Abstract
Abstract
The survival of preterm infants has steadily improved thanks to advances in perinatal and neonatal intensive clinical care. The focus is now on finding ways to improve morbidities, especially neurological outcomes. Although antenatal steroids and magnesium for preterm infants have become routine therapies, studies have mainly demonstrated short-term benefits for antenatal steroid therapy but limited evidence for impact on long-term neurodevelopmental outcomes. Further advances in neuroprotective and neurorestorative therapies, improved neuromonitoring modalities to optimize recruitment in trials, and improved biomarkers to assess the response to treatment are essential. Among the most promising agents, multipotential stem cells, immunomodulation, and anti-inflammatory therapies can improve neural outcomes in preclinical studies and are the subject of considerable ongoing research. In the meantime, bundles of care protecting and nurturing the brain in the neonatal intensive care unit and beyond should be widely implemented in an effort to limit injury and promote neuroplasticity.
Impact
With improved survival of preterm infants due to improved antenatal and neonatal care, our focus must now be to improve long-term neurological and neurodevelopmental outcomes.
This review details the multifactorial pathogenesis of preterm brain injury and neuroprotective strategies in use at present, including antenatal care, seizure management and non-pharmacological NICU care.
We discuss treatment strategies that are being evaluated as potential interventions to improve the neurodevelopmental outcomes of infants born prematurely.
Introduction
The cost and deleterious impact of preterm brain injury on individuals, their extended families and society cannot be overstated. Preterm neonates are among our most vulnerable citizens. Brain injury is far more common in preterm infants than at term and is associated with a greater risk of adverse outcomes.1 Preterm birth is one of the leading indicators of the health of a nation, as it is associated with high mortality and significant risks of brain injury and impaired brain development.2–6 In turn, this is associated with poor academic achievements and behavioral and mental health issues, all of which affect the quality of life and reduce earning capacity throughout life.5,7–9
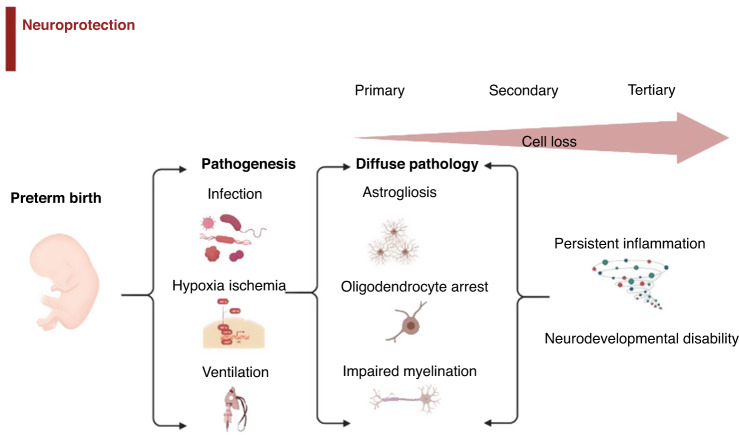
There is now considerable evidence that the pathogenesis of preterm brain damage is multifactorial, involving hypoxia-ischemia (HI), exposure to perinatal infection/inflammation, ventilation, and other technologies to support immaturity, and other perinatal events (Fig. 1).10–14 For example, in preterm infants, a 5-min Apgar score <7 is associated with a greater risk of death or cerebral palsy (CP) than a score ≥9. Equally, the extent and duration of inflammatory markers both at the time of birth and after are highly associated with severe neurodevelopmental disability.15
Fig. 1. Brain injury in the premature infant can be caused by a variety of factors including infection and hypoxic injury.
This can lead to diffuse damage, including cell loss via astrogliosis, oligodendrocyte arrest and impaired myelination. Intervention at each stage of injury and cell loss aims to prevent neurodevelopmental complications.
In modern cohorts, the most common underlying pathology is diffuse white matter injury (WMI), with astrogliosis, maturational arrest of oligodendrocytes and impaired myelination (Fig. 1).16–18 For example, in a large cohort of infants born before 30 weeks of gestation and/or less than 1500 g at birth, neurodevelopmental impairment at 7 years of age was associated with smaller total brain, cortical gray matter and white matter volumes on magnetic resonance imaging (MRI) at 7 years of age (n = 62, p < 0.05), and with reduced fractional anisotropy on diffusion-tensor imaging.19 The key mechanisms that underlie white matter dysmaturation in this context involve persistent neuroinflammation and loss of cellular trophic support.20–22
Although the incidence of CP has fallen in some populations of premature infants, absolute rates remain high, and overall neurodevelopmental outcomes have not clearly improved despite improvements in perinatal and neonatal clinical care.23–25 Structurally, CP is associated with diffuse white matter injury (WMI) and, in most severe cases, cystic WMI, previously called cystic periventricular leukomalacia.26–28 Experimentally, extensive cell loss most often occurred during the first few days after an injury, concomitant with secondary loss of cerebral oxidative metabolism.29 However, strikingly, in some studies, injury continued to evolve over days and weeks in a tertiary phase of injury.20,30 Consistent with this, clinically, cystic WMI can be seen on ultrasonography at a median of ~4 weeks after birth/injury, albeit more severe cysts tended to appear earlier.31,32 Non-cystic, diffuse WMI is most likely to be detected on MRI, although it can sometimes be identified on ultrasound. This apparent delay supports the hypothesis that both dysmaturation and, in some cases, severe injury may evolve over many weeks in the tertiary phase, potentially offering a very wide window of opportunity for intervention.33 Evidence gained from preclinical studies must be examined with precautions—while animals typically undergo a defined insult and then likely be sacrificed, injuries suffered by the human preterm infant are likely to be additive, with the risk for multiple, sequential insults in the same patient.
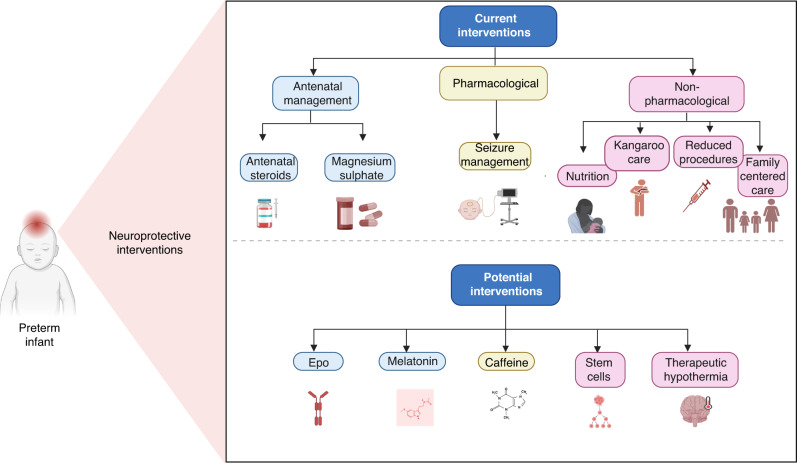
Interventions to protect the preterm brain with an aim to decrease long-term neurodevelopmental impairment can be divided into antenatal strategies, such as antenatal steroid use and magnesium sulfate, and postnatal treatment strategies, including those in routine use and potential agents for future use (Fig. 2).
Fig. 2.
Current and potential therapies to prevent and treat brain injury in the preterm infant.
Antenatal management
Antenatal steroids
The use of antenatal corticosteroids has strong short-term benefits and most likely improves the neurodevelopmental outcomes of preterm neonates. The primary aim is to accelerate fetal lung development but also, importantly, to stabilize the fetal neurovasculature.34,35 At present, a single course of antenatal steroids is recommended for all women at risk of delivering a premature infant by guidelines from all the major perinatal organizations, although there are minor differences in the recommendations for the upper and lower gestational age cutoffs.36–39 The Royal College of Obstetricians and Gynecologists (RCOG) suggests steroids in at-risk deliveries between 24+0 and 34+6 gestational ages. The American College of Obstetrics and Gynecology (ACOG) recommends steroids for pregnancies between 24+0 and 33+6 weeks gestation. They also recommend consideration to be given to providing steroids between 22+0 and 24+0 in consultation with the family and taking into consideration the family’s opinion on neonatal resuscitation. The World Health Organization (WHO) guidelines consider the setting of the pregnancy alongside gestational age, suggesting steroids are given between 24 and 34 weeks gestational age providing adequate care is available to the neonates. They acknowledge the potential for substantial clinical benefit in infants under 24 weeks and again advocate for shared decision-making between families and clinicians. In addition, recommendations vary for rescue dose after the initial course; the RCOG suggests considering it with caution only if the initial course was given at less than 26 weeks of gestation.36
A recent meta-analysis confirms that treatment with antenatal corticosteroids reduces the risk of perinatal death (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.77 to 0.93) and the risk of neonatal death and respiratory distress syndrome (RR 0.71, 95% CI 0.65 to 0.78). They also probably reduce the risk of intraventricular hemorrhage (IVH) (RR 0.58, 95% CI 0.45 to 0.75) and developmental delay in childhood (RR 0.51, 95% CI 0.27 to 0.97).40
By contrast, their use in moderate to late premature infants is highly controversial, as the potential for benefit is much lower, and so risks may outweigh the benefits. The Antenatal Late Preterm Steroids (ALPS) trial, a randomized trial of 2827 infants between 34 and 36+5 weeks gestation, showed a decrease in respiratory morbidity in steroid-treated late premature infants, alongside an increase in episodes of hypoglycemia.41 A Finnish cohort study of over 600,000 children showed a higher risk of mental health and behavioral disorders in steroid-exposed infants, including autism spectrum disorder and attention-deficit/hyperactivity disorder.42,43 While the findings of this study have been debated, they point to the importance of avoiding therapeutic drift, especially at later gestations, when benefits will be less pronounced.
Postnatally, steroids are primarily used to decrease respiratory morbidity in premature infants with prolonged and high ventilatory requirements or oxygen use.44,45 Their use has remained controversial due to an apparent increase in the risk of longer-term neurodevelopmental impairments.46 Dexamethasone tends to be the first-line postnatal steroid for the prevention of bronchopulmonary dysplasia in the second week of life, mainly based on the findings of the DART trial.47,48 While underpowered for their outcomes, the DART trial showed improvements in respiratory status but no statistical difference in long-term outcomes. Thus, in practice, the risk of neurodevelopmental impairments with steroid use must be balanced against the improvement in short-term respiratory status. The challenge for neonatologists is in identifying the infants at the highest risk of poor neurodevelopmental outcomes secondary to poor respiratory status, in whom the benefits of steroid use likely outweigh the risks.
