Abstract
Background.
Histoplasmosis is a fungal infection associated with exposure to bat guano. An outbreak of an unknown severe febrile illness occurred among tunnel workers in the Dominican Republic, and resulted in several deaths. We conducted an investigation to confirm etiology and recommend control measures.
Methods.
A case was defined as fever and ≥2 symptoms consistent with histoplasmosis in a tunnel worker, July–September 2015. We interviewed workers and family members, reviewed medical records, tested serum and urine for Histoplasma antigen/antibody, and conducted a cohort study to identify risk factors for histoplasmosis and severe infection (intensive care).
Results.
A crew of 36 male workers removed large amounts of bat guano from tunnels without respiratory protection for a median of 24 days per worker (range, 1–25 days). Median age was 32 years (range, 18–62 years); none were immunocompromised. Thirty (83%) workers had illness that met the case definition, of whom 28 (93%) were hospitalized, 9 (30%) required intensive care, 6 (20%) required intubation, and 3 (10%) died. The median time from symptom onset to antifungal treatment was 6 days (range, 1–11 days). Twenty-two of 34 (65%) workers had laboratory evidence of infection.
Conclusions.
Severe illnesses and death likely resulted from exposure to large inocula of Histoplasma capsulatum spores in an enclosed space, lack of respiratory protection, and delay in recognition and treatment. Clinician education about histoplasmosis, improved laboratory capacity to diagnose fungal infections, and occupational health guidance to protect workers against endemic fungi are recommended in the Dominican Republic.
Keywords: fungi, histoplasmosis, outbreak, Dominican Republic, occupational
Histoplasma capsulatum, the causative agent in histoplasmosis, is often found in association with bird or bat droppings [1]. Histoplasma capsulatum is present throughout the Americas and the Caribbean. Exposure to H. capsulatum typically occurs by inhalation of fungal spores, specifically the microconidia, following disruption of soil or other contaminated material. Once at body temperature (37°C), it transforms into the yeast phase. The average incubation period is 1–3 weeks, and clinical manifestations can range from asymptomatic infection to severe, disseminated disease [1]. Acute pulmonary histoplasmosis is the most common symptomatic manifestation and is often self-limited, especially among healthy persons.
On 16 September 2015, the Dominican Republic Ministry of Health (DR MoH) requested assistance from the US Center for Disease Control and Prevention (CDC) with the investigation of an unknown severe febrile illness among several male tunnel workers. All men were members of a work crew tasked with removing bat guano from access tunnels to a hydroelectric dam. Workers were initially treated for leptospirosis, which is endemic to the area. Histoplasmosis was later considered when examination of one patient’s bronchoalveolar lavage (BAL) specimen demonstrated yeast cells, consistent with histoplasmosis; however, there was no local laboratory capacity to confirm the diagnosis. Three men had died and 25 others were hospitalized. Although histoplasmosis is endemic to the Americas, including other Caribbean islands such as Puerto Rico and Jamaica, cases had never been diagnosed in the DR [2]. Local physicians were unfamiliar with diagnosis and management of histoplasmosis. CDC and the DR MoH investigated to confirm the etiology of the outbreak, elucidate clinical and occupational risk factors for histoplasmosis and severe disease, assess treatment outcomes, and identify control measures.
METHODS
Descriptive Epidemiology and Cohort Study
We defined a case of histoplasmosis as fever and ≥2 symptoms (chills, night sweats, weakness, joint pain, cough, headache, generalized malaise, dyspnea, myalgias, difficulty breathing, diarrhea, and vomiting) in a person who worked in the tunnels during 30 July–2 September 2015; this time period included all work performed on the tunnels. We reviewed company payroll records to identify all persons who had worked in the tunnels during that time period and interviewed workers to identify any additional persons exposed to the tunnels who may not have been on the official company records (eg, temporary substitutes for regular workers).
We interviewed tunnel workers in person in Spanish using a standardized questionnaire. For the 3 workers already deceased at the time of the interview, we spoke with an immediate family member. Worker interviews addressed demographic characteristics, underlying medical conditions, general information about the tunnels, number of days spent in the tunnels, tasks performed, and use of personal protective equipment.
We reviewed medical records from the local hospital where workers were initially hospitalized, and regional hospitals, where they were later transferred for care, using a standardized case report form that included clinical information, details of the hospital stay, treatment, and outcome.
