Abstract
Germfree suckling rats were infected with an SA11 rotavirus strain. Infected pups developed diarrhea associated with histopathological changes. The virus was detected in feces and in the small intestine. Cellular vacuolation was observed in the villi of the jejunum. These results provide a new model for further investigations of group A rotavirus infection.
Group A rotavirus is the leading cause of diarrhea among 6- to 24-month-old children worldwide. In industrialized countries, the illness is usually self-limiting, with a mean duration of 4 to 7 days (28). However, it is estimated that 870,000 deaths/year are caused by rotavirus-associated diarrhea, principally in developing countries (10). The primary symptom is watery diarrhea, frequently associated with severe dehydration (5). Limited investigation by mucosal biopsy of infected infants has shown that rotavirus principally infects the cells of the small intestine.
The physiopathology of rotavirus-associated diarrhea has been experimentally studied in several animal species, including monkeys (22), calves (19), dogs (14), pigs (31), and rabbits (6). However, most of these animals are costly and cumbersome to maintain (4).
To date, the murine model has been the most often used model for studying rotavirus infection. However, difficulties in growing murine rotavirus strains to high concentrations in tissue culture have limited their use (21). The use of heterologous rotavirus, particularly the simian SA11, strain, has led to physiopathological symptoms in mice identical to those produced by the murine strain (3, 17, 18, 21) and may limit cross contamination among animals bred in the laboratory (6).
Surprisingly few studies of rotavirus infections have been conducted with rats, even though this animal is commonly used in the experimental field. To our knowledge, these experiments have described only homologous models using rats infected with group B rotavirus (24, 27, 29, 30).
The purpose of the present study was to investigate a new simplified model for rotavirus investigation. Clinical and histopathological symptoms of SA11 rotavirus strain-associated diarrhea in germfree suckling rats are described.
Animal inoculation.
The use of animals maintained in sterile isolators prevents the exposure to extraneous rotavirus or other enteric pathogens (31). Furthermore, intestinal microflora can enhance the defense mechanisms of the host against pathogens (25) and can influence rotavirus infection, as described previously for mice (13). Under such conditions, the germfree status makes it possible to study rotavirus infection without any disturbing interference by other microorganisms. In our experiment, rats (Fischer F344 strain) were delivered and maintained in sterile isolators until the age of inoculation, and then they were housed in separate isolators. Germfree pups were orally inoculated at 2, 5, or 8 days of age. Inoculation was performed with a graduated plastic Pasteur pipette (Miniliquipette; Prolabo, Fontenay sous bois, France). After inoculation, infant rats were returned to their dams and allowed to suckle. Feng et al. (9) have demonstrated that diarrhea was dose dependent according to the rotavirus strain. The minimal dose of rotavirus provoking diarrhea in mice is 105-fold higher with heterologous strains than with homologous ones. SA11-infected mice developed diarrhea if the rotavirus dose was higher than 4 × 105 PFU/ml (17). Therefore, we chose to infect the rats with a high concentration of SA11 rotavirus to ensure that diarrhea would occur. Rats received a single 0.1-ml dose of either virus suspension (1.6 × 109 PFU/ml), prepared as described by Jourdan et al. (15), or modified Eagle medium (MEM) as a control.
Clinical signs of diarrhea.
Diarrhea was assessed in 23 to 35 infected rats and 16 to 25 control rats per age group. Rats were checked for diarrhea by gentle massage of the abdomen. Diarrhea was diagnosed when poorly formed yellow-green feces occurred immediately upon palpation. Control rats were treated identically to infected ones. Individual stool specimens were carefully collected in sterile plastic tubes on a weighed piece of plastic. Samples were stored at −80°C before analysis.
