Abstract
Background
Aggressive metastasis directed treatment of extracranial oligometastatic breast cancer with the aim of increasing disease-free survival has emerged as a new potential treatment paradigm, however there is currently a lack of data to assist in identifying the subset of patients who will potentially benefit most. This single-institute retrospective cohort study aimed to evaluate survival outcomes for patients with a solitary extracranial metastasis from breast cancer and to assess for significant prognostic factors.
Methods and materials
Medical records of 70 female breast cancer patients with a solitary extracranial metastasis actively managed at the Peter MacCallum Cancer Centre (PMCC) Melbourne Campus between 2000 and 2019 were reviewed. Kaplan-Meier curves were used to estimate overall survival (OS), local progression free survival (LPFS) and distant progression free survival (DPFS).
Results
Median follow-up period was 9.4 years. The study included 40 hormone receptor positive/HER2 negative (HR+HER2-), 14 hormone receptor positive/HER2 positive (HR+HER2+), 3 hormone receptor negative/HER2 positive (HR-HER2+), 9 triple negative (TNBC) and 4 unclassified breast cancer patients. 5-year OS rate for all patients was 46%, LPFS rate was 56% and DPFS was 20%. Tumour receptor group had a statistically significant association with OS and DPFS rates. TNBC patients had significantly poorer OS and DPFS rates in comparison to HR+HER2-patients.
Conclusion
Among patients with a solitary extracranial metastasis from breast cancer, TNBC was associated with the poorest OS and DPFS rates. Identification of other significant prognostic factors for oligometastatic breast cancer patients may inform guidelines for metastasis directed treatments.
Keywords: Oligometastatic, Solitary metastasis, Breast cancer
Highlights
-
•
Aggressive metastasis-directed treatment for OMBC is an area of active research.
-
•
Tumour receptor group is a prognostic factor for OMBC.
-
•
Identification of other prognostic factors may inform treatment guidelines.
1. Introduction
Breast cancer is among the most commonly diagnosed cancers in women with 3–6% of breast cancer cases metastatic at diagnosis [1]. The 5-year rate of distant recurrence in women with T1-T3 node negative and T4/node positive breast cancer has been reported as 4.4% and 10.4% respectively [2]. In Australia, the 5-year relative survival rate for metastatic breast cancer (MBC) is 32% [3]. Survival rates vary according to the presence or absence of hormone receptors (HR), which are the oestrogen (ER) and progesterone (PR) receptors, as well as the human epidermal growth factor receptor 2 (HER2) receptor with TNBC associated with the poorest overall and progression free survival rates [4].
Oligometastatic breast cancer (OMBC) represents a subset of MBC and is defined in the 5th ESO-ESMO international consensus guidelines for advanced breast cancer as low-volume metastatic disease with limited number and size of metastatic lesions (up to 5 and not necessarily in the same organ), potentially amenable for local treatment aimed at achieving a complete remission status [5]. Oligometastatic disease can be further categorised according to guidelines from the EORTC and ESTRO, for instance into synchronous and metachronous according to timing of metastasis identification in relation to diagnosis of the primary [6]. Among OMBC patients, the total number of metastases has been reported as a significant prognostic factor for progression free survival rate [7]. For patients with a solitary metastasis from breast cancer, survival rates vary according to metastasis site with a reported median overall survival of 7.00–7.54 years for bone [8,9] and of 20 months for brain [10].
For some extracranial OMBC cases, aggressive metastasis directed treatments are justifiable given the possibility of improving progression free survival rates. This might include treatment with surgery, stereotactic ablative radiotherapy (SABR) or radio-frequency ablation. However, there is a knowledge gap in identifying which breast cancer patients with extracranial metastases are most suitable for aggressive treatment. Treating a solitary metastasis from breast cancer with curative intent was favoured by an expert panel at the St Gallen/Vienna 2023 Consensus Conference on Early Breast Cancer Treatment Standards, while palliative treatment was recommended for patients with multiple metastases [11].
The aim of this single-institute retrospective cohort study was to compare outcomes including OS, LPFS and DPFS rates among patients with a solitary extracranial metastasis from breast cancer and to assess the significance of several potentially important prognostic factors.