Finally, it is important to consider preclinical evidence that antenatal corticosteroids could have adverse effects for some premature infants exposed to acute HI. For example, maternal dexamethasone given 15 min after asphyxia in fetal sheep was associated with more severe damage in the hippocampus and basal ganglia and exacerbated loss of oligodendrocytes.49 Moreover, a single intramuscular injection of maternal dexamethasone 4 h before umbilical cord occlusion in preterm fetal sheep was associated with cystic brain injury, with increased numbers of seizures, worse brain activity and increased arterial glucose levels compared to preterm fetal sheep treated with vehicle before occlusion.50 These findings could be replicated by glucose infusions before asphyxia, suggesting that dexamethasone-induced hyperglycemia may transform diffuse injury into cystic brain injury after asphyxia, at least in the preterm fetal sheep. Unfortunately, acute asphyxia in a fetus after administration of antenatal steroids is typically unanticipated and unpreventable.
Magnesium sulfate (MgSO4)
Antenatal magnesium sulfate (MgSO4) given prior to preterm birth for fetal neuroprotection is widely used, based on meta-analyses of trials of pre-eclampsia treatment or fetal neuroprotection involving 6131 neonates, including a recent individual participant meta-analysis (IPD-MA). Use of MgSO4 was associated with reduced risk of CP (RR 0.68, 95% confidence interval (CI) 0.54 to 0.87, 4601 neonates, 5 trials, number needed to benefit of 46).51,52 However, it is important to appreciate that, overall, MgSO4 did not significantly improve the risk of the composite outcome of death or CP (RR 0.94, 95% CI 0.85 to 1.05). Exclusion of a trial of treatment of maternal pre-eclampsia suggested, in the remaining four trials, a reduction in CP (RR 0.68, 95% CI 0.53 to 0.87) and in the combined risk of death or CP (RR 0.86, 95% CI 0.75 to 0.99). The findings from the most recent meta-analysis (5917 women giving birth to 6037 infants), including trial sequential analysis (TSA) of all RCTs of neuroprotective and other intent, provided additional evidence that antenatal MgSO4 in women at imminent risk for preterm delivery was associated with decreased risk of CP,53 but again the risk of death or CP was not significantly different (17.1% versus 18.3%, RR 0.88, 95% CI 0.75–1.02). There was no apparent benefit for short-term outcomes, such as severe intraventricular hemorrhage or periventricular leukomalacia.52 The specific causes of infant death in these studies are unknown, but could include redirection to comfort care in agreement between family and caregivers.
Overall, MgSO4 appears to be safe. There was no increase in the primary outcome of severe adverse maternal events related to treatment (including death or respiratory or cardiac arrest in 2 studies that reported this in a total of 1635 women). However, smaller studies suggest possible adverse intestinal effects in neonates that should be investigated carefully. For example, there was an apparent increase in spontaneous intestinal perforation in neonates born <26 weeks after the introduction of MgSO4.54 Further, in a subset of neonates <26 weeks from the BEAM trial, antenatal MgSO4 was associated with death and severe NEC.55 Moreover, the Korean neonatal research network reported that antenatal MgSO4 exposure was associated with meconium-related ileus in infants <25 weeks gestation.56 A recent meta-analysis of 38 non-randomized studies and six randomized trials involving 51,466 preterm infants did not, however, show an increase in gastro-intestinal pathology.57
Even if we consider the reduction in risk of CP to be the true effect of the intervention, there are concerns that this effect is small,58 with a risk reduction of all grades of CP from 6.7% to 4.7% (absolute reduction 2%, relative reduction 30%).52 The effect size for CP reduction is larger in extremely preterm infants, with an NNT of 31 below 28 weeks gestation. Recently, in a trial of treatment for preterm delivery at 30 to 34 gestation, MgSO4 was associated with a trend toward worse outcomes, with adjusted RR for death or disability of 1.19 [95% CI, 0.65 to 2.18],59 suggesting that we should consider limiting the use of MgSO4 to the highest risk gestations. Moreover, there was no improvement in school-age outcomes in magnesium sulfate-treated cohorts, albeit these studies were not powered to detect small effects and were challenged by loss to follow-up that might bias results.60,61
Importantly, there is a notable lack of consistent in vivo evidence demonstrating neuroprotection. A systematic review of animal studies found that the effect of MgSO4 treatment before or shortly after acute HI at term-equivalent gestation was inconsistent between studies.62 In rodent studies showing benefits, the majority were of extremely preterm gestation and used MgSO4 before the inflammatory/HI insult and did not report controlling temperatures. Given that magnesium is a potent vasodilator, this raises the possibility that it could induce neuroprotective hypothermia. MgSO4 given pre-insult, using a clinically relevant higher dose, induced strong preconditioning of the immature rat brain, which provided resistance to HI- and excitotoxicity-mediated injury.63 A small incremental benefit of MgSO4 + hypothermia (TH) compared to TH was found in term gestation piglets, with reduced total cell death and increased oligodendrocyte survival but no improvement in aEEG recovery or magnetic resonance spectroscopy (MRS) at 48 h.64 By contrast, in preterm fetal sheep (0.7 of gestation, 28–30 weeks equivalent), an infusion of MgSO4 before acute HI somewhat improved white and gray matter gliosis and myelin density but did not improve long-term (3 weeks) EEG maturation or neuronal or oligodendrocyte survival.65
Postnatal management
Therapeutic hypothermia for preterm hypoxia-ischemia
The etiology of preterm brain injury is unequivocally multifactorial13, and in most cases, disability is not simply related to acute HI. Nevertheless, the risk of acute HI in preterm infants is greater than at term and highly associated with adverse outcomes.1 The use of therapeutic hypothermia (TH) in term infants has been proven in RCTs and meta-analyses to be safe and effective, improving disability-free survival at 18–24 months of age and reducing the risk of death or CP or IQ score <55 in 6- to 7-year-old children in high-income countries.66–69. In preterm infants, there is concern that TH might exacerbate complications of prematurity, including postnatal hypotension, intracranial bleeding, and respiratory compromise.70 In a cohort of neonates cooled outside standard TH criteria (n = 36, e.g., infants at 34–35 weeks gestation, with postnatal collapse or cardiac disease), compared with infants cooled as per protocol (n = 129), complication rates and neurologic outcomes at 18–20 months were similar; five neonates developed intracranial hemorrhage and had poor outcomes.71 In a retrospective cohort of 31 preterm infants born at 34–35 weeks gestation, TH appeared to be associated with increased mortality (12.9% vs. 0%, p = 0.04) and increased white matter damage (66.7% vs. 25.0%, p = 0.001), compared to 32 term infants.72 By contrast, a retrospective cohort study of preterm neonates (gestational age range 33–35 weeks) with HIE who underwent TH found similar rates of death or moderate-severe neurodevelopmental impairment in 11/22 cases73 compared to those in treated term infants in the large controlled TH trials.67
Preclinical evidence supports similar benefits and constraints in preterm animals. In preterm-equivalent fetal sheep, cerebral cooling for 72 h (with extradural temperature titrated to 29.5 ± 2.6 °C) started 90 min after severe asphyxia was associated with basal ganglia and hippocampal neuroprotection, protection of immature oligodendrocytes in periventricular and parasagittal white matter, and reduced overall microgliosis and apoptosis.74,75 Similarly, mild systemic hypothermia (37.2 ± 0.3 °C vs 39.5 ± 0.1 °C in normothermic controls) started 30 min after severe asphyxia, and continued until 72 h, reduced neuronal loss and microglial induction in the striatum, with faster recovery of spectral edge frequency, reduced seizure burden, and less suppression of EEG amplitude (p < 0.05).76 However, no benefit was seen when TH was commenced 5 h after HI.
Many questions remain to be answered for preterm neonates. It is plausible that TH may alleviate brain damage after acute perinatal asphyxia but not after chronic insults such as inflammation. Thus, the high rates of prenatal and postnatal infection/inflammation in preterm neonates might impair responses to TH.77 In postnatal day 7 rats exposed to HI, hemispheric and hippocampal protection with systemic cooling was lost after pre-insult sensitization with gram-negative lipopolysaccharide, but not a gram-positive mimic, PAM3CSK4,78 suggesting a pathogen-dependent effect. It is unknown whether these studies are relevant to the typical long-standing mixed effects of chorioamnionitis and postnatal inflammation. Combination therapies are promising but are largely unproven. Finally, the ideal protocol for identifying at-risk premature infants with acute neonatal encephalopathy remains unclear.
One multi-center randomized controlled trial is currently in progress testing the safety, feasibility and efficacy of TH within 6 h of birth in preterm infants at 33–35 weeks gestation with moderate to severe HIE (ClinicalTrials: NCT01793129). Publication of final results are awaited. Targeted use of TH for extremely preterm infants seems unlikely given the multiple morbidities (IVH, NEC, late-onset sepsis, BPD, apnea) during a prolonged hospitalization, which increases the risk of death or disability at 2 years of age. Moderate and late preterm infants are more likely to have identifiable, timed insults and may allow a focused use of TH similar to infants ≥36 weeks gestation at birth.
Pluripotential (stem) cells
There is now considerable evidence from modern cohorts that neurodevelopmental disability after preterm birth is commonly associated with diffuse WMI, reflecting a persistently impaired white matter maturation.16 Persistent (tertiary) loss of trophic support and inflammation are key mechanisms.20,30 Exogenous pluripotential cells (stem cells) may promote angiogenesis, neurogenesis, synaptogenesis and neurite outgrowth, and so may have the potential to improve outcomes after preterm brain injury. Although its use to date has been primarily preclinical, a meta-analysis of neonatal rodent studies suggested that overall neural stem cells significantly reduced brain infarct size and improved motor and cognitive function.79 Many questions remain to be resolved, including the optimal dose, type of stem cells, and window of opportunity to start treatment.
