Abstract
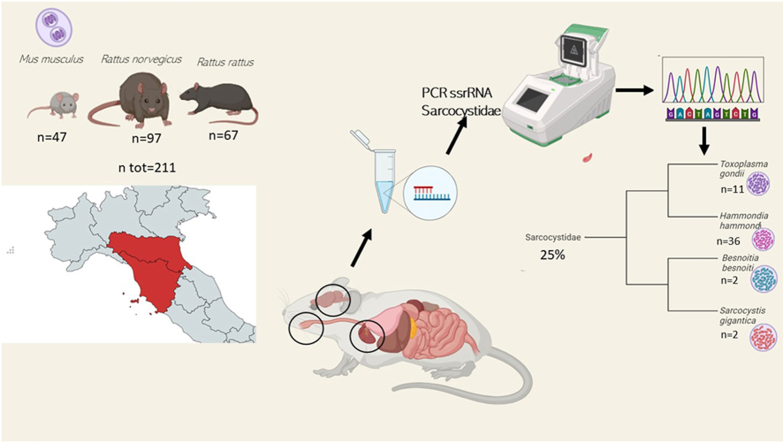
Synanthropic rodents play a crucial role in maintaining the life cycle of Toxoplasma gondii in anthropized regions and can serve as indicators of environmental oocyst contamination. This investigation aimed to explore the occurrence of T. gondii infection within synanthropic rodent populations using a molecular diagnostic technique targeting the 18S rDNA gene, which is generic for Coccidia, with subsequent specific PCR confirmation. We examined 97 brown rats (Rattus norvegicus), 67 black rats (R. rattus), 47 house mice (Mus musculus), and 1 common shrew (Sorex araneus). PCR tests were conducted on the brain, heart, and tongue tissues. PCR tested positive in at least one of the examined tissues in 26 R. norvegicus (26.8%), 13 R. rattus (19.4%), and 13 M. musculus (27.6%). Sequencing comparisons by BLAST allowed us to identify four different species of cyst-forming Apicomplexa. In particular, T. gondii DNA was detected in 13 (6.1%) rodents, Hammondia hammondi (including H. hammondi-like organisms) in 36 (17%) subjects, Besnoitia sp. (in two cases identified as B. besnoiti) in 8 (3.7%), and Sarcocystis gigantea in two (0.94%). Rodents from peri-urban and urban environments can act as indicators of environmental contamination by oocysts of apicomplexan parasites with cats as definitive hosts, such as T. gondii, H. hammondi, and S. gigantea, the latter of which has never been previously recorded in rodents. Moreover, the presence of B. besnoiti, a parasite with an unidentified definitive host in Europe, sheds light on the potential role of these hosts as infection sentinels.
Keywords: Toxoplasmosis, Zoonosis, Rodentia, Apicomplexa, Hammondia hammondi, Besnoitia sp, Sarcocystis gigantea
Graphical abstract
Highlights
-
•
Molecular prevalence of Sarcocystidae in synanthropic rodents is assessed.
-
•
Hammondia hammondi and Toxoplasma gondii are the most prevalent Sarcocystidae.
-
•
Besnoitia besnoiti first detection in rodents in Italy.
-
•
Sarcocystis gigantea may infect also rodents in anthropized settings.
1. Introduction
Synanthropism, as defined by Klegarth (2016), encompasses the behaviour of wildlife (or flora) thriving within the shared ecosystems of humans. This behaviour, in turn, drives an increase in population density, reproduction rates, and survival advantages among these synanthropic species. Conversely, their territorial range diminishes due to their reliance on centralized anthropogenic resources (Gehrt et al., 2011; Hulme-Beaman et al., 2016).
From the time of the Neolithic Revolution, human activities have led to profound and enduring impacts on the natural environment. This process of settling down and adopting agricultural practices created a stable ecological niche that ensured sustenance over extended periods. Consequently, this environment began to attract initial rodent populations, as documented by Frynta et al. (2005) and Cucchi et al. (2005), which in turn drew the interest of subsequent cat populations (Krajcarz et al., 2022). Through the passing decades and centuries, these modest human settlements gradually expanded into villages and towns, forming the earliest instances of urban settings inhabited by synanthropic species (Baumann, 2023).