2. Materials and methods
2.1. Patient eligibility criteria
The eligibility criteria for this study were: female sex, metastatic breast cancer, a solitary extracranial metastasis and receipt of active management at the PMCC from the year 2000–2019. Active management was defined as having attended the PMCC (Melbourne Campus) for at least two clinic appointments. Patients may have received any form of treatment, including palliative intent treatment.
2.2. Patient identification
The medical records of 70 female breast cancer patients fitting eligibility criteria were reviewed. These patients were identified in a search of electronic medical records for references to “solitary,” “single,” or “isolated,” “metastasis” and cross-referenced with references to a breast cancer diagnosis. As a result of this search strategy 82 patients who were potentially eligible for inclusion in this study were identified. Further review of each patient's medical record was performed to confirm eligibility. During this process twelve patients were identified who did not fit the inclusion criteria. Nine patients had multiple metastases at MBC diagnosis, while one had an adrenal mass initially thought to represent breast cancer metastasis, but subsequent biopsy confirmed as clear cell carcinoma. One patient had an erroneous medical record entry of breast cancer diagnosis. One patient had cervical vertebral imaging changes initially thought to represent a metastasis but subsequently confirmed to be inflammatory.
2.3. Data collection
Data obtained from medical records was recorded using a Research Electronic Data Capture (REDCap) database. Breast cancer oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status was recorded as HR+HER2- (ER+ and/or PR+ and HER2-), HR+HER2+ (ER+ and/or PR+ and HER2+), triple negative (ER-PR-HER2-) or HR-HER2+ (ER-PR-HER2+). For cases where documentation of tumour receptor group was missing from medical records, tumour receptor group was recorded as unclassified. Single extracranial metastasis site was recorded into the categories of distant nodal, bone, liver, lung and other. Bone metastasis site was further delineated as sternal, spine, pelvic girdle, rib, femur, humerus or other. Patient treatment with systemic therapy, radiotherapy or surgery within 2 months was recorded. Systemic treatment included bone modifying agents, endocrine therapy, chemotherapy, targeted therapy or immunotherapy.
2.4. Endpoints
OS rate was measured from the date of MBC diagnosis to the date of death due to any cause. LPFS was measured from date of MBC diagnosis to date of local progression of the solitary extracranial metastasis. The date of local progression was based on the dates of imaging and clinical documentation. DPFS was measured from the date of MBC diagnosis to the date of distant progression (excluding the solitary extracranial metastasis) based on the dates of imaging and clinical documentation. Potential prognostic factors for OS, LPFS and DPFS that were assessed in this study included age at diagnosis of MBC, synchronous metastasis, loco-regional breast cancer in situ at time of MBC diagnosis, systemic treatment within 2 months of MBC diagnosis, metastasis directed surgery within 2 months of MBC diagnosis, metastasis directed radiotherapy within 2 months of MBC diagnosis and tumour receptor group. In the assessment of prognostic factors HR+HER2+ and HR-HER2+ patients were grouped together into one HER2+ tumour receptor group. In this study there were very low numbers of patients with a solitary metastasis at sites other than bone. Furthermore, for those with bone metastases there were very low numbers of patients within each bone subsite other than spine. As a result, meaningful statistical analysis of the differences in survival rates was not able to be performed comparing each metastasis subsite.
2.5. Statistical analysis
For breast cancer patients included in this study the Kaplan-Meier estimator was used to estimate the time-to-event outcomes, which were OS, LPFS and DPFS. Cox proportional hazard models were used to assess associations between the potential prognostic factors listed above and OS, LPFS and DPFS. A p-value of less than 0.05 on multivariable analysis with a hazard ratio 95% confidence interval (CI) not crossing 1.0 was considered statistically significant. All statistical analyses were performed in R (R version 4.0.3 (2020-10-10)).
Ethics approval
Approval for this study was received from the research ethics committee of the Peter MacCallum Cancer Centre (HREC/67310/PMCC-2020).
