Abstract
Background:
Women with medical conditions associated with increased risk for thrombosis generally should not use estrogen-containing contraceptives; however, less is known about progestin-only contraceptives (POCs) and thrombosis risk.
Objectives:
The objective was to identify evidence regarding the risk of venous thromboembolism (VTE) or arterial thromboembolism [stroke or acute myocardial infarction (AMI)] among women using POCs.
Methods:
We searched the PubMed database for all articles published from database inception through January 2016 for studies examining thrombosis among women using POCs. We included studies which examined women with medical conditions associated with thrombosis risk, as well as studies of women in the general population (either without these conditions or who were not specified to have these conditions). Hormonal contraceptives of interest included progestin-only pills (POPs), injectables, implants and levonorgestrel-releasing intrauterine devices (LNG-IUDs). Outcomes of interest included VTE, stroke and AMI.
Results:
There were 26 articles of good to poor quality that met inclusion criteria; 9 studies examined women with medical conditions and 20 examined women in the general population. Two studies found that, among smokers and women with certain thrombogenic mutations, use of depot medroxyprogesterone acetate (DMPA) had elevated odds of VTE compared with nonsmokers or those without mutations, although confidence intervals were wide and overlapped with odds among nonusers. One study found that, among women with previous VTE, use of POCs (including DMPA) was associated with a nonsignificant increased odds of recurrent VTE (all of which were among DMPA users); two other studies that examined POCs other than DMPA did not observe an association with recurrent VTE. Two studies found that use of DMPA among healthy women was also associated with increased odds of VTE. Two studies found that use of POCs for therapeutic indications was associated with increased odds of VTE. Studies did not find increased odds of VTE with POPs for contraceptive purposes, implants or LNG-IUDs nor were there increased odds of stroke or AMI with any POCs.
Conclusion:
The majority of evidence identified by this systematic review did not suggest an increase in odds for venous or arterial events with use of most POCs. Limited evidence suggested increased odds of VTE with use of injectables (three studies) and use of POCs for therapeutic indications (two studies, one with POCs unspecified and the other with POPs). Any increase in risk likely translates to a small increase in absolute numbers of thrombotic events at the population level.
Keywords: Progestin-only contraception, Venous thromboembolism, Stroke, Myocardial infarction, Systematic review
1. Introduction
The association between combined hormonal contraceptives and thrombosis is well established. Combined hormonal contraceptives, containing estrogen and progestin, are associated with a 2- to 3-fold increased risk of venous thromboembolism (VTE), including deep venous thrombosis and pulmonary embolism (PE), compared with nonuse [1,2]. Combined hormonal contraceptives are also associated with a 2-fold increased risk of arterial thromboembolism (ATE), including stroke and acute myocardial infarction (AMI), compared with nonuse [2,3]. Although these events are overall rare among women of reproductive age [VTE 5–10/10,000 women years (WY), stroke 21/100,000 WY and AMI 10/100,000 WY], they can have devastating complications associated with significant morbidity [2,3]. Development of thrombosis is most likely due to estrogen effects on the coagulation system [4]. Historically, progestin-only contraceptives (POCs) were not thought to be linked with thrombosis. However, evidence has demonstrated that combined oral contraceptives with the same estrogen dose but different progestins are associated with differential VTE risk, suggesting that the progestin component may play a role in thrombosis development [1]. In addition, few recent studies have found an elevated risk of VTE with use of POCs, specifically with use of depot medroxyprogesterone acetate (DMPA) that delivers a relatively higher dose and potency of progestin [5,6].
The US Medical Eligibility Criteria for Contraceptive Use, 2010 (US MEC) provides guidance for safety of contraceptive methods among women with certain characteristics or medical conditions and includes guidance for many conditions associated with increased risk of thrombosis such as postpartum, history of thrombosis, thrombogenic mutations, systemic lupus erythematosus, diabetes, hypertension and others [7]. This systematic review was conducted to identify evidence on thrombosis risk associated with POC use among women with medical conditions that increase their baseline risk for thrombosis, as well as among women in the general population. This review was conducted as part of the process of updating the US MEC.
2. Materials and methods
We conducted this systematic review according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [8].
2.1. Literature search
We searched the PubMed database for all relevant articles published from database inception through January 2016 (see Appendix A for search strategy). We searched for all primary research articles published in any language. We also searched reference lists of identified articles and relevant review articles for additional citations of interest. We did not consider unpublished studies, abstracts of conference presentations or dissertations.
2.2. Selection criteria
Articles were included in this review if they were primary research articles on VTE or ATE among women using POCs. We included studies examining women with certain characteristics or medical conditions that put them at increased risk for VTE or ATE, such as history of VTE or ATE, thrombogenic mutations, postpartum, sickle cell disease, hypertension, diabetes, smoking or systemic lupus erythematosus. We also included studies examining women in the general population (those without these conditions or who were not specified to have the conditions) to get a better understanding of their thrombosis risk with POC use. The contraceptive methods of interest included all POCs [progestin-only pills (POPs), injectables, implants and levonorgestrel-releasing intrauterine device (LNG-IUD)]. The reference group of interest was use of nonhormonal contraceptives or no contraceptive method. The outcomes of interest were VTE, stroke or AMI.
2.3. Study quality assessment and data synthesis
Three authors (NT, MW and KC) summarized and systematically assessed the evidence. We considered several study features that could impact study quality and potential biases. Related to the study population, we assessed whether studies excluded women with or controlled for important risk factors such as history of thrombosis or recent pregnancy. Related to contraceptive use, we assessed whether use was current (within 3 months of the thrombosis event) and was confirmed by medical records or review of pill packs. Related to outcomes of interest, we assessed whether diagnoses were confirmed by physician or medical record review. We assessed the quality of each individual piece of evidence using the system developed by the United States Preventive Services Task Force [9]. Summary measures were not calculated.
3. Results
The search identified 1035 articles, of which 21 met inclusion criteria (Fig. 1) [3,5,6,10–27]. Most excluded articles addressed combined hormonal contraceptives only, did not have a comparison group of nonusers, were review articles or otherwise did not address the question of interest. Five additional articles were identified from reference lists of included articles or relevant reviews [28–32]. Therefore, a total of 26 articles, describing 9 cohort studies [3,12,19–22,25,27,32] and 17 case–control studies [5,6,10,11,13–18,23,24,26,28–31], were included in this review. Nine studies addressed VTE or ATE among women with specific medical conditions or characteristics associated with risk of thrombosis, including systemic lupus erythematosus [22], hypertension [10,14], smoking [10,14,29], thrombophilia [5,12] and history of VTE [12,25,27,32] (Table 1). Twenty studies addressed VTE or ATE among women in the general population (Table 2) [3,5,6,10,11,13–21,23,24,26,28,30,31]. Most of these studies excluded women with significant risk factors for thrombosis, such as history of thrombosis or recent pregnancy or controlled for risk factors in analyses.
Fig. 1.
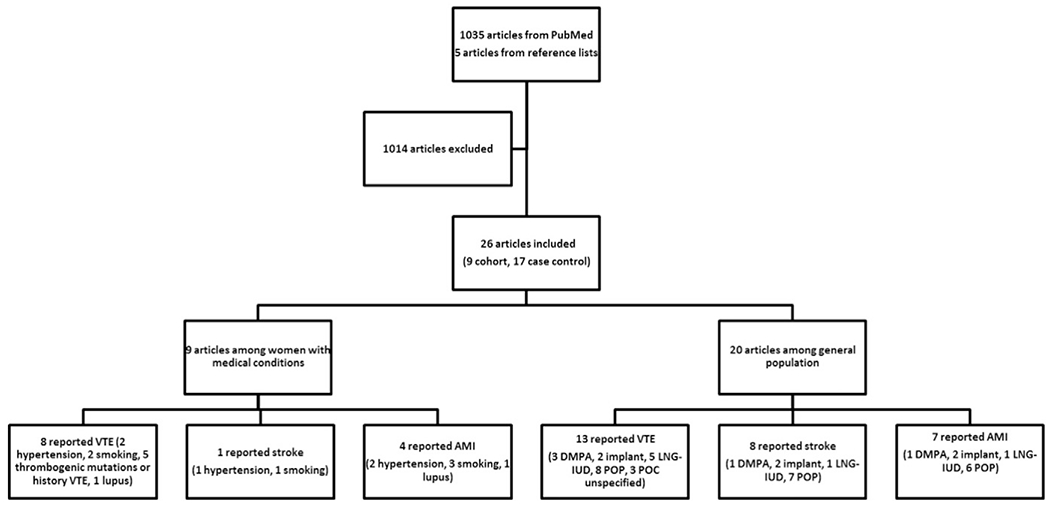
Flow chart of systematic review. Abbreviations: AMI, acute myocardial infarction; DMPA, depot medroxyprogesterone acetate; IUD, intrauterine device; LNG, levonorgestrel; MTHFR, methylenetetrahydrofolate reductase; POC, progestin-only contraceptive; POP, progestin-only pill; VTE, venous thromboembolism.
Table 1.
