Abstract
Background
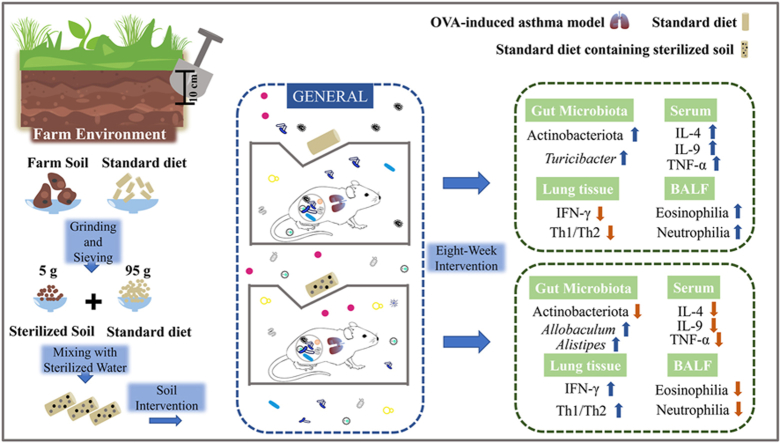
A low-clean living environment (LCLE) can increase gut microbial diversity and prevent allergic diseases, whereas gut microbial dysbiosis is closely related to the pathogenesis of asthma. Our previous studies suggested that soil in the LCLE is a key factor in shaping intestinal microbiota. We aimed to explore whether sterilized soil intake as a prebiotic while being incubated with microbes in the air can attenuate mouse asthma inflammation by modifying gut microbiota.
Methods
16S rRNA gene sequencing was used to analyze the gut microbial composition, in combination with immune parameters measured in the lung and serum samples.
Results
16S rRNA gene sequencing results showed significant differences in the fecal microbiota composition between the test and control mice, with a higher abundance of Allobaculum, Alistipes, and Lachnospiraceae_UCG-001, which produce short-chain fatty acids and are beneficial for health in the test mice. Soil intake significantly downregulated the concentrations of IL-4 and IL-9 in serum and increased the expression of IFN-γ, which regulated the Th1/Th2 balance in the lung by polarizing the immune system toward Th1, alleviating ovalbumin-induced asthma inflammation. The effect of sensitization on gut microbiota was greater than that of air microbes and age together but weaker than that of soil.
Conclusions
Soil intake effectively reduced the expression of inflammatory cytokines in asthmatic mice, possibly by promoting the growth of multiple beneficial bacteria. The results indicated that the development of soil-based prebiotic products might be used for allergic asthma management, and our study provides further evidence for the hygiene hypothesis.
Keywords: Gut microbiota, Soil, Prebiotic, Asthma, Allergy, Prevention and control, Hygiene hypothesis
Graphical abstract
Introduction
Asthma is a common chronic respiratory inflammatory disease with symptoms including shortness of breath, chest tightness, and coughing. It affects over 300 million people worldwide, a number that is expected to increase to 400 million by 2025.1
Numerous epidemiological studies have shown that low-cleanliness living environment (LCLE) exposure can protect against asthma and atopic diseases.2,3,4 A previous study showed that mice housed in cages with soil, house dust, and decaying plant material had higher gut microbial diversity and lower serum immunoglobulin E (IgE) levels than specific pathogen-free (SPF) mice after administration of 2,4-dinitrofluorobenzene.5 Similarly, Ottman et al found that mice housed in LCLE altered gut microbial composition and alleviated Th2-type allergic responses.6 In addition, LCLE exposure resulted in faster gut microbiota maturation in weaned piglets.7 Furthermore, keeping antibiotic-treated mice in LCLE accelerated gut microbial recovery.8 Recently, human studies showed that direct contact with the varied microbial community of soil- and plant-based material stabilized human gut microbes, triggering a beneficial immune response in allergic diseases.9
Targeting the gut microbiota using probiotics,10,11 prebiotics,12 and fecal microbiota transplantation13,14 positively affects asthma. Due to the diverse composition of LCLE, it remains unclear which factors play a key role in its protective effect against asthma. Our previous study indicated that soil is a crucial factor in shaping intestinal microecology and that exposing mice to sterilized soil significantly changed the gut microbial composition.15 Furthermore, ingestion of sterilized soil, while inhaling microbes in the air, reduced serum IgE levels by increasing gut microbial diversity.16 However, it is unknown whether the intake of sterilized soil as a prebiotic can achieve a therapeutic effect against ovalbumin (OVA)-induced asthma by changing the intestinal microbiota.
To explore the therapeutic effect of oral sterilized soil prebiotics on asthma and its effects on intestinal microorganisms and immune function, we established OVA-induced mouse asthma models in an SPF animal room. These mice were transferred to the general animal room, where they could be continuously inoculated with the microorganisms in the air. Mice in the test group were fed 5% sterilized soil. After 8 weeks of soil intervention, 16S rRNA gene sequencing was used to analyze the intestinal microbiota composition. The effect of soil intake on the immune function of asthmatic mice was investigated using pathological section examinations, flow cytometry analyses, and reverse transcription-quantitative polymerase chain reaction (RT‒qPCR). As increasing evidence shows the bidirectional communication between the gut microbiota and systemic immunity along the gut–lung axis,17,18 we also further examined the differences in the intestinal microbiota composition between the steady-state and asthmatic mice.
Methods
Animals and asthma model
Adult male and female C57 Bl/6 mice with the same genetic background were purchased from B&K Universal (Shanghai, China) and housed in the SPF animal facility. All animal experiments were approved by the National Institute of Bioscience and the Advisory Committee on Animal Health Research (Approval number: 2019101522). Male mice (n = 23, 4 weeks old) were selected from the offspring and co-housed until 8 weeks of age. Then, they were randomly divided into P_Asthma (n = 15) and P_PBS (n = 8) groups. After completing asthma modeling, we randomly shuffled 15 asthmatic mice into Asthma (n = 7) and Soil (n = 8) groups to eliminate cage effects for subsequent experiments, while the control group mice (P_PBS) were re-labeled as the PBS group (n = 8). Detailed information regarding the mice and treatment is described in Table E1.
We employed the asthma modeling method proposed by Noora et al6 with appropriate modifications (Fig. E1). P_Asthma group mice were exposed to the murine asthma model protocol, receiving 2 intraperitoneal injections of 50 μg OVA emulsified in 2.25 mg of alum in a total volume of 100 μL of PBS on days 0 and 14, followed by a 5% aerosolized OVA challenge from days 21–27 for 40 min each time. P_PBS group mice received 2 intraperitoneal phosphate-buffered saline (PBS) injections and were challenged with PBS only. The above operations were conducted in the SPF animal room.
