Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) has the potential for curative outcomes for a variety of hematologic malignancies. Current allo-HCT studies often describe the outcomes and costs in the near term; however, research on the lifetime economic burden post-allo-HCT remains limited. This study was conducted to estimate the average total lifetime direct medical costs of an allo-HCT patient and the potential net monetary savings from an alternative treatment associated with improved graft-versus-host disease (GVHD)-free, relapse-free survival (GRFS). A disease-state model was constructed using a short-term decision tree and a long-term semi-Markov partitioned survival model to estimate the average per-patient lifetime cost and expected quality-adjusted life years (QALYs) for an allo-HCT patient from a US healthcare system perspective. Key clinical inputs included overall survival, GRFS, incidence of both acute and chronic GVHD, relapse of the primary disease, and infections. Cost results were reported as ranges based on varying the percentage of chronic GVHD patients that remained on treatment after 2 years (15% or 39%). Over a lifetime, the average per-patient medical cost of allo-HCT was estimated to range from $942,373 to $1,247,917. The majority of the costs were for chronic GVHD treatment (37% to 53%), followed by the allo-HCT procedure (15% to 19%). The expected lifetime QALYs of an allo-HCT patient were estimated as 4.7. Lifetime per-patient treatment costs often exceed $1,000,000 for allo-HCT patients. Innovative research efforts focused on the reduction or elimination of late complications, particularly chronic GVHD, may provide the greatest value to improved patient outcomes.
Keywords: Allogeneic, HCT, Total costs, Lifetime burden, GVHD, Health economics
INTRODUCTION
Allogeneic hematopoietic cell transplantation (allo-HCT) has the potential for curative outcomes in a variety of hematologic malignancies [1,2]. The number of allo-HCTs performed in the United States has increased every year over the last 20 years with the exception of 2020, likely due to Coronavirus disease 2019) [3,4]. In 2020, a reported 8326 allo-HCTs were performed in the United States, with the most common indications including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndromes (MDS), accounting for 76% of these procedures [3]. The use of allo-HCT to treat malignant diseases in patients age ≥65 years also has increased; in 2020, 27% of allo-HCT recipients were ≥65 [3]. Despite the ability to increase survivorship among its recipients, in many cases cure or control of the underlying disease by allo-HCT is not accompanied by full restoration of health [5].
Long-term side effects after allo-HCT include nonmalignant organ or tissue dysfunction, changes in quality of life, infections related to abnormal immune reconstitution, and secondary cancers. Moreover, mortality rates among allo-HCT recipients remain 4- to 9-fold higher than the expected population rate for at least 30 years after transplantation, with many of these events and complications attributed to graft-versus-host disease (GVHD), specifically chronic GVHD [5,6].
Allo-HCT management has evolved with the emergence of molecular assays to detect relapse at previously unmeasurable levels and therapies to treat post-HCT complications such as GVHD. This expansion of diagnostic analyses and healthcare resource utilization may potentially increase the economic burden on healthcare systems. Historical assessments of allo-HCT have examined details of the associated expenditures. A report from the Agency for Healthcare Research and Quality showed that HCT had the most rapidly increasing expenditures among medical procedures between 2004 and 2007, with an increase of 84.9% and a total of US $1.3 billion spent on HCT in 2007 [7,8]. More recently, in an update of previous data, Bentley et al. [9] reported that in the United States in 2020, the average billed cost of bone marrow allo-HCT for the first 180 days post-transplantation was $1,071,700. In a retrospective cohort study using claims data, Perales et al. [10] reported that among allo-HCT recipients, those with complications had significantly higher mean total costs compared with those without complications during the 12-month post-HCT period ($533,999 versus $325,365). Current economic allo-HCT studies often describe the outcomes and costs associated with recipients in the near term, and research on the long-term outcomes and, more specifically, the lifetime economic burden associated post allo-HCT remains limited. Therefore, a disease-state model was constructed to estimate (1) the average total lifetime medical costs of allo-HCT and (2) the potential net monetary savings from an alternative treatment associated with improved GVHD-free, relapse-free survival (GRFS).
METHODS
Model Structure
The economic model contains 2 parts that link short-term outcomes to long-term costs and consequences. A short-term decision tree calculates the costs and consequences from the allo-HCT hospital admission up to 100 days during which patients could experience GVHD, relapse of the primary disease, GRFS, or death. After the first 100 days, the patients were assigned to 1 of 3 mutually exclusive health states in a semi-Markov partitioned survival model for the remainder of their lifetime: (1) GRFS, (2) progressed and/or GVHD, or (3) death. The projected time spent in each health state was derived from data provided by the Center for International Blood and Marrow Transplant Research (CIBMTR). The GRFS health state consisted of patients who did not experience acute GVHD grade III-IV, chronic GVHD of any severity, relapse, or death. Patients transitioned between health states in 1-year cycles. Costs and outcomes were discounted at 3% per year [11]. Total life-years (LYs), quality-adjusted life-years (QALYs), and direct medical costs were estimated over a lifetime horizon. Cost results were reported as a range based on varying the percentage of chronic GVHD patients that remained on treatment after 2 years (15% or 39%) as derived from the published literature [12,13]. Scenario analyses were conducted to address whether allo-HCT complications were reduced by improving GRFS outcomes. Additionally, a broadened societal perspective that included components for productivity loss was analyzed. Figure 1 illustrates the model structure used to estimate costs and health outcomes.
Figure 1.