We conducted a cohort study to identify risk factors for developing histoplasmosis and severe disease (defined as admission to the intensive care unit [ICU]). The cohort included all workers exposed to the tunnels during 30 July–2 September 2015.
Laboratory Analysis
We collected serum and urine samples from tunnel workers and sent them to the CDC Mycotic Diseases Branch laboratory (Atlanta, Georgia) for analysis. Environmental sampling is not routinely performed and given the volume of guano involved in this outbreak, processing of environmental samples was not feasible. Histoplasma capsulatum antigen detection was performed on both urine and serum samples using an enzyme immunoassay (EIA) employing Histoplasma monoclonal analyte-specific reagents (IMMY, Norman, Oklahoma), with a cutoff value of ≥0.5 ng/mL for a positive result. Before performing the assay, we treated serum with pronase at 56°C for 30 minutes followed by boiling for 5 minutes. We tested urine undiluted. Optical density EIA results were analyzed against a 7-point standard curve to provide a quantitative result [3]. EIA was chosen over other molecular methods as it can be performed on specimens that do not require invasive collection procedures, such as BAL or tissue biopsy. We performed qualitative H. capsulatum antibody detection using immunodiffusion on serum samples. In the outbreak setting, we considered an M band sufficient to conclude a positive result.
Statistical Analysis
We calculated medians and ranges for continuous variables, and frequencies and percentages for categorical variables. We evaluated unadjusted associations between demographic, occupational, exposure, and clinical variables using the outcomes of histoplasmosis and severe disease. We used exact logistic regression models to estimate exact odds ratios and exact 95% confidence intervals (CIs). We assumed linear relationships for continuous variables, but categorized them with category boundaries set either at the median or quartiles when the Akaike information criterion indicated that a linear term was not the best fit [4, 5]. We considered exact 2-sided P values of ≤.05 to be statistically significant. Due to the small size of our study, we also considered P values between .05 and .1 to be “borderline significant.” Statistical analyses were performed with SAS version 9.3 software (SAS Institute, Cary, North Carolina).
Ethics Approval
A local ethics committee in the DR and designated ethics officers at CDC determined that this was an emergency public health investigation and did not meet criteria for research.
RESULTS
Background on Tunnel Work
The dam was constructed in 1972 and provides hydroelectric energy to most of the surrounding communities. This embankment dam had 5 tunnels, each approximately 1–2 km long, which allow access to the dam for inspection and maintenance. Tunnel entrances were small (approximately 3 m wide and tall) (Figure 1). The tunnels lacked ventilation or illumination and were inhabited by large bat colonies. Bat guano up to 1 m deep had accumulated since the tunnels were last accessed approximately 30 years ago.
Figure 1.
Entrance to a tunnel associated with severe histoplasmosis outbreak, Dominican Republic.
A private company was contracted to clean the tunnels and recruited workers informally in a nearby town center. A total of 36 workers were exposed to the tunnels during July–September. Each worker was provided with a pair of knee-high rubber boots, a hard hat with an attached personal headlamp, and a shovel. Additionally, some workers received loose-fitting paper surgical masks. Workers were responsible for filling wheelbarrows with bat guano, transporting it outside, and depositing it immediately near the tunnel entrance. They worked for 3–4 hours daily, 5 days per week, usually in the mornings. Work cleaning 2 of the tunnels began 30 July 30 and stopped 2 September, when a number of workers became ill.
Worker Interviews
All workers were male and the median age was 32 years (range, 18–62 years); 5 (15%) reported having asthma, 15 (43%) were current cigarette smokers, and 12 (39%) used illicit drugs (Table 1). Fifteen workers (43%) reported shoveling guano as their sole task, 5 (14%) reported only transporting the wheelbarrows containing guano, 11 (31%) engaged in both tasks, 3 (9%) supervised the work of others, and 1 (3%) performed other work (eg, holding a light). Ten (29%) workers worked in tunnel 1 only, 7 (20%) worked in tunnel 2 only, and 18 (51%) worked in both tunnels (4 workers worked 1–4 days in a third tunnel in addition to working in tunnels 1, 2, or both, but we did not consider the third tunnel in analyses because it could not have accounted for the large number of workers who became ill). The median number of days per worker spent in the tunnels was 24 (range, 1–25 days). Workers reported oppressive heat and difficulty breathing inside the tunnels while wearing the masks. Sixteen (48%) workers never used the masks, 14 (42%) used them sometimes, and 3 (9%) reported using them always (Table 1).