We compared the baseline gut microbiota levels after asthma modeling, and based on unweighted distance results, there were no differences between the Soil and Asthma groups mice (Fig. E2). Then, all mice were transferred to the general animal room. Asthma and PBS group mice were fed a standard diet, while Soil group mice were fed a standard diet containing 5% sterilized soil. All conditions, including gender, age, house temperature, and humidity, were equal for the 3 groups. After 8 weeks of soil intervention, Asthma and Soil group mice were challenged with 5% aerosolized OVA for 40 min, while PBS group mice were challenged with PBS. Feces, lung, serum, and bronchoalveolar lavage fluid (BALF) samples were collected the next day for subsequent experiments. The detailed timeline of the experimental design is shown in Fig. E3.
Fecal collection
All fecal collection procedures were performed on an ultra-clean bench in the animal room. The anus of mice was wiped with alcohol before sampling, after which mouse feces were collected into sterilized 1.5 mL centrifuge tubes as fast as possible. Specifically: lifted the mouse's tail to expose the anus, disinfected it with 70% alcohol, and waited for the mouse to defecate naturally. When the feces were about to be expelled, immediately positioned the sterilized 1.5 mL centrifuge tube close to the mouse's anus to collect them into the sterile tube. Each tube contained 1–2 pellets, which were then immediately transferred to a −80°C freezer for storage until analysis. During this process, any contaminated feces were discarded.
Soil and feed
The soil used in the experiment was obtained from the top 10 cm at Xiaozhuang farm. The area is rich in vegetation, including various plants such as shrubs, vines, and willows. Our previous soil analysis results indicate that there is no heavy metal contamination.16 The area has never been planted with crops, hence chemical substances such as herbicides or pesticides have not been used. Additionally, there are no chemical factories surrounding the farm; thus there is also no presence of organic pollutants. The collected soil was ground into powder, filtered, and then sterilized. Sterilization was performed twice by autoclaving at 121°C for 30 min. Standard feed for mice (Xietong Pharmaceutical Bioengineering Company) was ground into powder using a flour mill. Sterilized soil (5 g) and standard feed (95 g) were weighed and then mixed with sterilized water on a super-clean bench. See Table S3 for a detailed soil composition analysis.16
DNA extraction and sequencing
DNA sequencing was performed at Shanghai Lingen Biotechnology Co., Ltd. Fecal microbial DNA was extracted using a fecal DNA kit (Omega Bio-tek, Norcross, GA, USA). The V4–V5 region of the bacterial 16S ribosomal RNA gene was amplified using polymerase chain reaction (PCR) and 515F 5′-barcode-GTGCCAGCMGCCGCGG-3′ and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′ primers. PCR products were quantified using the QuantiFluor™-ST Blue Fluorescence Quantification System (Promega). After Illumina PE250 library construction, sequencing of PCR product libraries was performed on the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA). The paired-end (PE) reads obtained from sequencing were spliced into a sequence according to the overlapping relationship between them, while the sequence quality was controlled and filtered. Using the QIIME software package (V1.9.0 http://qiime.org/scripts/assign_taxonomy.html), sequences with similarities ≥97% were clustered into an operational taxonomic unit (OTU) and analyzed taxonomically for species.
Real-time quantitative PCR
Total RNA was extracted from mouse lung tissue using an RNA extraction kit according to the manufacturer's instructions (BioMiGA, China). All materials required for the experiment were treated with diethyl pyrocarbonate (DEPC) water to remove RNA enzymes (BioMiGA, China). cDNA was obtained using reverse transcription PCR (Yugong Biolabs, China). The reaction system was prepared according to the kit's requirements (Yugong Biolabs), and fluorescence quantitative analysis was performed using the ABI 7500 PCR system (Applied Biosystems). Conditions were as follows: 30 s at 95°C, 40 cycles of 10 s at 95°C, and 30 s at 60°C, followed by 15 s at 95°C, 60 s at 60°C, and 15 s at 95°C. The cytokine mRNA expression levels were calculated according to the 2−ΔΔCT formula. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control.
Flow cytometry
The LEGENDplex™ MU Th Panel kit (Biolegend) was used to detect serum cytokine concentrations using flow cytometry (FACS Calibur). Quantitative analyte analysis was performed using LEGENDplex v8.0 software.
Results
Soil intake shapes the gut microbial composition and promotes beneficial bacterial growth
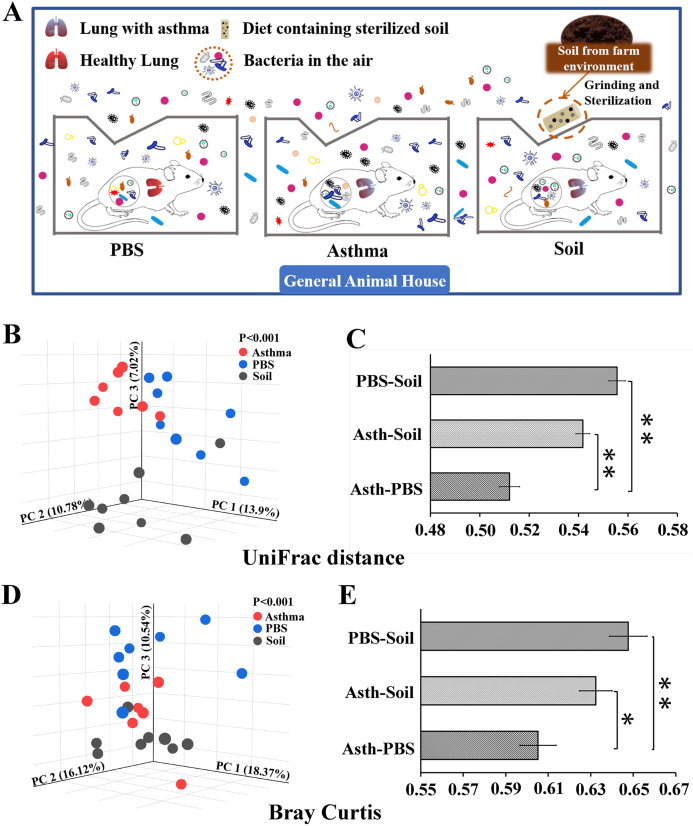
Mice were transferred to the general animal room, where they were incubated with air microorganisms (Fig. 1A). Soil group mice were simultaneously fed 5% sterilized soil. After the 8-week soil treatment, feces were collected for 16S rRNA gene sequencing.