Evidence for risk of venous or arterial thrombosis among women with medical conditions using POCs
| Author, published, location, support | Study design, years | Population | Medical condition | Exposure | Outcome | Results | Strengths/limitations | Quality grading | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mintz [22], 1984 Mexico; Funding not stated | Prospective cohort 1981–1983 | Ages 18–40 years Cohort: using POP or NET-EN Comparison group: using IUD, sterilization, no sexual activity Exclusions: liver abnormalities, history thrombosis, abnormal uterine bleeding, breast or uterine neoplasm Follow-up time not reported | Lupus Inactive for at least 6 months Confirmed by study investigators | POP (LNG) NET-EN Timing of use not stated (AMI was within 13 weeks after first NET-EN injection) Study investigators | PE AMI Study investigators |
|
Strengths: Outcomes confirmed by study investigators Limitations: No information on source of study population or response rates Unclear whether contraceptive use was measured only at baseline or throughout study Follow-up time for specific events not reported PE and AMI assessment not described Small sample size, no assessment of study power Statistical tests for comparisons of interest not reported; no adjustment for potential confounders Large and differential loss to follow-up: 40% for NET-EN, 80% for POP and not reported among comparison group |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WHO [10], 1998 Multiple countries in Africa, Asia, Europe and Latin America; Funding: World Bank, NIH | Case–control 1989–1993 | Ages 20–44 years Cases (N= 1137 VTE, 2196 stroke, 364 AMI): admitted to hospital, identified by physicians Controls (hospital) (N=9997): hospitalized at same hospitals, matched by 5-year age band Exclusions: TIA, death within 24 h of admission, history of VTE, stroke or AMI, menopause, pregnancy, bedrest or surgery | HTN Smoking Participant questionnaire [45] | POP POI (most DMPA) Use within 3 months [45] Participant questionnaire | VTE Stroke AMI Ascertained by recruitment system at hospitals [45] Diagnosis verified by medical records and imaging |
*
Adjusted for BMI
†
Adjusted for marital status, smoking
‡
Adjusted for diabetes, smoking
NR=not reported due to no cases in certain cells
*
Adjusted for BMI
†
Adjusted for HTN, marital status
‡
Adjusted for HTN, diabetes
|
Strengths: Multiple countries Proxy interviews for deceased cases [45] VTE, stroke and AMI diagnoses confirmed with medical records Matched on age and adjusted for confounding; excluded women with recent pregnancy Limitations: Hospital controls Participation rate of controls not reported (97% participation rate of cases) [45] Contraceptive use and medical conditions not verified Contraceptive formulations not specified < 10 events for some comparisons of interest |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heinemann [14], 1999 Europe; Funding: Schering AG | Case–control 1993–1996 | Ages 16–44 years Cases (N=394): hospitalized and death reports [46] Controls (hospital and community) (N=2366): hospital controls and community controls (from general practice or neighborhood) [47], matched on age Exclusions: history thrombosis, hysterectomy, pregnancy, surgery, severe trauma | HTN Smoking Participant questionnaire [47] | POP Use within 3 months Participant questionnaire [46], confirmed by examining pill packs | VTE Ischemic stroke AMI Obtained from hospitals and death reports, confirmed by radiology results and necropsy reports [46] |
*
Adjusted for BMI
†
Adjusted for alcohol, smoking
‡
Adjusted for diabetes, education, smoking
*
Adjusted for BMI
†
Adjusted for HTN, alcohol
‡
Adjusted for HTN, diabetes, education
|
Strengths: Multinational Use of both hospital and community controls (combined, but results similar when examined separately) Proxy interviews for deceased cases [47] POP use verified VTE, stroke and AMI diagnoses confirmed by radiology and necropsy Matched on age and adjusted for confounders; excluded women with recent pregnancy Limitations: <10 POP users for some comparisons of interest |
II-2, good | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rosenberg [29], 2001 United States; Funding: NIH | Case–control 1985–1999 | Ages <45 years Cases: hospitalized Controls (hospital): admission lists or patient files from same hospitals, admitted with diagnosis unrelated to OC use | Smoking ≥25 cigarettes per day | POP Use within 1 month before admission Participant questionnaire Inpatient interviews prompted with pictures Validation of some participants by physicians (86% agreement for hospital interviews, 81% agreement for phone interviews) | AMI Phone call to collaborating hospitals, confirmed by review of records |
|
Strengths: POP use confirmed AMI diagnosis confirmed by medical records Limitations: 1 case and 1 control using POPs OR not calculated for comparison of interest |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conard [12], 2004 France;Funding source not stated | Retrospective cohort 1992–1997 | Ages 15–50 years Cohort (N=102): women using POP referred to thrombosis clinic, mean follow-up 31.2 months Comparison group (N=102 with VTE): nonhormonal, matched on age, date of first visit and thrombosis risk factors; mean follow-up 35 months | Thrombophilia (blood test by study investigators) History VTE (questionnaire) | POP (chlormadinone acetate) Current use at beginning of follow-up Participant questionnaire | VTE Ascertained by study investigators in thrombosis clinic |
* Adjusted for age, thrombophilia, BMI
|
Strengths: Exposed and unexposed from same clinic population VTE diagnoses confirmed by follow-up in clinic Matched on age and probable personal VTE history; adjusted for age, BMI, thrombophilia Limitations: <10 VTEs in users and nonusers Did not account for recent pregnancy Did not measure contraceptive use during follow-up Unable to examine risk separately for thrombophilias or history VTE, due to small numbers No information on initial response rates; median follow-up times similar between groups |
I-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Christiansen [32], 2010 The Netherlands; Funding:Netherlands Heart Foundation, Prevention Fund/ZonMW | Prospective cohort 1998–2000 | Ages 16–48 years Cohort (N=12): POC users with previous VTE during OC use or pregnancy, followed in anticoagulant clinic; total follow-up 52 WY Comparison group (N=80): currently not using hormonals and not pregnant, previous VTE during OC use or pregnancy, followed in anticoagulant clinic; total follow-up 760 WY Exclusions: recent cancer | History VTE while using COCs or pregnant Initial VTE confirmed by imaging | POP DMPA Use within 1 month Participant questionnaire | Recurrent VTE Ascertained by participant questionnaire, confirmed by medical records and radiology results |
* Adjusted for age
|
Strengths: Exposed and unexposed from same clinic population VTE diagnoses confirmed by imaging Limitations: <10 VTEs in users and nonusers POP formulations not specified All POCs analyzed together |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vaillant-Roussel [25], 2011 France; Funding: Public funds | Retrospective cohort 1995–2008 | Ages 17–53 years Cohort (N=34): POP users, with previous VTE during COC use, from hospital records and referred to clinic Comparison group (N=126): nonusers, with previous VTE from same hospital Median follow-up 74 months | History VTE while using COCs Hospital records | POP Use within 1 month Participant questionnaire | Recurrent VTE Ascertained by hospital or office records, confirmed by radiology results |
|
Strengths: Exposed and unexposed from same patient population VTE diagnoses confirmed with radiology results Limitations: POP use not confirmed; type unknown <10 recurrent VTE among POP users Did not adjust for potential confounders |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bergendal [5], 2014 Sweden; Funding: Janssen-Cilag, Novartis, Organon, Schering, Wyeth, AFA Insurance, Karolinska Instituet, Medical Products Agency | Case–control 2003–2009 | Ages 18–54 years Cases: inpatient and outpatient Controls (community): from population register, matched on age Exclusions: history thrombosis, pregnancy within 3 months, current cancer | Thrombogenic mutations Blood sample by investigators | POP DMPA Implant LNG-IUD Use within 3 months Participant questionnaire | VTE Diagnosis confirmed by medical records and imaging Included only if initiated anticoagulation |
* Adjusted for age, smoking, BMI, immobilization
FVL and DMPA: OR 16.7 (95% CI 2.4–714) FVL and LNG-IUD: OR 3.2 (95% CI 1.2–10.4)
* No longer significant when excluded obese and severely immobilized women
No statistically significant elevated odds of VTE among women with prothrombin gene mutation, factor XIII, glycoprotein IIIa, endothelial nitric oxide synthase, plasminogen activator inhibitor |
Strengths: Population-based VTE diagnoses confirmed with medical records Adjusted for confounders, excluded women with recent pregnancy Response rate high (>88%) in both groups Limitations: POC use not confirmed <10 POC users for some comparisons of interest |
II-2, good | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Le Moigne [27], 2016 France; Support not stated | Prospective cohort 1992–2013 | Ages ≤50 years Cohort (N=163): POC users, with previous VTE, from hospital records; median duration of follow-up 5.1 years after stopping anticoagulation Comparison group (N=256): nonusers, with previous VTE from same hospital; median duration of follow-up 3.8 years after stopping anticoagulation Exclusions: active cancer, hormone replacement, anticoagulant therapy, periods of exposure to combined hormonal contraception or pregnancy | History VTE Hospital records | POP (LNG or DSG) ETG implant LNG-IUD Timing of use not stated Ascertainment not stated | Recurrent VTE Ascertained by review of hospital diagnoses, confirmed by imaging |
* Adjusted for age
|
Strengths: Exposed and unexposed from same patient population VTE diagnoses confirmed by imaging Limitations: Method of ascertainment of POC use not stated Timing of POC use relative to VTE not stated Comparison group may have used POCs during follow-up All POCs analyzed together <10 recurrent VTE among POC users |
II-2, poor |
Abbreviations: AMI, acute myocardial infarction; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; DSG, desogestrel; HR, hazard ratio; HTN, hypertension; IRR, incidence rate ratio; IUD, intrauterine device; LNG, levonorgestrel; MTHFR, methyl tetrahydrofolate reductase; NET-EN, norethisterone enanthate; NIH, National Institutes of Health; PE, pulmonary embolism; POI, progestin-only injectable; POP, progestin-only pill; TIA, transient ischemic attack; VTE, venous thromboembolism.