Table 1.
Characteristics of Tunnel Workers (n = 36) and Tunnel Work in a Histoplasmosis Outbreak, Dominican Republic, 2015
| Characteristic | No. (%) | Missing, No. |
|---|---|---|
|
| ||
| Male sex | 36 (100) | |
| Age, ya | 1 | |
| 18–32 | 18 (51) | |
| 33–62 | 17 (49) | |
| Asthma | 5 (15) | 3 |
| Current cigarette smoking | 15 (43) | 1 |
| Illicit drug use (noninjection) | 12 (39) | 5 |
| Type of work | 1 | |
| Shoveling/filling only | 15 (43) | |
| Transporting wheelbarrow only | 5 (14) | |
| Shoveling/filling and transporting wheelbarrow | 11 (31) | |
| Supervising | 3 (9) | |
| Other | 1 (3) | |
| Tunnel of work | 1 | |
| Tunnel 1 only | 10 (29) | |
| Tunnel 2 only | 7 (20) | |
| Tunnels 1 and 2 | 18 (51) | |
| Median cumulative days worked in tunnels (range) | 24 (1–25) | 1 |
| Personal protective equipment use: surgical mask | 3 | |
| Never | 16 (48) | |
| Sometimes | 14 (42) | |
| Always | 3 (9) | |
Category boundary set at the median among all workers.
Clinical Presentation
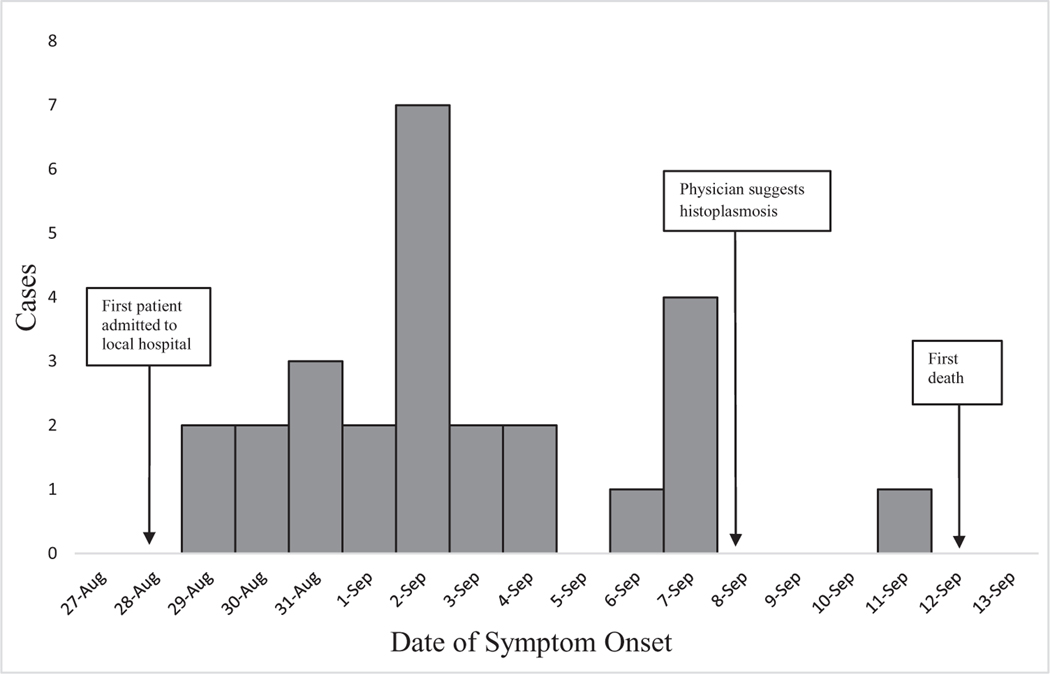
The first ill worker presented to the local hospital on 28 August with an unknown febrile illness (Figure 2). By 4 September, 14 workers had been admitted to the local hospital. Leptospirosis was initially suspected as the cause of the outbreak because it is endemic to the region. However, the workers did not improve with penicillin, the treatment for leptospirosis. Physicians noted the common exposure to tunnel work among the admitted patients and reported the illnesses to the local health authorities. On 8 September, all 19 workers who had been admitted to the local hospital were transferred to regional hospitals, where a higher level of care was available to manage their unknown illness. Two of the 19 (11%) workers transferred from the local hospital to regional facilities required intubation within 1 day of arrival. At one regional facility, an astute physician, who had treated cases of histoplasmosis while training in Mexico, suggested the diagnosis of histoplasmosis given its association with exposure to bat guano. The same day, a pathologist noted yeast cells, suggestive of H. capsulatum by microscopy on a BAL specimen.