In this context, the establishment of a predator-prey relationship has given rise to the development of a peri-domestic life cycle of predation-associated parasites (Mendoza Roldan and Otranto, 2023). The process of predation stands out as one of the most effective mechanisms for facilitating the transmission of parasites, offering a direct pathway for the parasite to fulfil its life cycle within the trophic chain (Johnson et al., 2010; Médoc and Beisel, 2011). Parasites transmitted through trophic interactions have, in various instances, undergone adaptations to optimize predation through manipulation of their host preys (Seppälä et al., 2008). A pertinent illustration of this phenomenon can be observed in Toxoplasma gondii (Eucoccidiorida: Sarcocystidae), which relies on the predatory behaviour of felines (definitive hosts of the parasite), that include the consumption of small rodents and other prey species, to successfully conclude its life cycle (Vyas et al., 2007; Dubey et al., 2021). The reproductive fitness of Toxoplasma is intricately linked to the predation patterns exhibited by felids. Disruption of the innate aversion mechanism, caused by the interaction of the parasite with the SNC of the host, heighten predation rates, thereby increasing the reproductive fitness of the parasite. This stance aligns with the ‘behavioural manipulation’ hypothesis, postulating that T. gondii can induce alterations in host behaviour that directly contribute to the enhancement of their own reproductive success, as proposed by Vyas et al. (2007) and Webster (2007).
Rodents play for this reason a crucial part in upholding the lifecycle of T. gondii and influencing the spread of toxoplasmosis. This significance is particularly pronounced in species residing near human settlements. The establishment of an infection transmission cycle via rodents (and other small preys) results in the release of millions of unsporulated oocysts by cats, that can therefore be found in various environmental matrices where, upon sporulation, become infectious and can remain viable up to several years (Dubey et al., 2021). Consequently, this process heightens the risk of infection for all hosts of the parasite in the ecosystem, most notably humans within these habitats (Mercier et al., 2013).
Apart from their crucial role in maintaining the life cycle of T. gondii in anthropized regions, synanthropic rodents, due to their feeding behaviour that predominantly facilitate oral transmission of sporulated oocysts within the environment, can be regarded as indicative of environmental oocyst contamination. Consequently, the finding of T. gondii in wild rodent populations might reflect the dissemination of the parasitic environmental phase within a specific geographical area (Dubey et al., 2021).
The main aim of this study is to investigate the prevalence of T. gondii infection among synanthropic rodent populations in a specific area of Italy that has been previously studied to assess prevalence rates in animals and humans. These earlier studies revealed a consistent presence of the parasite in the region (Dini et al., 2023a, Dini et al., 2023b; Dini et al., 2024a, Dini et al., 2024b). The central aim of this study is to ascertain the potential environmental significance of the parasite within urbanized settings. This is achieved by evaluating the occurrence of infections in rodents residing in the peri-domestic environment, utilizing molecular diagnostic techniques.
2. Materials and methods
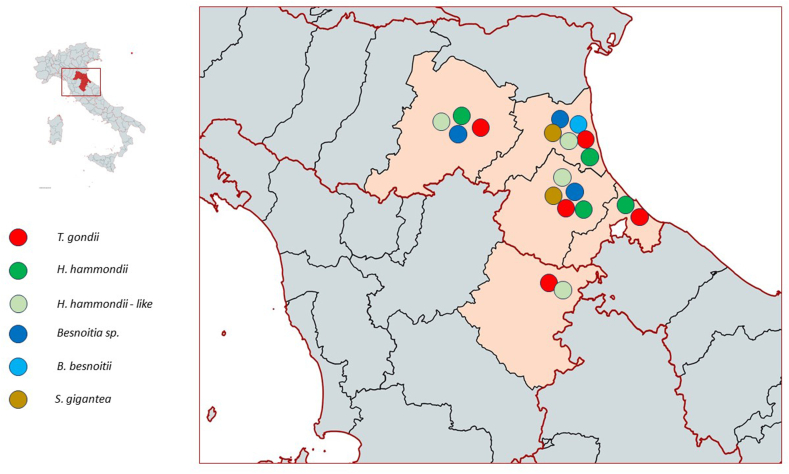
From June 2019 to March 2023, 212 carcasses of adults or subadults peridomestic rodent were collected and stored at −20 °C by professional rodent control services during pest control programs from urban and rural areas in the provinces of Ferrara, Forlì-Cesena, Ravenna, Bologna (Emilia Romagna Region) and Arezzo (Toscana Region) (Fig. 1). In detail, 97 brown rats (Rattus norvegicus), 67 black rats (Rattus rattus), 47 house mouse (Mus musculus) and 1 common shrew (Sorex araneus) were sampled. The carcasses were identified morphologically according to CDC (2006); sex and weight of each rodents were recorded. Sampling was performed with sterile surgical instruments and, according to the state of the carcasses, 25–50 mg of tissue were collected from heart, 25–200 mg from brain and 25 mg from tongue muscle. Samples were placed in sterile 1.5 ml tubes and stored at −20 °C until DNA extraction. In total, 209 brains (due to poor condition of 3 R. rattus), 212 tongue, and 180 heart samples (poor condition of 19 R norvegicus, 10 R. rattus, and 3 M. musculus) were collected.