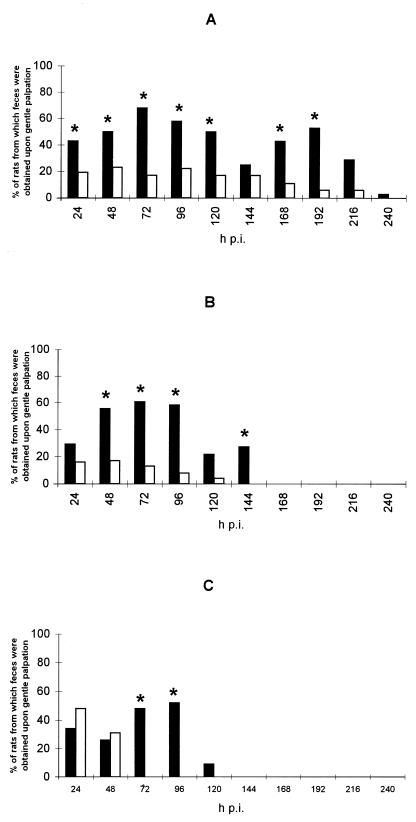
Our results demonstrate that diarrhea was induced in young germfree rats by inoculation with 108 PFU of SA11 rotavirus. The infection was self-limiting, and clinical signs were easy to interpret since diarrheal feces occurred spontaneously in infected pups as described previously for rat rotavirus-infected rats (24). Although feces occurred in control pups upon gentle palpation as well, samples were obtained from <20% of these animals (Fig. 1) except at 24 and 48 h postinfection (hpi) for the control rats inoculated at 8 days. Moreover, stools differed in color and weight. Control pups produced dark and small feces. The weight of stool samples (mean ± standard deviation) collected from 5-day-old infected rats was significantly greater (8 ± 4 mg) than the weight of stool samples collected from control rats (4 ± 4 mg) (P = 0.04 [t test]). The percentage of diarrheal samples was significantly higher from 24 to 192 hpi in pups infected at 2 days, from 48 to 144 hpi in pups infected at 5 days, and from 72 to 96 hpi in pups infected at 8 days (Fig. 1). The susceptibility of germfree rats was age dependent. The younger the rats were, the longer the period during which they developed diarrhea was. We found that germfree rats were susceptible to rotavirus infection for at least 8 days after birth. Vonderfecht et al. (30) have demonstrated that conventional rats were susceptible to a rotavirus-like agent up to the age of 14 days. In these authors’ experiments, clinical symptoms of rotavirus diarrhea were defined as liquid or poorly formed content in the distal colon associated with lesions of the perianal skin (29, 30). The relationship of age to the susceptibility to rotavirus infection may be due to the maturation changes in the gut and to the availability of rotavirus-specific receptors on the enterocytes (28).
FIG. 1.
Percentage of rats delivering feces immediately upon gentle palpation. Rats were inoculated with either SA11 rotavirus (solid bars) or MEM as a control (open bars). An asterisk denotes a percentage of samples significantly different from that of the control group (P < 0.05 [χ2 test]). Rats were infected at the age of 2 days (infected group, n = 35; control group, n = 20) (A), 5 days (infected group, n = 34; control group, n = 25) (B), or 8 days (infected group, n = 23; control group, n = 16) (C).
Rotavirus shedding.
Virus antigen load was determined by a double sandwich enzyme-linked immunosorbent assay (ELISA) adapted from a previous study (12): each fecal sample was diluted in 170 μl of phosphate-buffered saline (PBS) (pH 7.2). Negative and positive control tests were included in each plate. The baseline was determined with PBS. Samples obtained from noninfected pups produced optical densities (OD) between 0.01 and 0.06. Positive tests consisted of serial dilutions of the viral inoculum (from 1.6 × 108 to 1.9 × 105 PFU/ml). Assays were performed in duplicate. The viral antigen load in samples was determined relative to the standard curve and the dilution of samples in each plate. Crude OD are not relevant to this study since they are not really comparable. Indeed, the weights of feces were different whereas all feces were diluted in the same buffer volume. Therefore, dilutions and consequently OD were different from one sample to another, although viral antigen loads could be similar. Using this assay, we have been able to detect rotavirus antigen in virus stock with a titer of 106 PFU/ml.