Figure 2.
Dates of histoplasmosis symptom onset for 26 workers exposed to bat guano during maintenance of tunnels of a hydroelectric dam, Dominican Republic, 2015 (symptom onset dates are not shown for 4 workers due to 3 missing data and 1 extreme outlier).
Thirty of the 36 (83%) exposed workers had illnesses that met the case definition. Symptom onset ranged between 21 August and 11 September 2015 (Figure 2). Twenty-eight (93%) workers were hospitalized, 9 (30%) required ICU admission, 6 (20%) were intubated, and 3 (10%) died (Table 2). The 3 workers who died were 21–36 years of age, had no known medical comorbidities, and were nonsmokers. All 3 received voriconazole and intravenous steroids.
Table 2.
Clinical Characteristics of Workers Who Met the Case Definition (n = 30) in Histoplasmosis Outbreak in the Dominican Republic, 2015
| Characteristic | No. (%) | Missing, No. |
|---|---|---|
|
| ||
| Level of medical care | ||
| Inpatient hospitalization | 28 (93) | |
| Intensive care unit | 9 (30) | |
| Mechanical ventilation | 6 (20) | |
| Deaths | 3 (10) | |
| Symptoms | ||
| Fever | 25 (83) | |
| Cough | 23 (77) | |
| Headache | 21 (70) | |
| Generalized malaise | 15 (50) | |
| Difficulty breathing | 11 (37) | |
| Myalgias | 11 (37) | |
| Diarrhea | 8 (27) | |
| Diagnostics | ||
| CXR | 20 (67) | |
| Bilateral infiltrates, No. (%) of workers who had a CXR | 17 (85) | |
| Interstitial consolidation, No. (%) of workers who had a CXR | 14 (70) | |
| CT chest scan | 12 (40) | |
| Bilateral infiltrates, No. (%) of workers who had a CT chest scan | 11 (100) | 1 |
| Interstitial consolidation, No. (%) of workers who had a CT chest scan | 11 (100) | 1 |
| Bronchoscopy | 9 (30) | |
| BAL, No. (%) of workers who had a bronchoscopy | 7 (100) | 2 |
| Evidence of histoplasmosis on pathology, No. (%) of workers who had a BAL | 2 (67) | 4 |
| Bacterial culture consistent with ventilator–associated pneumonia, No. (%) of workers who had a BAL | 6 (86) | |
| Laboratory | ||
| Leukocytosis (WBC >12 × 109/L) | 19 (68) | 2 |
| AST or ALT >120 U/L | 10 (36) | 2 |
| HIV (type unknown) | 0 (0) | 15 |
| Leptospirosis (type unknown) | 0 (0) | 7 |
| Treatment | ||
| Any antifungal | 28 (93) | |
| Voriconazole, No. (%) of workers who had any antifungal | 22 (79) | |
| Itraconazole, No. (%) of workers who had any antifungal | 14 (50) | |
| Fluconazole, No. (%) of workers who had any antifungal | 9 (33) | 1 |
| Amphotericin B, No. (%) of workers who had any antifungal | 8 (29) | |
| Days from symptom onset to first antifungal treatment, No. (%) of workers who had any antifungala | 4 | |
| 0–4 | 7 (29) | |
| 5–6 | 6 (25) | |
| 7–8 | 6 (25) | |
| 9–11 | 5 (21) | |
| Any corticosteroid | 26 (87) | |
| Corticosteroids prior to antifungal treatment, No. (%) of workers who had any antifungal and any corticosteroid | 1 | |
| No | 12 (48) | |
| Corticosteroids received on same day as antifungals | 9 (36) | |
| Yes | 4 (16) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAL, bronchoalveolar lavage; CT, computed tomography; CXR, chest radiograph; HIV, human immunodeficiency virus; WBC, white blood cell count.
