Extended Data Figure 8. Relative binding affinities between fragments of TIM and CRY H377L by the SWFTI assay.
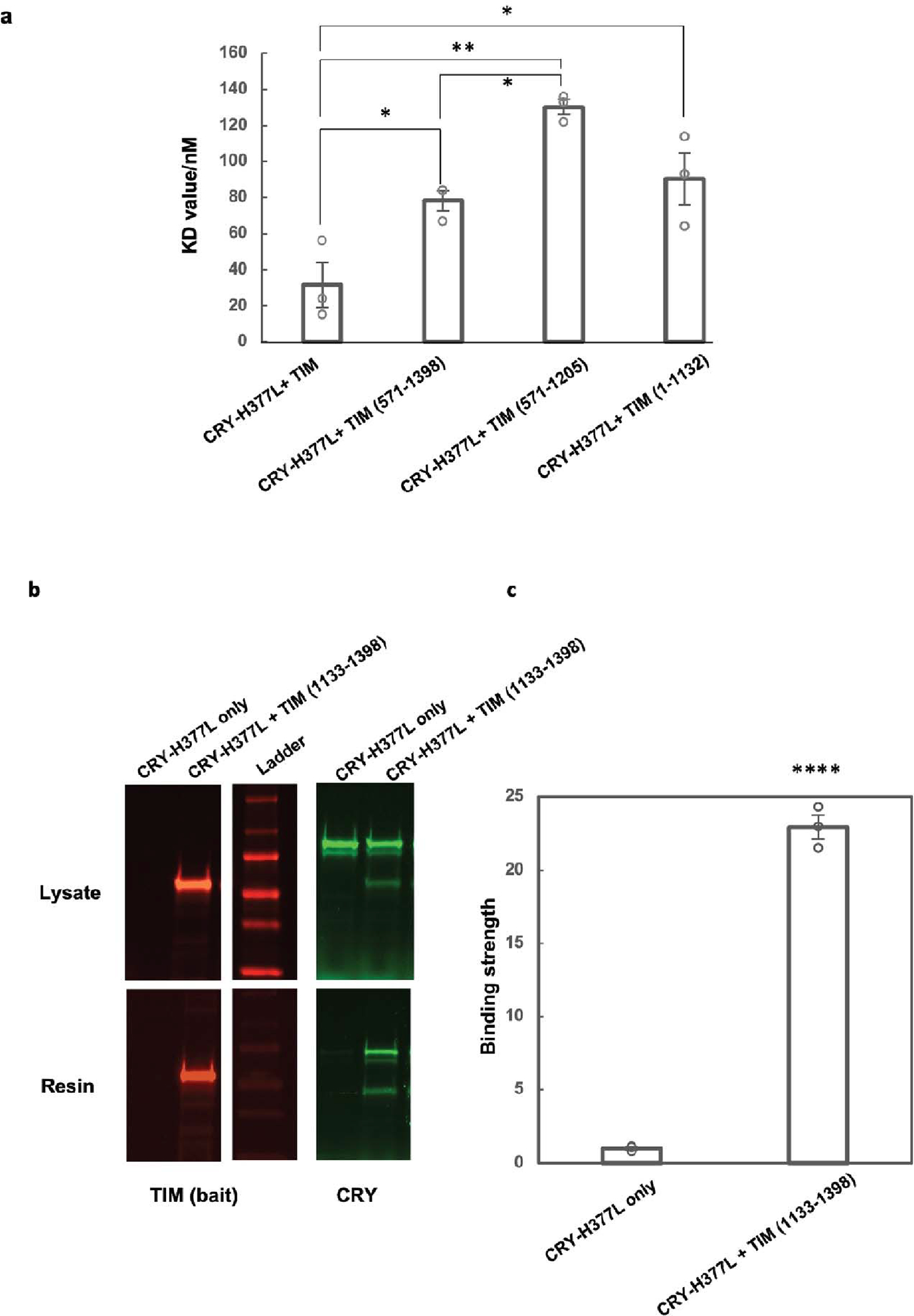
(a) The CRY H377L variant acts as a constitutive light-activated state and enhances binding affinity for TIM by stabilizing the TIM-binding conformation of CRY16. H377L thereby increases TIM expression levels and facilitates detection of weaker binding variants. Mean ± standard error is shown for sample size = 3, from different biological replicates. One-way ANOVA with post-hoc Tukey HSD test was used to determine p values. (b) Pull down results of CRY-H377L with TIM-1133–1398. CRY-H377L interacts with the C-terminal regions of TIM. Gel lanes containing replicates or unrelated samples are not shown. (c) Quantification of the binding strength between CRY-H377L and TIM (1133–1398) compared to the negative control (CRY-H377L only with the same amount of resin). The binding strength is defined as the amount of CRY on the resin divided by the amount of CRY in the lysate sample. The negative control is normalized to 1. Mean ± standard error is shown for sample size = 3, from different biological replicates. Two-tailed unpaired t-test was used to determine p value. Binding affinities could not be derived from this experiment owing to the challenge of producing excessive CRY relative to TIM 1133–1398.