3.1. Women with medical conditions that carry risk of thrombosis
3.1.1. VTE among women with medical conditions
Eight studies reported odds of VTE among women with certain medical conditions using POCs (Table 1) [5,10,12,14,22,25,27,32]. Two studies assessed odds of VTE among women with hypertension using POPs (Fig. 2) [10,14]. Both studies found that, among women with hypertension using POPs, odds of VTE were not statistically significantly elevated compared with nonusers without hypertension [odds ratio (OR) 1.2, 95% confidence interval (CI) 0.1–23.7 in one study; OR 2.3, 95% CI 0.2–28.1 in the other study].
Fig. 2.
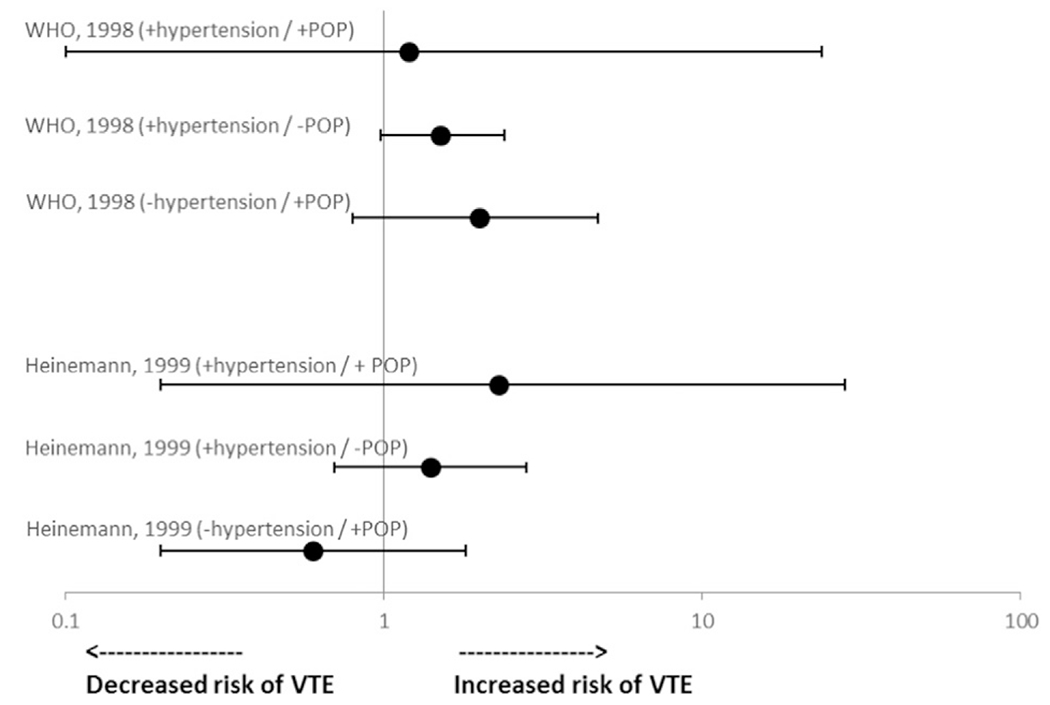
Risk* of VTE among women with hypertension using POCs. Abbreviations: POP, progestin-only pill; VTE, venous thromboembolism. *Reference group is nonusers without hypertension.
The same two studies discussed above also assessed odds of VTE among women who smoked and used POPs (Fig. 3) [10,14]. Neither study found a statistically significantly elevated odds of VTE among smokers using POPs (OR 2.4, 95% CI 0.7–8.3 in one study and OR 0.95, 95% CI 0.2–6.0 in the other study, both compared with nonusers who did not smoke). One of the studies also assessed smokers using injectables (mostly DMPA) and found that, compared with nonsmokers who did not use injectables, the OR for VTE was 7.0 (95% CI 0.4–138), which was higher than the ORs for VTE for smoking with no injectable use (OR 1.3, 95% CI 0.97–1.6) and injectable use with no smoking (OR 1.9, 95% CI 0.5–7.0), although CIs were wide and not statistically significant [10].
Fig. 3.
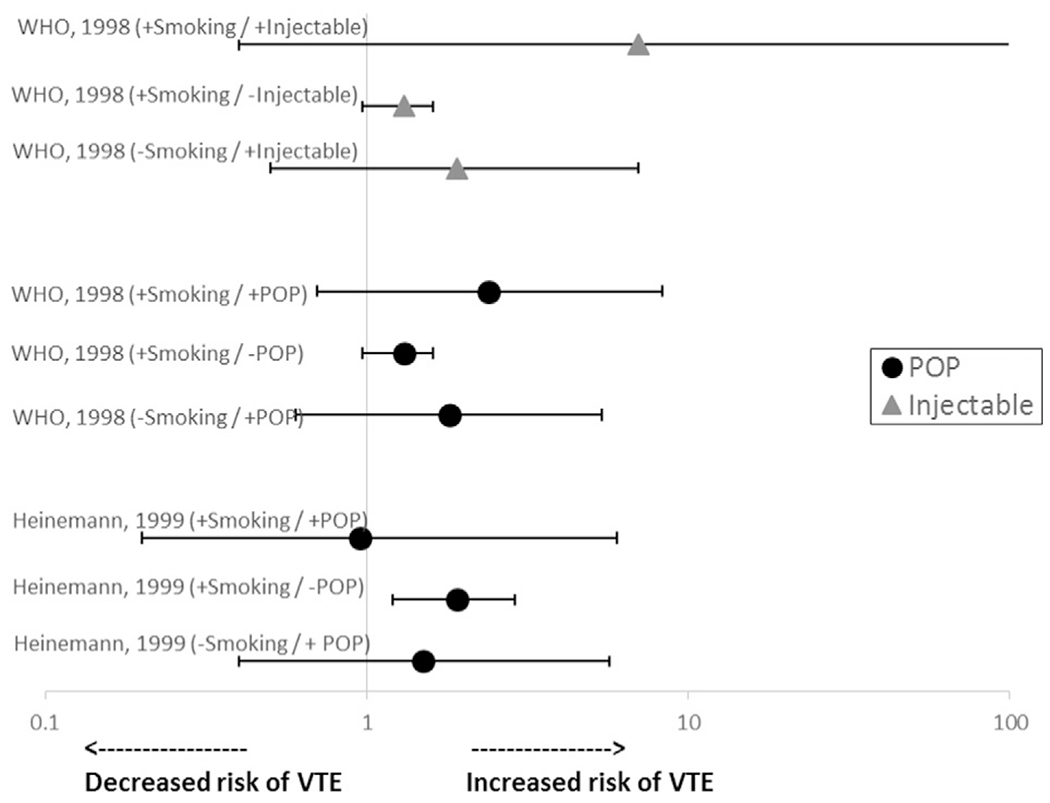
Risk* of VTE among smokers using POCs. Abbreviations: POP, progestin-only pill; VTE, venous thromboembolism. *Reference group is nonusers who did not smoke.
Five studies examined women with thrombogenic mutations or a history of VTE (Fig. 4) [5,12,25,27,32]. One of the studies examined women with thrombogenic mutations using DMPA and found that the odds of VTE were elevated among women with the factor V Leiden (FVL) mutation using DMPA compared with nonusers without the mutation (OR 16.7, 95% CI 2.4–714) [5]. The OR was also elevated among women with FVL not using POCs (OR 2.6, 95% CI 1.8–3.7) but odds were not reported for use of DMPA compared with nonuse among women without FVL. This study also examined the association of VTE with LNG-IUD use among women with FVL. Relative to nonusers without FVL, similar ORs were detected for women with FVL using LNG-IUDs (OR 3.2, 95% CI 1.2–10.4) and women with FVL without POC use (OR 2.6, 95% CI 1.8–3.7) [5]; odds among women without FVL using LNG-IUDs were not reported. Odds of VTE among women with FVL were also increased among all POC users analyzed together (OR 5.4, 95% CI 2.5–13) and among nonusers (OR 2.6, 95% CI 1.8–3.7), compared with nonusers without FVL. This study also detected an elevated unadjusted OR for VTE among women with the methylenetetrahydrofolate reductase (MTHFR) polymorphism and POC use as compared to nonusers without MTHFR (OR 3.6, 95% 2.5–5.4); however, the OR was no longer significant after excluding obese and severely immobilized women. Odds of VTE were not elevated among women with MTHFR not using POCs (OR 0.9, 95% CI 0.7–1.1). Increased odds of VTE were not detected in this study among women with other thrombogenic mutations using POCs. One study examined women with a personal or family history of VTE or hereditary thrombophilia and found no association between POP use and VTE (OR 0.8, 95% CI 0.2–3.9) [12]. Three studies examined risk for recurrent VTE. One study found that, among women with history of VTE, odds of recurrent VTE were elevated among women using POCs (all recurrent VTEs occurred among DMPA users) compared with nonhormonal users; however, this did not reach statistical significance (OR 3.6, 95% CI 0.7–17.3) [32]. However, two studies examined use of POPs or non-DMPA POCs (POPs, LNG-IUDs and implants) analyzed together and neither found statistically significantly elevated odds of VTE compared with nonhormonal users (OR 1.3, 95% CI 0.5–3.0 and OR 0.6, 95% CI 0.3–1.5) [25,27].
Fig. 4.