Category boundaries set at the quartiles among all workers who met the case definition and who had any antifungal.
Nine of 30 (30%) case patients underwent bronchoscopy; 7 of these had BAL samples collected and 6 (86%) had BAL cultures positive for bacteria, consistent with ventilator-associated pneumonia. These samples were not available for further evaluation for H. capsulatum (Table 2).
Nineteen (68%) case patients had leukocytosis (white blood cell count >12 × 109/L) and 10 (36%) had aspartate aminotransferase or alanine aminotransferase >120 U/L. Human immunodeficiency virus testing was performed for 15 (50%) case patients, and none were positive. Testing for leptospirosis, the original suspected pathogen, was performed for 23 (77%) case patients, and none were positive (Table 2).
Twenty-eight (93%) case patients received an antifungal, and the median time from symptom onset to first antifungal treatment was 6 days (range, 0–11 days). Voriconazole was the first antifungal administered to 16 (62% of those with data) case patients and 17 (61%) received >1 antifungal (not shown). Overall, 22 (79%) case patients received voriconazole, 14 (50%) received itraconazole, 9 (33%) received fluconazole, and 8 (29%) received amphotericin B during their treatment course. Twenty-six (87%) case patients received corticosteroids and 4 (16%) received corticosteroids at least 1 day before treatment with antifungals (Table 2).
Laboratory Analysis
Thirty-four of the 36 exposed workers provided samples; we obtained 34 unique serum and 29 unique urine specimens. Urine and serum were available for 28 workers. Time from symptom onset to collection of specimen ranged from 5 to 33 days, with a median of 14 days. Eighteen (53%) serum and 13 (45%) urine samples were positive for H. capsulatum antigen. Additionally, immunodiffusion was performed on 31 serum samples, and 11 (35%) were positive. In total, 22 of the 34 (65%) workers tested had laboratory evidence of H. capsulatum infection.
Cohort Study
None of the variables examined in the cohort analysis—including age, presence of comorbidities, type of work, tunnel of work, days worked in tunnels, personal protective equipment use, days from symptom onset to antifungal treatment (ICU admission only), symptoms (ICU admission only), and laboratory results (ICU admission only)—were significantly associated with histoplasmosis or severe disease (ICU admission) (Tables 3 and 4; and Supplementary Table 1). However, days worked in tunnels (P = .06) and difficulty breathing (P = .07) had borderline significant positive associations with severe disease.
Table 3.
Associations Between Characteristics and Developing Histoplasmosis Among Tunnel Workers (n = 36) in the Dominican Republic, 2015
| Characteristic | Histoplasmosis |
||||
|---|---|---|---|---|---|
| Yes | No | Unadjusted Odds Ratio | (Exact 95% CI) | Exact P Value | |
|
| |||||
| Age, ya | |||||
| 18–32 | 14 (48) | 4 (67) | 1.00 | Reference | Reference |
| 33–62 | 15 (52) | 2 (33) | 2.10 | (.3–26.65) | .72 |
| Asthma | 4 (15) | 1 (17) | 0.87 | (.06–51.28) | >.99 |
| Current cigarette smoking | 12 (41) | 3 (50) | 0.71 | (.08–6.27) | >.99 |
| Illicit drug use (noninjection) | 9 (35) | 3 (60) | 0.37 | (.03–3.81) | .56 |
| Type of work | |||||
| Shoveling/filling only | 13 (45) | 2 (33) | 1.00 | Reference | Reference |
| Transporting wheelbarrow only | 2 (7) | 3 (50) | 0.12 | (.01–1.71) | .15 |
| Shoveling/filling and transporting wheelbarrow | 10 (34) | 1 (17) | 1.51 | (.07–99.71) | >.99 |
| Supervising | 3 (10) | 0 (0) | 0.49b | (.05–∞) | >.99 |
| Other | 1 (3) | 0 (0) | 0.14b | (.01–∞) | >.99 |
| Tunnel of work | |||||
| Tunnel 1 only | 10 (34) | 0 (0) | 3.39b | (.53–∞) | .30 |
| Tunnel 2 only | 5 (17) | 2 (33) | 0.72 | (.07–10.36) | >.99 |
| Tunnels 1 and 2 | 14 (48) | 4 (67) | 1.00 | Reference | Reference |
| Cumulative days worked in tunnels (per additional day worked) | 1.04 | (.93–1.15) | .46 | ||
| Personal protective equipment use: surgical mask | |||||
| Never | 13 (48) | 3 (50) | 1.00 | Reference | Reference |
| Sometimes | 11 (41) | 3 (50) | 0.85 | (.09–7.71) | >.99 |
| Always | 3 (11) | 0 (0) | 0.8b | (.09–∞) | >.99 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: CI, confidence interval.