3. Results
3.1. Patient demographics and clinicopathological characteristics
Table 1 shows the characteristics of patients included in this study. The median age at diagnosis of metastatic breast cancer was 50 years. Metastatic disease was more commonly metachronous, diagnosed more than 6 months following the primary breast cancer diagnosis (n = 46, 66%). HR+HER2-breast cancer was the most common tumour receptor group (n = 40, 57%) followed by HR+ HER2+ (n = 14, 20%), triple negative (n = 9, 13%) and HR-HER2+ (n = 3, 4%). The most common site of metastasis was bone (n = 53, 76%) with spinal metastasis being the most common bone subsite (n = 22, 31%). The characteristics of treatment are described in Table 2. Most patients (n = 60, 86%) received systemic treatment such as endocrine therapy, chemotherapy, bone-modifying agent, targeted therapy or immunotherapy within 2 months of metastatic breast cancer diagnosis. Most patients received metastasis directed therapy at some point in their treatment (n = 58, 83%). For non-stereotactic radiotherapy median dose was 20 Gy (range, 8–50 Gy) and the median fraction number was 5 (range, 1–25). For stereotactic radiotherapy the median dose was 20 Gy (range 20–24) and the median fraction number was 1 (range 1–2).
Table 1.
Patient characteristics.
| Characteristic | Total (n = 70) |
|---|---|
| Age at diagnosis MBC (years) | |
| Median [range] | 50.0 [28.0–77.0] |
| Synchronous Metastasis | |
| Yes | 24 (34%) |
| No | 46 (66%) |
| Tumour receptor | |
| HR + HER2- | 40 (57%) |
| HR + HER2+ | 14 (20%) |
| HER2+HR- | 3 (4%) |
| Triple Negative | 9 (13%) |
| Unclassified | 4 (6%) |
| Solitary extracranial metastasis site | |
| Bone | 53 (76%) |
| Distant Nodal | 2 (3%) |
| Liver | 6 (9%) |
| Lung | 6 (9%) |
| Other | 3 (4%) |
| Solitary extracranial metastasis bone subsite | |
| Spine | 22 (31%) |
| Pelvic girdle | 8 (11%) |
| Sternum | 8 (11%) |
| Rib | 6 (9%) |
| Femur | 5 (7%) |
| Humerus | 1 (1%) |
| Other | 3 (4%) |
| Loco-regional breast cancer in situ at time of MBC diagnosis (within 2 months) | |
| Yes | 25 (36%) |
| No | 45 (64%) |
Table 2.
Treatment characteristics.
| Systemic treatment at MBC diagnosis (within 2 months) | |
| Yes | 60 (86%) |
| No | 10 (14%) |
| Metastasis directed treatment at MBC diagnosis (within 2 months) | |
| Surgery | 12 (17%) |
| SABR | 6 (9%) |
| Non-stereotactic radiotherapy | 17 (24%) |
| None | 37 (53%) |
| Metastasis directed treatment at any time | |
| Surgery | 18 (26%) |
| SABR | 15 (21%) |