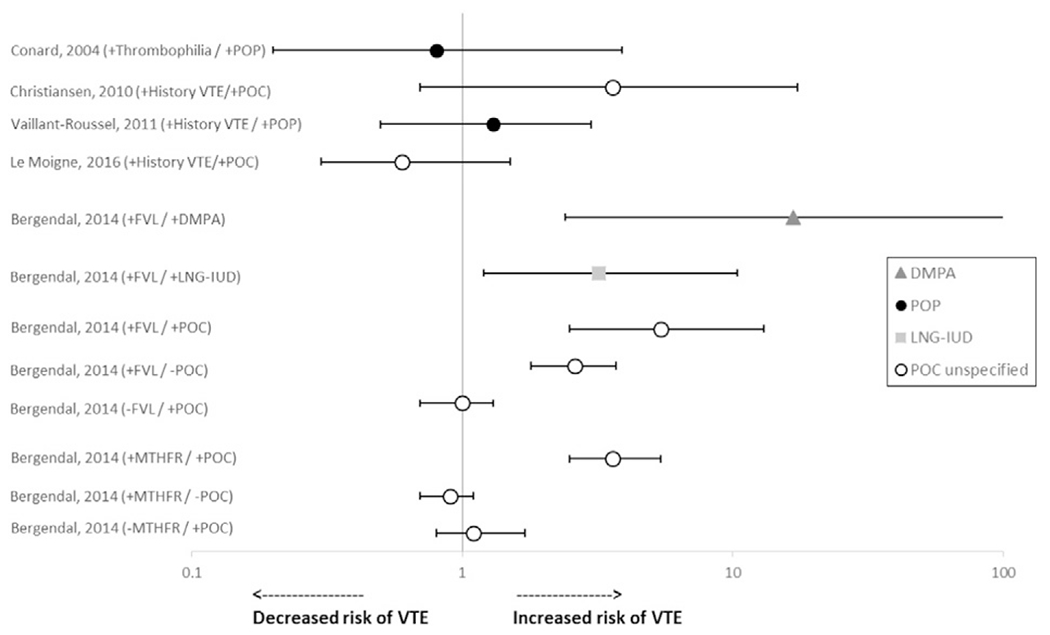
Risk* of VTE among women with thrombogenic mutations or history of VTE using POCs. Abbreviations: DMPA, depot medroxyprogesterone acetate; FVL, Factor V Leiden; IUD, intrauterine device; LNG, levonorgestrel; MTHFR, methylenetetrahydrofolate reductase; POC, progestin-only contraceptive; POP, progestin-only pill; VTE, venous thromboembolism. *Reference group is nonusers with thrombogenic mutations or history of VTE, except Bergendal (reference group nonusers without thrombogenic mutations).
One study addressed women with systemic lupus erythematosus using POCs (POPs containing LNG or NET-EN) [22]. There was 1 PE among 15 women using POPs (6.7%) and 1 PE among 18 women using nonhormonal or no method (5.6%). Statistical tests were not conducted.
3.1.2. Stroke among women with medical conditions
One study examined odds of stroke among women with hypertension using POPs [10]. The study found that, among women with hypertension, odds of stroke were increased among women using POPs (OR 10.9, 95% CI 3.6–33.8) and not using POPs (OR 7.2, 95% CI 6.1–8.5) but were not elevated among women without hypertension using POPs (OR 0.95, 95% CI 0.5–1.8), compared with nonusers without hypertension.
The same study also examined women who smoked and found that odds of stroke were not statistically significantly elevated among smokers using POPs compared with nonusers who did not smoke [10].
3.1.3. AMI among women with medical conditions
Two studies assessed odds of AMI among women with hypertension using POPs [10,14]. Neither study found a statistically significantly increased odds of AMI among women with hypertension using POPs, compared with nonusers without hypertension (OR 1.9, 95% CI 0.1–38.4 in one study; OR 0.8, 95% CI 0.03–20.4 in the other study).
Three studies examined women who smoked [10,14,29]. One study found that odds of AMI were elevated but not statistically significant among smokers using POPs (OR 7.2, 95% CI 0.7–74.6) but were significant among smokers not using POPs (OR 5.0, 95% CI 3.4–7.3), both compared to nonsmokers not using POPs [10]. Another study found that odds of AMI were statistically significantly elevated but similar among smokers using POPs (OR 10.4, 95% CI 1.1–98.8) and not using POPs (OR 10.2, 95% CI 5.0–20.6), both compared with nonusers who did not smoke [14]. A third study reported that, among women who smoked ≥ 25 cigarettes per day, there was one case (0.16%) using POPs and one control (0.03%) using POPs; however, statistical tests for this comparison were not calculated [29].
One study reported instances of AMI among women with lupus [22]. There was one AMI among ten women using NET-EN (10%). There were no AMIs among women using POPs or among the comparison group of nonhormonal users.
3.2. Women in the general population
3.2.1. VTE among women in the general population
The search identified 13 publications from 11 studies that reported odds of VTE among women in the general population using POCs compared with nonuse of hormonal contraception (Table 2 and Fig. 5) [5,6,10,11,14,16,17,19–21,24,26,28]. While these studies represented women in the general population, several attempted to create a “healthy” cohort by excluding women with risk factors for VTE, such as previous VTE or recent pregnancy.
Fig. 5.
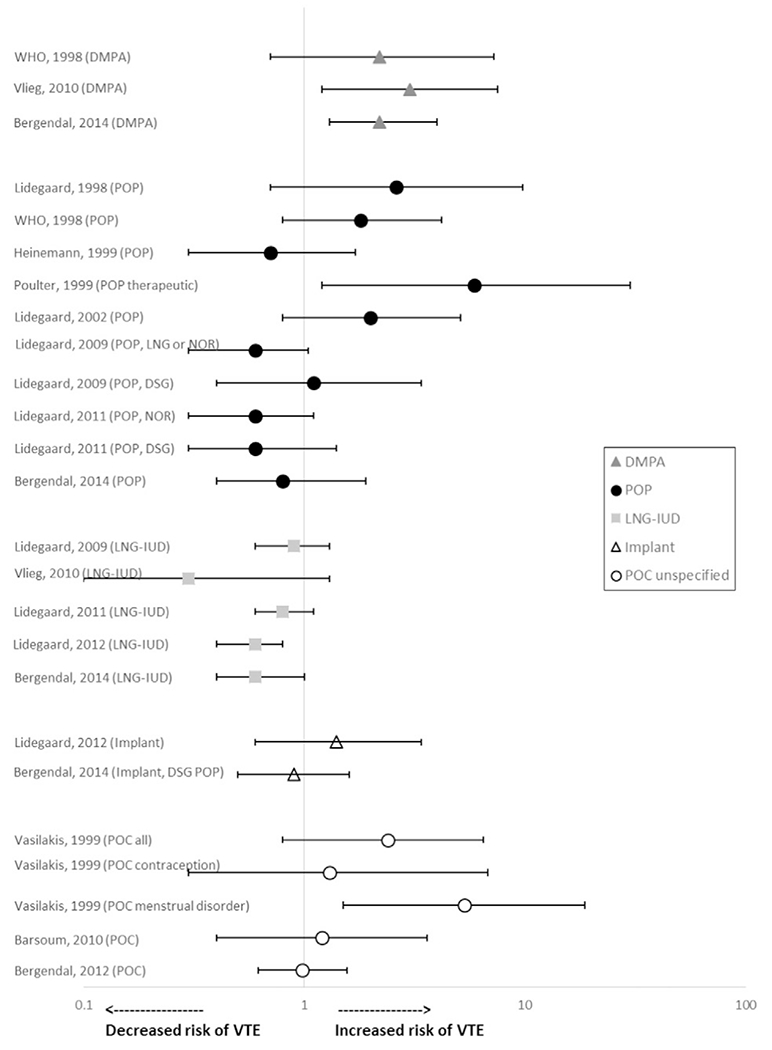
Risk* of VTE among women in the general population using POCs. Abbreviations: DMPA, depot medroxyprogesterone acetate; DSG, desogestrel; IUD, intrauterine device; LNG, levonorgestrel; NOR, norethindrone; POC, progestin-only contraceptive; POP, progestin-only pill; VTE, venous thromboembolism. *Reference group is nonusers.
Three studies reported odds of VTE among women using DMPA [5,6,10]. Two of these studies found statistically significantly increased odds of VTE among women using DMPA compared with nonusers. Van Hylckama Vlieg found that women using DMPA had a statistically significant increased odds of VTE compared with nonusers (OR 3.0, 95% CI 1.2–7.5) [6]. Bergendal found that women using DMPA had statistically significant increased odds of VTE compared with nonusers (OR 2.2, 95% CI 1.3–4.0) [5]. The remaining study found a similar magnitude of risk that did not reach statistical significance with use of progestin-only injectables, reported to be mostly DMPA (OR 2.2, 95% CI 0.7–7.3) [10].
Two studies reported risk of VTE among women using implants [5,20]. Lidegaard found that the adjusted rate ratio of all VTEs was statistically significantly elevated among women using implants compared with past or never users (rate ratio 2.1, 95% CI 1.3–3.5) [20]. This risk was attenuated and no longer significant when the analysis was limited to confirmed VTEs (rate ratio 1.4, 95% CI 0.6–3.4). Bergendal did not find increased odds of VTE with use of implants and desogestrel POPs combined, compared with nonhormonal users [5].
Five studies reported odds of VTE among women using LNG-IUDs compared with nonhormonal users [5,6,19–21]. None of the studies found increased odds of VTE with use of LNG-IUDs compared with nonuse (OR ranged from 0.3 to 0.9).
Eight articles from seven studies reported odds of VTE among women using POPs compared with nonusers [5,10,14,16,17,19,21,24]. Studies did not find increased odds with use of POPs for contraceptive purposes compared with nonuse (OR ranged from 0.6 to 2.6; none reached statistical significance). Poulter found that odds of VTE were elevated among women using POPs for therapeutic indications compared with nonusers (OR 5.9, 95% CI 1.2–30.1), although conditions being treated were not stated and formulations and doses of POPs were not reported [24].