Some conclusions can be drawn from the preclinical literature. Critically, it is very likely that multiple doses will be needed to see benefits. In term-equivalent, postnatal day 10 (P10) neonatal rats, for example, a single dose of umbilical cord blood (UCB) cells at P11 did not improve behavioral or neuropathological outcomes, whereas 3 doses (at P11, 13, 20) of UCB cells improved brain weight and behavioral deficits.80 Similar findings were seen in another term-equivalent study in P9 rats.81 In very preterm-equivalent rats, who underwent HI on postnatal day 3 and received 4 doses of human UCB cells over the next 3 days, UCBs were associated with improved behavioral outcomes at 27 days post HI.82 Consistent with this, in preterm fetal sheep, a single infusion of human amnion epithelial cells (hAECs) up to 24 h after HI was anti-inflammatory, anti-gliotic, and neuroprotective in the hippocampus, but had limited effect on numbers of oligodendrocytes or the proportion of immature/mature numbers of oligodendrocytes after 7 days recovery and had no effect on recovery of EEG activity.83 By contrast, in the same paradigm, repeated and delayed intranasal infusion of hAECs at 1, 3 and 10 days after umbilical cord occlusion for 25 min was associated with improved brain weight and restoration of immature/mature oligodendrocytes and fractional area of myelin basic protein, with reduced microglia and astrogliosis. Neuronal survival in deep gray matter nuclei was improved, with reduced microglia, astrogliosis and cleaved-caspase-3-positive apoptosis. Functionally, cortical EEG frequency distribution was partially improved.84
Further, hAECs may alleviate inflammatory injury. In preterm fetal sheep, after lipopolysaccharide (LPS) infusions for 3 consecutive days at 109, 110 and 111 days of gestation, 3 intravenous doses of 60 million hAECs given starting at 110 days were associated with attenuation of activated microglia, reduced number of pyknotic cells and significantly increased number of oligodendrocytes and myelin basic protein compared to animals treated with LPS only.85 Thus, overall, there is good large animal evidence supporting the benefit of a reasonably wide window of opportunity with intermittent infusions of stem cells, although more detailed studies are needed to establish the ideal protocol.
There have been several rather heterogeneous Phase I/II clinical trials in preterm infants as recently reviewed.86 Many were focused on treating chronic deficits rather than perinatal injury. Overall, these studies support the general safety of stem cells and that there may be potential benefits, but definitive studies are still lacking. A safety and feasibility study in infants under 28 weeks gestation, using autologous UCB mononuclear cells via intravenous injection at a dose of 25–50 million cells/kg in 20 infants, is underway.87
Anti-inflammatory interventions
There is growing evidence that there is a tertiary phase of ongoing inflammation and apoptosis for weeks, months or even years after perinatal injury and that this likely contributes to diffuse injury and dysmaturation of the developing brain.20 For example, in postmortem studies, premature infants exposed to HI can have stalled maturation of oligodendrocytes and chronic inflammation16,88 and impaired connectivity on MRI studies.89–91 Notably, many clinical cases of cystic WMI are not seen until several weeks after birth.31,32 This delayed damage could, in principle, reflect the slow evolution of injury or multiple injurious inputs over time. Both are likely contributing, as suggested by a cohort study that found that combinations of HI, infection and inflammation could all ultimately be associated with injury.92
Preclinical studies strongly support the concept that cystic WMI can develop in the tertiary phase even after a single episode of HI. For example, in P7 neonatal rats, necrotic brain lesions were found to evolve from 24 h to 21 days after.93 Moderate HI was associated with the slowest evolution of brain necrosis. Similarly, in preterm fetal sheep, at 3 and 7 days after global HI induced by complete occlusion of the umbilical cord for 25 min, HI led to diffuse WMI injury, with selective loss of mature oligodendrocytes, increased microglia and impaired myelination, like the typical pattern of diffuse WMI in most recent human postmortem studies.28,94 However, unexpectedly, 14 to 21 days after HI, cystic lesions developed in the temporal white matter.30 Intriguingly, the regional distribution of this cystic white matter degeneration at 21 days after HI was closely related to the location of dense microglial aggregates visualized at earlier time points, raising the possibility that exuberant inflammation may contribute to late necrosis. Strongly supporting this concept, in a subsequent study, intracerebroventricular infusion of the soluble tumor necrosis factor (TNF) inhibitor, etanercept, at 3, 8 and 13 days after umbilical cord occlusion attenuated cystic WMI and restored oligodendrocyte maturation and deficits in myelin protein expression.95 These encouraging results suggest that, although far more knowledge is needed, delayed anti-inflammatory interventions may be a promising strategy to reduce neurodevelopmental disabilities. Erythropoietin, azithromycin, melatonin, and caffeine citrate have been the subject of extensive preclinical and clinical studies and will be discussed in detail. Other repurposed anti-inflammatory medications are also under investigation, both preclinically and clinically. This includes anakinra, an interleukin 1 receptor antagonist, which is the subject of a safety and feasibility study in preterm infants between 24 and 28 weeks gestation, that aims to decrease the morbidity and mortality of prematurity attributed to inflammation, including bronchopulmonary dysplasia, gut injury and necrotizing enterocolitis, and brain injury.96
Erythropoietin
Erythropoietin (Epo) is an endogenous growth factor whose primary function is to stimulate erythropoiesis. However, Epo receptors (EpoR) are also present on neuron progenitor cells,97 neurons,98 astrocytes,99 oligodendrocytes,100 microglia,101 and endothelial cells.97 Epo-bound receptors dimerize to activate anti-apoptotic pathways via phosphorylation of JAK2, phosphorylation and activation of MAPK, ERK1/2, as well as the PI3K/Akt pathway and STAT5, which are critical in cell survival.101,102 Epo also has indirect effects, increasing iron utilization by increasing erythropoiesis and by decreasing inflammation103,104 and oxidative injury.105,106
In preclinical models of neonatal brain injury, recombinant human Epo has anti-inflammatory,103,104,107–111 anti-excitotoxic,112 antioxidant,113 and anti-apoptotic effects on neurons and oligodendrocytes, and promotes neurogenesis and angiogenesis,114 which are essential for injury repair and normal neurodevelopment. Epo effects are dose-dependent, and repeated doses are more effective than single doses115–117 at reducing learning impairments following brain injury.118,119 Encouragingly, in P7 rats, although starting Epo 48 h after HI did not improve the area of infarction, it improved behavioral outcomes, with enhanced neurogenesis, increased axonal sprouting, and reduced WMI.120 However, this should be interpreted with caution. In preterm fetal sheep, a high dose infusion of Epo from 30 min after asphyxia until 72 h was associated with partial neuroprotection, with improved electrophysiological and cerebrovascular recovery after 1 week of recovery and reduced apoptosis and inflammation.121 When the infusion was delayed until 6 h after asphyxia, there was no histological or electrophysiological protection.122 Of concern, in the same paradigm, a clinical regime of high-dose intermittent boluses of Epo started at 6 h was associated with impaired EEG recovery and bilateral cystic injury of temporal lobe intragyral white matter in the majority of fetuses,122 suggesting that unless Epo can be started immediately, it is unlikely to be beneficial for preterm HI brain injury.
Clinical evidence supports this concern. Five clinical trials of Epo for neuroprotection of infants <37 weeks have now been published,123–127 four of which were reviewed in a meta-analysis by Fischer et al., suggesting that prophylactic Epo improved the cognitive development of preterm infants.128 However, significant differences in the patient populations (mean gestational age 25.9 to 30.4), Epo dose (400 U/kg to 3000 U/kg) and duration of therapy (3 days to 12 weeks) make it difficult to reach definitive conclusions. Critically, the two largest trials, the Swiss trial125 and the Preterm Epo Neuroprotection Trial (PENUT)127 did not show any improvement in the combined outcome of death or severe neurodevelopmental impairments at 2 years of age. Epo also did not affect inflammatory markers in the PENUT trial, despite data in rodents that this was one of the mechanisms of action.129 Potential reasons why Epo was ineffective include important differences between preterm infants and preclinical models, incorrect timing of the intervention, incorrect dosing schedule or the possibility that iron deficiency in the Epo-treated group contributed to the lack of benefit in children enrolled in the PENUT Trial.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is a naturally occurring indolamine secreted by the pineal gland to regulate circadian rhythm.130 Melatonin is safe in pregnancy and in neonates, readily crosses the placenta and blood-brain barrier, and is a potent antioxidant with potential for neuroprotection after inflammation and HI in high-risk preterm fetuses and infants.130
In preterm fetal sheep at 0.7 gestation (98–99 days of gestation, term gestation being 147 days), a maternal infusion of low-dose melatonin dissolved in ethanol from 15 min before umbilical cord occlusion until 6 h after was associated with faster recovery of EEG activity, delayed onset of seizures, improved survival of mature oligodendrocytes, and reduced microglial activation in the periventricular white matter.131 The ethanol vehicle was independently associated with reduced duration of fetal seizures and improved neuronal survival in the striatum, but worse neuronal survival in the hippocampus and less white matter proliferation compared to saline treatment. Similarly, fetal infusion of melatonin dissolved in ethanol over 24 h in preterm fetal sheep, starting 2 h after HI, was associated with region-specific improvements in white matter damage 10 days after HI.132 Similarly, independent and combined neuroprotective effects of melatonin and ethanol were confirmed in term neonatal piglets.133,134 The EMA and FDA have recognized that ethanol is an active or adjuvant excipient for melatonin and is likely to be safe at levels below that recommended by the FDA. The American Academy of Pediatrics Committee on Drugs recommended that the amount of ethanol in any preparation should not lead to blood concentration >0.25 g/L after a single dose in term infants.135 A multicentre international phase I dose escalation study of melatonin as an adjunct with TH (ACUMEN Study—Acute high dose Melatonin for Encephalopathy of the Newborn) is starting using a melatonin/ethanol formulation in term infants. As the oral bioavailability of melatonin is around 15%, intravenous administration is necessary.136
Small clinical studies suggest that, in preterm infants, oral melatonin is safe and may improve survival in septic shock and reduce lung injury associated with ventilation.137,138 Further, in a randomized double-blind placebo-controlled pilot study, oral melatonin given once a day was associated with lower levels of F2-isoprostanes at 48 h.139 This result may be significant given the association of increased levels of F2-isoprostanes with abnormal white matter injury scores at term-corrected age.140 Long-term follow-up has not been reported and is essential.