| Non-stereotactic radiotherapy | 33 (47%) |
| None | 12 (17%) |
3.2. Survival rate according to tumour receptor group
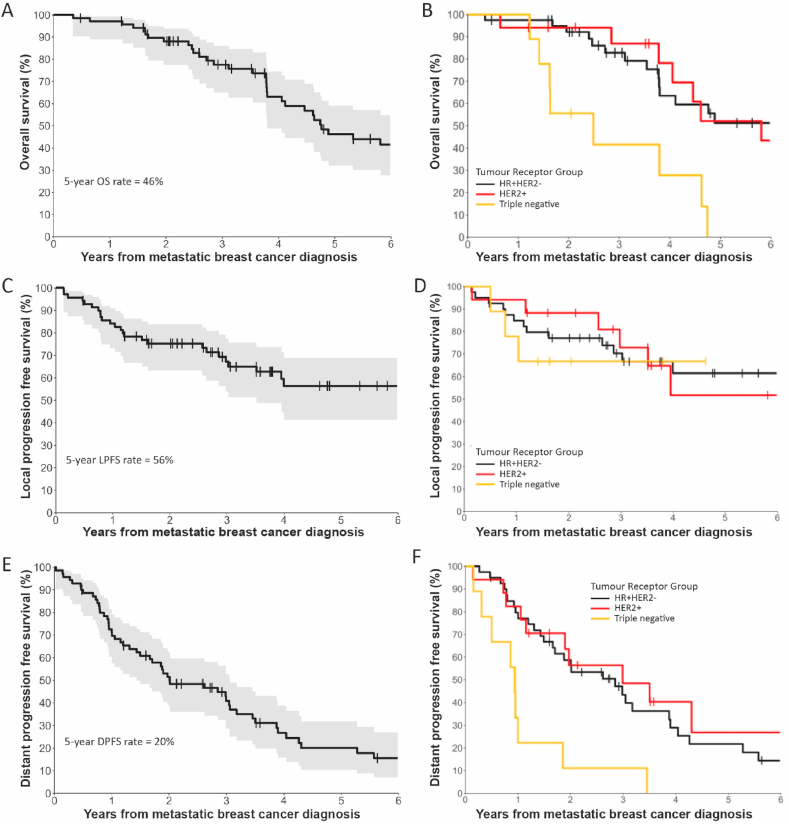
Kaplan-Meier curves comparing OS, DPFS and LFPS rates between tumour receptor groups are depicted in Fig. 1. The median OS for all 70 patients was 4.8 years, for triple negative breast cancer was 2.5 years, for HR+HER2-breast cancer was 7.4 years and for HER2+ breast cancer was 5.8 years (Fig. 1A and B). The 5-year LPFS rate was 56% for all patients, 61% for HR+HER2-breast cancer and 64% for HER2+ breast cancer (Fig. 1C and D). Median DPFS for all patients was 2.0 years, for triple negative breast cancer was 0.9 years, for HR+HER2-breast cancer was 2.8 years and for HER2+ breast cancer was 3.0 years (Fig. 1E and F).
Fig. 1.
A) Overall survival, C) Local progression free survival and E) Distant progression free survival for all 70 patients included in the study. The shaded area represents 95% confidence interval. B) Overall survival, D) Local progression free survival and F) Distant progression free survival according to tumour receptor group. Vertical ticks represent censored patients.
3.3. Cox regression analysis of potential prognostic factors
There was a significant association between tumour receptor group and OS (p = 0.012). There was no significant difference in OS of HER2+ and the reference group HR+HER2-but there was a significant difference in OS between TNBC and HR+HER2-breast cancer patients with a hazard ratio of 4.4 (95% confidence interval 1.6–12.0). Similarly, tumour receptor group was a statistically significant prognostic factor for DPFS (p = 0.008), with no significant difference in DPFS between HER2+ and HR+HER2-breast cancer, but a statistically significant difference between TNBC and HR+HER2-breast cancer patients. Tumour receptor group was not significantly associated with LPFS (p = 0.264). Other potential prognostic factors assessed in this study included age at diagnosis of metastatic breast cancer, synchronous metastasis, loco-regional breast cancer in situ at time of metastatic breast cancer diagnosis, systemic treatment within 2 months of metastatic breast cancer diagnosis, metastasis directed surgery within 2 months of MBC diagnosis and metastasis directed radiotherapy within 2 months of MBC diagnosis. None of these factors were found to have a statistically significant association with OS, LPFS or DPFS (see Table 3).
Table 3.
Multivariable Cox regression analysis of potential prognostic factors for OS, LPFS and DPFS.