Three studies reported risk of VTE among women using all POCs combined or unspecified [11,26,28]. None of the studies found increased odds of VTE among women using POCs for contraceptive purposes compared with nonusers (OR ranged from 0.98 to 1.2). Vasilakis found that women using POCs (not specified) had an elevated but not statistically significant increased relative risk (RR) of VTE, compared with nonusers (RR 2.4, 95% CI 0.8–6.5) [26]. When stratified by indication for use, women using POCs for therapeutic reasons (primarily menstrual disorders) had a significantly increased RR of VTE compared with nonusers (RR 5.3, 95% CI 1.5–18.7).
3.2.2. Stroke among women in the general population
Eight articles from seven studies reported odds of stroke (ischemic or unspecified) among women using POCs (Table 2) [3,10,14,15,18,23,24,31]. Among these studies, one reported on progestin-only injectables (primarily DMPA) [10], two reported on implants [3,23], one reported on the LNG-IUD [3] and seven reported on POPs (including unspecified doses used for therapeutic reasons) [3,10,14,15,18,24,31]. None of the studies found elevated odds of stroke with use of POCs compared with nonuse (OR ranging from 0.7 to 1.6).
3.2.3. AMI among women in the general population
Seven articles from six studies reported odds of AMI among women using POCs (Table 2) [3,10,13,14,23,24,30]. Among these studies, one study reported on progestin-only injectables (primarily DMPA) [10], two studies reported on implants [3,23], one study reported on the LNG-IUD [3] and six studies reported on POPs (including unspecified doses used for therapeutic reasons) [3,10,13,14,24,30]. Petitti found that odds of AMI were elevated among implant (Norplant®) users compared with nonusers, but the CI was wide and crossed 1 (OR 3.5, 95% CI 0.2–56.5) [23]. Lidegaard also found that odds of AMI were elevated among implant users compared with nonusers, but the CI crossed 1 (OR 2.1, 95% CI 0.7–6.7) [3]. The remaining studies did not find elevated odds of AMI with use of progestin-only injectables, LNG-IUD or POPs compared with nonuse (OR ranging from 0.7 to 1.5).
4. Discussion
Evidence identified by this systematic review generally found that any risk of venous or arterial thrombosis among women with certain medical conditions was not further elevated with use of POCs. Among women with hypertension, odds of stroke were higher with use of POPs compared with nonuse, but CIs overlapped. Similar results were found among smokers using POPs. One study suggested that injectable use among women who smoke increased the odds of VTE compared with smokers who did not use injectables [10]. Another study suggested significantly elevated odds of VTE among women with FVL who used DMPA compared with nonuse [5]. The same study also found a significantly elevated odds of VTE among women with the MTHFR polymorphism who used POCs compared with nonuse; however, this risk was attenuated and no longer significant when excluding obese and severely immobilized women. One study suggested an elevated odds of recurrent VTE among women with a history of VTE using POCs (all of which occurred in DMPA users); however, this did not reach statistical significance [32]; two other studies examining non-DMPA POCs found no increased odds of recurrent VTE.
Evidence identified by this systematic review generally found no statistically significant increased odds of arterial or venous thrombosis among women in the general population using POPs, implants or LNG-IUDs, compared with nonuse. However, among the three studies examining use of DMPA, the two more recent studies suggested elevated odds of VTE with use of DMPA [5,6]. The only other study to specifically report VTE risk with use of injectables was an older study published in 1998 that included other types of injectables and found an elevated OR that did not reach statistical significance [10]. In addition, this systematic review identified two studies that suggested increased odds of VTE with use of POCs for therapeutic indications for which the dose is typically higher [24,26]. These results suggest the potential for a dose–response relationship between progestin dose and VTE risk; alternatively, they may suggest residual confounding associated with women who choose to use DMPA or, in the studies of therapeutic use, residual confounding related to the underlying condition being treated.
The current review expands on several previously published metaanalyses assessing POC use, by including more recent studies and including results for women with medical conditions. A metaanalysis that included four studies on the risk of VTE among women using POCs (all types combined) found that odds were not significantly elevated (OR 1.45, 95% CI 0.92–2.26) [33]. A more recent metaanalysis that included eight studies on the risk of VTE similarly found that odds were not significantly elevated with use of all POCs (OR 1.03, 95% CI 0.76–1.39) or with use of POPs or LNG-IUDs [34]. However, use of injectables was associated with an elevated risk of VTE, when combined odds were calculated from two studies (OR 2.67, 95% CI 1.29–5.53) [6,10]. A metaanalysis that included six studies on the risk of stroke among women using POCs found that the combined OR was not elevated (OR 0.96, 95% CI 0.70–1.31) [35]. The estimated odds did not change when injectables were excluded. A metaanalysis that included six studies on the risk of AMI among women using POCs also found that odds were not significantly elevated (OR 1.07, 95% CI 0.62–1.84) [36]. The estimated odds did not change when injectables were excluded.
The mechanism of thrombosis is complex and involves alterations to many different components of the hemostatic system. Potential biological effects of POCs on the hemostatic system are not well understood. Progestins have variable effects on clotting factors, and in addition, these effects likely vary with progestin type, potency, dose and route of administration. Studies have generally not found deleterious effects on coagulation parameters, including fibrinogen, clotting factors and platelet function, with use of POCs [36–39]. However, progestins may have certain effects on vasculature that could potentially reduce blood flow, including increased distensibility in veins and increased vasoconstriction in arteries [38]. Progestins have been found to have varying effects on other biological parameters. Studies have shown that different POCs can cause changes in lipid parameters in both positive and negative directions [36]. DMPA and certain POPs have been associated with decreased HDL, increased total cholesterol and increased triglycerides [36]. Studies have also found increased insulin resistance with use of DMPA and etonogestrel implants [36]. However, it is not clear how these biological effects associated with POCs translate into clinical thrombosis outcomes and whether there are any markers predictive of these outcomes.
Any potential risk of thrombosis with use of POCs may be compounded in women with certain medical conditions that elevate the risk of thrombosis. Even if POCs confer no or small increased risk of thrombosis among healthy women, there is theoretical concern that use among women already at elevated risk could further increase that risk to a concerning level. The US MEC includes recommendations for many conditions and characteristics associated with increased risk of thrombosis [7]. The risk of VTE among postpartum women is 2.5–84 times higher than that among nonpregnant nonpostpartum women [40]. Certain inherited conditions, such as antithrombin deficiency, protein C deficiency, protein S deficiency and FVL, confer a relative risk for VTE of 5–15 times higher compared with individuals without the condition [41]. Individuals with systemic lupus erythematosus have 3–8 times higher risk of VTE and individuals with inflammatory bowel disease have 3–4 times higher risk [42]. Obesity, hypertension, diabetes and smoking confer 1.5–2 times higher risk of VTE [42,43]. The risk of thrombosis also increases with increasing age [42]. One study of postmenopausal women (mean age 58 years) did not find increased risk of VTE with use of POCs (all analyzed together) [28]. Another study of women aged > 50 years found increased risk of VTE with DMPA use; however, exact OR and CI were not reported [44]. It is not clear whether these findings can be extrapolated to older women of reproductive age, however. Therefore, the present systematic review identified little direct evidence on further increases in risk related to POC use among women with significant thrombosis risk factors.
There are several limitations of this body of evidence, which was of good to poor quality. Most of the studies had small numbers for analyses of interest, and therefore, the studies had low power to detect differences between groups and some elevated point estimates with wide CIs were difficult to interpret. Several studies obtained information on contraceptive use from patient report or proxy report, which was not further verified and may be subject to recall bias [5,6,10,13–16,23,25]. Some studies did not verify thrombosis diagnoses with medical records or other measures, which may lead to misclassification of individuals who were evaluated for thrombosis but ultimately ruled out [3,19,22]. This might bias results if individuals using contraception are more likely to be referred for evaluation of symptoms of thrombotic events. Several studies reported results for all POCs combined or did not specify formulation and dose of POC [11,24,26–28,32]. Combining all POCs together in analyses might result in attenuated risk estimates. Several studies did not report statistical tests for the comparison of POC use versus nonuse [22,29,30,44]. Some studies did not account for recent pregnancy or other important risk factors such as history of thrombosis [12,17,18,23,25,26,31].
In conclusion, this systematic review identified level II-2, good to poor quality evidence on the risk of venous and arterial thrombosis among women using POCs who also have medical conditions or characteristics associated with thrombosis risk. Evidence did not suggest increased risk of venous or arterial thrombosis among women with hypertension or lupus using POCs. Limited evidence from three studies suggested increased risk of VTE with use of injectables among smokers, women with thrombogenic mutations and women with a history of VTE. This review also identified level II-2, good to poor quality evidence on the risk of venous and arterial thrombosis among women in the general population using POCs. Evidence did not suggest increased risk of VTE with use of POPs, implants or LNG-IUDs. Evidence also did not suggest increased risk of stroke or AMI with use of any POCs. Limited evidence from four studies suggested increased risk of VTE with use of injectables and use of POCs for therapeutic indications. Any elevated risk is likely small and represents only a slight increase in absolute numbers of thrombotic events at the population level.
Table 2.