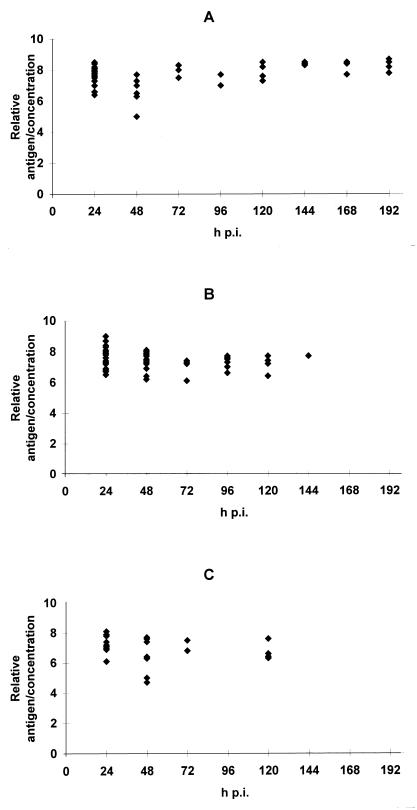
Rotavirus was detected in fecal samples for 192, 144, or 120 hpi in pups infected at 2, 5, or 8 days, respectively (Fig. 2). Direct detection of rotavirus in the intestinal segments of rats demonstrated that it remained in the small intestine for at least 5 days postinfection (p.i.). Five-day-old pups were inoculated with 0.1 ml of a mixture containing rotavirus (1.6 × 109 PFU/ml) and spores of Bacillus subtilis (3 × 106 CFU/ml), usually used as an intestinal transit marker (7, 8). At selected time intervals, two to three pups were sacrificed. The entire intestinal tract of each pup was removed and divided into stomach, small intestine, and colon segments. The segments were separately diluted in sterile water (stomach and small intestine, 1/10 [wt/vol]; colon, 1/100 [wt/vol]). Then, they were homogenized in an Ultra-turrax homogenizer (Bioblock, Paris, France) and tested for the presence of rotavirus and spores. Rotavirus was tested by ELISA. B. subtilis spores were numerated on agar medium (meat extract [8 g/liter], yeast extract [2 g/liter], manganese sulfate [0.04 g/liter], glucose [1 g/liter], Bi-Tek agar [28 g/liter]) after incubation at 53°C for 24 h under aerobic conditions. The count was expressed as log10 CFU/milliliter. Rotavirus and bacterial spores were found in the stomach immediately after inoculation but not at any time point thereafter. Spores were found decreasingly from 12 to 120 hpi in the colon but were not found in the small intestine during this period. By contrast, rotavirus remained in the small intestine at 12 to 120 hpi and was found in the colon (data not shown). Similar experiments with mice have shown that murine rotavirus was detected in mucosal homogenates from germfree mice at 8 days p.i. or from conventional mice at 13 days p.i. (13). Rotavirus was present for 7 days p.i. in the intestinal contents of conventional mice (16).
FIG. 2.
Relative viral antigen load/virus concentration ratio determined in feces of infected pups. Rats were inoculated at the age of 2 (A), 5 (B), or 8 (C) days. Feces were sampled and assayed for rotavirus antigens by ELISA. A standard curve obtained from the viral inoculum was determined for each plate. The viral antigen load in feces was determined conventionally relative to the standard curve. Data represent individual values obtained for fecal specimens.
Histological examination.