| Overall Survival |
Local Progression Free Survival |
Distant Progression Free Survival |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Level | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age at MBC diagnosis | Per year increase | 1.0 (1.0, 1.1) | 0.299 | 1.0 (1.0, 1.0) | 0.807 | 1.0 (1.0, 1.0) | 0.810 |
| Tumour receptor group | HR+HER2- | reference | 0.012 | reference | 0.264 | reference | 0.008 |
| HER2+ | 0.8 (0.3, 1.8) | 0.7 (0.2, 2.3) | 0.9 (0.4, 1.8) | ||||
| Triple Negative | 4.4 (1.6, 12.0) | 2.8 (0.7, 11.2) | 4.4 (1.8, 10.7) | ||||
| Loco-regional breast cancer in situ at time of MBC diagnosis | No | reference | 0.131 | reference | 0.335 | reference | 0.399 |
| Yes | 0.3 (0.0, 1.8) | 0.4 (0.1, 2.6) | 0.6 (0.2, 2.2) | ||||
| Systemic treatment at MBC diagnosis | No | reference | 0.229 | reference | 0.146 | reference | 0.475 |
| Yes | 2.0 (0.6, 6.4) | 3.8 (0.5, 31.8) | 1.4 (0.5, 3.6) | ||||
| Metastasis directed radiotherapy at MBC diagnosis | No | reference | 0.975 | reference | 0.286 | reference | 0.657 |
| Yes | 1.0 (0.4, 2.4) | 0.6 (0.2, 1.7) | 0.9 (0.4, 1.7) | ||||
| Metastasis directed surgery at MBC diagnosis | No | reference | 0.120 | reference | 0.043 | reference | 0.178 |
| Yes | 2.2 (0.8, 6.0) | 0.2 (0.0, 1.4) | 1.8 (0.8, 4.0) | ||||
| Synchronous metastasis | No | reference | 0.092 | reference | 0.729 | reference | 0.851 |
| Yes | 4.7 (0.6, 35.1) | 0.7 (0.1, 4.6) | 1.1 (0.3, 4.3) | ||||
Reference denotes the patient group which was the reference for calculation of the hazard ratio.
HR = Hazard Ratio, CI = Confidence interval.
4. Discussion
Metastatic breast cancer patients with few sites of metastasis may be considered for aggressive local treatment even when these metastases are asymptomatic, not only to promote local control but with the aim of achieving increased disease free and overall survival rates [12,13].
It is currently unclear which subgroups among OMBC patients should be considered for aggressive treatment. These local treatments to single site metastases for other cancer types have been shown to be able to confer an overall survival benefit, for example resection of liver metastases in cases of colorectal cancer [14]. A similar benefit has not yet been identified in randomised prospective clinical trials for oligometastatic breast cancer [15].
The SABR-COMET phase II randomised trial assessed OS rates among patients with a controlled primary malignancy and 1–5 metastases who received either metastasis directed SABR vs standard of care [16]. Multiple different histologies were included in the study with breast the primary for 18 of the 99 patients. A significant improvement in the 5-year OS rate for patients treated with SABR was reported. However, an imbalance in the number of patients with a solitary metastasis for each group (30 of 66 in the SABR group and 12 of 33 in the standard of care group) may have been a confounding factor. A further evaluation of the effect of metastasis directed SABR on survival rates among patients with oligometastatic disease is currently underway in larger phase III randomised trials including SABR-COMET-3 and SABR-COMET-10 [17,18].
In contrast to the SABR-COMET trial, the results of the breast cancer specific NRG-BR002 trial indicated that although metastasis directed SABR and surgery improve local control, there may be no impact on overall or progression free survival rates in the setting of OMBC. 125 OMBC patients with 1–4 extracranial metastases were included in this trial and initial analysis of survival rates compared patients who received standard of care systemic therapy with or without treatment to all metastatic sites with SABR or surgical resection [19]. The rate of progression at the treated/index sites of metastasis was lower in the SABR/surgery arm compared to standard of care (6.7% vs 29.2%). There was no significant improvement in median progression free survival or overall survival rates identified in the phase II part of the trial. As a result, the phase III component did not proceed. A large proportion of the patients included in this study had favourable prognostic features with respect to tumour receptor status and number of metastases: 79% had HR+HER2-breast cancer and 60% had a solitary metastasis. However, median follow-up was relatively short at 30 months, and it is possible that this follow-up period was too limited to identify a subset of patients who may have benefitted from aggressive metastasis directed treatment.