Evidence for risk of venous or arterial thrombosis among women with no or unspecified medical conditions using POCs
| Author, publication, location, support | Study design, years | Population | Exposure | Outcome | Results | Strengths/limitations | Quality grading | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thorogood [30], 1991 England and Wales; Funding: British Heart Foundation, Committee on Safety of Medicines, Family Health International | Case–control 1986–1988 | Ages <40 years Cases: death certificates Controls (community): living controls from same general practitioner, matched on age (5-year age groups) and marital status Exclusions: resident of institution for 1 month, pregnant within 2 months, another life threatening illness such as cancer | POP Use within 1 month Physician questionnaire | Death due to AMI Diagnosis codes from death certificates, confirmed by post-mortem reports and medical records |
|
Strengths: AMI diagnoses confirmed by medical records Limitations: Use of deceased cases and living controls <10 cases using POPs OR not reported for comparison of interest |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [15], 1993 Denmark; Support not stated | Case–control 1985–1989 | Ages 15–44 years Cases: first episode identified from national registry using discharge diagnosis codes Controls (community): randomly selected from national registry, matched on age Exclusions: history thromboembolism, current pregnancy, HTN; controls also excluded if they had migraine or other pre-disposing medical factor | POP Use at time of event Participant questionnaire | Ischemic stroke Identified by diagnosis codes Confirmed by physician or subject confirmation or CT scan |
* Adjusted for age, smoking, education
|
Strengths: National dataset Stroke diagnoses confirmed by physician or subject or radiology results Limitations: <10 cases using POPs POP use not confirmed Included TIA Did not account for recent pregnancy |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tzourio [31], 1995 France; Funding: GLAXO France | Case–control 1990–1993 | Ages 18–44 years Cases (N=41): hospitalized with first episode, diagnosis codes Controls (hospital) (N=62): randomly selected from same hospitals, orthopedic or benign rheumatologic illnesses Exclusions: not stated (no participants had history of stroke) | POP Use within 1 month Participant questionnaire | Ischemic stroke Identified by diagnosis codes Confirmed by radiology results |
* Reference group not clearly stated
|
Strengths: Stroke diagnoses confirmed by radiology results Limitations: <10 cases and controls using POPs POP use not confirmed Did not account for recent pregnancy or other confounding factors Reference group not clearly stated Did not report CI |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [16], 1998 Denmark; Funding: Organon, Wyeth-Ayerst, Schering AG | Case–control 1994–1995 | Ages 15–44 years Cases: identified from national registry using discharge diagnostic codes Controls (community): randomly selected from national registry Exclusions: history VTE or AMI, pregnancy at time of thrombosis | POP Current use (at time of admission for cases and time of interview for controls) Participant questionnaire | VTE Identified by diagnosis codes and physician confirmation Classified as uncertain, probable or certain (based on radiologic results and/or anticoagulation); all analyzed together |
* Adjusted for age, age at first birth, smoking
|
Strengths: High response rate for cases and controls (approximately 90%)High percent (>95%) VTE diagnoses confirmed by radiologic results and/or anticoagulation Limitations: <10 cases and controls using POPs POP use not confirmed Did not account for recent pregnancy |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Petitti [23], 1998 United States; Funding: NIH | Pooled case–control 1991–1995 (stroke) [48,49] 1991–1994 (AMI) [50] | Ages 18–44 years Stroke [48,49]: Cases: discharge diagnoses from hospitals, death registers Controls (community): random-digit dialing of residents of same counties as cases or randomly selected from insurance plan and matched on age and location Exclusions: history heart disease, stroke AMI [50]: Cases: discharge diagnoses from hospital, emergency rooms Controls (community): randomly selected from insurance plan, matched on age and location Exclusions: history heart disease |
Implant (Norplant®) Use within 1 month [48–50] Participant questionnaire | Ischemic stroke AMI Diagnosis codes, confirmed by physician review of records |
* Adjusted for age
* Adjusted for age
|
Strengths:Stroke and AMI diagnoses confirmed by medical records Limitations:Implant use obtained from proxys for deceased cases and not confirmed < 5 cases and controls using implants Did not account for pregnancy/postpartum or other important confounders |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WHO [10], 1998 Multiple countries in Africa, Asia, Europe and Latin America; Funding: World Bank, NIH | Case–control 1989–1993 | Ages 20–44 years Cases: admitted to hospital, identified by physicians Controls (hospital): hospitalized at same hospitals, matched by 5-year age band Exclusions: TIA, death within 24 h of admission, history of VTE, stroke or AMI, menopause, pregnancy, bedrest or surgery | POP POI (most DMPA) Use within 3 months [45] Participant questionnaire | VTE Stroke AMI Ascertained by recruitment system at hospitals [45] Diagnosis verified by medical records and imaging |
* Adjusted for BMI
* Adjusted for HTN, marital status, smoking
* Adjusted for HTN, diabetes, smoking
All results similar when reported separately for Europe and developing countries |
Strengths: Multiple countries Proxy interviews for deceased cases [45] VTE, stroke and AMI diagnoses confirmed with medical records Matched on age and adjusted for confounding; excluded women with recent pregnancy Limitations: Hospital controls Participation rate of controls not reported (97% participation rate of cases) [45] Contraceptive use and medical conditions not verified Contraceptive formulations not specified < 10 events for some comparisons of interest |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dunn [13], 1999 England, Scotland, Wales; Funding: NV Organon, Schering AG | Case–control 1993–1995 | Ages 16–44 years Cases: hospitals or death register Controls (community): same general practice as cases, matched on age Exclusions: History AMI, pregnancy within 6 wks, menopause, hysterectomy, oophorectomy, breast or ovarian cancer |
POP Use within 3 months Participant or proxy (for deceased cases) questionnaire; validated with records from general practitioner or family planning clinic | AMI Diagnosis codes from hospital records or death records, confirmed by physician review of medical records |
* Adjusted for smoking, BMI, blood pressure measurement in past year, diabetes, angina, hypertension, family hx ischemic heart disease, use of other drugs, additional variables from univariate analysis; interview and medical record data
|
Strengths: AMI diagnosis confirmed by medical records ORs adjusted for important confounders Limitations: <10 cases using POPs |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heinemann [14], 1999 Europe; Funding: Schering AG | Case–control 1993–1996 | Ages 16–44 years Cases: hospitalized and death reports [46] Controls (hospital and community): hospital controls and community controls (from general practice or neighborhood) [47], matched on age Exclusions: history thrombosis, hysterectomy, pregnancy, surgery, severe trauma | POP Use within 3 months Participant questionnaire [46], confirmed by examining pill packs | VTE Ischemic stroke lAMI Obtained from hospitals and death reports, confirmed by radiology results and necropsy reports [46] |
* Adjusted for BMI
* Adjusted for HTN, alcohol, smoking
* Adjusted for HTN, diabetes, education, smoking
|
Strengths: Multinational Use of both hospital and community controls (combined, but results similar when examined separately) Proxy interviews for deceased cases [47] POP use verified VTE, stroke and AMI diagnoses confirmed by radiology and necropsy Matched on age and adjusted for confounders; excluded women with recent pregnancy Limitations: <10 POP users for some comparisons of interest |
II-2, good | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poulter [24], 1999 (Same study as WHO, 1998 [10]) Multiple countries in Africa, Asia, Europe and Latin America; Funding: World Bank, NIH | Case–control 1989–1995 | Ages 20–49 years Cases: admitted to collaborating hospitals Controls (hospitalized): hospitalized, matched by 5-year age band Exclusions: TIA, history thrombosis, natural or surgical menopause, pregnant or postpartum, prolonged bed rest, surgery | POP for therapeutic reasons Use within 3 months [45] Participant questionnaire [45] | VTE Stroke AMI Ascertained by recruitment system at hospitals [45] Diagnosis confirmed by medical records and imaging |
* Adjusted for BMI
* Adjusted for hypertension, marital status, smoking
* Adjusted for hypertension, diabetes, smoking
|
Strengths: VTE, stroke and AMI diagnoses confirmed by medical records Limitations: Formulation and dose of POPs not stated Conditions being treated not reported <10 cases using POPs |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vasilakis [26], 1999 United Kingdom; Funding: WHO | Nested case–control 1993–1997 | Ages <50 years Cases: computer records from hospital admissions Controls (community): matched on age and general practice Exclusions [51]: history VTE, stroke, AMI, cancer, epilepsy, diabetes, hypertension, hyperlipidemia, cystic fibrosis | POCs (not specified) “Current” use, not further defined Computer record | VTE Computer hospital records, some validated by physician |
* Adjusted for BMI and smoking
|
Strengths: VTE diagnoses confirmed with medical records Limitations: Formulation and dose of POCs not specified |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [17,18], 2002 Denmark; Funding: Organon, Wyeth-Ayerst, Schering AG | Case–control 1994–1998 | Ages 15–44 years Cases: diagnosis codes from national registry of hospital discharges Controls (community): randomly selected from national registry, matched on age Exclusions: history thrombosis, pregnant at time of diagnosis | POP Current use (at time of admission for cases and time of interview for controls) Participant questionnaire | VTE Ischemic stroke + TIA Discharge diagnosis codes, confirmed by physician and patient |
* Adjusted for age, year, BMI, education, coagulation disturbances, family VTE, previous birth, diabetes, smoking
* Adjusted for age, year, smoking, migraine, education
|