Infected and control pups were inoculated at 5 days. Three pups were sacrificed 3 days p.i. Jejunal segments were removed. The cell morphology was observed by light microscopy. Fresh tissues were fixed in slightly modified Carnoy fixative (absolute ethanol–30% formaldehyde–acetic acid [6:3:1; vol/vol/vol]), dehydrated, and embedded in paraffin. Paraffin sections (6 μm) were cut on a microtome (Leitz, Wetzlar, Germany) and were polychromatically stained. Acid and neutral mucin were stained with Alcian blue and periodic acid-Schiff stain, respectively. Cellular nuclei were stained with Hansen ferric trioxyhematein, muscular fibers were stained with picric acid, and collagen and basement membranes were stained with indigo carmine. Care was taken that only longitudinal sections cut perpendicularly to the muscular mucosae were studied. Villus height, crypt depth, and the number of mucus-containing cells were determined for 10 different villi from the same intestinal section.
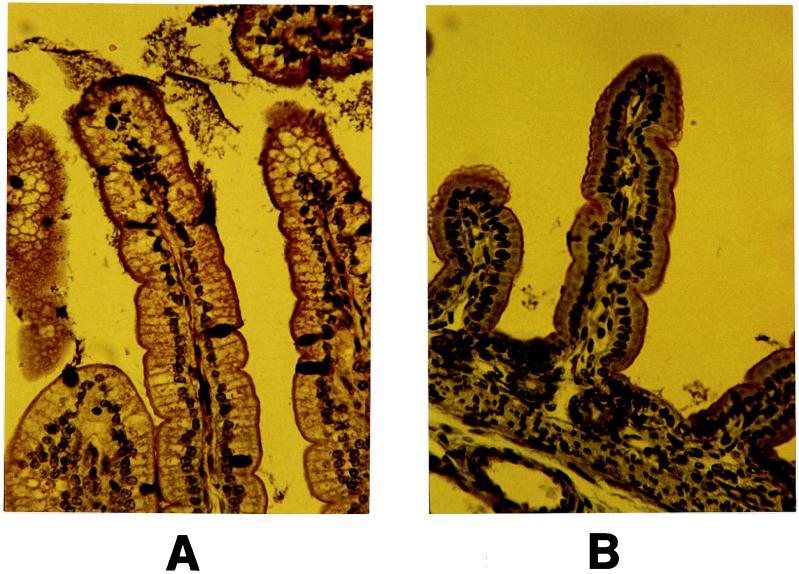
Morphologic changes associated with rotavirus infection were limited to cellular vacuolation. The control section presented cells characterized by nuclei localized at their base and by a large supranuclear area occupying almost the whole apical cytoplasm. Nuclear polarity and cellular morphology of the villus basis were not affected by the virus. By contrast, large areas of cellular vacuolation were observed in infected intestinal sections at 3 days p.i. (Fig. 3). These lesions were revealed in the enterocytes localized at the upper one-third of the intestinal villi. The villus height/crypt depth and number of mucus-containing cells/villus height ratios of infected animals were not significantly different from those of control animals (data not shown). Mucus in infected villi was not completely released on the intestinal lumen, as suggested by the presence of dark stained goblet cells. In rats infected with a rotavirus-like agent, histological changes were defined in the distal small intestine by villus shortening, syncytial cell formation, and crypt elongation but limited vacuolation at the tip of the villus (30). The damage intensities caused by rotavirus infection in the different parts of the small intestine differ according animals and rotavirus strains as reported by different authors. Some studies have reported greater changes in the ileum than in the jejunum in gnotobiotic dogs infected with a canine rotavirus (14) or in suckling mice infected with nonmurine rotavirus strains (23). By contrast, Kubelka et al. (17) have observed more changes in the jejunum in SA11-infected mice. Others have demonstrated similar changes in the ileum and jejunum in SA11-infected mice (18) or in group B rotavirus-infected rats (24). Heyman et al. (13) have reported the appearance of vacuoles in enterocytes of the villi of the proximal small intestine associated with a release of mucus in the lumen of mice infected with a murine strain.
FIG. 3.