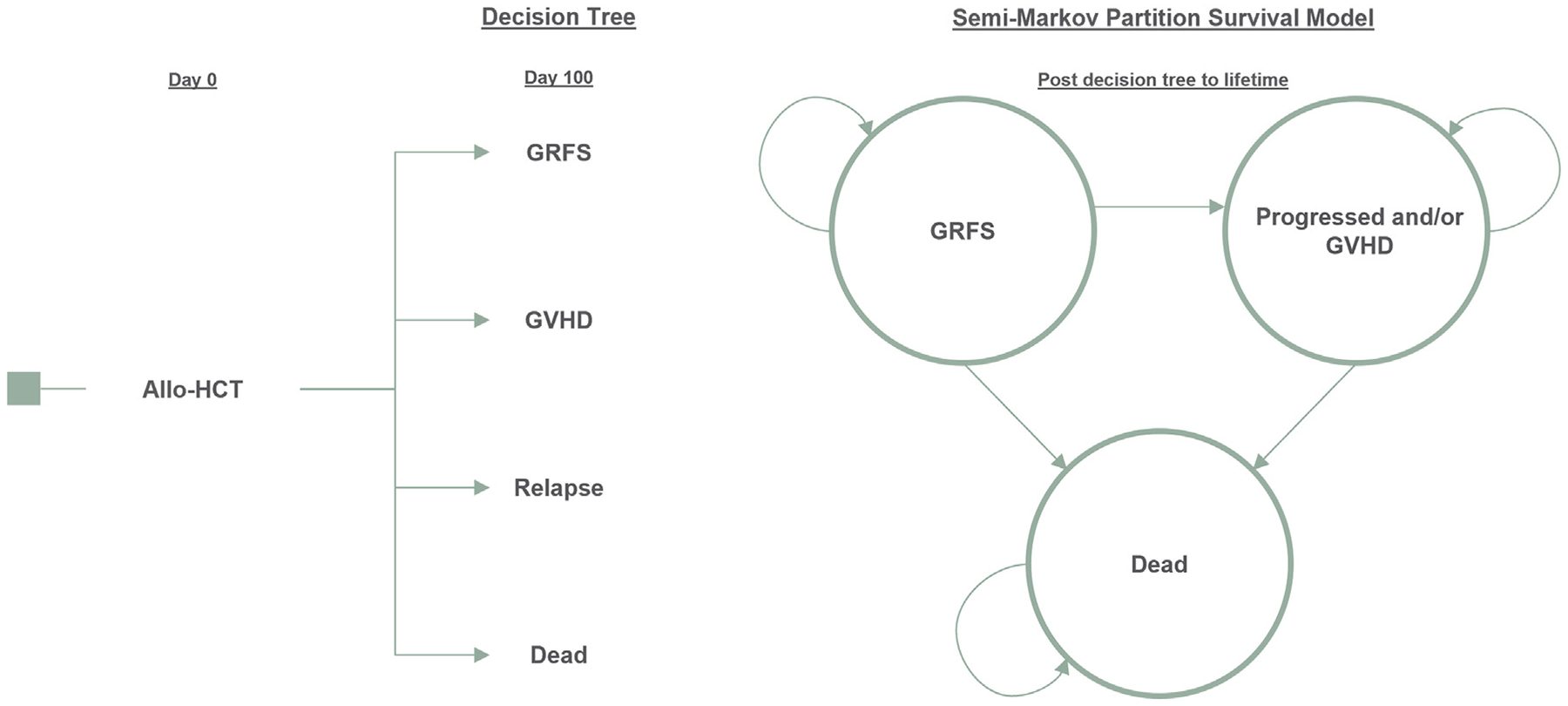
Model diagram. Decision-tree mapping: GRFS patients would continue in the GRFS health state in the semi-Markov partition survival model. GVHD and relapse patients were assigned to the Progressed or GVHD health state in the semi-Markov partition survival model. Any patients who died in the decision tree remained in the all-absorbing dead state in the partition survival model.
Inputs and Assumptions
Population Characteristics
Patient characteristics included in the model were age at transplantation, sex, donor type, graft source, and underlying malignancy/disease mix. For simplicity, the model focused on a weighted average of the 3 most common indications accounting for the majority of allo-HCTs: AML, ALL, and MDS. Patient characteristics were based on data from the CIBMTR and are presented in Table 1 [3,14].
Table 1.
Population Inputs
| Model Parameter | Value | Source |
|---|---|---|
| Population inputs | ||
| Age at transplantation, yr | 53 | CIBMTR [3] |
| Male sex, % | 58 | CIBMTR [14] |
| Malignancy or underlying disease, % | ||
| AML | 51 | CIBMTR [3] |
| ALL | 21 | |
| MDS | 28 | |
| SCT donor type and graft source, % | ||
| Related | 32 | |
| Bone marrow | 8 | |
| PBSCs | 92 | |
| Unrelated | 68 | |
| Bone marrow | 9 | |
| PBSCs | 91 | |
| Healthcare utilization costs, $ | ||
| Cost of allo-HCT | 182,642 | Grubb et al. 2016 [21] |
| Acute GVHD | ||
| 100 days | 79,197 | Yu et al. 2020 [22] |
| 1 year | 158,938 | |
| Chronic GVHD (1 year) | 220,202 | Bachier et al. 2021 [19] |
| Relapse episode | 206,003 | Pandya et al. 2019 [24] |
| Infection-related hospitalization | 53,214 | Godara et al. 2021 [25], Schuster et al. 2017 [26] |
| Maintenance therapy | ||
| Annual cost of sorafenib | 277,765 | IBM Redbook [29], Nexavar PI [30] |
| Annual cost of imatinib mesylate | 184,861 | IBM Redbook [29], Gleevec PI [31] |
| End of life | 174,102 | Mau et al. 2020 [32], Scitovsky et al. 2005 [33] |
| Patient utilities and disutilities | ||
| Utilities | ||
| Post-HCT without GVHD | 0.86 | Perić et al. 2016 [35], Forsythe et al. 2018 [36] |
| Post-HCT with GVHD | 0.69 | |
| Relapse/progressed | ||
| AML | 0.53 | Pan et al. 2010 [42] |
| ALL | 0.74 | Delea et al. 2017 [43] |
| MDS | 0.60 | Pan et al. 2010 [42] |
| Disutilities | ||
| Infection | −0.23 | Sarkar et al. 2019 [37] |
All costs are inflation-adjusted to 2021 US dollars using the using the medical care consumer price index from the Bureau of Labor Statistics [34]. PBSCs indicates peripheral blood stem cells.
Allo-HCT Survival and GRFS
Overall survival (OS) and GRFS outcomes over 15 years were obtained from the CIBMTR targeting adults age ≥18 years who underwent allo-HCT for AML, ALL, or MDS. 2016–2019 transplant data for AML, MDS, and ALL for patients age ≥18 years were extracted from digitized survival curves from the CIBMTR’s US Summary Slides and used for the first 3 years of the model [3]. To estimate outcomes in years 4 to 15 for OS and years 2 to 15 for GRFS, the HCT curves were assumed to follow the same relative trend as observed in a CIBMTR cohort of 8934 transplantations performed in the United States during 2000 to 2005 [14]. This assumption takes a conservative approach, suggesting that there are no ongoing improvements in supportive care. For GRFS, the reported probability in year 1 was adjusted upward to reflect current trends reported in the literature of 31% to 34% GRFS in year 1 post-HCT [15,16]. OS and GRFS data were generated for each underlying malignancy/disease and then weighted by the patient characteristics as described previously.