Strengths: Contraceptive type prompted by list of available OCs VTE and stroke diagnoses confirmed by physician and patient High response rate (approx. 90%) Limitations: Did not account for recent pregnancy <10 VTE and stroke cases using POPs Included TIA (36% of cases), analyzed together with cerebral infarction |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [19], 2009 Denmark; Funding: Gyn Clinic, Rigshospitalet | Retrospective cohort 1995–2005 | Ages 15–49 years Cohort (>10 million WY): national registry Exclusions: cancer, history cardiovascular event, pregnant or postpartum | POP LNG-IUD Current use (valid prescription at hospital admission) Obtained from filled prescriptions | VTE Diagnosis codes (validated in previous study, 10% uncertain) |
* Adjusted for age, calendar year, education
|
Strengths: Large national cohort Limitations:VTE diagnoses not confirmed <10 VTEs among users of some POPs No information on BMI |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Barsoum [11], 2010 United States; Funding: NIH, US Public Health Service, Mayo Foundation | Case–control 1998–2000 | All ages Cases: inpatient, outpatient, emergency, nursing home, autopsy, death certificate Controls (community): from population, matched on age, medical care within 1 year of case event date, medical record number close to case’s number | POC (not specified, some receiving noncontraceptive doses and formulations) Use within 3 months Obtained from medical record | VTE Diagnosis confirmed by medical records and imaging |
* Adjusted for BMI, hospitalization, nursing home, trauma/fracture, cancer, leg paresis, varicose veins, pregnancy
|
Strengths: VTE diagnoses confirmed with medical records Limitations: Contraceptive types not specified Analysis may have included postmenopausal women <10 POC users among controls Did not account for history of VTE |
II-2, poor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vlieg [6], 2010 Netherlands; Funding: Netherlands Heart Association, Dutch Cancer Foundation, Netherlands Organization for Scientific Research | Case–control 1999–2004 | Ages 18–50 years Cases: anticoagulant clinics Controls (community): partners of patients or random-digit dialing and matched on age Exclusions: history DVT, OC users, postmenopausal, pregnant or postpartum, severe psychiatric problems, non-Dutch speaking | DMPA LNG-IUD “Current use” not further defined [52] Participant questionnaire | VTE Diagnosis from anticoagulation clinic |
* Adjusted for age, BMI, family history DVT, smoking
|
Strengths: Accounted for important confounders VTE diagnoses confirmed by clinics Limitations: DMPA and LNG-IUD use not confirmed <10 cases using LNG-IUD |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [21], 2011 Denmark; Funding: Bayer Schering | Retrospective cohort 2001–2009 | Ages 15–49 years Cohort (<8 million WY): national registry Exclusions: history thrombosis, cancer, hysterectomy, oophorectomy, sterilization, pregnant or postpartum, coagulation disorder | POP (NOR or DSG) LNG-IUD Use within 4 weeks Obtained from filled prescriptions | VTE Discharge diagnosis codes from national registry, confirmed with prescription for anticoagulant 200 randomly selected women validated by medical records |
* Adjusted for age, year, and education
No significant differences when stratified by whether anticoagulation was used (confirmed VTEs) or not recorded |
Strengths: Large national cohort VTE diagnoses confirmed by anticoagulant prescription, subset validated by medical records Limitations: <10 VTEs among users of each POP No information on BMI |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bergendal [28], 2012 Sweden; Funding: Janssen-Cilag, Novartis, Organon, Schering, Wyeth, AFA, Center for Gender Medicine | Case–control 2003–2009 | Premenopausal Cases: hospitals Controls (community): randomly selected from population register, matched on age Exclusions: history thrombosis, pregnant or postpartum, current cancer | DMPA Implant LNG-IUD Use within 3 months Telephone interview | VTE Identified from study coordinator at hospitals, confirmed with radiology results |
* Adjusted for age, BMI, smoking, use of hormones, bedrest/minor trauma, surgery, cast, surgery and cast, prothrombin mutation, factor V Leiden
|
Strengths: Contraceptive information prompted by pictures VTE diagnoses confirmed by radiology results Limitations: Differential participation rate between cases (90%) and controls (69%) All POCs analyzed together |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [3], 2012 Denmark; Funding: Danish Heart Association | Retrospective cohort 1995–2009 | Ages 15–49 years Cohort (>14 million WY): national registry Exclusions: history thrombosis, cancer, hysterectomy, oophorectomy, sterilization, pregnant or postpartum, coagulation disorder | POP (NOR, LNG or DSG) LNG-IUD Implant Use within 4 weeks [21] Obtained from filled prescriptions | Ischemic stroke AMI Discharge diagnosis codes from national registry |
* Adjusted for age, calendar year, education, HTN, heart disease, diabetes, hyperlipidemia
* Adjusted for age, calendar year, education, HTN, heart disease, diabetes, hyperlipidemia
|
Strengths: Large national cohort Limitations: Stroke and AMI diagnoses not confirmed <10 strokes and AMI among users of most POCs No information on BMI |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lidegaard [20], 2012 Denmark; Funding: Gyn Clinic, Rigshospitalet | Retrospective cohort 2001–2010 | Ages 15–49 years Cohort (>9 million WY):National registry Exclusions: history thrombosis, cancer, hysterectomy, oophorectomy, sterilization, pregnant or postpartum, coagulation disorder | LNG-IUD Implant Use within 4 weeks Obtained from filled prescriptions | VTE Discharge diagnosis codes from national registry, confirmed with prescription for anticoagulant |
*
Adjusted for age, calendar year, education
†
Confirmed by prescription for anticoagulant
|
Strengths: Large national cohort VTE diagnoses confirmed by anticoagulant prescription Limitations: >10 VTEs among implant users No information on BMI |
II-2, fair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bergendal [5], 2014 (same study as Bergendal, 2012 [28]) Sweden; Funding: Janssen-Cilag, Novartis, Organon, Schering, Wyeth, AFA Insurance, Karolinska Instituet, Medical Products Agency | Case–control 2003–2009 | Ages 18–54 years Cases: inpatient and outpatient Controls (community): from population register, matched on age Exclusions: history thrombosis, pregnancy within 3 months, current cancer | High dose: DMPA Medium dose: implants, oral DSG Low dose:Oral LNG, LYN, NET Very low dose: LNG-IUD Use within 3 months Participant questionnaire | VTE Diagnosis confirmed by medical records and imaging Included only if initiated anticoagulation |
* Adjusted for smoking, BMI, immobilization
|
Strengths: Population-based VTE diagnoses confirmed with medical records Adjusted for confounders, excluded women with recent pregnancy Response rate high (>88%) in both groups Limitations: POC use not confirmed |
II-2, good | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abbreviations: AMI, acute myocardial infarction; aOR, adjusted odds ratio; aRR, adjusted relative risk; BMI, body mass index; CI, confidence interval; DMPA, depot medroxyprogesterone acetate; DSG, desogestrel; DVT, deep venous thrombosis; GPRD, General Practices Research Database; HTN, hypertension; IR, incidence rate; IUD, intrauterine device; LNG, levonorgestrel; LYN, lynestrenol; NR, not reported; NET, norethisterone; NIH, National Institutes of Health; NOR, norethindrone; OC, oral contraceptive; POC, progestin-only contraception; POI, progestin-only injectable; POP, progestin-only pill; TIA, transient ischemic attack; VTE, venous thromboembolism; WY, women years.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Appendix A. Search strategy for POCs and VTE or ATE
((((((((progestin* OR progestins[MeSH] OR Progesterone[MeSH] OR progesterone OR progestogen* OR progestagen* OR “Levonorgestrel”[Mesh] OR Levonorgestrel OR “Norgestrel”[Mesh] OR norgestrel OR etonogestrel) AND contracept*) OR dmpa OR “depo medroxyprogesterone” OR “depo provera” OR “net en” OR “norethisterone enanthate” OR “norethindrone enanthate” OR (contracept* AND (inject* OR implant)) OR ((levonorgestrel OR etonogestrel) AND implant) OR implanon OR nexplanon OR jadelle OR norplant OR uniplant OR sino-implant OR (levonorgestrel-releasing two-rod implant))) OR ((levonorgestrel AND (intrauterine devices[mesh] OR iud OR iucd OR ius OR iuc OR intrauterine system OR intra-uterine system OR intrauterine device OR intra-uterine device OR intrauterine contraceptive OR intrauterine contraception)) OR mirena OR skyla))) OR (((“levonorgestrel”[Mesh] OR levonorgestrel) AND (emergency OR “morning after” OR postcoit*)) OR (“Contraception, Postcoital”[Mesh] OR “Contraceptives, Postcoital”[MeSH] OR emergency contracept* OR “morning after pill” OR postcoital contracept* OR “plan b”)))) AND ((((“venous thrombosis”[MeSH Terms] OR (“venous”[All Fields] AND “thrombosis”[All Fields]) OR “venous thrombosis”[All Fields] OR (“deep”[All Fields] AND “vein”[All Fields] AND “thrombosis”[All Fields]) OR “deep vein thrombosis”[All Fields]) OR DVT[All Fields] OR (“venous thromboembolism”[MeSH Terms] OR (“venous”[All Fields] AND “thromboembolism”[All Fields]) OR “venous thromboembolism”[All Fields]) OR ((“veins”[MeSH Terms] OR “veins”[All Fields] OR “venous”[All Fields]) AND (“thromboembolism”[MeSH Terms] OR “thromboembolism”[All Fields] OR (“thromboembolic”[All Fields] AND “event”[All Fields]) OR “thromboembolic event”[All Fields])) OR VTE[All Fields] OR PE[All Fields] OR (“pulmonary”[All Fields] AND “embolus”[All Fields]) OR “pulmonary embolus”[All Fields])) OR ((“cerebrovascular disorders”[MeSH Terms] OR (“cerebrovascular”[All Fields] AND “disorders”[All Fields]) OR “cerebrovascular disorders”[All Fields]) OR (“stroke”[MeSH Terms] OR “stroke”[All Fields]) OR (((“brain”[MeSH Terms] OR “brain”[All Fields]) OR (“cerebrum”[MeSH Terms] OR “cerebrum”[All Fields] OR “cerebral”[All Fields] OR “brain”[MeSH Terms] OR “brain”[All Fields])) AND ((“infarction”[MeSH Terms] OR “infarction”[All Fields]) OR (“ischaemia”[All Fields] OR “ischemia”[MeSH Terms] OR “ischemia”[All Fields]) OR (“embolism”[MeSH Terms] OR “embolism”[All Fields]) OR (“thrombosis”[MeSH Terms] OR “thrombosis”[All Fields]))) OR (“myocardial infarction”[MeSH Terms] OR (“myocardial”[All Fields] AND “infarction”[All Fields]) OR “myocardial infarction”[All Fields] OR (“heart”[All Fields] AND “attack”[All Fields]) OR “heart attack”[All Fields]) OR (“myocardial infarction”[MeSH Terms] OR (“myocardial”[All Fields] AND “infarction”[All Fields]) OR “myocardial infarction”[All Fields]))).