Histological aspects of jejunum villi of germfree suckling rats examined on day 3 p.i. Five-day-old rats were inoculated with either SA11 rotavirus (A) or MEM as a control (B). Intestinal sections were polychromatically stained and observed by light microscopy. Magnification, ×300.
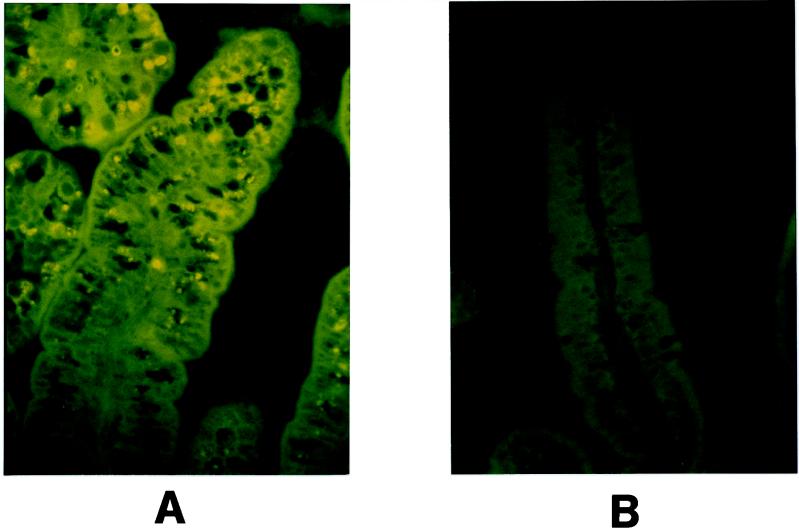
Rotavirus structural proteins in the cells were detected by immunofluorescence. Paraffin sections were deparaffinized with toluene and rehydrated with decreasing concentrations of ethanol (100, 95, 90, and 70%). The slides were washed in water and then in PBS. After drying at room temperature, the preparations were incubated for 1 h at 37°C with a hyperimmune rabbit antirotavirus serum. The serum was diluted 1/100 in PBS containing 3% bovine serum albumin. The slides were again washed with PBS and incubated for 30 min at 37°C with a goat anti-rabbit immunoglobulin-fluorescein isothiocyanate conjugate (Boehringer SA, Meylan, France) diluted 1/100 in PBS containing 3% bovine serum albumin. After final washings with PBS, fluorescence was examined by UV light microscopy. Greenberg et al. (11) have reported that rotavirus has a tissue tropism limited to the upper part of the villus. Our observations from 3 days p.i. are in agreement with this result. Rotavirus antigen was detected by immunofluorescence in differentiated cells at the tip of the villus, although adjacent vacuolated cells closer to the crypt did not show any fluorescence (Fig. 4). Similar results have been reported in infant rats infected with a rotavirus-like agent (30): virus particles in the upper one-third of villi of the small intestine have been found to be associated with histological changes.
FIG. 4.
Detection of rotavirus antigens in jejunum cells of germfree suckling rats 3 days p.i. Five-day-old rats were inoculated with either SA11 rotavirus (A) or MEM as a control (B). Rotavirus structural proteins were labeled with a hyperimmune rabbit antirotavirus serum and then a goat anti-rabbit immunoglobulin-fluorescein isothiocyanate. Rotavirus antigens were detected under UV light by immunofluorescence. Magnification, ×400.
Minimal replication and histological lesions have been associated with diarrhea in animal models, suggesting a toxin-like effect of rotavirus (1, 26). In our experiment, diarrhea occurred whereas enterocyte morphology presented mild modifications and vacuolation was observed in cells in which no rotavirus structural antigens were detected. This result might be explained by the mechanism of infection proposed by Ball et al. (1). Rotavirus particles bind some cells, resulting in virus entry and gene expression at the tip of the villus. Then, the nonstructural protein NSP4, expressed in infected cells, is released into the lumen and interacts with a specific receptor on adjacent cells, increasing the endogenous secretory pathway.