Survival beyond 15 years was based on US Life Tables [17], with modifications taking into account the excess mortality experienced by long-term survivors of allo-HCT. Additionally, patients who experience GVHD have a higher mortality rate (hazard ratio, 1.6) [6]. Therefore, the ratios were applied to the life table estimates to account for the excess mortality observed in post-allo-HCT patients and patients who experienced GVHD.
Clinical Events
The projected time spent in each health state was derived from data provided by the CIBMTR. The Progressed and/or GVHD health state includes acute GVHD grade III-IV, chronic GVHD, and relapse. The number of clinical events experienced by a patient who progressed and/or had GVHD was based on incidence data obtained from the CIBMTR and published literature. CIBMTR data included the earliest GRFS event incidence for patients for up to 15 years. Competing events data from Veltri et al. [18] were also incorporated to account for the potential for concurrent events in which a patient may experience a relapse and a GVHD event in a single year (19% for acute GVHD grade III-IV and 17% for chronic GVHD) [18]. To account for the proportion of patients who develop chronic GVHD after experiencing acute GVHD (66%), data from Bachier et al. [19] were used [19]. Clinical event incidence data were weighted by graft source, donor relationship, primary disease, and health state occupancy for incorporation into the model. Finally, patients in either health state also were at risk for infection in the first year post-allo-HCT. A 100-day post-allo-HCT infection rate (73%) obtained from the CIBMTR was used as a proxy for the proportion incurring infection in the first year post-allo-HCT.
Healthcare Utilization and Costs
Costs were estimated from a US healthcare system perspective. Costs pertaining to allo-HCT and associated complications were obtained from the published literature based on medical claims analyses of payments (paid amounts) and were converted into costs using the American Hospital Association’s payment-to-cost ratios [20]. The following costs were included in the model: allo-HCT, acute GVHD, chronic GVHD, relapse, infection, maintenance therapy, and end of life.
The cost per allo-HCT was based on the mean total cost of an allo-HCT index admission with no GVHD [21]. Acute GVHD costs incremental to the allo-HCT were calculated by taking the difference between the mean total costs for patients who experienced acute GVHD and those who did not have an event [22]. The mean annual cost of chronic GVHD was estimated as $202,202 based on the analysis by Bachier et al. [19] Most patients were modeled to remain on chronic GVHD treatment for 2 years to align with the median durations of treatment reported in the literature [12,23]. However, the literature also reports a subset of chronic GVHD patients who require treatment well beyond 2 years, and thus cost results were presented as a range based on a varying percentage of chronic GVHD patients who remained on treatment after 2 years (15% or 39%), as derived from the published literature [12,13]. These patients continued treatment for the remainder of the model and/or died. For costs associated with relapse, mean total costs from the relapsed/refractory without HSCT arm in the study by Pandya et al. 2019 [24] were used to estimate the incremental costs attributable to a relapse event. After converting to a healthcare system perspective and adjusting for inflation, the mean total cost per relapse episode was estimated to be $206,003. To calculate infection costs, the average incremental cost due to bacterial sepsis, Clostridium difficile, invasive fungal infection, and cytomegalovirus were estimated and weighted based on data describing the incidence of infection in allo-HCT recipients [25,26].
Maintenance therapy costs for FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD)-positive AML patients and Philadelphia chromosome-positive (Ph+) ALL patients were based on published literature, expert opinion, and prescribing information. The model assumed that 25% of AML patients had FLT3-ITD as reported by Cohen et al. [27]. All FLT3-ITD positive patients were assumed to have been treated with off-label sorafenib (400 mg twice daily) maintenance therapy for a period of 24 months, based on the improved observed outcomes [27]. Among the ALL population, 30% were modeled to be Ph+ [28]. All Ph+ patients were assumed to have been treated with off-label imatinib mesylate (600 mg daily) post-HCT for a period of 24 months based on expert opinion from the authors (R.T.M. and M-A.P.). Drug acquisition costs were based on the wholesale acquisition costs obtained from REDBOOK and prescribing information [29–31].
A one-time end-of-life cost of $174,102 was applied to patients who died to approximate the costs incurred in the last 3 months of life. End-of-life costs were based on the average costs attributable to the last year of life estimated from Mau et al. [32] and the proportion of costs incurred in the last 3 months of life derived from Scitovsky et al. [33] using a loga-rithmic regression analysis.
All costs were inflation-adjusted to 2021 US dollars using the using the medical care consumer price index from the Bureau of Labor Statistics and are presented in Table 1 [34].
LYs and QALYs
The model estimates the expected LYs and QALYs over a lifetime horizon. Utilities were based on data reported in the literature and are displayed in Table 1. The utility associated with the GRFS state was based on a post-HCT without GVHD value obtained from Forsythe et al. [35] that estimates utilities by mapping from QLQ-C30 scores obtained from a study evaluating quality of life in patients with chronic GVHD [36].
The utility score for the progressed and/or GVHD state was estimated based on a weighted average of a post-HCT with GVHD utility as well as relapse utilities specific to the underlying malignancy/disease mix as outlined in Table 1. The weighted proportions applied were based on the occurrence rates of the GVHD and relapse events.
Across the health states, a disutility was applied for patients who incurred an infection [37]. The infection disutility was incurred over a period of 10 days. To calculate the number of days in which the infection disutility was applicable, the average incremental length of stay for bacterial sepsis, C. difficile, invasive fungal infection, and cytomegalovirus were estimated and weighted based on data describing the incidence of infection in allo-HCT recipients [25,26].
Ethical Considerations
This study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline for economic evaluations [38] and was based on an economic model that uses secondary literature to inform model inputs. Only hypothetical patients and facilities were assessed. Therefore, Institutional Review Board approval and informed consent were not required according to 45 CFR 46.101(b)(4), because no primary data from actual patients were collected or evaluated.
Data Sharing Statement
For original data, please contact jbadaracco@bluepathsolutions.com
RESULTS
Base-Case Analysis
Total LYs, QALYs, and direct medical costs were estimated over a lifetime horizon. Cost results are reported as a range based on varying the percentage of chronic GVHD patients remaining on treatment after 2 years (15% or 39%) (Table 2). In the base case analysis over a lifetime, the estimated average per-patient medical cost of allo-HCT ranged from $942,373 to $1,247,917. In a breakdown of those total costs, 23% to 30% were incurred within the first 100 days and 42% to 56% within the first year. Of the medical costs estimated, the majority were for chronic GVHD treatment (37% to 53%), followed by the allo-HCT procedure (15% to 19%). The expected lifetime LYs and QALYs of an allo-HCT recipient were estimated as 6.4 and 4.7, respectively.