A recent review discusses the preclinical evidence for melatonin as a therapeutic agent for preterm brain injury and calls for well-designed preclinical research to understand the dosing and timing of administration and neonates who might benefit from melatonin.141 Nevertheless, there is intense interest in the entrainment of the circadian rhythm in high-risk neonates in the NICU and its effect on later cognitive development; this is being studied in the “Circa Diem” Study.142
Caffeine citrate
Caffeine citrate, a methylxanthine, is routinely used in preterm infants with the primary intention of preventing apnea of prematurity (AOP) and in an attempt to decrease the risk of unsuccessful extubation attempts. These indications are based on the results of the CAP (Caffeine for Apnea of Prematurity),143 other caffeine RCTs and resulting Cochrane meta-analyses.144,145 The CAP trial randomized 2006 infants less than 1250 g to caffeine citrate or placebo and performed rigorous follow-up of recruited infants. Caffeine citrate for AOP decreased bronchopulmonary dysplasia and time requiring respiratory support with no significant side effects. Caffeine given for AOP reduced the incidence of CP (4.4% vs. 7.3%; adjusted odds ratio, 0.58; 95% CI, 0.39 to 0.87; p = 0.009) and of cognitive delay (33.8% vs. 38.3%; adjusted odds ratio, 0.81; 95% CI, 0.66 to 0.99; p = 0.04) at 18 to 21 months.146 The significant improvement in disability-free survival was not seen when infants were followed at 5 years of age.147 At 11 years of age, neurobehavioral outcomes were similar between groups, but the caffeine-treated group performed better in fine motor coordination (mean difference (MD) = 2.9; p = 0.01), visuomotor integration (MD = 1.8; p < 0.05), visual perception (MD = 2.0; p = 0.02) and visuospatial organization (MD = 1.2; p = 0.003).148 The caffeine-treated group also had a reduced risk of motor impairment.149 A number of mechanisms underlie the potential neuroprotective effects of caffeine citrate in premature infants. Caffeine is an adenosine receptor antagonist and, by this mechanism, increases respiratory neural output and also enhances CO2 responsiveness.150 Preclinical studies testing caffeine after HI in a neonatal premature model demonstrated improved function, reduced brain injury, decreased apoptosis, anti-inflammatory actions, increase in myelination and promotion of oligodendroglial in treated animals.151–154
Seizure management
Preterm neonates have high rates of clinical and subclinical seizures.155,156 Neonatal seizures are associated with poor neurodevelopment, and this may be even more severe in preterm than term infants.157 In experimental studies, there is often little relationship between seizure control and neurohistological outcomes.158 For example, in preterm fetal sheep, intravenous infusion of MgSO4 for 24 h before asphyxia until 24 h after asphyxia was associated with a marked reduction of the post-asphyxial seizure burden, confirming its physiological anti-excitotoxic role but did not improve neuronal survival and indeed exacerbated loss of oligodendrocytes after 7 days.159
Of further concern, in animal studies, many anticonvulsant drugs are neurotoxic at clinical and subclinical levels.160 For example, in P7 rats, when brains are equivalent to the late preterm infant, clinically relevant doses of phenytoin, phenobarbital, diazepam, clonazepam, vigabatrin and valproate were associated with widespread apoptotic degeneration, reduced expression of pro-survival neurotrophins, and a significant reduction in brain weight after eight days. Further, in P7 rats, clinical doses of phenobarbitone, phenytoin and lamotrigine, but not levetiracetam, were associated with impaired striatal synaptic development between P10 and P18.161 The optimal management of seizures in preterm neonates is a high priority for research.
Non-pharmacological neuroprotection
Beyond medications that can be prescribed or novel procedures that can be performed, the care practices that are provided daily have an impact on both the prevention of acquired brain injury and the promotion of healthy brain maturation. Models of care that reduce separation, promote family integration into care, and focus on creating a neuro-supportive environment are rooted in evidence and, at the same time, require further study to be able to optimize outcomes for preterm infants and their family units.162
Specifically, immediate skin-to-skin care, otherwise known as kangaroo care, has been shown to reduce preterm mortality globally,163 and has been linked with improved biomarkers of resilience and stress reduction in both mothers and infants. Skin-to-skin care has also shown benefits for microbiome development and stabilization of respiratory physiology, both of which may also influence neurodevelopment.164,165 Early and frequent skin-to-skin care for preterm infants results in decreased length of exposure to mechanical ventilation and oxygen and overall decreased length of stay for the initial neonatal hospitalization.166
Nutrition is critical to brain development. Promotion of maternal breast milk or donor breast milk if maternal breast milk is unavailable, early enteral nutrition and early fortification have been linked to reduction of growth failure and improved brain health outcomes and should be an important focus on neuroprotective care.167,168
Evidence supports that both pain and our treatment of pain impact brain development and neurodevelopmental outcomes in preterm infants. Both prophylactic and procedural opioid exposure specifically has been linked to cerebellar injury, decreased cerebellar volume, and poorer neurodevelopmental outcomes in early childhood.169–171 Treating pain non-pharmacologically as well as reducing pain have shown to be neuroprotective. Efforts should be made to minimize unnecessary procedures, particularly skin breaks, in premature neonates, as these have been linked to altered thalamic and microstructural development and have an independent impact on childhood cognitive outcomes.172–175 Recognizing that all pain is not avoidable in our efforts to support the health and development of preterm children, many neuro-supportive care practices can also be used to minimize the impact of pain, including maternal breast milk and skin-to-skin care.176 Pharmacological agents such as sucrose can be effective in reducing the pain experienced by neonates undergoing mildly painful procedures.177 Sucrose should be used judiciously as the underlying mechanism is still unknown, and the long-term effects on development and potential for hyperalgesia are unknown.172 The preterm infant spends most of its time asleep, and unlike term and young infants, the majority of time is spent in active sleep (AS). The ultradian cycling between AS and quiet sleep (QS) is thought to be important in brain development and interrupted sleep is associated with adverse neurodevelopmental outcomes in both preterm human infants and experimental animal models.178 In addition, improving the timing of hands-on care or interventions in order to safeguard sleep has also been linked to improved brain health and development.179
Models of care, such as family-centered care (FCC)180 or family-integrated care (FiCARE),181 both of which are centered around the concept of Families as Partners (VON),182 are linked to intermediate outcomes of better family engagement, which can in turn lead to improved neurodevelopment.
Together, these care practices have an opportunity to optimize brain development and are within the control of neonatal intensive care providers and units to a growing body of literature demonstrating the feasibility of implementation.183
Neuroprotection and neuroplasticity bundles
Preterm brain injury is a complex and multifactorial process.184 Genetic, epigenetic, metabolic, prenatal, perinatal and postnatal factors interact to protect, cause or exaggerate neonatal brain injury.185–188 Given the complexity of the issue, there is an increasing interest in a “bundled” approach using quality improvement methodology to prevent acute preterm brain injury.189 Neuroprotection bundles can be divided into (1) acute brain injury prevention and (2) neuroplasticity bundles.
The key interventions in the preterm acute brain injury prevention bundles are early identification of high-risk pregnancies and in-utero transfer,190 prevention of fluctuation in physiologic parameters such as partial pressure of carbon dioxide, blood pressure, and temperature at birth,191–193 prevention of acidosis,194 midline head position,195 minimizing painful procedures,196 and maintenance of electrolyte balance (especially serum sodium).197 Recent studies showed the implementation of such neuroprotection bundles targeting some or all those key elements using Quality Improvement (QI) methodology decreased the incidence of acute brain injury and improved long-term neurodevelopment outcomes in extremely premature infants born at and outside tertiary centers.198–202
Neuroplasticity interventions, unlike those for acute brain injury, target potential brain injury and growth well beyond the first few days of birth and after discharge.203 The key elements of such bundles include the non-pharmacological interventions discussed in detail above, including empowering families through family-centered care model;181 optimizing nutrition;204 developmental care;205 skin-to-skin care and massage therapy;206,207 positive stimulating sounds such as music therapy and reading programs,208 parental voice,209,210 minimizing disturbing noises,211 enhancing physiologic sleep-wake cycles212 and encouraging positive social interaction.213
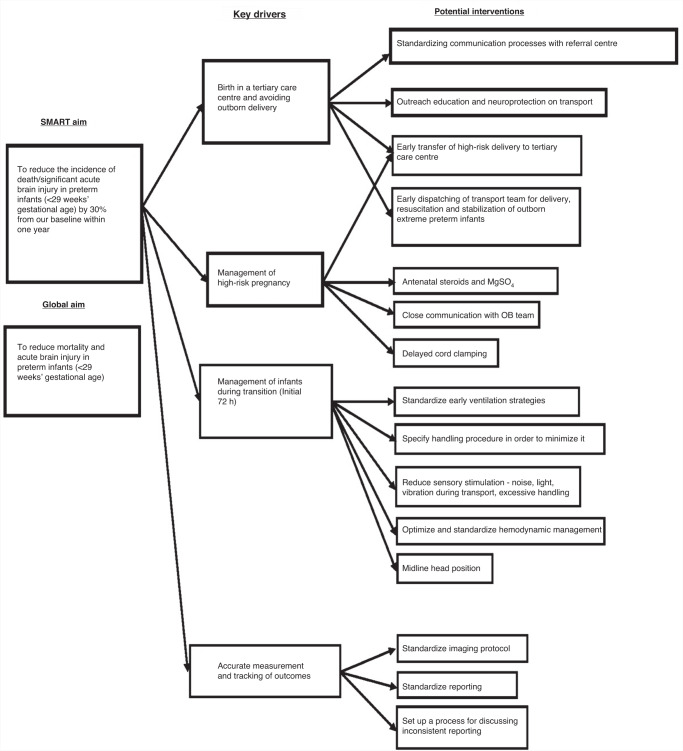
Compared to acute brain injury prevention bundles, there is emerging evidence that parental involvement214 and neuroplasticity interventions may improve long-term cognitive and motor outcomes.206–208 These studies were relatively small, and studies with a larger sample size are now needed to confirm these encouraging preliminary findings. Figure 3 shows an example of using QI methodology for premature infants, with a driver diagram and smart aims to reduce IVH.