Fig. 1.
Geographical distribution of PCR positive rodents.
Genomic DNA was purified using Pure Link ® Genomic DNA Mini kit (Invitrogen by Thermo Fisher), according to the manufacturer's protocol. Initial end-point PCR targeting 18S rDNA gene of Coccidia was performed on all the samples with the primers COC-1 and COC-2 as described by Ho et al. (1996) following some modification by Hornok et al. (2015). Briefly, a reaction volume of 25 μl, containing 12.5 μl 2x Dream Taq Hot Start Green PCR Master Mix (Thermo Scientific), 9.5 μl ddH2O, 0.25 μl (1 μM final concentration) of each primer, and 2.5 μl template DNA were used. For amplification, an initial denaturation step at 94 °C for 10 min was followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s and extension at 72 °C for 30 s. Final extension was performed at 72 °C for 10 min. In all the PCRs, sterile water was included as negative control.
An additional species-specific PCR have been carried out to validate the species identification obtained through sequencing the 18S rDNA fragment amplified with COC primers. Specifically, for Besnoitia sp. specimens, we employed an end-point PCR targeting 231 bp of the ITS-1 rDNA following the method of Cortes et al. (2007).
To confirm the Sarcocystis gigantea samples, an end-point PCR assay was used with Sarcocystis genus-specific primers: sarF (TGGCTAATACATGCGCAAATA) (Vangeel et al., 2007) and sarcoREV (AACCCTAATTCCCCGTTA) (Chiesa et al., 2013), amplifying an expected region of 240 bp following the protocol reported by Rubiola et al. (2020).
Finally, to confirm Toxoplasma gondii and Hammondia hammondi positive samples, we employed a semi-nested PCR targeting the ITS rDNA as described by Sreekumar et al. (2005).
Amplifications were performed in a T-personal thermal cycler (Biometra, Göttingen, Germany). The PCR products were electrophoresed on 1.5% agarose gel stained with SYBR Safe DNA Gel Stain (Thermo Fisher Scientific, Carlsbad, CA, USA) in 0.5 × TBE. For sequencing, the amplicons were excised and purified by Nucleo-Spin Gel and PCR Clean-up (Mackerey-Nagel, Düren, Germany), and sequenced with an ABI 3730 DNA analyzer (StarSEQ, Mainz, Germany).
The trace files were assembled with Contig Express (VectorNTI Advance 11 software, Invitrogen, Carlsbad, CA, USA), and the consensus sequences were compared with published data by BLAST tools (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence alignments were carried out by BioEdit 7.2.5 (Hall, 1999), while p-distance and maximum-likelihood (ML) tree (GTR + G + I substitution model for both genes and bootstrap of 1000 replicate) were calculated by MEGA 7 (Kumar et al., 2016).
Student t-test was used to compare the average weight of male and female; Pearson's χ2 test was used to associate sex with prevalence data. Differences were considered significant when P ≤ 0.05. The Sample Size Calculator (https://www.surveysystem.com/sscalc.htm) was used to calculate 95% confidence intervals (CIs) for the observed prevalence values.