Category boundary set at the median among all workers.
Median unbiased estimate.
Table 4.
Associations Between Characteristics and Developing Severe Histoplasmosis (Defined as Admission to Intensive Care Unit) Among Tunnel Workers (n = 36) in the Dominican Republic, 2015
| Characteristic | ICU Admission |
||||
|---|---|---|---|---|---|
| Yes | No | Unadjusted Odds Ratio | (Exact 95% CI) | Exact P Value | |
|
| |||||
| Age, ya | |||||
| 18–32 | 3 (33) | 15 (58) | 1.00 | Reference | Reference |
| 33–62 | 6 (67) | 11 (42) | 2.65 | (.45–20.07) | .38 |
| Asthma | 0 (0) | 5 (20) | 0.41b | (.00–2.52) | .45 |
| Current cigarette smoking | 3 (33) | 12 (46) | 0.59 | (.08–3.54) | .79 |
| Illicit drug use (noninjection) | 1 (13) | 11 (48) | 0.16 | (<.01–1.63) | .17 |
| Type of work | |||||
| Shoveling/filling only | 5 (56) | 10 (38) | 1.00 | Reference | Reference |
| Transporting wheelbarrow only | 1 (11) | 4 (15) | 0.52 | (.01–7.42) | >.99 |
| Shoveling/filling and transporting wheelbarrow | 2 (22) | 9 (35) | 0.46 | (.04–3.73) | .69 |
| Supervising | 1 (11) | 2 (8) | 1.00 | (.01–23.97) | >.99 |
| Other | 0 (0) | 1 (4) | 2.20b | (.00–41.80) | >.99 |
| Tunnel of work | |||||
| Tunnel 1 only | 4 (44) | 6 (23) | 3.18 | (.40–28.95) | .36 |
| Tunnel 2 only | 2 (22) | 5 (19) | 1.94 | (.13–22.90) | .87 |
| Tunnels 1 and 2 | 3 (33) | 15 (58) | 1.00 | Reference | Reference |
| Cumulative days worked in tunnels (per additional day worked) | 1.18 | (1.00–1.59) | .06 | ||
| Personal protective equipment use: surgical mask | |||||
| Never | 4 (50) | 12 (48) | 1.00 | Reference | Reference |
| Sometimes | 3 (38) | 11 (44) | 0.82 | (.10–6.14) | >.99 |
| Always | 1 (13) | 2 (8) | 1.47 | (.02–36.16) | >.99 |
| Days from symptom onset to first antifungal treatmentc,d | |||||
| 0–4 | 0 (0) | 7 (47) | 0.16a | (.00–1.22) | .13 |
| 5–6 | 4 (44) | 2 (13) | 1.89 | (.12–37.87) | >.99 |
| 7–8 | 3 (33) | 3 (20) | 1.00 | Reference | Reference |
| 9–11 | 2 (22) | 3 (20) | 0.69 | (.03–12.15) | >.99 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval, ICU, intensive care unit.
Category boundary set at the median among all workers.
Median unbiased estimate.
Among only the 28 workers who had received any antifungal.
Category boundaries set at the quartiles among all workers who met the case definition and who had received any antifungal.
DISCUSSION
This is the first report of an outbreak of histoplasmosis in the DR. Histoplasma capsulatum is endemic to the Caribbean region, and outbreaks have been reported throughout Latin America [2, 6–8]. Sporadic cases of histoplasmosis attributed to exposure in the DR have been diagnosed in travelers returning to their countries of origin [7]. Clinicians in the DR were largely unfamiliar with histoplasmosis and laboratories did not have the capacity to definitively diagnose the disease. It is possible that there have been previous cases and outbreaks of histoplasmosis in the DR that have gone unrecognized as the illness is often self-limited. The high mortality in a young healthy population likely brought more attention to this outbreak, and the investigation led to the confirmation of histoplasmosis in the DR.