Our study aimed to assess the outcomes of breast cancer patients with a solitary extracranial metastasis and to identify prognostic factors with a significant association with OS, DPFS and LPFS. For patients included in this study the 5-year OS rate was 46% and the median OS was 4.8 years. These rates are favourable when compared to those reported in other studies for breast cancer with metastases of any site or number. A 2010 study performed by Dawood et al. retrospectively reviewed a large database of MBC patients and reported median overall survival rate of 39.2 months for patients with de novo MBC and 27.2 months for patients with distant recurrence of previously non-MBC [20]. The 5-year OS rate of 46% was close to those reported in other small single-institute retrospective studies of oligometastatic breast cancer. Lan et al. (2020) reported a 5-year OS rate of 58% for patients with extracranial oligometastatic breast cancer (1–3 lesions), the majority of which had a solitary extracranial metastasis (42/50 = 84% of the patient cohort) [21]. Yoo et al. (2015) reported a 5-year OS rate of 61.6% for 50 patients with a solitary bone metastasis from breast cancer which was treated with radiotherapy [22].
In this study multi-variable Cox regression analysis demonstrated that among the potential prognostic factors assessed, only tumour receptor group had a statistically significant association with OS and DPFS, with TNBC patients experiencing a significantly poorer OS and DPFS rates in comparison to HR+HER2-breast cancer patients. Despite the higher rate of distant progression the LPFS rate among TNBC patients was comparable to the other tumour receptor groups assessed. Apart from tumour receptor group, few prognostic factors have been consistently reported for patients with OMBC. Several additional potential prognostic factors were assessed in this study, none of which achieved a statistically significant association with survival rates.
4.1. Study limitations
This study was single institute and retrospective, reducing the generalisability of the results presented here. Furthermore, the patient population was heterogenous with respect to treatment modality, treatment intent and metastasis site. The overall sample size was 70 patients, which is relatively small, potentially resulting in an inability to detect significant associations. In addition, the solitary metastasis was not confirmed on PET scan in all patients, which is now Medicare funded in Australia for breast cancer staging. Of the 70 patients included in this study, only 32 patients had records of a PET scan performed within 2 months of metastasis identification. Finally, patients were included from a relatively long time period over which management of metastatic breast cancer has changed with new systemic agents and increasing use of SABR.
5. Future directions
Understanding which prognostic factors have a significant influence on survival rates for OMBC patients remains an important area of ongoing research. Large, multicenter studies are needed to inform guidelines regarding the most appropriate selection of patients for aggressive metastasis directed therapies. The TAORMINA phase III randomised controlled trial will assess treatment with SABR and systemic therapy in comparison to systemic therapy alone among patients with 1–5 extracranial metastases from breast cancer of any subtype [23]. Another phase III trial, STEREO-SEIN is assessing the benefit of SABR in addition to systemic therapy for breast cancer patients with extracranial oligometastases but excludes TNBC [24]. Prospective registry studies such as the OligoCare cohort study are currently underway, which aim to assess the utility of metastasis directed therapies for oligometastatic cancers [25].
Another potential prognostic factor for breast cancer patients is circulating tumour DNA (ctDNA) levels [26]. A 2020 systematic review and meta-analysis of data from 8 separate studies reported a significant association between elevated ctDNA levels and poorer relapse-free survival rates in early stage, locally advanced and metastatic breast cancer [27]. Evaluating whether measuring or monitoring ctDNA levels has a role in risk-stratifying patients more likely to progress with distant metastases is an area of important research interest and high potential for clinical translation. The Consolidative Use of Radiotherapy to Block (CURB) Oligoprogression Trial investigated the effect of metastasis-directed SABR on progression free survival rates and ctDNA metrics among 59 non-small cell lung cancer (NSCLC) and 47 breast cancer patients with oligo-progressive disease. The majority of patients included in the CURB trial had more than 1 metastasis (75%) and 34% of breast cancer patients included had TNBC. Both a significant increase in progression free survival and reduction in ctDNA was demonstrated among NSCLC patients treated with SABR. In contrast, among breast cancer patients treated with SABR there was no significant improvement in progression free survival compared to standard of care (median progression free survival of 4.4 months and 4.2 months respectively, p = 0.43) or reduction in ctDNA [28].
6. Conclusion
In this study, MBC patients with a solitary extracranial metastasis had a median OS of 4.8 years. This is similar to OS rates reported in other small, single-institute retrospective studies and is favourable in comparison to those previously reported for MBC in general. Tumour receptor group was shown to be a significant prognostic factor with HR + HER2-patients demonstrating significantly higher OS and DPFS rates than TNBC patients, however additional significant prognostic factors were not identified.