Table 2.
Base Case Results: Average Per-Patient Medical Cost of Allo-HCT
| Category | 15% Remaining on Chronic GVHD Treatment | 39% Remaining on Chronic GVHD Treatment |
|---|---|---|
| Costs | ||
| HCT costs | $182,642 | $182,642 |
| Maintenance therapy | $59,097 | $59,097 |
| Acute GVHD | $34,260 | $34,260 |
| Chronic GVHD | $351,260 | $656,804 |
| Relapse | $123,835 | $123,835 |
| Infection | $54,836 | $54,836 |
| End of life | $136,443 | $136,443 |
| Total costs | $942,373 | $1,247,917 |
| Total LYs | 6.4 | 6.4 |
| Total QALYs | 4.7 | 4.7 |
All values are rounded to the nearest whole number.
Scenario Analyses
Net Monetary Benefit: Improvement in GRFS
Scenario analyses were conducted to estimate the potential clinical and economic value that may be realized by improving GRFS outcomes. The net monetary benefit analyses focused on testing various improvements in GRFS. Scenario 1 was modeled as a 50% improvement in GRFS (52% GRFS in year 1 as opposed to 34% modeled in the base case) with 39% of chronic GVHD patients remaining on treatment after 2 years. Scenario 2 was modeled as doubled improvement in GRFS (69% GRFS in year 1) with 39% of chronic GVHD patients remaining on treatment after 2 years.
In both scenarios, the difference in expected QALYs between the allo-HCT patients and patients treated with improved outcomes were used to calculate the number of QALYs gained. The estimated QALYs gained were then multiplied by a willingness-to-pay threshold of $150,000 to monetize or value the gain in the quality of life. The willingness-to-pay threshold used for this analysis aligns within commonly cited cost-effectiveness thresholds in the United States of $50,000 to $150,000 per QALY gained [39]. In the scenario analyses, the net monetary savings achieved by improving GRFS outcomes (via the medical cost offsets and QALYs gained) was associated with a net monetary value ranging from $548,178 to $911,062. Disaggregated incremental medical costs savings showing the contribution of the cost categories are presented in Figure 2A,B. Of the estimated net monetary value, 88% to 90% was driven by direct medical cost savings, and the remaining 12% to 10% was due to value of the gain in expected QALYs.
Figure 2.
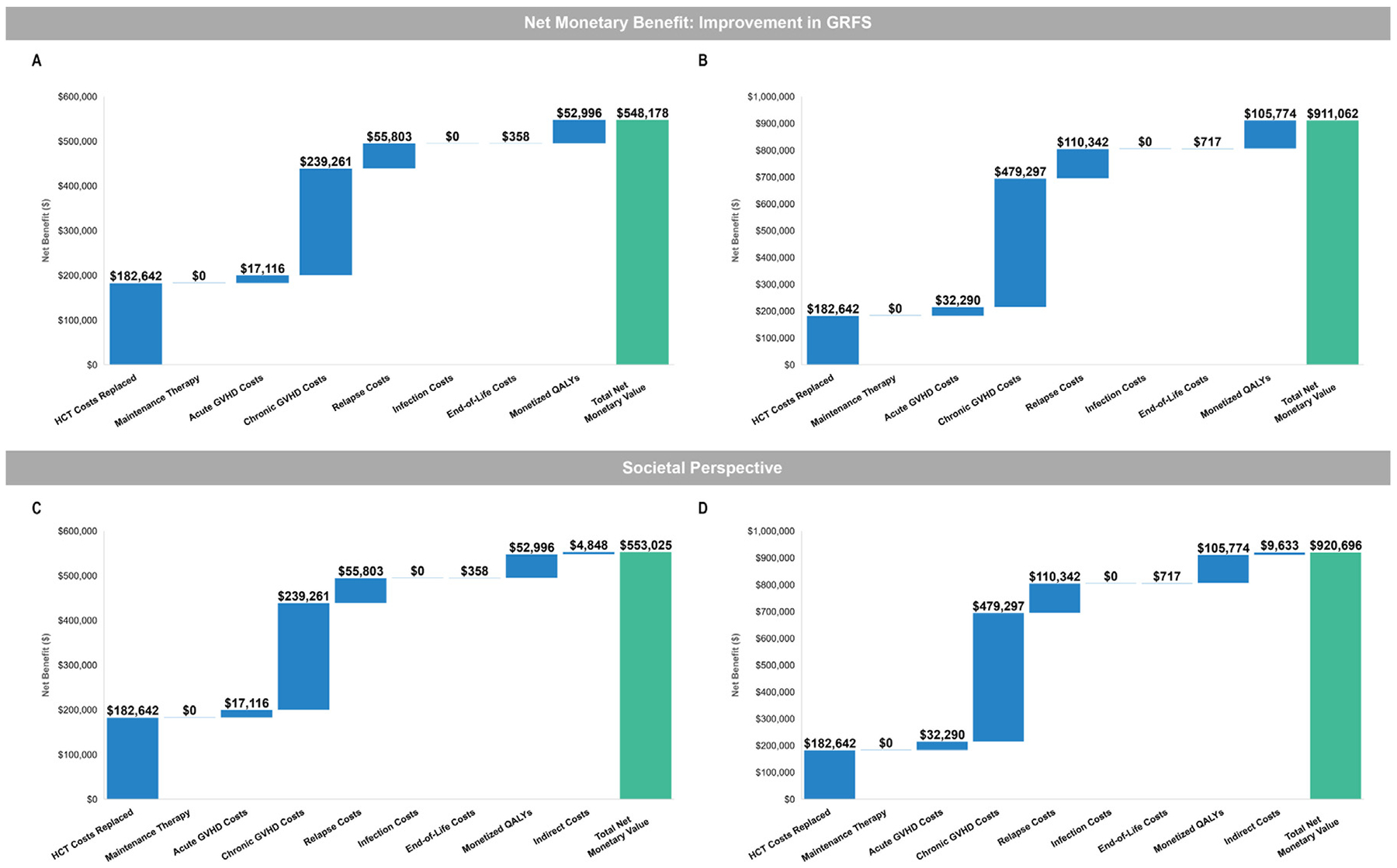
Net monetary benefit of improving GRFS versus standard allo-HCT. (A) Cost analysis with 50% improvement in GRFS. (B) Cost analysis with doubled improvement in GRFS. (C) Societal perspective that included indirect costs with 50% improvement in GRFS. (D) Societal perspective that included indirect costs with doubled improvement in GRFS. All analyses were performed with 39% of patients with cGVHD remaining on treatment.