Fig. 1.
Schematic diagram of experimental treatments and gut microbial beta diversity.
A. Soil treatment mode for asthmatic mice: after completing the asthma model in the SPF animal room, all 3 groups of mice were transferred to the general animal room with microbes in the air. Sterilized soil (5%) was added to the feed of the Soil group. B. PCoA of unweighted UniFrac distance. C. Each bar represents the mean ± SEM. D. PCoA of Bray‒Curtis distance. E. Each bar represents the mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. According to the nonparametric Kruskal‒Wallis test with Bonferroni post hoc and one-way ANOVA. n = 7–8.
Principal coordinates analysis (PCoA) of the unweighted UniFrac distance showed that the gut microbial composition differed significantly among the 3 groups and that soil intervention contributed to the unique microbial structure and composition (Fig. 1B). The distance between the Soil group and Asthma group or PBS group was significantly greater than that between the PBS and Asthma groups (Fig. 1C and Table E2), suggesting that the similarity between the Asthma and PBS groups was the highest, while the difference between the intestinal microorganisms in the Soil group and Asthma and PBS groups was more pronounced, indicating that soil significantly affected the gut microbial composition. Furthermore, the PCoA of the Bray–Curtis distance was similar to that of the unweighted UniFrac distance (Fig. 1D and E).
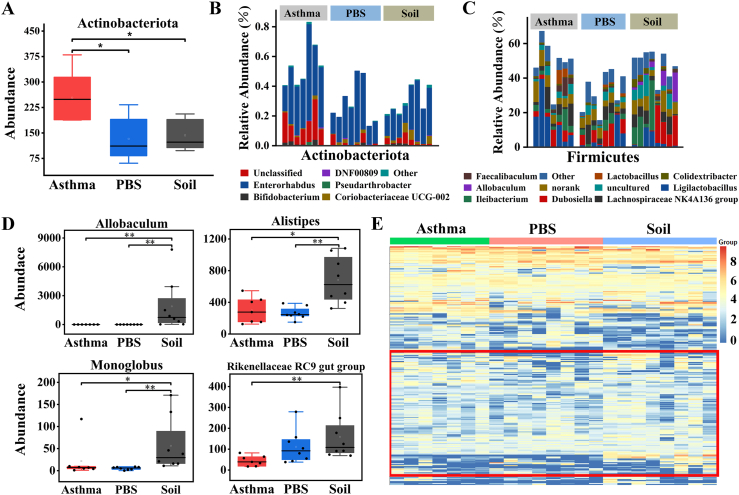
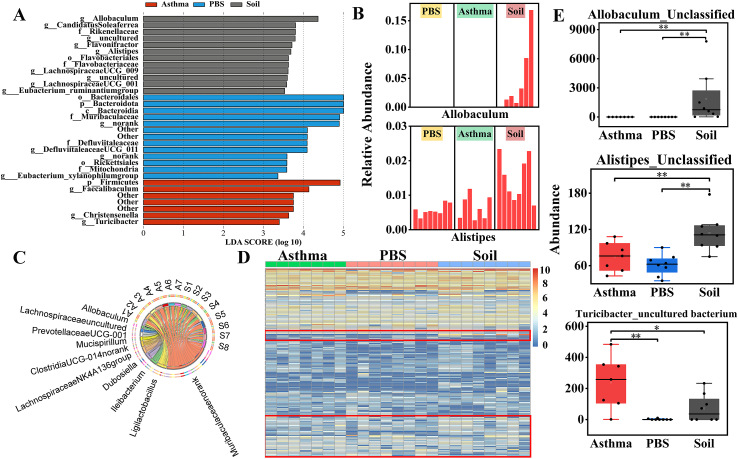
At the phylum level, the gut microbial communities of all 3 groups of mice were dominated by Bacteroidetes and Firmicutes (Fig. E4B and Table E3), and the Bacteroidetes/Firmicutes ratio of the Soil and Asthma groups was significantly lower than that of the PBS group, but no significant differences were found between the Asthma and Soil groups (Fig. E4C). Compared to the Asthma group, the abundance of Actinobacteria was significantly lower in both the PBS and Soil groups (Fig. 2A). Significant changes were found in the genus composition of Actinobacteria (Fig. 2B), Firmicutes (Fig. 2C), and Bacteroidetes (Fig. E4D). Soil group mice showed a significant change in the percentage of genus composition with a higher abundance of Allobaculum, Alistipes, Monoglobus, Rikenellaceae_RC9_gut_group, and Christensenellaceae R-7 group. (Fig. 2D and E4E and Table E4). The heatmap of the top 250 OTUs revealed that soil intake had a significant effect on OTUs in asthmatic mice and that it promoted the growth of some bacterial OTUs (Fig. 2E).
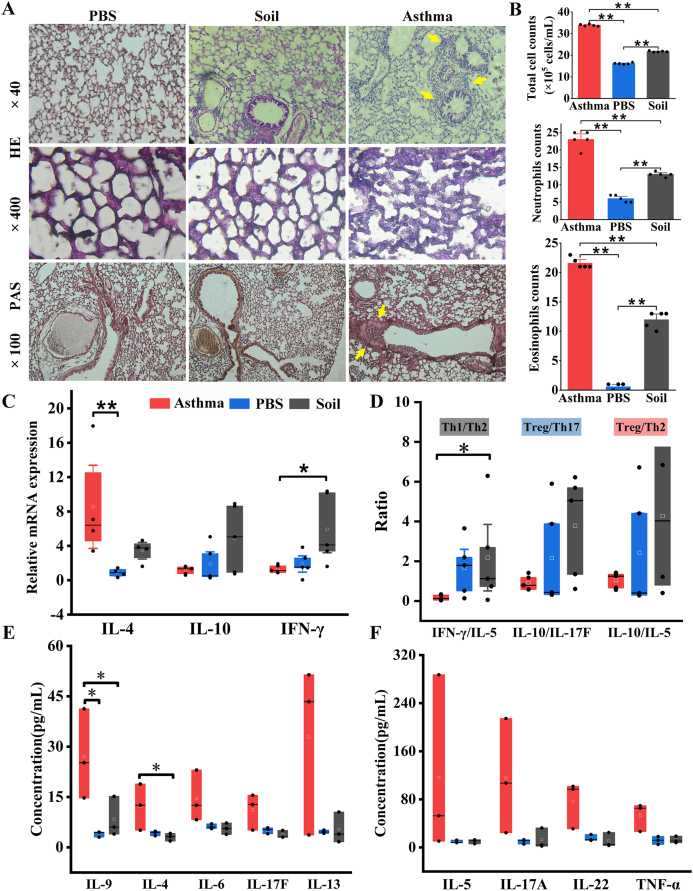
Fig. 4.