References
- [1].Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama VA, Helmerhorst FM, Stijnen T, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and metaanalysis. Obstet Gynecol 2013;122:380–9. [DOI] [PubMed] [Google Scholar]
- [3].Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New Engl J Med 2012;366:2257–66. [DOI] [PubMed] [Google Scholar]
- [4].Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res 2010;126:5–11. [DOI] [PubMed] [Google Scholar]
- [5].Bergendal A, Persson I, Odeberg J, Sundstrom A, Holmstrom M, Schulman S, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol 2014;124:600–9. [DOI] [PubMed] [Google Scholar]
- [6].van Hylckama VA, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depotmedroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol 2010;30:2297–300. [DOI] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep 2010;59(RR-4):1–86. [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- [10].Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, caseontrol study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Contraception 1998;57:315–24. [PubMed] [Google Scholar]
- [11].Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case–control study. Thromb Res 2010;126:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Conard J, Plu-Bureau G, Bahi N, Horellou MH, Pelissier C, Thalabard JC. Progestogen-only contraception in women at high risk of venous thromboembolism. Contraception 2004;70:437–41. [DOI] [PubMed] [Google Scholar]
- [13].Dunn N, Thorogood M, Faragher B, de Caestecker L, MacDonald TM, McCollum C, et al. Oral contraceptives and myocardial infarction: results of the MICA case–control study. BMJ 1999;318:1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heinemann LA, Assmann A, DoMinh T, Garbe E. Oral progestogen-only contraceptives and cardiovascular risk: results from the transnational study on oral contraceptives and the health of young women. Contracept Reprod Health Care 1999;4:67–73. [DOI] [PubMed] [Google Scholar]
- [15].Lidegaard O. Oral contraception and risk of a cerebral thromboembolic attack: results of a case–control study. BMJ 1993;306:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lidegaard O, Edstrom B, Kreiner S. Oral contraceptives and venous thromboembolism. A case–control study. Contraception 1998;57:291–301. [DOI] [PubMed] [Google Scholar]
- [17].Lidegaard O, Edstrom B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case–control study. Contraception 2002;65:187–96. [DOI] [PubMed] [Google Scholar]
- [18].Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case–control study. Contraception 2002;65:197–205. [DOI] [PubMed] [Google Scholar]
- [19].Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ 2009;339:b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ 2011;343:d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mintz G, Gutierrez G, Deleze M, Rodriguez E. Contraception with progestagens in systemic lupus erythematosus. Contraception 1984;30:29–38. [DOI] [PubMed] [Google Scholar]
- [23].Petitti DB, Siscovick DS, Sidney S, Schwartz SM, Quesenberry CP, Psaty BM, et al. Norplant implants and cardiovascular disease. Contraception 1998;57:361–2. [DOI] [PubMed] [Google Scholar]
- [24].Poulter NR, Chang CL, Farley TM, Meirik O. Risk of cardiovascular diseases associated with oral progestagen preparations with therapeutic indications. Lancet 1999;354:1610. [DOI] [PubMed] [Google Scholar]
- [25].Vaillant-Roussel H, Ouchchane L, Dauphin C, Philippe P, Ruivard M. Risk factors for recurrence of venous thromboembolism associated with the use of oral contraceptives. Contraception 2011;84:e23–30. [DOI] [PubMed] [Google Scholar]
- [26].Vasilakis C, Jick H, del Mar Melero-Montes M. Risk of idiopathic venous thromboembolism in users of progestagens alone. Lancet 1999;354:1610–1. [DOI] [PubMed] [Google Scholar]
- [27].Le Moigne E, Tromeur C, Delluc A, Gouillou M, Alavi Z, Lacut K, et al. Risk of recurrent venous thromboembolism on progestin-only contraception: a cohort study. Haematologica 2016;101:e12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bergendal A, Bremme K, Hedenmalm K, Larfars G, Odeberg J, Persson I, et al. Risk factors for venous thromboembolism in pre- and postmenopausal women. Thromb Res 2012;130:596–601. [DOI] [PubMed] [Google Scholar]
- [29].Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low-dose oral contraceptive use and the risk of myocardial infarction. Arch Intern Med 2001;161:1065–70. [DOI] [PubMed] [Google Scholar]
- [30].Thorogood M, Mann J, Murphy M, Vessey M. Is oral contraceptive use still associated with an increased risk of fatal myocardial infarction? Report of a case–control study. Brit J Obstet Gynaecol 1991;98:1245–53. [DOI] [PubMed] [Google Scholar]
- [31].Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, d’Anglejan-Chatillon J, et al. case–control study of migraine and risk of ischaemic stroke in young women. BMJ 1995;310:830–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Christiansen SC, Lijfering WM, Helmerhorst FM, Rosendaal FR, Cannegieter SC. Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. J Thromb Haemost 2010;8:2159–68. [DOI] [PubMed] [Google Scholar]
- [33].Bergendal A, Odlind V, Persson I, Kieler H. Limited knowledge on progestogen-only contraception and risk of venous thromboembolism. Acta Obstet Gynecol Scand 2009;88:261–6. [DOI] [PubMed] [Google Scholar]
- [34].Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ 2012;345:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chakhtoura Z, Canonico M, Gompel A, Thalabard JC, Scarabin PY, Plu-Bureau G. Progestogen-only contraceptives and the risk of stroke: a meta-analysis. Stroke 2009;40:1059–62. [DOI] [PubMed] [Google Scholar]
- [36].Chakhtoura Z, Canonico M, Gompel A, Scarabin PY, Plu-Bureau G. Progestogen-only contraceptives and the risk of acute myocardial infarction: a meta-analysis. J Clin Endocrinol Metab 2011;96:1169–74. [DOI] [PubMed] [Google Scholar]
- [37].Alhenc-Gelas M, Plu-Bureau G, Guillonneau S, Kirzin JM, Aiach M, Ochat N, et al. Impact of progestagens on activated protein C (APC) resistance among users of oral contraceptives. J Thromb Haemost 2004;2:1594–600. [DOI] [PubMed] [Google Scholar]
- [38].Kuhl H. Effects of progestogens on haemostasis. Maturitas 1996;24:1–19. [DOI] [PubMed] [Google Scholar]
- [39].Vieira CS, Ferriani RA, Garcia AA, Pintao MC, Azevedo GD, Gomes MK, et al. Use of the etonogestrel-releasing implant is associated with hypoactivation of the coagulation cascade. Hum Reprod 2007;22:2196–201. [DOI] [PubMed] [Google Scholar]
- [40].Jackson E, Curtis KM, Gaffield ME. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol 2011;117:691–703. [DOI] [PubMed] [Google Scholar]
- [41].Blanco-Molina MA, Lozano M, Cano A, Cristobal I, Pallardo LP, Lete I. Progestin-only contraception and venous thromboembolism. Thromb Res 2012;129:e257–62. [DOI] [PubMed] [Google Scholar]
- [42].Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis - current understanding from an epidemiological point of view. Brit J Haematol 2010;149:824–33. [DOI] [PubMed] [Google Scholar]
- [43].Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a metaanalysis. Circulation 2008;117:93–102. [DOI] [PubMed] [Google Scholar]
- [44].Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost 2013;11:124–31. [DOI] [PubMed] [Google Scholar]
- [45].A multinational case-control study of cardiovascular disease and steroid hormone contraceptives. Description and validation of methods. World Health Organization Collaborative Study of Cardiovascular and Steroid Hormone Contraception. J Clin Epidemiol 1995;48:1513–47. [DOI] [PubMed] [Google Scholar]
- [46].Spitzer WO, Lewis MA, Heinemann LA, Thorogood M, MacRae KD. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case–control study. Transnational research group on oral contraceptives and the health of young women. BMJ 1996;312:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lewis DM, Assmann A, Heinemann L, Spitzer WO. Interim review of the transnational case–control study of oral contraceptives and health: approved protocol revisions through September 1995. Pharmacoepidemiol Drug Saf 1996;5:43–51. [DOI] [PubMed] [Google Scholar]
- [48].Schwartz SM, Siscovick DS, Longstreth WT Jr, Psaty BM, Beverly RK, Raghunathan TE, et al. Use of low-dose oral contraceptives and stroke in young women. Ann Intern Med 1997;127:596–603. [DOI] [PubMed] [Google Scholar]
- [49].Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. Med 1996;335:8–15. [DOI] [PubMed] [Google Scholar]
- [50].Sidney S, Petitti DB, Quesenberry CP Jr, Klatsky AL, Ziel HK, Wolf S. Myocardial infarction in users of low-dose oral contraceptives. Obstet Gynecol 1996;88:939–44. [DOI] [PubMed] [Google Scholar]
- [51].Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet 1995;346:1589–93. [DOI] [PubMed] [Google Scholar]
- [52].van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case–control study. BMJ 2009;339:b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]