Societal Perspective
A societal perspective that included additional components for productivity loss was performed. To account for lost productivity during allo-HCT and acute GVHD, we assumed the amount of time missed from work was equal to the total number of days in which inpatient care was provided. For allo-HCT and acute GVHD, the days missed from work equated to the time spent hospitalized. For chronic GVHD and relapse, indirect costs were calculated based on the percentage of patients who could not return to workforce due to permanent disability and lost wages due to death from GVHD and/or relapse. An average hourly wage of $28.01 was used as the unit cost for 1 hour of work missed based on the Occupational Employment and Wage Statistics Survey from the Bureau of Labor Statistics [40]. Jones et al. [41] reported that 25% of chronic GVHD patients are unable to return to the workforce due to permanent disability. To estimate the years of lost wages due to death from GVHD and/or relapse, the total number of patient years lost up to the end of their assumed working years (age ≤65) was multiplied by the average annual wage and the percentage of patients employed. The additional parameters incorporated for the societal perspective are displayed in Table 3.
Table 3.
Societal Perspective Inputs
| Model Parameter | Value | Source |
|---|---|---|
| Employment inputs | ||
| Employment rate | 58% | Bureau of Labor Statistics [40] |
| Average hourly wage | $28 | |
| Average annual wage | $58,260 | |
| Hospital days–initial transplantation and acute GVHD | ||
| From day 0 to day 100 | ||
| Allo-HCT | 26 | Yu et al. 2020 [22] |
| Incremental LOS due to acute GVHD | 11 | |
| From day 101 to1 year | ||
| Allo-HCT | 2 | |
| Incremental LOS due to acute GVHD | 11 | |
| Chronic GVHD | ||
| Percentage of chronic GVHD patients unable to return to the workforce | 25% | Jones et al. 2016 [41] |
All values are rounded to the nearest whole number.
Improving GRFS outcomes (via the medical cost offsets, QALYs gained, and indirect costs offsets) was associated with a net monetary savings ranging from $553,025 to $920,696. Of the estimated net monetary value, 87% to 90% was driven by direct medical cost savings, and the remaining 10% to 13% was due to value of the gain in expected QALYs and indirect costs. Disaggregated incremental cost savings showing the contribution of the cost categories are presented in Figure 2C,D.
Sensitivity Analyses
One-way sensitivity analyses were conducted to identify the key drivers of the modeled outcomes, using available measures of parameter uncertainty (ie, standard errors) or reasonable ranges (±25% when confidence intervals were not present). This was presented as a change from the base case total cost of allo-HCT, as well as the change from the base-case total expected QALYS. Sensitivity analysis results from the base case healthcare system perspective are presented as tornado diagrams (Figures 3 and 4). The analyses showed that changes in the chronic GVHD occurrence rate and the percentage who remained on chronic GVHD after 2 years had the greatest impact on the estimated total medical costs. The expected QALYs ranged from 4.16 to 5.41 (versus the base case total of 4.7) under variations tested in the sensitivity analyses.
Figure 3.
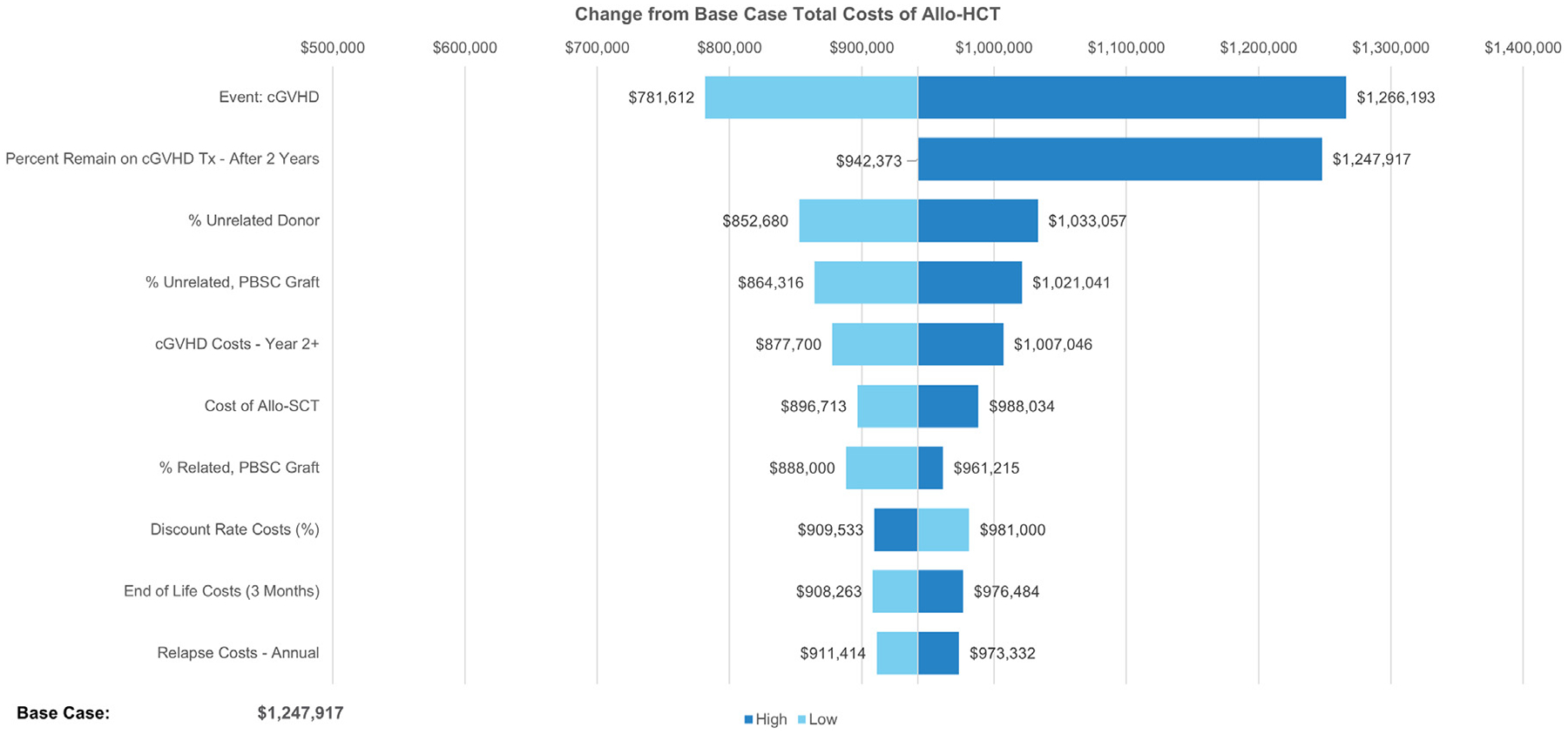
Deterministic sensitivity analysis on the impact on estimated total lifetime medical costs versus base case. cGVHD, chronic graft-versus-host disease; PBSCs, peripheral blood stem cells.