Funding
Funding for this project was obtained from the Radiation Oncology Department of the Peter MacCallum Cancer Centre.
CRediT authorship contribution statement
Patrick Dyer: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Data curation. Jing Xie: Writing – review & editing, Validation, Software, Resources, Methodology, Formal analysis. Phillip K. Tran: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Resources, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Keelan Byrne: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
References
- 1.Daily K., Douglas E., Romitti P.A., Thomas A. Epidemiology of de novo metastatic breast cancer. Clin Breast Cancer. 2021 Aug 1;21(4):302–308. doi: 10.1016/j.clbc.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Lord S.S., Daniels B., Kiely B.E., O’Connell D.L., Beith J., Pearson S., Chiew K.L., Bulsara M.K., Houssami N. Long term risk of distant metastasis in women with non‐metastatic breast cancer and survival after metastasis detection: a population‐based linked health records study. Med J Aust. 2022 Oct 17;217(8):402–409. doi: 10.5694/mja2.51687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canberra, Australia: 2021. Cancer incidence and survival by stage data visualisation [Internet]https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-incidence-and-survival-by-stage-data-visualisation [Last updated: 24 Aug 2023; cited 15 Oct 2023]. Available from: [Google Scholar]
- 4.Lobbezoo D.J., van Kampen R.J., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J., van de Wouw A.J., Peters F.P., van Riel J.M., Peters N.A., de Boer M. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013 Oct;141:507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., Barrios C.H., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., Nandita M.D., Dingemans A.M., Fournier B., Hurkmans C., Lecouvet F.E., Meattini I. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and Oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020 Jan 1;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 7.Nagasaki E., Kudo R., Tamura M., Hayashi K., Uwagawa T., Kijima Y., Nogi H., Takeyama H., Suzuki M., Nishikawa M., Yano S. Long-term outcomes of oligometastatic breast cancer patients treated with curative intent: an updated report. Breast Cancer. 2021 Sep;28:1051–1061. doi: 10.1007/s12282-021-01240-1. [DOI] [PubMed] [Google Scholar]
- 8.Karatas M., Zengel B., Durusoy R., Tasli F., Adibelli Z., Simsek C., Uslu A. Clinicopathologic features of single bone metastasis in breast cancer. Medicine. 2021;100(1) doi: 10.1097/MD.0000000000024164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkes A., Warneke C.L., Clifton K., Al‐Awadhi A., Oke O., Pestana R.C., Alhalabi O., Litton J.K., Hortobagyi G.N. Prognostic factors in patients with metastatic breast cancer with bone‐only metastases. Oncologist. 2018 Nov 1;23(11):1282–1288. doi: 10.1634/theoncologist.2018-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niwińska A., Pogoda K., Murawska M., Niwiński P. Factors influencing survival in patients with breast cancer and single or solitary brain metastasis. Eur J Surg Oncol (EJSO) 2011 Jul 1;37(7):635–642. doi: 10.1016/j.ejso.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Curigliano G., Burstein H.J., Gnant M., Loibl S., Cameron D., Regan M.M., Denkert C., Poortmans P., Weber W.P., Thürlimann B., Aebi S. Understanding breast cancer complexity to improve patient outcomes: the St Gallen international consensus conference for the primary therapy of individuals with early breast cancer 2023. Ann Oncol. 2023 Nov 1;34(11):970–986. doi: 10.1016/j.annonc.2023.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Pagani O., Senkus E., Wood W., Colleoni M., Cufer T., Kyriakides S., Costa A., Winer E.P., Cardoso F. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Institute. 2010 Jan 1;102(7):456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Lascio S., Pagani O. Oligometastatic breast cancer: a shift from palliative to potentially curative treatment? Breast Care. 2014 Jan 1;9(1):7–14. doi: 10.1159/000358750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Primrose J.N. Surgery for colorectal liver metastases. Br J cancer. 