Outbreaks of histoplasmosis tend to involve small numbers of people and fatalities are rare, even in resource-poor settings [6]. Several factors may have contributed to the high proportion of hospitalizations and deaths observed in this outbreak. Although local physicians and public health authorities quickly recognized that the ill workers had been exposed to tunnels, recognition of histoplasmosis and initiation of antifungal treatment were delayed. Furthermore, amphotericin B, the recommended treatment for severe pulmonary histoplasmosis, was not administered in the majority of cases despite decompensation, and when administered, it was delayed [9]. The causes of this delay were likely multifactorial, and due to both unfamiliarity of physicians with guidelines and lack of access to the medication. Paucity of serologic or urine diagnostic capacity likely also contributed to the delay in definitive diagnosis and subsequent treatment. Aside from the delayed diagnosis and treatment, probable exposure to high H. capsulatum inocula in the tunnels, coupled with poor ventilation and inadequate occupational precautions, contributed to the outbreak’s severity. Earlier diagnosis and treatment of histoplasmosis, combined with appropriate occupational precautions, might have prevented the 3 deaths observed in this outbreak.
A greater number of days spent in the tunnels was associated with increased risk of severe disease, although this association was only borderline statistically significant, likely due to the small sample size. It is known that H. capsulatum can exist in “hotspots,” or pockets within the environment. When these pockets are disrupted, large amounts of spores can be released into the air [1]. We suspect that such events occurred during this outbreak, exposing workers to large inocula within short periods of time. Workers’ proximity to these “hotspots” rather than their cumulative time spent in the tunnels may have determined their risk of acquiring histoplasmosis. This hypothesis is supported by the clustering of symptom onset during a 2-week period rather than over the entire duration of the tunnel work.
Increased education and awareness of histoplasmosis among clinicians is needed to respond to cases and future outbreaks in the DR, as early treatment with an appropriate antifungal can reduce morbidity and mortality [1]. As elsewhere in Latin America and the Caribbean, histoplasmosis is likely an important cause of disease. In fact, we suspect that had convalescent testing been performed, we would have detected exposure and antibody response in even more of the tunnel workers. In the acute setting, antibodies may not yet have formed and antigen-based testing can be falsely negative. Unfortunately, we were unable to collect convalescent sera in this outbreak investigation [10]. Enhanced availability of histoplasmosis diagnostics may help uncover an unrecognized burden of illness. Historically, antibody and antigen testing has been performed by only a limited number of laboratories worldwide. Newer diagnostic technologies, such as point-of-care loop-mediated isothermal amplification or lateral flow assays, could facilitate rapid detection and treatment, especially in resource-limited settings [11].
Because tunnels involved in this outbreak could not be closed for access, as they are needed for continued maintenance of the dam, using the occupational health and safety hierarchy of controls will be important for preventing additional illnesses [12].
Development of a site safety plan is an important step in minimizing exposure, and provides direction for continued access and work in the tunnels. Additional methods, such as moistening material prior to translocation, can reduce dust generation and spore dispersal [12]. Given the likely spore burden present in the guano, when removed it should be treated as biohazard waste to minimize further disease [12]. Worker training is another key component and should address heat exhaustion, health risk communication, appropriate use of personal protective equipment, and compliance with occupational health and environmental safety recommendations [12]. The US National Institute for Occupational Safety and Health (NIOSH) has developed recommendations for the prevention of histoplasmosis in occupational settings [12]. NIOSH considers disposable N95 respirators to be the lowest acceptable level of protection needed when working in areas with the potential for H. capsulatum exposure [12]. In this outbreak, the tunnel workers were provided with paper surgical masks that were not consistently worn and would not have provided adequate protection.
Applying the safe work practices discussed above to the setting of this outbreak may be challenging given the tropical climate and limited resources. The tunnels are poorly ventilated and hot, likely limiting extended use of any type of respirator; furthermore, personal protective equipment can be costly. Specialists, such as an industrial hygienist, could help determine the most appropriate and feasible options (see Supplementary Materials for full details).