Figure 4.
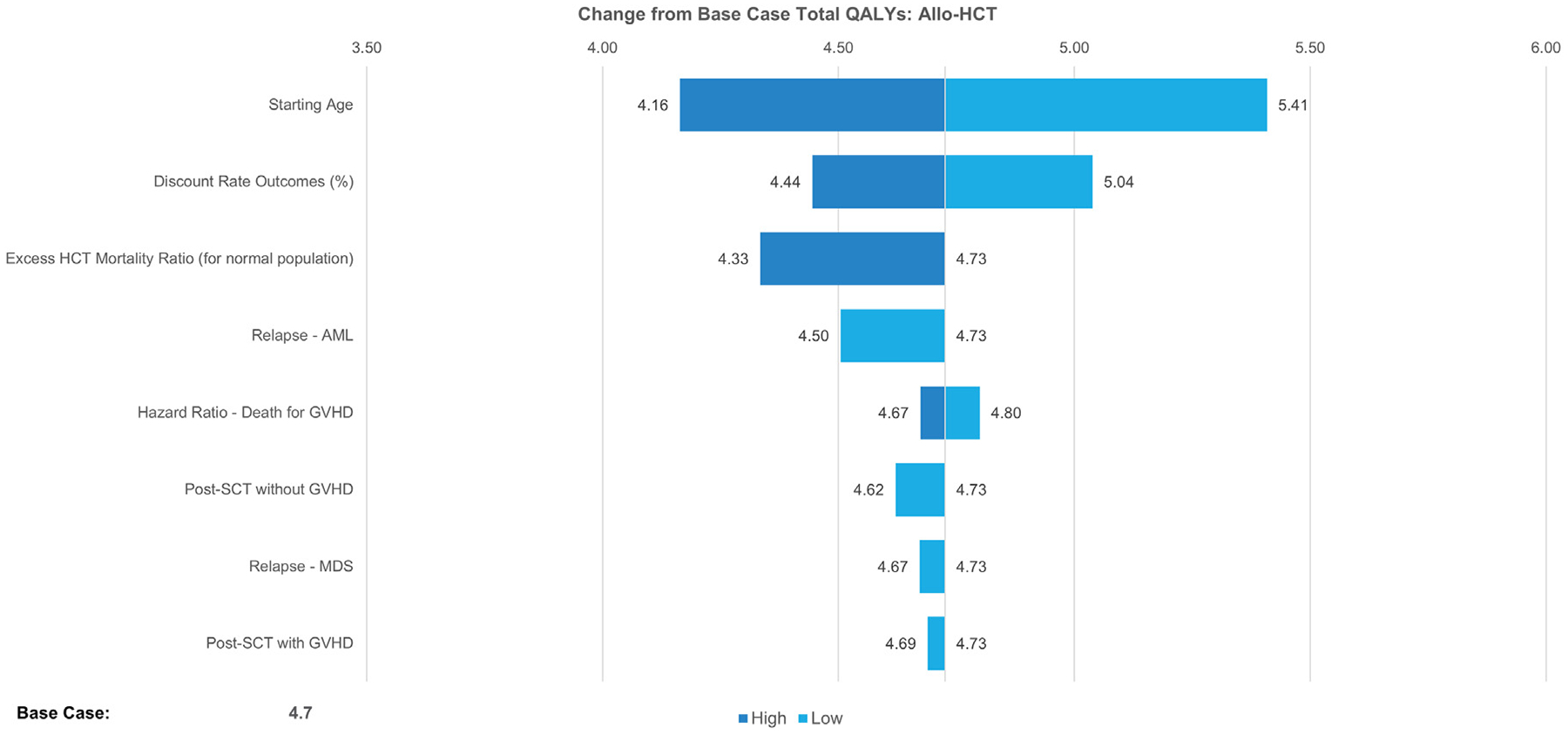
Deterministic sensitivity analysis of the impact on estimated total QALYs versus the base case.
DISCUSSION
Allo-HCT can be a lifesaving and curative procedure, but it does carry associated risks of morbidity and mortality with often significant costs to the healthcare system. To better understand this latter issue, we have developed a long-term analysis of transplantation and its economic impact. One benefit of this analysis is that it attempts to bridge the gap between understanding the short-term outcomes associated with allo-HCT with the long-term consequences that these patients continue to endure throughout life. The costs of allo-HCT have been reported previously [9,10]; however, current studies often describe the outcomes and costs associated with allo-HCT in the near term, and evidence on the long-term outcomes and, more specifically, the lifetime economic burden post-allo-HCT remains limited and unexamined. The advantage of the current analysis is the focus on a comprehensive outlook of allo-HCT outcomes and costs well beyond the standard 1- to 2-year horizon. Patients who survive for at least 5 years after transplantation without recurrence of the primary disease have a high probability of surviving for an additional 15 years, but their life expectancy is often not normalized [6]. To realistically measure such an effort with real-world evidence alone may not be possible given the time horizon to follow patients, the diversity in patient types, and the lag in obtaining current evidence reflecting today’s treatment patterns. In the absence of long-term clinical and economic data, this model attempts to estimate the lifetime economic burden of these allo-HCT patients to fully appreciate the loss in overall health that can be experienced after transplantation.