Soil intake attenuated allergic asthma inflammation and decreased levels of asthma-related markers.
A. Representative micrographs of HE- and PAS-stained lung tissue. Glycogen and other PAS-positive substances in the tissue were conspicuously red in PAS. B. Numbers of total cells, eosinophils, and neutrophils in BALF. A total of 200 cells were counted per slide when counting eosinophils and neutrophils. C, D. Concentrations of TNF-α, IL-22, IL-6, IL-4 and IL-17F in serum. E. Lung mRNA levels of IL-4, IL-10, and IFN-γ. F. Effects of soil ingestion on the balance of Th1/Th2, Treg/Th17, and Treg/Th2. ∗P < 0.05, ∗∗P < 0.01. One-way ANOVA and the non-parametric Kruskal-Wallis test with Bonferroni post hoc were used. n = 7–8
Fig. 2.
Comparison of intestinal microbial composition and structure.
A. Actinobacteriota abundance. B, C. Detailed relative abundance of bacterial genera in the phyla Actinobacteriota and Firmicutes. D. Abundance of bacterial genera. E. Heatmap of the impact of sterilized soil intake on OTUs at a 97% threshold. ∗P < 0.05, ∗∗P < 0.01. According to the non-parametric test of Kruskal-Wallis with Bonferroni correction. n = 7–8.
Linear discriminant analysis effect size (LEfSe) analysis was performed to identify the most differentially abundant taxa between Asthma and Soil groups. LEfSe analysis showed a significant increase in the phylum Actinobacteria in the Asthma group compared to that in the Soil group. Furthermore, Allobaculum, Alistipes (Fig. 3A and B), Lachnospiraceae UCG-001, and Flavonifractor were significantly enriched in the Soil group, while Turicibacter (Fig. E4F) dominated in the Asthma group mice. To reveal the relationship between samples and bacterial abundance, Circos analysis was performed. The Circos plot demonstrated that Lachnospiraceae_NK4A136_group, lleibacterium, and Allobaculum were more abundant in the Soil group, while the association with Ligilactobacillus appeared to be clearer in the Asthma group (Fig. 3C). Interestingly, growth-promoting bacteria with soil, including Allobaculum, Alistipes, Lachnospiraceae_UCG-001, Rikenellaceae_RC9_gut_group, and Christensenellaceae_R-7_group, could produce short-chain fatty acids (SCFAs),19, 20, 21, 22, 23 belonging to beneficial bacteria in the intestine, while Turicibacter, whose growth is inhibited, was previously shown to be a conditionally pathogenic bacterium24 and the core genus in an house-dust-mite (HDM)-induced asthma model.25
Fig. 3.
Soil intake effects on OTUs, genera, and species in asthmatic mice.
A. The most differentially abundant taxa identified by LEfSe in the 3 groups at the genus level. Soil-enriched taxa are labeled in grey, and taxa enriched in the Asthma group are labeled in red. Only taxa meeting an LDA significance threshold of >3.0 are shown. B. Relative abundance of biomarkers identified by LFfSe in mice of each group. C. Circos plot. The left semi-circle is the distribution of the more dominant bacterial genus in each sample, and each bacterial genus was assigned a specific color. The right semi-circle represents different samples. S: Soil; A: Asthma. D. Heatmap of the impact of sterilized soil intake at the species level. E. Differential species among the 3 groups. ∗P < 0.05, ∗∗P < 0.01. According to the non-parametric Kruskal-Wallis test with Bonferroni post hoc. n = 7–8
Heatmap analysis at the species level revealed that the species composition of the intestinal bacterial community changed after soil intake (Fig. 3D). Specifically, soil ingestion boosted the growth of Allobaculum_Unclassified, Alistipes_Unclassified, Rikenellaceae RC9 gut group_unculutured bacterium, and Romboustia_unculutured bacterium, whereas the abundance of [Eubacterium] xylanophilum group_uncultured bacterium, Ligilactobacillus_Unclassified, and Atopobiaceae_Unclassified decreased in the Soil group (Fig. 3E–E4G and Table E5).
Hence, soil intake significantly altered the mouse gut microbial structure and composition and promoted the growth of various beneficial bacteria. OVA sensitization also significantly affected the mouse intestinal microbial composition, but the effect was not as strong as that of soil intervention.
Soil intake attenuated lung inflammation and alleviated Th2-type allergic responses
To study the therapeutic effect of soil intake on OVA-induced lung inflammation, we examined the immune status of the lung, BALF, and serum. Hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) staining results showed that the alveolar structure was intact in the PBS group, with no evident inflammatory cell infiltration or mucus in the lung. In contrast, Asthma group mice developed severe inflammation with more inflammatory cell infiltration and lung tissue mucus, while soil ingestion significantly reduced the numbers of total cells, eosinophils, and neutrophils, and lung inflammation was improved in the Soil group (Fig. 4A and B).
To further explore the underlying mechanisms, the gene expression levels of asthma-related cytokines in the lung were investigated. Soil intake upregulated IFN-γ and downregulated IL-4 in the lung, which altered the Th1/Th2 balance and skewed the immune system toward a higher level of anti-inflammatory signaling, attenuating OVA-induced allergic asthma responses (Fig. 4C and D and Table E6). IL-10 levels in the lung were relatively higher than those of IL-17F and IL-5 in the Soil group compared with the PBS and Asthma groups, although this difference was not significant (Fig. 4D and Table E6). Furthermore, we examined the changes in serum immune parameters. Soil intake significantly decreased the serum concentrations of IL-4 and IL-9, which can elevate the asthma exacerbation risk (Fig. 4E). We also observed a decrease in the concentrations of other asthma-related inflammatory cytokines, including IL-6, IL-17F, IL-13, IL-5, IL-17A, IL-22, and TNF-α, in the serum after soil intervention (Fig. 4E and F). However, probably due to the small sample size, this difference was not statistically significant.
Thus, soil intervention effectively reduces asthma pro-inflammatory cytokine expression levels, alleviating asthma inflammation. A reduced Th2-type allergic response was one of the underlying mechanisms. In contrast, no significant effect on asthma was found with simple air microorganism exposure in the general animal rooms.