Group A rotavirus is a significant human and veterinary pathogen in terms of morbidity, mortality, and economic loss (4). Bass et al. (2) mentioned that natural group A rotavirus receptors had never been observed in rats. To our knowledge this is the first report describing diarrhea in germfree rats infected with a group A rotavirus strain. The suckling rat model presents almost the same advantages as mouse or rabbit models in terms of cost, diarrhea intensity and duration, and histopathological changes. However, one advantage of the rat model over the other small-animal models is found with regard to the clinical signs. In rabbits, both infected and control animals normally excrete soft, moist feces (6). It is of interest that our present model may further the knowledge of rotavirus infection. This report clarifies the previously held concept that group A rotavirus does not infect rats (2).
To date rotavirus pathogenesis remains poorly understood (1). The new animal model presented here may be useful for further investigations of group A rotavirus infection.
Acknowledgments
We thank M. K. Estes (Baylor College of Medicine, Houston, Tex.) for kindly providing the rotavirus strain.
This work was supported in part by the CIRDC, Danone, France.
REFERENCES
- 1.Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 2.Bass D M, Mackow E R, Greenberg H B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991;183:602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- 3.Bell L M, Clark H F, O’Brien E A, Kornstein M J, Plotkin S A, Offit P A. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc Soc Exp Biol Med. 1987;184:127–132. doi: 10.3181/00379727-184-rc2. [DOI] [PubMed] [Google Scholar]
- 4.Burns J W, Krishnaney A A, Vo P T, Rouse R V, Anderson L J, Greenberg H B. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 5.Burns J W, Greenberg H B. Viral gastroenteritis. Infect Dis Clin Pract. 1994;3:411–417. [Google Scholar]
- 6.Conner M E, Estes M K, Graham D Y. Rabbit model of rotavirus infection. J Virol. 1988;62:1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contrepois M, Gouet P. Utilisation d’une technique microbiologique pour la mesure de la vitesse de transit des microparticules dans le tractus digestif des ruminants. C R Acad Sci Paris. 1969;268:1757–1759. [Google Scholar]
- 8.Ducluzeau R, Bellier M, Raibaud P. Transit through the digestive tract of the inocula of several bacterial strains inoculated “per os” into axenic and “holoxenic” mice. The antagonistic effect of the microflora of the gastrointestinal tract. Zentralbl Bakteriol Abt 1 Orig. 1970;213:533–548. [PubMed] [Google Scholar]
- 9.Feng N, Burns J W, Bracy L, Greenberg H B. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass R I, Gentsch J R, Ivanoff B. New lessons for rotavirus vaccines. Science. 1996;272:46–48. doi: 10.1126/science.272.5258.46. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg H B, Clark H F, Offit P A. Rotavirus pathology and pathophysiology. Curr Top Microbiol Immunol. 1994;185:255–283. doi: 10.1007/978-3-642-78256-5_9. [DOI] [PubMed] [Google Scholar]
- 12.Guerin-Danan C, Andrieux C, Popot F, Charpilienne A, Vaissade P, Gaudichon C, Pedone C, Bouley C, Szylit O. Pattern of metabolism and composition of the fecal microflora in infants 10 to 18 months old from day care centers. J Pediatr Gastroenterol Nutr. 1997;25:281–289. doi: 10.1097/00005176-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Heyman M, Corthier G, Petit A, Meslin J C, Moreau C, Desjeux J F. Intestinal absorption of macromolecules during viral enteritis: an experimental study on rotavirus-infected conventional and germ-free mice. Pediatr Res. 1987;22:72–78. doi: 10.1203/00006450-198707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson C A, Snider T G, Henk W G, Fulton R W. A scanning and transmission electron microscopic study of rotavirus-induced intestinal lesions in neonatal gnotobiotic dogs. Vet Pathol. 1986;23:443–453. doi: 10.1177/030098588602300415. [DOI] [PubMed] [Google Scholar]
