Welch MJ, Chohan B, Mandaliya K, et al. Hormonal contraception, vitamin a deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet 1997;350:922–7. [DOI] [PubMed] [Google Scholar]
- [23].Kakaire O, Byamugisha JK, Tumwesigye NM, Gemzell-Danielsson K. Intrauterine contraception among women living with human immunodeficiency virus: a randomized controlled trial. Obstet Gynecol 2015; 126:928–34. [DOI] [PubMed] [Google Scholar]
- [24].Huchko MJ, Woo VG, Liegler T, Leslie H, Smith-McCune K, Sawaya GF, et al. Impact of loop electrosurgical excision procedure for cervical intraepithelial neoplasia on HIV-1 genital shedding: a prospective cohort study. Brit J Obstet Gynaecol 2013;120:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kietpeerakool C, Srisomboon J, Suprasert P, Phongnarisorn C, Charoenkwan K, Cheewakriangkrai C, et al. Outcomes of loop electrosurgical excision procedure for cervical neoplasia in human immunodeficiency virus-infected women. Int J Gynecol Cancer 2006;16:1082–8. [DOI] [PubMed] [Google Scholar]
- [26].Kietpeerakool C, Suprasert P, Srisomboon J. Outcome of loop electrosurgical excision for HIV-positive women in a low-resource outpatient setting. Int J Gynecol Obstet 2009;105:10–3. [DOI] [PubMed] [Google Scholar]
- [27].Stuart GS, Sheffield JS, Hill JB, McIntire DD, McElwee B, Wendel GD. Morbidity that is associated with curettage for the management of spontaneous and induced abortion in women who are infected with HIV. Am J Obstet Gynecol 2004;191:993–7. [DOI] [PubMed] [Google Scholar]
- [28].Julkunen HA, Kaaja R, Friman C. Contraceptive practice in women with systemic lupus erythematosus. Brit J Rheumatol 1993;32:227–30. [DOI] [PubMed] [Google Scholar]
- [29].Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010;82:102–12. [DOI] [PubMed] [Google Scholar]
- [30].Sanchez-Guerrero J, Uribe AG, Jimenez-Santana L, Mestanza-Peralta M, Lara-Reyes P, Seuc AH, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. New Engl J Med 2005;353:2539–49. [DOI] [PubMed] [Google Scholar]
- [31].Achilles SL, Creinin MD, Stoner KA, Chen BA, Meyn L, Hillier SL. Changes in genital tract immune cell populations after initiation of intrauterine contraception. Am J Obstet Gynecol 2014;211:489.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wright TC Jr, Subbarao S, Ellerbrock TV, Lennox JL, Evans-Strickfaden T, Smith DG, et al. Human immunodeficiency virus 1 expression in the female genital tract in association with cervical inflammation and ulceration. Am J Obstet Gynecol 2001;184:279–85. [DOI] [PubMed] [Google Scholar]
- [33].Chung MH, McKenzie KP, Richardson BA, John-Stewart GC, Coombs RW, De Vuyst H, et al. Cervical HIV-1 RNA shedding after cryotherapy among HIV-positive women with cervical intraepithelial neoplasia stage 2 or 3. AIDS 2011;25:1915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007. Available at http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf [Accessed June 15, 2016].
- [35].CDC. Revised surveillance case definition for HIV infection–United States, 2014. MMWR Recomm Rep 2014;63(RR-03)1–10. [PubMed] [Google Scholar]