Influence of OVA sensitization, air microbes, and age on the gut microbial composition
The “gut-lung axis” states that sensitization processes contribute to changes in intestinal microorganisms and that gut microbial dysbiosis can in turn aggravate lung inflammation.26 To investigate the effect of OVA sensitization on intestinal microbial composition in mice, we analyzed fecal microbiota. PCoA based on the unweighted UniFrac distance showed obvious differences between the P_Asthma and P_PBS groups (Fig. 5A). Furthermore, nonmetric multidimensional scaling analysis was similar to that of the unweighted UniFrac distance matrix (Fig. 5B).
Fig. 5.
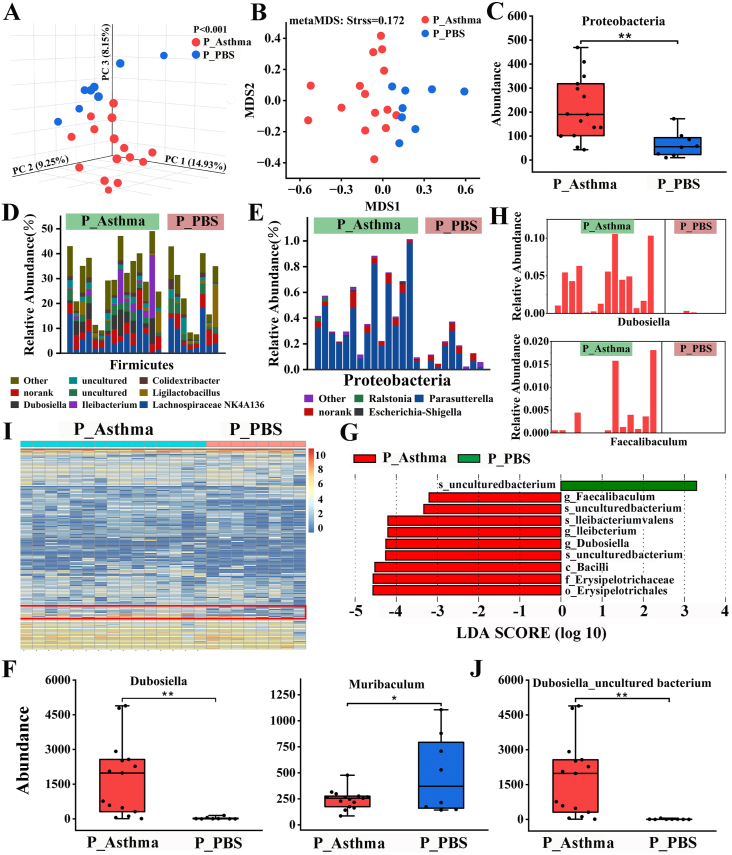
Sensitization altered gut microbial composition.
A. PCoA of unweighted UniFrac distance. B. Nonmetric multidimensional scaling of Bray–Curtis distance. C. The abundance of the fecal bacterial phylum Proteobacteria. D, E. Detailed relative abundance of bacterial genera in the phyla Firmicutes and Proteobacteria. F. Differential genera. G. LEfSe analysis for characteristic microbial genera in the P_Asthma and P_PBS groups with an LDA score >3.2. H. Relative abundance of the most differentially abundant genera identified by LFfSe. I. Heatmap at the species level. J. Differential species. ∗P < 0.05, ∗∗P < 0.01. According to the two-tailed least significant difference test. n = 8–15.
OVA sensitization changed the microbial communities at the phylum level, with a lower abundance of Proteobacteria (Fig. 5C and Table E7). The gut microbial communities of both P_Asthma and P_PBS mice were dominated by Bacteroidetes and Firmicutes (Fig. E5a), and sensitization did not change the Bacteroidetes/Firmicutes ratio (Fig. E5B). Detailed genera compositions of Firmicutes (Fig. 5D), Proteobacteria (Fig. 5E), and Bacteroidata (Fig. E5C) are shown. At the genus level, a higher abundance of Dubosiell, Parasutterell, Rikenella, and Catenibacterium was observed in the P_Asthma group. In contrast, the abundance of Muribaculum, Staphylococcus, and Butyricicoccus decreased in asthmatic mice (Fig. 5F–E5D, and Table E8). LEfSe results identified 3 bacterial genera (Dubosiella, Ileibacterium, and Faecalibaculum) enriched in the P_Asthma group with a linear discriminant analysis (LDA) score >3.2 (Fig. 5G and H and Fig. E5F). A heatmap at the species level revealed that sensitization changed the species composition of the intestinal bacterial community (Fig. 5I), and sensitization promoted the growth of Dubosiella_uncultured bacterium, Burkholderiales bacterium YL45, Bacteroides_uncultured bacterium, and Catenibacterium_uncultured bacterium, while the abundance of Muribaculum_uncultured bacterium, Staphylococcus_Unclassified, and Butyricicoccus_uncultured bacterium decreased in asthmatic mice (Fig. 5J–E5E and Table E9). Thus, sensitization has a significant effect on gut microbial composition and structure.
Several reports indicate that farm environmental microbes4,27 and age28,29 affect the gut microbiota. We investigated these findings, and the PCoA results (Fig. E6A) showed a significant difference between the P-Asthma and Asthma groups and between the P-PBS and PBS groups. However, the effect of air microorganisms and age on the mouse gut microbiota was weaker than that of OVA sensitization (Fig. E6B) and largely weaker than that of soil (Fig. E7). The LEfSe results showed (Fig. E8) that for asthmatic mice, air microbes and age significantly increased the phylum Firmicutes and genera Ligilactobacillus and Faecalibaculum, while for normal mice, air microbes and age significantly increased the genera Dubosiella, Ileibacterium, and Lactobacillus (Fig. E9).
In conclusion, air microorganisms and age and sensitization had significant effects on mouse gut microbes, with sensitization having greater effects on mouse gut microbes than air microorganisms and age together, but all these effects were weaker than those of soil.
Discussion
We used OVA-induced asthma model mice to investigate the effects of sterilized soil intake, as a prebiotic, accompanied by air microorganism exposure on asthmatic mice. We found that soil prebiotics significantly altered the gut microbial composition and attenuated inflammation in asthmatic mice. Further studies revealed that this improvement in inflammation was achieved by reducing the serum concentrations of Th2-related cytokines and regulating the Th1/Th2 balance by enhancing anti-inflammatory signaling in the lung.