An additional benefit of this analysis is that it helps identify the inputs that drive the uncertainty in the modeled estimates and further highlights the key drivers that affect the lifetime costs for an allo-HCT patient. Both the base case results and sensitivity analyses demonstrated that GVHD, specifically chronic GVHD, had a substantial impact on the overall economic burden. This observation is associated with a proportion of patients with chronic GVHD that require ongoing lifelong treatment. Most chronic GVHD patients are not adequately managed with first-line corticosteroids, and more than 50% of these patients cycle through several therapies, often with escalating costs the further through lines of therapy they progress [19]. The best path forward, and one that could potentially provide the healthcare system the greatest value, is to prevent or greatly reduce these chronic GVHD occurrences, as illustrated in the net monetary benefit analyses conducted in this study.
This economic model has a number of limitations that should be considered when interpreting the results. First, the 15-year allo-HCT outcomes used to inform the trend after the first 3 years post-HCT consisted of a cohort of recipients from the United States during 2000 to 2005. These data may not fully be representative of outcomes observed today as diagnostic tools, treatment, and care continue to evolve and improve. Given that the initial years were still based on more recent data from 2016 to 2019, we hope that this shortcoming is somewhat mitigated while still acknowledging that the limitation is noteworthy. Additionally, the cost inputs in this analysis were obtained from published claims analyses and reflect averages of various populations, which can introduce heterogeneity into the model. Although most of the inputs came from separate publications, all were US-based, and all payments were converted back into healthcare costs prior to incorporation into the model. This allowed for the inputs to remain consistent with the intended perspective and relevant to the modeled population.
It is also important to note that off-label maintenance therapy for FLT3-ITD+ and Ph+ patients is not yet broadly adopted in clinical practice so not all FLT3-ITD+ and Ph+ patients may receive this kind of treatment or receive the treatment for the proposed duration that was modeled. Incorporating the costs of such treatment may result in an overestimation of the total lifetime costs of allo-HCT. Also, infection rates were only available up to 100 days post allo-HCT from the CIBMTR. Nonetheless, we acknowledge that there is an increased risk of infection among patients being treated for chronic GVHD in which it is assumed that the increased cost from chronic GVHD treatment also would capture any additional infection costs that are a result of immunosuppression.
Furthermore, the claims analysis used to inform chronic GVHD management costs was based on a time when ibrutinib, ruxolitinib, and belumosudil utilization was relatively low or absent [19]. The use of these high-cost therapies has been increasing (eg, 1 month of ruxolitinib 5 mg tablets twice daily costs $16,686 [29]), and other new emerging high-cost chronic GVHD treatments will likely come onto the market; therefore, the current modeled analysis potentially may be underestimating the true cost of chronic GVHD and thus the overall lifetime cost of an allo-HCT patient and the value that improved treatments may provide. In the societal perspective scenario analysis, we assumed that the amount of time missed from work was equal to the total number of days in which inpatient care was provided; however, we acknowledge that this may still underestimate productivity losses, as many patients may not return to work for 6 months or even up to a year post allo-HCT. Furthermore, the analysis did not contain other relevant societal costs, such as caregiver burden, transportation costs, government spending/work disability, and others, that may further exacerbate the economic burden of allo-HCT.
This analysis attempted to capture the total lifetime medical costs associated with an allo-HCT and to highlight where key areas of research and innovation could potentially focus to provide the greatest value in improved patient outcomes. Results of the scenario analyses suggest that there is great value if future treatments can be developed that improve outcomes and reduce complications currently associated with standard allo-HCT. Such an intervention would impact all patients, in which it will be important to ensure that the treatment can offset expenditures, recognizing that it might not reduce the overall cost of acquisition itself. This is why it is important to ensure that new interventions also can bring other forms of value by improving outcomes and increasing the quality and the overall quantity of a patient’s life. The current per-patient cost of allo-HCT carries a substantial medical cost burden, often exceeding $1M over a patient’s lifetime. Innovative research efforts focused on disease control and reduction or elimination of late complications, particularly chronic GVHD, may provide the greatest value to improving patient outcomes.
ACKNOWLEDGMENTS
Financial disclosure:
This study was funded by Orca Bio. All authors contributed to and approved the manuscript. The results presented were obtained using data from the Center for International Blood and Marrow Transplant Research. The analysis plan, results, and interpretation were not reviewed or approved by Statistical or Scientific Committees of the CIBMTR, and the CIBMTR cannot confirm their accuracy. M-A.P. also received support from National Cancer Institute Cancer Center Support Grant P30CA008748.
Conflict of interest statement:
R.T.M. has acted as a consultant and/or adviser for Artiva Therapeutics, Bristol-Myers Squibb/Celgene, CRISPR Therapeutics, Incyte, Kite, and Novartis and has received research funding from BMS and Novartis. He has served on data safety and monitoring boards for Novartis, Athersys, Vor Pharmaceuticals, and Century Therapeutics. S.D. has acted as a consultant and/or adviser for Orca Bio with payments received through the National Marrow Donor Program. L.P.G. has acted as a consultant and/or adviser for BluePath Solutions. I.A. is an employee and shareholder of Orca Bio. J.B. and M.G. are employees of BluePath Solutions, which received consulting fees from Orca Bio for conducting this analysis. M-A.P. reports honoraria from Adicet, Allogene, Allo-Vir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on data safety and monitoring boards for Cidara Therapeutics, Medigene, and Sellas Life Sciences and on the scientific advisory board of NexImmune. He has ownership interests in NexImmune, Omeros, and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis.
Footnotes
Presented in part as a poster at American Society of Hematology (ASH) 2022, New Orleans, Louisiana, 10–13 December 2022
Financial disclosure: See Acknowledgments on page 637.e8.
REFERENCES
- 1.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auletta JJ, Kou J, Chen M, et al. Real-world data showing trends and outcomes by race and ethnicity in allogeneic hematopoietic cell transplantation: a report from the Center for International Blood and Marrow Transplant Research. Transplant Cell Ther. 2023;29. 346.e1–346.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021.
- 4.Phelan R, Chen M, Bupp C, et al. Updated trends in hematopoietic cell transplantation in the United States with an additional focus on adolescent and young adult transplantation activity and outcomes. Transplant Cell Ther. 2022;28. 409.e1–409.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PJ, Counts GW Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yalniz FF, Murad MH, Lee SJ, et al. Steroid refractory chronic graft-versus-host disease: cost-effectiveness analysis. Biol Blood Marrow Transplant. 2018;24:1920–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Stranges E, Russo A, Friedman B. Statistical brief #82: Procedures with the most rapidly increasing hospital costs, 2004–2007. December 2009. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb82.jsp. Accessed September 4, 2017.