Our study had several limitations that may have interfered with our ability to detect associations. First, we interviewed workers several weeks after work in the tunnels concluded, potentially introducing recall bias. Second, it is conceivable that given the widespread medical evaluation of exposed workers, some may have overreported symptoms. This could increase the number of cases detected, biasing the risk ratio toward the null. Finally, the small sample size limited our ability to adjust for potential confounders or to find risk factors associated with developing histoplasmosis or severe disease.
This outbreak adds to evidence that histoplasmosis is underdiagnosed in Latin America and the Caribbean [7, 13–17]. Increased awareness of the disease among clinicians and public health officials, improved diagnostic capacity, and access to antifungals is essential in helping to prevent severe illness and death. Occupational health precautions during shigher-risk activities, particularly those involving disturbances to bird and bat guano, could reduce worker exposure to H. capsulatum. Because workers are often at higher risk of exposure than the general population, the identification of high-risk environments as well as the implementation of appropriate engineering and administrative controls and adequate personal protective equipment may help to prevent similar outbreaks in the future.
Supplementary Material
Acknowledgments
This work is written by (a) US Government employee(s) and is in the public domain in the US.
Disclaimer.
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or the National Institute for Occupational Safety and Health (NIOSH).
Financial support.
This work was supported by the CDC and NIOSH.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Richardson M, Warnock D. Histoplasmosis. In: Fungal infection: diagnosis and management. Oxford, UK: Wiley-Blackwell, 2012:304–21. [Google Scholar]
- 2.Handzel S, Jessamine AG. Imported histoplasmosis from Puerto Rico. Can J Public Health 1975; 66:393–5. [PubMed] [Google Scholar]
- 3.Theel ES, Harring JA, Dababneh AS, Rollins LO, Bestrom JE, Jespersen DJ. Reevaluation of commercial reagents for detection of Histoplasma capsulatum antigen in urine. J Clin Microbiol 2015; 53:1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 9:716–23. [Google Scholar]
- 5.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ Jr. Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calanni LM, Pérez RA, Brasili S, et al. Outbreak of histoplasmosis in province of Neuquén, Patagonia Argentina. Rev Iberoam Micol 2013; 30:193–9. [DOI] [PubMed] [Google Scholar]
- 7.Suzaki A, Kimura M, Kimura S, Shimada K, Miyaji M, Kaufman L. An outbreak of acute pulmonary histoplasmosis among travelers to a bat-inhabited cave in Brazil. Kansenshogaku Zasshi 1995; 69:444–9. [DOI] [PubMed] [Google Scholar]
- 8.Rocha-Silva F, Figueiredo SM, Silveira TT, et al. Histoplasmosis outbreak in Tamboril cave—Minas Gerais state, Brazil. Med Mycol Case Rep 2014; 4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheat LJ, Freifeld AG, Kleiman MB, et al. ; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 10.Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 2016; 62:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prattes J, Heldt S, Eigl S, Hoenigl M. Point of care testing for the diagnosis of fungal infections: are we there yet? Curr Fungal Infect Rep 2016; 10:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Occupational Safety and Health and National Center for Infectious Diseases. Histoplasmosis: protecting workers at risk. Revised ed. Cincinnati, Ohio: NIOSH/NCID, 2004. [Google Scholar]
- 13.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1–53. [DOI] [PubMed] [Google Scholar]
- 14.Mandell W, Goldberg DM, Neu HC. Histoplasmosis in patients with the acquired immune deficiency syndrome. Am J Med 1986; 81:974–8. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz B, Martínez MA, Palma G, et al. Molecular characterization of Histoplasma capsulatum isolated from an outbreak in treasure hunters Histoplasma capsulatum in treasure hunters. BMC Infect Dis 2010; 10:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay RJ, White HS, Fields PE, Quamina DB, Dan M, Jones TR. Histoplasmosis in the eastern Caribbean: a preliminary survey of the incidence of the infection. J Trop Med Hyg 1981; 84:9–12. [PubMed] [Google Scholar]
- 17.Gascón J, Torres JM, Luburich P, Ayuso JR, Xaubet A, Corachán M. Imported histoplasmosis in Spain. J Travel Med 2000; 7:89–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.