Soil intake decreased the expression level of inflammatory cytokines possibly by increasing the growth of a variety of beneficial commensal bacteria that are protective against allergies in the intestine, including Alistipes, Rikenellaceae_RC9_gut group, Allobaculum, Ileibacterium, and Christensenellaceae, and decreasing pathogenic bacteria abundance associated with asthma. Several studies have shown that a reduced abundance of Alistipes was observed in populations with asthma and food allergies30,31, and a higher abundance of Alistipes correlated with higher fecal and serum acetate and fecal butyrate levels.32 Similarly, the Rikenellaceae_RC9_gut group is positively correlated with butyric and valeric acid and can alleviate colitis symptoms by stimulating Treg cell differentiation.33,34 Lachnospiraceae UCG-00135 and Ileibacterium,36 which can produce SCFAs, have been reported to be beneficial against inflammation. Allobaculum was related to the increase in SCFA production, especially butyrate.19,20,37 Christensenellaceae is widespread in humans and animals, playing a key role in maintaining host health.38
The effect of soil ingestion on asthma-related pro-inflammatory cytokines is consistent with LCLE exposure. First, it can be seen from the HE staining and BALF that soil intake and keeping mice in an LCLE6 have similar protective effects against lung inflammation. Furthermore, asthma was prevented in mice housed in cages with soil with lower expression of IL-5 and IL-13 in the lung and higher expression of IFN-γ, altering the Th1/Th2 balance.6 Similarly, our results showed that the IFN-γ level was significantly higher in the lung, which altered the Th1/Th2 balance, skewing the immune system toward higher levels of anti-inflammatory signaling and thereby attenuating allergic responses. Exposure to forests, grasslands, climbing, and digging mud can increase the serum IL-10:IL-17A ratio among daycare children.39 Soil intake also affected the Treg/Th17 balance, and the expression of anti-inflammatory IL-10 relative to that of IL-5 in the lung was greater in the Soil group, which positively improved asthma symptoms, although the difference was not significant. González-Rodríguez et al found that exposure to dust derived from sterilized forest soil reduced the levels of IL-17F in serum and TNF-α in splenocytes.40 Similarly, in our study, reductions in the serum concentrations of IL-17F and TNF-α were observed after soil ingestion. A significant decrease in IL-9 and IL-4 was observed in serum after soil intervention. IL-9 can promote the proliferation and differentiation of mast cells and increase IgE production, while IL-4 can promote airway hyperresponsiveness, thereby exacerbating asthma symptoms.41,42 Furthermore, the concentrations of serum IL-6 and IL-22 were lower after soil intake. Increased serum IL-6 levels were observed in asthmatic patients and correlated with the risk of asthma exacerbation.43,44 IL-22, an important effector molecule produced by Th17 cells, can drive airway hyperresponsiveness in combination with IL-17.45 Although several pro-inflammatory cytokines did show a noticeable decreasing trend, the differences were not statistically significant, probably due to the small sample size. The reduction in the numbers of total cells, eosinophils, and neutrophils in BALF in the Soil group further supported the alleviation of asthma inflammation. Inflammatory cell infiltration in the lung was significantly reduced, the alveolar wall structure was more intact, and mucus secretion in the bronchi was considerably improved after soil intervention.
Although edible soil16 and LCLE exposure5,6 can effectively alleviate allergen-induced lung inflammation, edible soil has more advantages than LCLE treatment: (I) in contrast to LCLE, sterilized soil does not contain pathogens causing infections; (II) LCLE does not conform to the lifestyle of modern people; (III) taking soil as a prebiotic to treat asthma is more convenient; and (IV) edible soil is safe. Since prehistory, humans have taken mineral- and trace element-rich soil and used selected soils for medicinal purposes, usually for the treatment of gastrointestinal ailments.46 Some commercial medicines (e.g., kaopectate) contain soil components and are widely used to treat diarrhea, nausea, and other diseases. Moreover, soil contains a large number of natural clay minerals, such as montmorillonite and kaolinite,47 which are traditional Chinese medicines used to treat certain diseases. In addition, heat-killed bacteria48,49 and bacterial endotoxin50 can also effectively stimulate our immune response. Further study investigating the reasons for the immune changes caused by soil ingestion is warranted.
Soil ingestion altered the gut microbial composition in asthmatic mice, but no increase in intestinal microbial diversity or richness was found, which is inconsistent with the findings by Zhou et al.16 Presumably, this may be due to the influence of stress. In this experiment, mice were challenged with OVA in a confined space for 40 min to induce asthma symptoms, and our sampling time was 24 h post-challenge. The effects of stress on the gut microbiota have been previously reported51,52 and gut microbial diversity needs sufficient time to recover.8 Ottman et al did not observe changes in intestinal microbial diversity when the asthma model was established in mice raised in LCLE,6 consistent with our results.
It is unclear how soil supports the growth of a variety of beneficial commensal bacteria. Presumably, natural clay minerals that are widespread in soil, including montmorillonite, kaolinite, and illite, may play a key role in shaping the gut microbiota. Wavelength Dispersive X-Ray Fluorescence Spectrometer was used to analyze the soil composition we used in our previous study.16 The analysis results show that the main components in the soil are SiO2 and Al2O3, accounting for a high proportion of 72.07%. Some clay minerals, such as kaolinite, montmorillonite, and vermiculite, have SiO2 and Al2O3 as their main components.53 These clay minerals have a large specific surface area and cation exchange capacity per unit mass, so they can adsorb microbial cells and obtain nutrients (e.g., polysaccharides secreted by microorganisms) to interact with soil microorganisms to affect their growth and function and support biofilm formation,47 which is key to improving bacterial species colonization. Oral administration of montmorillonite can help probiotics, especially lactic acid bacteria (LABs), form biofilms on their surface and further promote the growth of LABs to enhance anti-tumor responses.54 The gut is a large reservoir inhabiting trillions of bacteria. Hence, we speculate that these microbes can interact extensively with clay minerals in the soil, and microorganisms in the intestine may form biofilms on the surface of these particles to modify the gut microbiota, which might be a potential mechanism of soil-induced changes in intestinal microbes. However, whether the specific cause of soil-induced changes in gut microbiota is attributed to clay minerals still requires further research for confirmation. In future studies, more refined research on soil, especially focusing on clay minerals, should be conducted to identify the key substances in soil shaping the gut microbiota.