Fig. 3.
Example of using QI methodology, a driver diagram and smart aim to reduce IVH.
Social determinants of neurodevelopmental outcomes
Evidence from multiple cohort studies and the resultant meta-analyses have shown that the impact of a number of social determinants has an impact on the neurodevelopmental outcomes of infants and that this risk is magnified in infants who are born preterm.7,215–217 A prospective cohort study of 226 infants born between 26 and 32 weeks gestation examined the impact of socio-economic status (SES) as a moderator of outcome, showed that SES had the same statistical association with cognitive outcome as WMI and that SES moderated the long-term outcomes of infants with brain injury and chronic lung disease.218 An MRI study of preterm infants, excluding those with severe brain injuries, showed regional differences in brain morphology associated both with premature birth and SES and that these factors interacted. Lower maternal education as a proxy for SES has been widely studied in this context, perceptions of neighborhood safety and the maternal vulnerability index, a composite measure that reflects physical, social, and health care needs, have also been associated with preterm birth, less use of prenatal care and poorer long-term outcomes.219,220 While the mechanisms underlying these associations are not fully elucidated, these findings suggest that strategies improving social determinants of health for premature infants may have a positive effect on their long-term health and neurodevelopmental outcomes221 and that, as such, advocacy by clinicians in this area is vital.
Conclusion
There has been little change in the rather high burden of lifelong disabilities after preterm birth in recent years, despite continuing improvement in survival. Developing therapeutic strategies for preterm brain injury is challenging because of the heterogeneous nature of preterm brain injury, including exposure to antenatal and postnatal hypoxia, acute perinatal asphyxia, prenatal and postnatal infection/inflammation, ventilation-induced brain injury and risk of side effects associated with treatments, including anticonvulsants and glucocorticoids. Each premature infant is likely exposed to different combinations of factors, As highlighted in this review, several potential neuroprotective interventions have shown promise in preclinical studies and/or in clinical trials. With further well-designed, translational research, these treatment strategies may help to reduce the high burden of brain damage and disability associated with preterm birth. Neurorestorative interventions targeting the repair of the injury should also be investigated. In the meantime, bundles of care protecting and nurturing the brain in the neonatal intensive care unit and beyond should be widely implemented in an effort to limit injury and promote neuroplasticity.
Author contributions
All authors (E.J.M., M.E., J.S., S.J., A.J.G., M.B., F.G., C.B., Y.W., N.J.R., M.C., A.B., T.H., S.T., A.L., T.A., K.M., E.R., K.L., P.W., S.L.B.) were involved in drafting the article and revising it critically for important intellectual content. Each author has approved the final version for publication.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Eleanor J. Molloy, Email: Eleanor.molloy@tcd.ie
on behalf of the Newborn Brain Society Guidelines and Publications Committee:
Sonia Lomeli Bonifacio, Pia Wintermark, Hany Aly, Vann Chau, Hannah Glass, Monica Lemmon, Courtney Wusthoff, Gabrielle deVeber, Andrea Pardo, Melisa Carrasco, James Boardman, Dawn Gano, and Eric Peeples
References
- 1.Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am. J. Obstet. Gynecol. 2016;215:103.e101–103.e114. doi: 10.1016/j.ajog.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarda SP, Sarri G, Siffel C. Global prevalence of long-term neurodevelopmental impairment following extremely preterm birth: a systematic literature review. J. Int. Med. Res. 2021;49:3000605211028026. doi: 10.1177/03000605211028026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016;21:74–79. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle LW, Spittle A, Anderson PJ, Cheong JLY. School-aged neurodevelopmental outcomes for children born extremely preterm. Arch. Dis. Child. 2021;106:834–838. doi: 10.1136/archdischild-2021-321668. [DOI] [PubMed] [Google Scholar]
- 7.Linsell L, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population-based cohort study. Arch. Dis. Child. 2018;103:363–370. doi: 10.1136/archdischild-2017-313414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone S, et al. Personality, emotional and cognitive functions in young adults born preterm. Brain Dev. 2020;42:713–719. doi: 10.1016/j.braindev.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Crump C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020;150:105187. doi: 10.1016/j.earlhumdev.2020.105187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates N, Gunn AJ, Bennet L, Dhillon SK, Davidson JO. Preventing brain injury in the preterm infant-current controversies and potential therapies. Int. J. Mol. Sci. 2021;22:1671. doi: 10.3390/ijms22041671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ophelders D, et al. Preterm brain injury, antenatal triggers, and therapeutics: timing is key. Cells. 2020;9:1871. doi: 10.3390/cells9081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg H, et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galinsky R, et al. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev. Med. Child Neurol. 2018;60:126–133. doi: 10.1111/dmcn.13629. [DOI] [PubMed] [Google Scholar]
- 14.Dean JM, et al. What brakes the preterm brain? An arresting story. Pediatr. Res. 2014;75:227–233. doi: 10.1038/pr.2013.189. [DOI] [PubMed] [Google Scholar]
- 15.Kuban KC, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 2015;52:42–48. doi: 10.1016/j.pediatrneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back SAWhite. Matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134:331–349. doi: 10.1007/s00401-017-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider J, Miller SP. Preterm Brain injury: white matter injury. Handb. Clin. Neurol. 2019;162:155–172. doi: 10.1016/B978-0-444-64029-1.00007-2. [DOI] [PubMed] [Google Scholar]
- 18.Verney C, et al. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 2012;71:251–264. doi: 10.1097/NEN.0b013e3182496429. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy E, Poppe T, Tottman A, Harding J. Neurodevelopmental impairment is associated with altered white matter development in a cohort of school-aged children born very preterm. Neuroimage Clin. 2021;31:102730. doi: 10.1016/j.nicl.2021.102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 21.Chakkarapani AA, et al. Therapies for neonatal encephalopathy: targeting the latent, secondary and tertiary phases of evolving brain injury. Semin. Fetal Neonatal Med. 2021;26:101256. doi: 10.1016/j.siny.2021.101256. [DOI] [PubMed] [Google Scholar]
- 22.Fleiss B, Murray DM, Bjorkman ST, Wixey JA. Editorial: Pathomechanisms and treatments to protect the preterm, fetal growth restricted and neonatal encephalopathic brain. Front. Neurol. 2021;12:755617. doi: 10.3389/fneur.2021.755617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheong JL, Spittle AJ, Burnett AC, Anderson PJ, Doyle LW. Have outcomes following extremely preterm birth improved over time? Semin. Fetal Neonatal Med. 2020;25:101114. doi: 10.1016/j.siny.2020.101114. [DOI] [PubMed] [Google Scholar]
- 24.Australian Cerebral Palsy Register. Report of the Australian Cerebral Palsy Register, Birth Years 1995–2016. https://cpregister.com/ (2023).
- 25.Cheong JLY, et al. Temporal trends in neurodevelopmental outcomes to 2 years after extremely preterm birth. JAMA Pediatr. 2021;175:1035–1042. doi: 10.1001/jamapediatrics.2021.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch. Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 27.Hamrick SE, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J. Pediatr. 2004;145:593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Gano D, et al. Diminished white matter injury over time in a cohort of premature newborns. J. Pediatr. 2015;166:39–43. doi: 10.1016/j.jpeds.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassink G, et al. A working model for hypothermic neuroprotection. J. Physiol. 2018;596:5641–5654. doi: 10.1113/JP274928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lear BA, et al. Tertiary cystic white matter injury as a potential phenomenon after hypoxia-ischaemia in preterm f sheep. Brain Commun. 2021;3:fcab024. doi: 10.1093/braincomms/fcab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierrat V, et al. Ultrasound diagnosis and neurodevelopmental outcome of localised and extensive cystic periventricular leucomalacia. Arch. Dis. Child Fetal Neonatal Ed. 2001;84:F151–F156. doi: 10.1136/fn.84.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S, et al. Outcome of preterm infants with transient cystic periventricular leukomalacia on serial cranial imaging up to term equivalent age. J. Pediatr. 2018;195:59–65.e53. doi: 10.1016/j.jpeds.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lear, B. A. et al. Is late prevention of cerebral palsy in extremely preterm infants plausible? Dev. Neurosci.44, 177–185 (2021). [DOI] [PubMed]
- 34.Vinukonda G, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson R, Monaghan-Nichols AP, DeFranco DB, Rudine AC. Effects of antenatal glucocorticoids on the developing brain. Steroids. 2016;114:25–32. doi: 10.1016/j.steroids.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stock SJ, Thomson AJ, Papworth S. Antenatal corticosteroids to reduce neonatal morbidity and mortality: Green-top Guideline No. 74. BJOG. 2022;129:e35–e60. doi: 10.1111/1471-0528.17027. [DOI] [PubMed] [Google Scholar]
- 37.Committee on Obstetric Practice. Committee Opinion No. 713: Antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 2017;130:e102–e109. doi: 10.1097/AOG.0000000000002237. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO Recommendations on Antenatal Corticosteroids for Improving Preterm Birth Outcomes (World Health Organization, 2022). [PubMed]
- 39.Cahill, A. G., Kaimal, A. J., Kuller, J. A. & Turrentine, M. A. Use of antenatal corticosteroids at 22 weeks of gestation. American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine Accessed 14/12/23 https://www.bing.com/ck/a?!&&p=30a56bad003914b7JmltdHM9MTcwMjUxMjAwMCZpZ3VpZD0wZWIzY2VlOC1jNWQ0LTZmM2ItM2ZhYi1kZWI5YzQ3ZjZlMmEmaW5zaWQ9NTE3OQ&ptn=3&ver=2&hsh=3&fclid=0eb3cee8-c5d4-6f3b-3fab-deb9c47f6e2a&psq=antenatal+steroids+at+22+weeks+COG&u=a1aHR0cHM6Ly93d3cuYWNvZy5vcmcvY2xpbmljYWwvY2xpbmljYWwtZ3VpZGFuY2UvcHJhY3RpY2UtYWR2aXNvcnkvYXJ0aWNsZXMvMjAyMS8wOS91c2Utb2YtYW50ZW5hdGFsLWNvcnRpY29zdGVyb2lkcy1hdC0yMi13ZWVrcy1vZi1nZXN0YXRpb24&ntb=1 (2022).