- 9.Bentley TS, Ortner N. 2020 U.S. organ and tissue transplants: cost estimates, discussion, and emerging issues February 18, 2020. Available at: https://www.milliman.com/en/insight/2020-us-organ-and-tissue-transplants. Accessed August 25, 2022.
- 10.Perales MA, Bonafede M, Cai Q, et al. Real-world economic burden associated with transplantation-related complications. Biol Blood Marrow Transplant. 2017;23:1788–1794. [DOI] [PubMed] [Google Scholar]
- 11.eds. In: Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, eds. Cost Effectiveness in Health and Medicine. 2nd ed. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 12.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104: 3501–3506. [DOI] [PubMed] [Google Scholar]
- 13.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofe-til for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for International Blood and Marrow Transplant (CIBMTR). Total cost of allogeneic stem cell transplant: effectiveness and value. Prepared for Orca. Revised March 11, 2022. [Google Scholar]
- 15.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeg RT, Moroz A, Gandhi A, et al. Orca-T results in high Gvhd-free and relapse-free survival following myeloablative conditioning for hematological malignancies: results of a single center phase 2 and a multicenter phase 1b study. Blood. 2021;138(suppl 1):98. [Google Scholar]
- 17.Arias E, Bastian B, Xu JQ, Tejada-Vera B. U.S. state life tables, 2018. National Vital Statistics Reports: vol. 70 no 1 Hyattsville, MD: National Center for Health Statistics; 2021. [PubMed] [Google Scholar]
- 18.Veltri L, Regier M, Cumpston A, et al. Incidence and pattern of graft-versus-host disease in patients undergoing allogeneic transplantation after nonmyeloablative conditioning with total lymphoid irradiation and antithymocyte globulin. Bone Marrow Res. 2013;2013: 414959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachier CR, Aggarwal SK, Hennegan K, et al. Epidemiology and treatment of chronic graft-versus-host disease post-allogeneic hematopoietic cell transplantation: a US claims analysis. Transplant Cell Ther. 2021;27. 504. e1–504.e6. [DOI] [PubMed] [Google Scholar]
- 20.American Hospital Association. Trendwatch Chartbook 2020. Appendix. Available at: https://www.aha.org/system/files/media/file/2020/10/TrendwatchChartbook-2020-Appendix.pdf. Accessed February 28, 2022.
- 21.Grubb WW, Huse S, Alam N, et al. Economic burden of acute graft-versus-host disease (GvHD) following allogeneic hematopoietic cell transplant (HCT) for hematologic malignancies. Blood. 2016;128:1187. [Google Scholar]
- 22.Yu J, Lal L, Anderson A, DuCharme M, Parasuraman S, Weisdorf D. Healthcare resource utilization and costs associated with acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Support Care Cancer. 2020;28:5491–5499. [DOI] [PubMed] [Google Scholar]
- 23.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with acute myeloid leukemia (AML) in the USA: a retrospective analysis of a commercial payer database. Adv Ther. 2019;36:1922–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godara A, Siddiqui NS, Munigala S, et al. Length of stay and hospital costs for patients undergoing allogeneic stem-cell transplantation. JCO Oncol Pract. 2021;17:e355–e368. [DOI] [PubMed] [Google Scholar]
- 26.Schuster MG, Cleveland AA, Dubberke ER, et al. Infections in hematopoietic cell transplant recipients: results from the Organ Transplant Infection Project, a multicenter, prospective, cohort study. Open Forum Infect Dis. 2017;4:ofx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J, Maziarz RT. Post-allogeneic stem cell transplant FLT3-targeted maintenance therapy: updates and considerations for clinical practice. Arch Stem Cell Ther. 2022;3:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu-Dumlao T, Kantarjian H, Thomas DA, O’Brien S, Ravandi F. Philadel phia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep. 2012;14:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IBM Micromedex® REDBOOK®. IBM Watson Health, an IBM Company; 2022. Available at: https://www.ibm.com/products/micromedex-red-book. Accessed June 23, 2022. [Google Scholar]
- 30.NEXAVAR® [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2020. [Google Scholar]
- 31.GLEEVEC® [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 32.Mau LW, Preussler JM, Burns LJ, et al. Healthcare costs of treating privately insured patients with acute myeloid leukemia in the United States from 2004 to 2014: a generalized additive modeling approach. Pharmacoeconomics. 2020;38:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scitovsky AA. “The high cost of dying”: what do the data show? 1984. Mil-bank Q. 2005;83:825–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Bureau of Labor Statistics. Consumer Price Index for all urban consumers (CPI-U): US city average, Medical care. 2021. Available at: https://www.bls.gov/bls/news-release/cpi.htm. Accessed February 28, 2022.
- 35.Perić Z, Desnica L, Duraković N, et al. Which questionnaires should we use to evaluate quality of life in patients with chronic graft-vs-host disease? Croat Med J. 2016;57:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsythe A, Brandt PS, Dolph M, Patel S, Rabe APJ, Tremblay G. Systematic review of health state utility values for acute myeloid leukemia. Clinicoe-con Outcomes Res. 2018;10:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ollendorf DA, Chapman R, Pearson SD, Institute for Clinical and Economic Review. Assessing the effectiveness and value of drugs for rare conditions: a technical brief for the ICER Orphan Drug Assessment & Pricing Summit May 2017. Available at: https://icer.org/wp-content/uploads/2020/10/ICER_Assessing-the-Value-of-Drugs-for-Rare-Conditions_051017-1.pdf. Accessed November 30, 2022.
- 40.US Bureau of Labor Statistics. Occupational employment and wage statistics (OEWS). Vol. 2022; 2021. [Google Scholar]
- 41.Jones CA, Fernandez LP, Weimersheimer P, et al. Estimating the burden of cost in chronic graft-versus-host disease: a human capital approach. J Health Econ Outcomes Res. 2016;4:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan F, Peng S, Fleurence R, Linnehan JE, Knopf K, Kim E. Economic analysis of decitabine versus best supportive care in the treatment of intermediate- and high-risk myelodysplastic syndromes from a US payer perspective. Clin Ther. 2010;32:2444–2456. [DOI] [PubMed] [Google Scholar]
- 43.Delea TE, Amdahl J, Boyko D, et al. Cost-effectiveness of blinatumomab versus salvage chemotherapy in relapsed or refractory Philadelphia-chromosome-negative B-precursor acute lymphoblastic leukemia from a US payer perspective. J Med Econ. 2017;20:911–922. [DOI] [PubMed] [Google Scholar]