In our study, both sensitization and air microorganisms and age had a significant effect on mouse intestinal microbes. Two possible factors affect intestinal microorganisms during sensitization: the sensitization-induced inflammatory state and stress responses. Both the inflammatory state55 and stress51 affect mouse gut microbes, consistent with our results. However, further experiments are needed to prove which factor is more influential. Furthermore, air microorganisms and age also significantly affected the intestinal microorganisms in mice. Both air microorganisms4 and age28 had an effect on gut microorganisms, but in this study, the effect of age may be smaller because the samples were collected twice in the adult stage, and the microbiota displays a stable homeostatic state in adulthood.56 Thus, air microorganisms may have a greater effect on mouse gut microorganisms than age, but their effects are significantly smaller than those of soil. Surprisingly, the effect of sensitization on gut microbes was greater than that of air microbes and age together.
Conclusion
Soil intake significantly reduced the expression of asthma-related proinflammatory cytokines in mouse models, which is associated with promoting the growth of gut-beneficial bacteria. The protective effects and mechanism of soil intake on asthma are consistent with those of LCLE, but edible soil is easier to implement in the clinic. The results indicated that the development of soil-based prebiotic products might be used for allergic asthma intervention.
Abbreviations
OVA, Ovalbumin; OUT, Operational taxonomic unit; PCoA, Principal coordinates analysis; LEfSe, Linear discriminant analysis effect size; SPF, Specific pathogen-free; BALf, Bronchoalveolar lavage fluid; PAS, Periodic acid-Schiff; PBS, phosphate-buffered saline; PCR, polymerase chain reaction (PCR); SCFA, Short-chain fatty acid; TNF-α, Tumour Necrosis Factor-alpha.
Funding
This work was supported by The Natural Science Foundation of China (grant no. 31770540), The Key Research Program of Jiangsu (grant no. BE2018663) and the Fundamental Research Funds for the Central Universities (grant nos. 2242021k30014 and 2242021k30059).
Availability of data and materials
The raw data of 16S rRNA gene sequences were deposited in the NCBI Sequence Read Archive (SRA) database (project number, PRJNA877056), and other relevant data supporting the findings are available within the supplementary file.
Author contributions
DZ (Corresponding Author): Conceptualization, Supervision, Funding Acquisition, Resources, Writing - Review & Editing, Project Administration. ML (First Author): Methodology, Experiments, Formal Analysis, Writing-Original Draft, Data Curation, Visualization, Writing - Review & Editing; NL and YD: Methodology, Formal Analysis; HZ, ZB, RZ, ZF, WZ, PX and XS: Methodology, Resources. All authors read and approved the final manuscript.
Ethics approval
All animal experiments were performed in strict accordance with the guidelines of the Ethical Committee on Animal Research of Southeast University, and this study obtained ethics approval from the National Institute of Bioscience and the Advisory Committee on Animal Health Research of Southeast University (Approval number: 2019101522).
Authors’ consent for publication
I confirm that each of the authors has reviewed this paper and approved submission for publication to the World Allergy Organization Journal.
Declaration of competing interest
The authors declare no competing financial or nonfinancial interests.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100897.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lin C.H. Treatment of hypertension in patients with asthma. N Engl J Med. 2019;381(23):2278–2279. doi: 10.1056/NEJMc1913646. [DOI] [PubMed] [Google Scholar]
- 2.Ege M.J., Mayer M., Normand A.C., et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 3.Stein M.M., Hrusch C.L., Gozdz J., et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375(5):411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depner M., Taft D.H., Kirjavainen P.V., et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med. 2020;26(11):1766–1775. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D., Zhang H., Bai Z., et al. Exposure to soil, house dust and decaying plants increases gut microbial diversity and decreases serum immunoglobulin E levels in BALB/c mice. Environ Microbiol. 2016;18(5):1326–1337. doi: 10.1111/1462-2920.12895. [DOI] [PubMed] [Google Scholar]
- 6.Ottman N., Ruokolainen L., Suomalainen A., et al. Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model. J Allergy Clin Immunol. 2019;143(3):1198–1206.e12. doi: 10.1016/j.jaci.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Vo N., Tsai T.C., Maxwell C., Carbonero F. Early exposure to agricultural soil accelerates the maturation of the early-life pig gut microbiota. Anaerobe. 2017;45:31–39. doi: 10.1016/j.anaerobe.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Li N., Zhang H., Bai Z., et al. Soil exposure accelerates recovery of the gut microbiota in antibiotic-treated mice. Environ Microbiol Rep. 2021;13(5):616–625. doi: 10.1111/1758-2229.12959. [DOI] [PubMed] [Google Scholar]
- 9.Nurminen N., Lin J., Grönroos M., et al. Nature-derived microbiota exposure as a novel immunomodulatory approach. Future Microbiol. 2018;13:737–744. doi: 10.2217/fmb-2017-0286. [DOI] [PubMed] [Google Scholar]
- 10.Forsythe P., Inman M.D., Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175(6):561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 11.Huang C.F., Chie W.C., Wang I.J. Efficacy of lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial. Nutrients. 2018;10(11) doi: 10.3390/nu10111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wawryk-Gawda E., Markut-Miotła E., Emeryk A. Postnatal probiotics administration does not prevent asthma in children, but using prebiotics or synbiotics may be the effective potential strategies to decrease the frequency of asthma in high-risk children - a meta-analysis of clinical trials. Allergol Immunopathol. 2021;49(4):4–14. doi: 10.15586/aei.v49i4.69. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Chen Y., Li Q., et al. Tetrahydrocurcumin alleviates allergic airway inflammation in asthmatic mice by modulating the gut microbiota. Food Funct. 2021;12(15):6830–6840. doi: 10.1039/d1fo00194a. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y., Cai Y. Future prospect of faecal microbiota transplantation as a potential therapy in asthma. Allergol Immunopathol. 2018;46(3):307–309. doi: 10.1016/j.aller.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D., Bai Z., Zhang H., Li N., Lu Z. Soil is a key factor influencing gut microbiota and its effect is comparable to that exerted by diet for mice. F1000 Res. 2018;7:1588. [Google Scholar]
- 16.Zhou D., Li N., Yang F., et al. Soil causes gut microbiota to flourish and total serum IgE levels to decrease in mice. Environ Microbiol. 2022 doi: 10.1111/1462-2920.15979. Published online March 22. [DOI] [PubMed] [Google Scholar]