2010 Apr;102(9):1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makhlin I, Fox K. Oligometastatic breast cancer: is this a curable entity? A contemporary review of the literature. Curr Oncol Rep. 2020 Feb;22:15. doi: 10.1007/s11912-020-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., Mulroy L., Lock M., Rodrigues G.B., Yaremko B.P., Schellenberg D. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020 Sep 9;38(25):2830. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson R., Mathews L., Liu M., Schellenberg D., Mou B., Berrang T., Harrow S., Correa R.J., Bhat V., Pai H., Mohamed I. Stereotactic ablative radiotherapy for the comprehensive treatment of 1–3 Oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020 Dec;20(1):1–2. doi: 10.1186/s12885-020-06876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palma D.A., Olson R., Harrow S., Correa R.J., Schneiders F., Haasbeek C.J., Rodrigues G.B., Lock M., Yaremko B.P., Bauman G.S., Ahmad B. Stereotactic ablative radiotherapy for the comprehensive treatment of 4–10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019 Dec;19(1):1–5. doi: 10.1186/s12885-019-5977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chmura S.J., Winter K.A., Woodward W.A., Borges V.F., Salama J.K., Al-Hallaq H.A., Matuszak M., Milano M.T., Jaskowiak N.T., Bandos H., Bazan J.G. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer ( NCT02364557) J Clin Oncol. 2022;40(16_suppl) [Google Scholar]
- 20.Dawood S., Broglio K., Ensor J., Hortobagyi G.N., Giordano S.H. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010 Nov 1;21(11):2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan B., Abudureheiyimu N., Zhang J., Wang C., Jiang S., Wang J., Ma F., Luo Y., Chen S., Xu B., Fan Y. Clinical features and prognostic factors for extracranial oligometastatic breast cancer in China. Int J Cancer. 2020 Dec 1;147(11):3199–3205. doi: 10.1002/ijc.33152. [DOI] [PubMed] [Google Scholar]
- 22.Yoo G.S., Yu J.I., Park W., Huh S.J., Choi D.H. Prognostic factors in breast cancer with extracranial oligometastases and the appropriate role of radiation therapy. Radiat Oncol J. 2015 Dec;33(4):301. doi: 10.3857/roj.2015.33.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linderholm B.K., Valachis A., Flote V.G., Poortmans P., Person O.K., Niligal-Yam E., O'Reilly S., Duane F., Marinko T., Ekholm M., Andersson A. 259TiP Treatment of oligometastatic breast cancer (OMBC): a randomised phase III trial comparing stereotactic ablative radiotherapy (SABR) and systemic treatment with systemic treatment alone as first-line treatment-TAORMINA. ESMO Open. 2023 May 1;8(1) [Google Scholar]
- 24.Gustave Roussy CCGP. Trial of superiority of stereotactic body radiation therapy in patients with breast cancer. [cited 2024 March 17] ClinicalTrials.gov [Internet]. Identifier NCT02089100. Available from: https://clinicaltrials.gov/study/NCT02089100.
- 25.Greto D., Van Hemelrijck M., Oppong F., Lievens Y., Ratosa I., Jereczek-Fossa B., Stellamans K., Peulen H., Verbeke L., Hemmatazad H., Ramella S. 2140P Short-term quality-of-life after metastases-directed SBRT: results of the prospective ESTRO & EORTC OligoCare cohort. Ann Oncol. 2023 Oct 1;34:S1115. [Google Scholar]
- 26.Sant M., Bernat-Peguera A., Felip E., Margelí M. Role of ctDNA in breast cancer. Cancers. 2022 Jan 9;14(2):310. doi: 10.3390/cancers14020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullinane C., Fleming C., O’Leary D.P., Hassan F., Kelly L., O’Sullivan M.J., Corrigan M.A., Redmond H.P. Association of circulating tumor DNA with disease-free survival in breast cancer: a systematic review and meta-analysis. JAMA Network Open. 2020 Nov 2;3(11):e2026921. doi: 10.1001/jamanetworkopen.2020.26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai C.J., Yang J.T., Shaverdian N., Patel J., Shepherd A.F., Eng J., Guttmann D., Yeh R., Gelblum D.Y., Namakydoust A., Preeshagul I. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet. 2024;403(10422):171–182. doi: 10.1016/S0140-6736(23)01857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]