- 40.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020;12:Cd004454. doi: 10.1002/14651858.CD004454.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyamfi-Bannerman C, et al. Antenatal betamethasone for women at risk for late preterm delivery. N. Engl. J. Med. 2016;374:1311–1320. doi: 10.1056/NEJMoa1516783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323:1924–1933. doi: 10.1001/jama.2020.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Räikkönen K, Gissler M, Tapiainen T, Kajantie E. Associations between maternal antenatal corticosteroid treatment and psychological developmental and neurosensory disorders in children. JAMA Netw. Open. 2022;5:e2228518. doi: 10.1001/jamanetworkopen.2022.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021;11:Cd001145. doi: 10.1002/14651858.CD001145.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramaswamy VV, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. 2021;175:e206826. doi: 10.1001/jamapediatrics.2020.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2009;14:391–395. doi: 10.1016/j.siny.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Rademaker KJ, Groenendaal F, van Bel F, de Vries LS, Uiterwaal CS. The DART study of low-dose dexamethasone therapy. Pediatrics. 2007;120:689–690. doi: 10.1542/peds.2007-1646. [DOI] [PubMed] [Google Scholar]
- 48.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Outcome at 2 years of age of infants from the DART study: a multicenter, international, randomized, controlled trial of low-dose dexamethasone. Pediatrics. 2007;119:716–721. doi: 10.1542/peds.2006-2806. [DOI] [PubMed] [Google Scholar]
- 49.Koome ME, et al. Antenatal dexamethasone after asphyxia increases neural injury in preterm fetal sheep. PLoS ONE. 2013;8:e77480. doi: 10.1371/journal.pone.0077480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lear CA, et al. The effects of dexamethasone on post-asphyxial cerebral oxygenation in the preterm fetal sheep. J. Physiol. 2014;592:5493–5505. doi: 10.1113/jphysiol.2014.281253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doyle, L. W., Crowther, C. A., Middleton, P., Marret, S. & Rouse, D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst. Rev.18, Cd004661 (2009). [DOI] [PubMed]
- 52.Crowther CA, et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLoS Med. 2017;14:e1002398. doi: 10.1371/journal.pmed.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf HT, et al. Magnesium sulphate for fetal neuroprotection at imminent risk for preterm delivery: a systematic review with meta-analysis and trial sequential analysis. BJOG. 2020;127:1180–1188. doi: 10.1111/1471-0528.16238. [DOI] [PubMed] [Google Scholar]
- 54.Rattray BN, et al. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J. Perinatol. 2014;34:819–822. doi: 10.1038/jp.2014.106. [DOI] [PubMed] [Google Scholar]
- 55.Kamyar M, Clark EA, Yoder BA, Varner MW, Manuck TA. Antenatal magnesium sulfate, necrotizing enterocolitis, and death among neonates < 28 weeks gestation. AJP Rep. 2016;6:e148–e154. doi: 10.1055/s-0036-1581059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung SI, et al. Increased risk of meconium-related ileus in extremely premature infants exposed to antenatal magnesium sulfate. Neonatology. 2022;119:68–76. doi: 10.1159/000520452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasath A, Aronoff N, Chandrasekharan P, Diggikar S. Antenatal magnesium sulfate and adverse gastrointestinal outcomes in preterm infants-a systematic review and meta-analysis. J. Perinatol. 2023;43:1087–1100. doi: 10.1038/s41372-023-01710-8. [DOI] [PubMed] [Google Scholar]
- 58.Macones GA. MgSO4 for CP prevention: too good to be true? Am. J. Obstet. Gynecol. 2009;200:589. doi: 10.1016/j.ajog.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 59.Crowther CA, et al. Prenatal intravenous magnesium at 30–34 weeks’ gestation and neurodevelopmental outcomes in offspring: the MAGENTA randomized clinical trial. JAMA. 2023;330:603–614. doi: 10.1001/jama.2023.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA. 2014;312:1105–1113. doi: 10.1001/jama.2014.11189. [DOI] [PubMed] [Google Scholar]
- 61.Chollat C, et al. School-age outcomes following a randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. J. Pediatr. 2014;165:398–400.e393. doi: 10.1016/j.jpeds.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Galinsky R, et al. A systematic review of magnesium sulfate for perinatal neuroprotection: what have we learnt from the past decade? Front. Neurol. 2020;11:449. doi: 10.3389/fneur.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koning G, et al. Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. J. Cereb. Blood Flow Metab. 2019;39:1038–1055. doi: 10.1177/0271678X17746132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lingam I, et al. Short-term effects of early initiation of magnesium infusion combined with cooling after hypoxia-ischemia in term piglets. Pediatr. Res. 2019;86:699–708. doi: 10.1038/s41390-019-0511-8. [DOI] [PubMed] [Google Scholar]
- 65.Galinsky R, et al. Magnesium sulphate reduces tertiary gliosis but does not improve eeg recovery or white or grey matter cell survival after asphyxia in preterm fetal sheep. J. Physiol. 2023;601:1999–2016. doi: 10.1113/JP284381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs SE, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013;2013:Cd003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shankaran S, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azzopardi D, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 70.Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin. Perinatol. 2008;35:735–748. doi: 10.1016/j.clp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Smit E, Liu X, Jary S, Cowan F, Thoresen M. Cooling neonates who do not fulfil the standard cooling criteria—short- and long-term outcomes. Acta Paediatr. 2015;104:138–145. doi: 10.1111/apa.12784. [DOI] [PubMed] [Google Scholar]
- 72.Rao R, et al. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34–35 weeks gestational age with hypoxic-ischemic encephalopathy. J. Pediatr. 2017;183:37–42. doi: 10.1016/j.jpeds.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrera TI, et al. Outcomes of preterm infants treated with hypothermia for hypoxic-ischemic encephalopathy. Early Hum. Dev. 2018;125:1–7. doi: 10.1016/j.earlhumdev.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Barrett RD, et al. Destruction and reconstruction: hypoxia and the developing brain. Birth Defects Res. C Embryo Today. 2007;81:163–176. doi: 10.1002/bdrc.20095. [DOI] [PubMed] [Google Scholar]
- 75.Bennet L, et al. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J. Physiol. 2007;578:491–506. doi: 10.1113/jphysiol.2006.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wassink G, et al. Hypothermic neuroprotection is associated with recovery of spectral edge frequency after asphyxia in preterm fetal sheep. Stroke. 2015;46:585–587. doi: 10.1161/STROKEAHA.114.008484. [DOI] [PubMed] [Google Scholar]
- 77.Kuban KC, et al. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J. Pediatr. 2017;180:116–123.e111. doi: 10.1016/j.jpeds.2016.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falck M, et al. Hypothermic neuronal rescue from infection-sensitised hypoxic-ischaemic brain injury is pathogen dependent. Dev. Neurosci. 2017;39:238–247. doi: 10.1159/000455838. [DOI] [PubMed] [Google Scholar]
- 79.Smith MJ, et al. Neural stem cell treatment for perinatal brain injury: a systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med. 2021;10:1621–1636. doi: 10.1002/sctm.21-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penny TR, et al. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. 2020;1746:147001. doi: 10.1016/j.brainres.2020.147001. [DOI] [PubMed] [Google Scholar]
- 81.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu LH, et al. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21. doi: 10.1016/j.brainres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 83.Davidson JO, et al. Window of opportunity for human amnion epithelial stem cells to attenuate astrogliosis after umbilical cord occlusion in preterm fetal sheep. Stem Cells Transl. Med. 2021;10:427–440. doi: 10.1002/sctm.20-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Heuij LG, et al. Delayed intranasal infusion of human amnion epithelial cells improves white matter maturation after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2019;39:223–239. doi: 10.1177/0271678X17729954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yawno T, et al. Human amnion epithelial cells protect against white matter brain injury after repeated endotoxin exposure in the preterm ovine fetus. Cell Transpl. 2017;26:541–553. doi: 10.3727/096368916X693572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Passera S, et al. Therapeutic potential of stem cells for preterm infant brain damage: can we move from the heterogeneity of preclinical and clinical studies to established therapeutics? Biochem. Pharm. 2021;186:114461. doi: 10.1016/j.bcp.2021.114461. [DOI] [PubMed] [Google Scholar]
- 87.Malhotra A, Novak I, Miller SL, Jenkin G. Autologous transplantation of umbilical cord blood-derived cells in extreme preterm infants: protocol for a safety and feasibility study. BMJ Open. 2020;10:e036065. doi: 10.1136/bmjopen-2019-036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bennet L, et al. Chronic inflammation and impaired development of the preterm brain. J. Reprod. Immunol. 2018;125:45–55. doi: 10.1016/j.jri.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Huppi PS, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 90.Miller SL, Supramaniam VG, Jenkin G, Walker DW, Wallace EM. Cardiovascular responses to maternal betamethasone administration in the intrauterine growth-restricted ovine fetus. Am. J. Obstet. Gynecol. 2009;201:613 e611–613.e618. doi: 10.1016/j.ajog.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 91.Ment LR, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghotra S, Vincer M, Allen VM, Khan N. A population-based study of cystic white matter injury on ultrasound in very preterm infants born over two decades in Nova Scotia, Canada. J. Perinatol. 2019;39:269–277. doi: 10.1038/s41372-018-0294-5. [DOI] [PubMed] [Google Scholar]
- 93.Northington FJ, et al. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J. Cereb. Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buser JR, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lear CA, et al. Tumour necrosis factor blockade after asphyxia in foetal sheep ameliorates cystic white matter injury. Brain. 2023;146:1453–1466. doi: 10.1093/brain/awac331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green EA, et al. Anakinra Pilot—a clinical trial to demonstrate safety, feasibility and pharmacokinetics of interleukin 1 receptor antagonist in preterm infants. Front. Immunol. 2022;13:1022104. doi: 10.3389/fimmu.2022.1022104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 98.Wallach I, et al. Erythropoietin-receptor gene regulation in neuronal cells. Pediatr. Res. 2009;65:619–624. doi: 10.1203/PDR.0b013e31819ea3b8. [DOI] [PubMed] [Google Scholar]
- 99.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci. Res. 2002;44:391–403. doi: 10.1016/S0168-0102(02)00161-X. [DOI] [PubMed] [Google Scholar]