- 17.Lathrop S.K., Bloom S.M., Rao S.M., et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grigg J.B., Sonnenberg G.F. Host-Microbiota interactions shape local and systemic inflammatory diseases. J Immunol. 2017;198(2):564–571. doi: 10.4049/jimmunol.1601621. Baltim. Md 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Zhao Y., Zhang M., et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M., Yang S., Wang S., et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo M., Li Z. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019;10(10):6873–6881. doi: 10.1039/c9fo00296k. [DOI] [PubMed] [Google Scholar]
- 22.Holman D.B., Gzyl K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol Ecol. 2019;95(6) doi: 10.1093/femsec/fiz072. [DOI] [PubMed] [Google Scholar]
- 23.López-Montoya P., Cerqueda-García D., Rodríguez-Flores M., et al. Association of gut microbiota with atherogenic dyslipidemia, and its impact on serum lipid levels after bariatric surgery. Nutrients. 2022;14(17):3545. doi: 10.3390/nu14173545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangadoo S., Dinev I., Chapman J., et al. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biotechnol. 2018;102(3):1455–1466. doi: 10.1007/s00253-017-8688-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu W., Lu W., Li L., et al. Both living and dead Faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J Sci Food Agric. 2021;101(13):5563–5573. doi: 10.1002/jsfa.11207. [DOI] [PubMed] [Google Scholar]
- 26.Penders J., Stobberingh E.E., van den Brandt P.A., Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62(11):1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 27.Shimojo N., Izuhara K. Old friends, microbes, and allergic diseases. Allergol Int Off J Jpn Soc Allergol. 2017;66(4):513–514. doi: 10.1016/j.alit.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Yatsunenko T., Rey F.E., Manary M.J., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 30.Zolnikova O.Y., Potskhverashvili N.D., Kudryavtseva A.V., et al. [Changes in gut microbiota with bronchial asthma] Ter Arkh. 2020;92(3):56–60. doi: 10.26442/00403660.2020.03.000554. [DOI] [PubMed] [Google Scholar]
- 31.Chen C.C., Chen K.J., Kong M.S., Chang H.J., Huang J.L. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2016;27(3):254–262. doi: 10.1111/pai.12522. [DOI] [PubMed] [Google Scholar]
- 32.Medawar E., Haange S.B., Rolle-Kampczyk U., et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl Psychiatry. 2021;11(1):500. doi: 10.1038/s41398-021-01620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qing Y., Xie H., Su C., et al. Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection. Dig Dis Sci. 2019;64(7):1830–1843. doi: 10.1007/s10620-018-5428-2. [DOI] [PubMed] [Google Scholar]
- 34.Dubin K., Callahan M.K., Ren B., et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7 doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei X., Tao J., Xiao S., et al. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep. 2018;8:3685. doi: 10.1038/s41598-018-22094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Ablimit N., Zhang Y., et al. Novel β-mannanase/GLP-1 fusion peptide high effectively ameliorates obesity in a mouse model by modifying balance of gut microbiota. Int J Biol Macromol. 2021;191:753–763. doi: 10.1016/j.ijbiomac.2021.09.150. [DOI] [PubMed] [Google Scholar]
- 37.Balakrishnan B., Luckey D., Bodhke R., et al. Prevotella histicola protects from arthritis by expansion of allobaculum and augmenting butyrate production in humanized mice. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.609644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters J.L., Ley R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17(1):83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roslund M.I., Puhakka R., Grönroos M., et al. Biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children. Sci Adv. 2020;6(42) doi: 10.1126/sciadv.aba2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Rodríguez M.I., Nurminen N., Kummola L., et al. Effect of inactivated nature-derived microbial composition on mouse immune system. Immun Inflamm Dis. 2022;10(3) doi: 10.1002/iid3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farahani R., Sherkat R., Hakemi M.G., Eskandari N., Yazdani R. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res. 2014;3:127. doi: 10.4103/2277-9175.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammad H., Lambrecht B.N. The basic immunology of asthma. Cell. 2021;184(6):1469–1485. doi: 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama A., Kohno N., Fujino S., et al. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995;151(5):1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 44.Jackson D.J., Bacharier L.B., Calatroni A., et al. Serum IL-6: A biomarker in childhood asthma? J Allergy Clin Immunol. 2020;145(6):1701–1704.e3. doi: 10.1016/j.jaci.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehead G.S., Kang H.S., Thomas S.Y., et al. Therapeutic suppression of pulmonary neutrophilia and allergic airway hyperresponsiveness by a RORγt inverse agonist. JCI Insight. 2019;5 doi: 10.1172/jci.insight.125528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sing D., Sing C.F. Impact of direct soil exposures from airborne dust and geophagy on human health. Int J Environ Res Public Health. 2010;7(3):1205–1223. doi: 10.3390/ijerph7031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L., Gadd G.M., Li Z. Microbial biomodification of clay minerals. Adv Appl Microbiol. 2021;114:111–139. doi: 10.1016/bs.aambs.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Park S., Kim J.I., Bae J.Y., et al. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. J Microbiol Seoul Korea. 2018;56(2):145–149. doi: 10.1007/s12275-018-7411-1. [DOI] [PubMed] [Google Scholar]
- 49.Miyazawa K., Kawase M., Kubota A., et al. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef Microbes. 2015;6(4):441–449. doi: 10.3920/BM2014.0108. [DOI] [PubMed] [Google Scholar]
- 50.Schuijs M.J., Willart M.A., Vergote K., et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 51.Vlčková K., Shutt-Phillips K., Heistermann M., et al. Impact of stress on the gut microbiome of free-ranging western lowland gorillas. Microbiol Read Engl. 2018;164(1):40–44. doi: 10.1099/mic.0.000587. [DOI] [PubMed] [Google Scholar]
- 52.Bailey M.T., Dowd S.E., Parry N.M.A., Galley J.D., Schauer D.B., Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78(4):1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie W., Chen Y., Yang H. Layered clay minerals in cancer therapy: recent progress and prospects. Small Weinh Bergstr Ger. 2023;19(34) doi: 10.1002/smll.202300842. [DOI] [PubMed] [Google Scholar]
- 54.Han C., Song J., Hu J., et al. Smectite promotes probiotic biofilm formation in the gut for cancer immunotherapy. Cell Rep. 2021;34(6) doi: 10.1016/j.celrep.2021.108706. [DOI] [PubMed] [Google Scholar]
- 55.Arrieta M.C., Stiemsma L.T., Dimitriu P.A., et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307) doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 56.Laukens D., Brinkman B.M., Raes J., De Vos M., Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40(1):117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of 16S rRNA gene sequences were deposited in the NCBI Sequence Read Archive (SRA) database (project number, PRJNA877056), and other relevant data supporting the findings are available within the supplementary file.