- 15.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwar S S, Singh V, Vinayak V K, Malik A K, Mehta S K, Mehta S. Differential tropism of EB rotavirus (serotype 3) to small intestine of homologous murine model. Acta Virol. 1994;38:269–276. [PubMed] [Google Scholar]
- 17.Kubelka C F, Marchevsky R S, Stephens P R, Araujo H P, Oliveira A V. /1994. Murine experimental infection with rotavirus SA-11: clinical and immunohistological characteristics. Exp Toxicol Pathol. 1993;45:433–438. doi: 10.1016/S0940-2993(11)80375-1. [DOI] [PubMed] [Google Scholar]
- 18.Majerowicz S, Kubelka C F, Stephens P, Barth O M. Ultrastructural study on experimental infection of rotavirus in a murine heterologous model. Mem Inst Oswaldo Cruz (Rio de Janeiro) 1994;89:395–402. doi: 10.1590/s0074-02761994000300018. [DOI] [PubMed] [Google Scholar]
- 19.Mebus C A, Newman L E. Scanning electron, light, and immunofluorescent microscopy of intestine of gnotobiotic calf infected with reovirus-like agent. Am J Vet Res. 1977;38:553–558. [PubMed] [Google Scholar]
- 20.Offit P A, Clark H F. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J Infect Dis. 1985;152:1152–1158. doi: 10.1093/infdis/152.6.1152. [DOI] [PubMed] [Google Scholar]
- 21.Offit P A, Clark H F, Kornstein M J, Plotkin S A. A murine model for oral infection with a primate rotavirus (simian SA11) J Virol. 1984;51:233–236. doi: 10.1128/jvi.51.1.233-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petschow B W, Litov R E, Young L J, McGraw T P. Response of colostrum-deprived cynomolgus monkeys to intragastric challenge exposure with simian rotavirus strain SA11. Am J Vet Res. 1992;53:674–678. [PubMed] [Google Scholar]
- 23.Ramig R F. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb Pathog. 1988;4:189–202. doi: 10.1016/0882-4010(88)90069-1. [DOI] [PubMed] [Google Scholar]
- 24.Salim A F, Phillips A D, Walker-Smith J A, Farthing M J. Sequential changes in small intestinal structure and function during rotavirus infection in neonatal rats. Gut. 1995;36:231–238. doi: 10.1136/gut.36.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salminen, S., E. Isolauri, and T. Onnela. 1995. Gut flora in normal and disordered states. Chemotherapy 41(Suppl. 1):5–15. [DOI] [PubMed]
- 26.Shaw R D, Hempson S J, Mackow E R. Rotavirus diarrhea is caused by nonreplicating viral particles. J Virol. 1995;69:5946–5950. doi: 10.1128/jvi.69.10.5946-5950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thillainayagam A V, Dias J A, Salim A F, Mourad F H, Clark M L, Farthing M J. Glucose polymer in the fluid therapy of acute diarrhoea: studies in a model of rotavirus infection in neonatal rats. Clin Sci Colch. 1994;86:469–477. doi: 10.1042/cs0860469. [DOI] [PubMed] [Google Scholar]
- 28.Uhnoo I, Dharakul T, Riepenhoff-Talty M, Ogra P L. Immunological aspects of interaction between rotavirus and the intestine in infancy. Immun Cell Biol. 1988;66:135–145. doi: 10.1038/icb.1988.17. [DOI] [PubMed] [Google Scholar]
- 29.Vonderfecht S L, Eiden J J, Miskuff R L, Yolken R H. Kinetics of intestinal replication of group B rotavirus and relevance to diagnostic methods. J Clin Microbiol. 1988;26:216–221. doi: 10.1128/jcm.26.2.216-221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonderfecht S L, Huber A C, Eiden J, Mader L C, Yolken R H. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J Virol. 1984;52:94–98. doi: 10.1128/jvi.52.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan L, Ward L A, Rosen B I, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]