- 100.Nagai A, et al. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J. Neuropathol. Exp. Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 101.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharm. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 103.Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36:1672–1678. doi: 10.1161/01.STR.0000173406.04891.8c. [DOI] [PubMed] [Google Scholar]
- 104.Juul SE, et al. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr. Res. 2009;65:485–492. doi: 10.1203/PDR.0b013e31819d90c8. [DOI] [PubMed] [Google Scholar]
- 105.Kumral A, et al. Protective effects of erythropoietin against ethanol-induced apoptotic neurodegenaration and oxidative stress in the developing C57bl/6 mouse brain. Brain Res. Dev. Brain Res. 2005;160:146–156. doi: 10.1016/j.devbrainres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem. Pharm. 2000;59:419–425. doi: 10.1016/S0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 107.Gorio A, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl Acad. Sci. USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Villa P, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sirén AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl Acad. Sci. USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bian XX, Yuan XS, Qi CP. Effect of recombinant human erythropoietin on serum S100b protein and interleukin-6 levels after traumatic brain injury in the rat. Neurol. Med. Chir. 2010;50:361–366. doi: 10.2176/nmc.50.361. [DOI] [PubMed] [Google Scholar]
- 111.Yatsiv I, et al. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 112.Zacharias R, et al. Dose-dependent effects of erythropoietin in propofol anesthetized neonatal rats. Brain Res. 2010;1343:14–19. doi: 10.1016/j.brainres.2010.04.081. [DOI] [PubMed] [Google Scholar]
- 113.Kumral A, et al. Erythropoietin increases glutathione peroxidase enzyme activity and decreases lipid peroxidation levels in hypoxic-ischemic brain injury in neonatal rats. Biol. Neonate. 2005;87:15–18. doi: 10.1159/000080490. [DOI] [PubMed] [Google Scholar]
- 114.Gonzalez FF, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44:753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr. Res. 2007;61:451–455. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- 116.Gonzalez FF, et al. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev. Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gonzalez FF, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 118.Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr. Res. 2005;58:297–301. doi: 10.1203/01.PDR.0000169971.64558.5A. [DOI] [PubMed] [Google Scholar]
- 119.McPherson RJ, Demers EJ, Juul SE. Safety of high-dose recombinant erythropoietin in a neonatal rat model. Neonatology. 2007;91:36–43. doi: 10.1159/000096969. [DOI] [PubMed] [Google Scholar]
- 120.Iwai M, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wassink G, et al. Partial white and grey matter protection with prolonged infusion of recombinant human erythropoietin after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2017;37:1080–1094. doi: 10.1177/0271678X16650455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dhillon SK, et al. Adverse neural effects of delayed, intermittent treatment with rEPO after asphyxia in preterm fetal sheep. J. Physiol. 2021;599:3593–3609. doi: 10.1113/JP281269. [DOI] [PubMed] [Google Scholar]
- 123.Ohls RK, et al. Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114:1287–1291. doi: 10.1542/peds.2003-1129-L. [DOI] [PubMed] [Google Scholar]
- 124.Ohls RK, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–1030. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natalucci G, et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA. 2016;315:2079–2085. doi: 10.1001/jama.2016.5504. [DOI] [PubMed] [Google Scholar]
- 126.Song J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann. Neurol. 2016;80:24–34. doi: 10.1002/ana.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Juul SE, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 2020;382:233–243. doi: 10.1056/NEJMoa1907423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer, H. S., Reibel, N. J., Bührer, C. & Dame, C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics139, e20164317 (2017). [DOI] [PubMed]
- 129.Wood TR, et al. Early biomarkers of hypoxia and inflammation and two-year neurodevelopmental outcomes in the preterm erythropoietin neuroprotection (PENUT) trial. EBioMedicine. 2021;72:103605. doi: 10.1016/j.ebiom.2021.103605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robertson NJ, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J. Pediatr. 2012;160:544–552.e544. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drury PP, et al. Partial neural protection with prophylactic low-dose melatonin after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2014;34:126–135. doi: 10.1038/jcbfm.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yawno T, et al. The beneficial effects of melatonin administration following hypoxia-ischemia in preterm fetal sheep. Front. Cell Neurosci. 2017;11:296. doi: 10.3389/fncel.2017.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robertson NJ, et al. High-dose melatonin and ethanol excipient combined with therapeutic hypothermia in a newborn piglet asphyxia model. Sci. Rep. 2020;10:3898. doi: 10.1038/s41598-020-60858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robertson NJ, et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: a translational study. Neurobiol. Dis. 2019;121:240–251. doi: 10.1016/j.nbd.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 135.Ethanol in liquid preparations intended for children. Pediatrics73, 405–407 (1984). [PubMed]
- 136.DeMuro RL, Nafziger AN, Blask DE, Menhinick AM, Bertino JS., Jr. The absolute bioavailability of oral melatonin. J. Clin. Pharm. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 137.El-Gendy FM, El-Hawy MA, Hassan MG. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Fetal Neonatal Med. 2018;31:2299–2303. doi: 10.1080/14767058.2017.1342794. [DOI] [PubMed] [Google Scholar]
- 138.Gitto E, et al. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: improvement with melatonin treatment. J. Pineal Res. 2005;39:287–293. doi: 10.1111/j.1600-079X.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 139.Marseglia L, et al. Antioxidant effect of melatonin in preterm newborns. Oxid. Med. Cell Longev. 2021;2021:6308255. doi: 10.1155/2021/6308255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coviello C, et al. Isoprostanes as biomarker for white matter injury in extremely preterm infants. Front. Pediatr. 2020;8:618622. doi: 10.3389/fped.2020.618622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Häusler, S. et al. Melatonin as a therapy for preterm brain injury: what is the evidence? Antioxidants12, 1630 (2023). [DOI] [PMC free article] [PubMed]
- 142.Australian New Zealand Clinical Trials Registry (ANZCTR). Cognitive Improvement at 2 Years Corrected Postnatal Age through Early Restoration of Circadian Rhythms in Very Preterm Infants Via Environmental Modification from Birth until Discharge Home: The CIRCA DIEM Study.https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374490 (ANZCTR, 2018).
- 143.Schmidt B, et al. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 144.Henderson‐Smart, D. J. & De Paoli, A. G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev.8, CD000140 (2010). [DOI] [PubMed]
- 145.Bruschettini, M. et al. Caffeine dosing regimens in preterm infants with or at risk for apnea of prematurity. Cochrane Database Syst. Rev.4, CD013873 (2023). [DOI] [PMC free article] [PubMed]
- 146.Schmidt B, et al. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt B, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 148.Mürner-Lavanchy, I. M. et al. Neurobehavioral outcomes 11 years after neonatal caffeine therapy for apnea of prematurity. Pediatrics141, e20174047 (2018). [DOI] [PubMed]
- 149.Schmidt B, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 2017;171:564–572. doi: 10.1001/jamapediatrics.2017.0238. [DOI] [PubMed] [Google Scholar]
- 150.Parikka V, et al. The effect of caffeine citrate on neural breathing pattern in preterm infants. Early Hum. Dev. 2015;91:565–568. doi: 10.1016/j.earlhumdev.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 151.Back SA, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 152.McLeod, R. M., Rosenkrantz, T. S., Fitch, R. H. & Koski, R. R. Sex differences in microglia activation in a rodent model of preterm hypoxic ischemic injury with caffeine treatment. Biomedicines11, 185 (2023). [DOI] [PMC free article] [PubMed]
- 153.Alexander M, Smith AL, Rosenkrantz TS, Fitch RH. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013;3:177–190. doi: 10.3390/brainsci3010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kilicdag H, Daglioglu YK, Erdogan S, Zorludemir S. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J. Matern. Fetal Neonatal Med. 2014;27:1470–1475. doi: 10.3109/14767058.2013.878694. [DOI] [PubMed] [Google Scholar]
- 155.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J. Pediatr. 1999;134:71–75. doi: 10.1016/S0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- 156.Glass HC, et al. Seizures in preterm neonates: a multicenter observational cohort study. Pediatr. Neurol. 2017;72:19–24. doi: 10.1016/j.pediatrneurol.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 158.Zhou, K. Q. et al. Treating seizures after hypoxic-ischemic encephalopathy—current controversies and future directions. Int. J. Mol. Sci.22, 7121 (2021). [DOI] [PMC free article] [PubMed]
- 159.Galinsky R, et al. Magnesium sulfate reduces eeg activity but is not neuroprotective after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2017;37:1362–1373. doi: 10.1177/0271678X16655548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bittigau P, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl Acad. Sci. USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol. 2012;72:363–372. doi: 10.1002/ana.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Browne JV, Jaeger CB, Kenner C. Executive summary: standards, competencies, and recommended best practices for infant- and family-centered developmental care in the intensive care unit. J. Perinatol. 2020;40:5–10. doi: 10.1038/s41372-020-0767-1. [DOI] [PubMed] [Google Scholar]
- 163.WHO Immediate KMC Study Group. Impact of continuous kangaroo mother care initiated immediately after birth (iKMC) on survival of newborns with birth weight between 1.0 to < 1.8 kg: study protocol for a randomized controlled trial. Trials. 2020;21:280. doi: 10.1186/s13063-020-4101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cho ES, et al. The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal-infant attachment, and maternal stress. J. Pediatr. Nurs. 2016;31:430–438. doi: 10.1016/j.pedn.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 165.Gonya J, Ray WC, Rumpf RW, Brock G. Investigating skin-to-skin care patterns with extremely preterm infants in the NICU and their effect on early cognitive and communication performance: a retrospective cohort study. BMJ Open. 2017;7:e012985. doi: 10.1136/bmjopen-2016-012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marvin MM, Gardner FC, Sarsfield KM, Travagli RA, Doheny KK. Increased frequency of skin-to-skin contact is associated with enhanced vagal tone and improved health outcomes in preterm neonates. Am. J. Perinatol. 2019;36:505–510. doi: 10.1055/s-0038-1669946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Georgieff MK, Ramel SE, Cusick SE. Nutritional influences on brain development. Acta Paediatr. 2018;107:1310–1321. doi: 10.1111/apa.14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ottolini KM, Andescavage N, Keller S, Limperopoulos C. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediatr. Res. 2020;87:194–201. doi: 10.1038/s41390-019-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McPherson C, et al. Brain injury and development in preterm infants exposed to fentanyl. Ann. Pharmacother. 2015;49:1291–1297. doi: 10.1177/1060028015606732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giordano V, et al. Effect of increased opiate exposure on three years neurodevelopmental outcome in extremely preterm infants. Early Hum. Dev. 2018;123:1–5. doi: 10.1016/j.earlhumdev.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 171.Zwicker JG, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J. Pediatr. 2016;172:81–87.e82. doi: 10.1016/j.jpeds.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McPherson C, Miller SP, El-Dib M, Massaro AN, Inder TE. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 2020;88:168–175. doi: 10.1038/s41390-019-0744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duerden EG, et al. Association of early skin breaks and neonatal thalamic maturation: a modifiable risk? Neurology. 2020;95:e3420–e3427. doi: 10.1212/WNL.0000000000010953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brummelte S, et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 2012;71:385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vinall J, et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133:412–421. doi: 10.1542/peds.2013-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Campbell-Yeo M, et al. Sustained efficacy of kangaroo care for repeated painful procedures over neonatal intensive care unit hospitalization: a single-blind randomized controlled trial. Pain. 2019;160:2580–2588. doi: 10.1097/j.pain.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 177.Harrison D, Loughnan P, Manias E, Johnston L. Analgesics administered during minor painful procedures in a cohort of hospitalized infants: a prospective clinical audit. J. Pain. 2009;10:715–722. doi: 10.1016/j.jpain.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 178.Bennet L, Walker DW, Horne RSC. Waking up too early—the consequences of preterm birth on sleep development. J. Physiol. 2018;596:5687–5708. doi: 10.1113/JP274950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van den Hoogen A, et al. How to improve sleep in a neonatal intensive care unit: a systematic review. Early Hum. Dev. 2017;113:78–86. doi: 10.1016/j.earlhumdev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 180.Erdei C, Inder TE, Dodrill P, Woodward LJ. The Growth and Development Unit. A proposed approach for enhancing infant neurodevelopment and family-centered care in the neonatal intensive care unit. J. Perinatol. 2019;39:1684–1687. doi: 10.1038/s41372-019-0514-7. [DOI] [PubMed] [Google Scholar]
- 181.O’Brien K, et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc. Health. 2018;2:245–254. doi: 10.1016/S2352-4642(18)30039-7. [DOI] [PubMed] [Google Scholar]
- 182.Edwards EM, Horbar JD. Following through: interventions to improve long-term outcomes of preterm infants. Semin. Perinatol. 2021;45:151414. doi: 10.1016/j.semperi.2021.151414. [DOI] [PubMed] [Google Scholar]
- 183.Waddington C, van Veenendaal NR, O’Brien K, Patel N. Family integrated care: supporting parents as primary caregivers in the neonatal intensive care unit. Pediatr. Investig. 2021;5:148–154. doi: 10.1002/ped4.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Baier RJ. Genetics of perinatal brain injury in the preterm infant. Front. Biosci. 2006;11:1371–1387. doi: 10.2741/1890. [DOI] [PubMed] [Google Scholar]
- 186.Sparrow S, et al. Epigenomic profiling of preterm infants reveals DNA methylation differences at sites associated with neural function. Transl. Psychiatry. 2016;6:e716. doi: 10.1038/tp.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blüml S, Wisnowski JL, Nelson MD, Jr., Paquette L, Panigrahy A. Metabolic maturation of white matter is altered in preterm infants. PLoS ONE. 2014;9:e85829. doi: 10.1371/journal.pone.0085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin. Perinatol. 2008;35:777–792. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kofke WA. Incrementally applied multifaceted therapeutic bundles in neuroprotection clinical trials…time for change. Neurocrit. Care. 2010;12:438–444. doi: 10.1007/s12028-010-9332-7. [DOI] [PubMed] [Google Scholar]
- 190.Helenius K, Longford N, Lehtonen L, Modi N, Gale C. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678. doi: 10.1136/bmj.l5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fabres J, Carlo WA, Phillips V, Howard G, Ambalavanan N. Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics. 2007;119:299–305. doi: 10.1542/peds.2006-2434. [DOI] [PubMed] [Google Scholar]
- 192.Miller SS, Lee HC, Gould JB. Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J. Perinatol. 2011;31:S49–S56. doi: 10.1038/jp.2010.177. [DOI] [PubMed] [Google Scholar]
- 193.Vesoulis ZA, et al. Blood pressure extremes and severe IVH in preterm infants. Pediatr. Res. 2020;87:69–73. doi: 10.1038/s41390-019-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goswami IR, Abou Mehrem A, Scott J, Esser MJ, Mohammad K. Metabolic acidosis rather than hypo/hypercapnia in the first 72 h of life associated with intraventricular hemorrhage in preterm neonates. J. Matern. Fetal Neonatal Med. 2021;34:3874–3882. doi: 10.1080/14767058.2019.1701649. [DOI] [PubMed] [Google Scholar]
- 195.Malusky S, Donze A. Neutral head positioning in premature infants for intraventricular hemorrhage prevention: an evidence-based review. Neonatal Netw. 2011;30:381–396. doi: 10.1891/0730-0832.30.6.381. [DOI] [PubMed] [Google Scholar]
- 196.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol. Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 197.Lee HJ, et al. Early sodium and fluid intake and severe intraventricular hemorrhage in extremely low birth weight infants. J. Korean Med. Sci. 2015;30:283–289. doi: 10.3346/jkms.2015.30.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murthy P, et al. Neuroprotection care bundle implementation to decrease acute brain injury in preterm infants. Pediatr. Neurol. 2020;110:42–48. doi: 10.1016/j.pediatrneurol.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 199.de Bijl-Marcus K, Brouwer AJ, De Vries LS, Groenendaal F, Wezel-Meijler GV. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: a multicentre cohort study. Arch. Dis. Child Fetal Neonatal Ed. 2020;105:419–424. doi: 10.1136/archdischild-2018-316692. [DOI] [PubMed] [Google Scholar]
- 200.McLendon D, et al. Implementation of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics. 2003;111:e497–e503. doi: 10.1542/peds.111.SE1.e497. [DOI] [PubMed] [Google Scholar]
- 201.Benlamri A, et al. Neuroprotection care bundle implementation is associated with improved long-term neurodevelopmental outcomes in extremely premature infants. J. Perinatol. 2022;42:1380–1384. doi: 10.1038/s41372-022-01443-0. [DOI] [PubMed] [Google Scholar]
- 202.Mohammad K, et al. Impact of quality improvement outreach education on the incidence of acute brain injury in transported neonates born premature. J. Perinatol. 2022;42:1368–1373. doi: 10.1038/s41372-022-01409-2. [DOI] [PubMed] [Google Scholar]
- 203.Cramer SC, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.DeMaster D, et al. Nurturing the preterm infant brain: leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr. Res. 2019;85:166–175. doi: 10.1038/s41390-018-0203-9. [DOI] [PubMed] [Google Scholar]
- 205.Maguire CM, et al. Effects of individualized developmental care in a randomized trial of preterm infants <32 weeks. Pediatrics. 2009;124:1021–1030. doi: 10.1542/peds.2008-1881. [DOI] [PubMed] [Google Scholar]
- 206.Procianoy RS, Mendes EW, Silveira RC. Massage therapy improves neurodevelopment outcome at two years corrected age for very low birth weight infants. Early Hum. Dev. 2010;86:7–11. doi: 10.1016/j.earlhumdev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 207.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 208.Braid S, Bernstein J. Improved cognitive development in preterm infants with shared book reading. Neonatal Netw. 2015;34:10–17. doi: 10.1891/0730-0832.34.1.10. [DOI] [PubMed] [Google Scholar]
- 209.Chorna O, et al. Neuroprocessing mechanisms of music during fetal and neonatal development: a role in neuroplasticity and neurodevelopment. Neural Plast. 2019;2019:3972918. doi: 10.1155/2019/3972918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shellhaas, R. A., Burns, J. W., Barks, J. D. E., Hassan, F. & Chervin, R. D. Maternal voice and infant sleep in the neonatal intensive care unit. Pediatrics144, e20190288 (2019). [DOI] [PMC free article] [PubMed]
- 211.Smith SW, Ortmann AJ, Clark WW. Noise in the neonatal intensive care unit: a new approach to examining acoustic events. Noise Health. 2018;20:121–130. doi: 10.4103/nah.NAH_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep40, zsx144 (2017). [DOI] [PMC free article] [PubMed]
- 213.Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118:e107–e114. doi: 10.1542/peds.2005-1145. [DOI] [PubMed] [Google Scholar]
- 214.Benavente-Fernández I, Siddiqi A, Miller SP. Socioeconomic status and brain injury in children born preterm: modifying neurodevelopmental outcome. Pediatr. Res. 2020;87:391–398. doi: 10.1038/s41390-019-0646-7. [DOI] [PubMed] [Google Scholar]
- 215.Bills SE, Johnston JD, Shi D, Bradshaw J. [Formula: see text] Social-environmental moderators of neurodevelopmental outcomes in youth born preterm: a systematic review. Child Neuropsychol. 2021;27:351–370. doi: 10.1080/09297049.2020.1861229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169:1162–1172. doi: 10.1001/jamapediatrics.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Joseph RM, et al. Maternal social risk, gestational age at delivery, and cognitive outcomes among adolescents born extremely preterm. Paediatr. Perinat. Epidemiol. 2022;36:654–664. doi: 10.1111/ppe.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Benavente-Fernández I, et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open. 2019;2:e192914. doi: 10.1001/jamanetworkopen.2019.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Salazar EG, et al. County-level maternal vulnerability and preterm birth in the US. JAMA Netw. Open. 2023;6:e2315306. doi: 10.1001/jamanetworkopen.2023.15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carter JG, Feinglass JM, Yee LM. Perception of neighborhood safety and maternal and neonatal health outcomes. JAMA Netw. Open. 2023;6:e2317153. doi: 10.1001/jamanetworkopen.2023.17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mckinnon K, et al. Association of preterm birth and socioeconomic status with neonatal brain structure. JAMA Netw. Open. 2023;6:e2316067. doi: 10.1001/jamanetworkopen.2023.16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.