3. Results
Among the 212 rodents collected, 133 were males while 78 were females as detailed in Table 1 together with the species sex and weight. In each species no significant weight differences between sexes were detected by Student's t-test. The single shrew collected, was a male weighing 4 g.
Table 1.
Descriptive statistics and PCR results.
| Species |
n. |
Sex |
n. (%) |
Weight (g) |
PCR positive (%) |
CI 95% |
||
|---|---|---|---|---|---|---|---|---|
| min | max | median | ||||||
| Rattus norvegicus | 97 | M |
64 (66%) |
28.5 |
490 |
228.5 |
12 (18.7%) |
[9.19–28.31] |
| F | 33 (34%) | 45 | 440 | 188 | 14 (42.4%) | [25.56–59.28] | ||
| Rattus rattus | 67 | M |
41 (61.2%) |
6 |
165 |
90 |
8 (19.5%) |
[7.38–31.64] |
| F | 26 (38.81%) | 5.2 | 195 | 106 | 5 (19.2%) | [4.08–34.4] | ||
| Mus musculus | 47 | M |
28 (59.6%) |
4.6 |
70 |
14.6 |
9 (32.1%) |
[14.84–49.44] |
| F | 19 (40.4%) | 4.9 | 25 | 16 | 4 (21%) | [2.72–39.38] | ||
Overall, 53 rodents (25%) were PCR positive in at least one of the examined tissues; splitting the results: R. norvegicus 26 out of 97 (26.8%, CI 95% = 7.99–35.61), R. rattus 13 out of 67 (19.4%, CI 95% = 9.93–28.87) while M. musculus 13 out of 47 (27.6%, CI 95% = 14.87–40.45). In Table 1 the PCR results are also reported in relation to sex. Notably, in R. norvegicus, females showed a significantly higher positivity rate (42.4%) than males (18.7%) (Yates-corrected chi-square: 5.7; p = 0.0243), while no significant sex differences were observed for R. rattus and M. musculus.
Sequences comparison by BLAST allows to identify four different species of cyst-forming Apicomplexa (Sarcocystidae): Toxoplasma gondii, Hammondia hammondi (including H. hammondi-like organism), Besnoitia sp. and Sarcocystis gigantea.
In particular T. gondii was detected in 13 (6.1%) rodents, (similarities 99.7%–100%), H. hammondi (and H. hammondi-like organism) in 36 (17%) subject (similarities 99.3%–100%), Besnoitia sp. was found in 8 rodents (3.7%), with low sequence similarity (94%–95%). Notably, only two samples showed 100% similarity with B. besnoiti from cattle (XR 003828656-59, KJ746531, JF314861). Finally, S. gigantea was detected in two heart samples of R. norvegicus, constituting 0.94% of the total rodent population and 2% of the R. norvegicus samples. These samples displayed 100% sequence similarity with sequences of S. gigantea available in GenBank (MT026574, MK420020, OP550293, KC209733).
In the context of host species differentiation, within R. norvegicus, the most frequently encountered cyst-forming coccidia was H. hammondi (and H. hammondi-like) with a prevalence of 17.5%, followed by Besnoitia sp. at 7.2%. Among the latter only one sequence showed 100% similarity with B. besnoiti. Trailing behind in terms of prevalence were T. gondii at 4% and Sarcocystis gigantea at 2%. In R. rattus, only two Apicomplexan species were molecularly identified in the analysed tissues: H. hammondi/H. hammondi-like (11.9%), and T. gondii (9%). Concerning M. musculus, the most prevalent was H. hammondi/H. hammondi-like (23.4%), followed by T. gondii (4.2%). Intriguingly, in one instance, B. besnoiti was detected in a heart sample with 100% sequence similarity.
Regarding the distribution of positive matrices in relation to parasites, H. hammondi/H. hammondi-like DNA was more frequently observed in CNS and heart samples; T. gondii was equally distributed among all matrices, while B. besnoiti occurred only in heart samples.
Co-infections (referred to the detection of DNA of more than one parasite in the same host) were only observed in Rattus spp. and M. musculus. Among Rattus species, one R. norvegicus and one R. rattus exhibited co-infection by T. gondii/H. hammondi + H. hammondi-like. Additionally, two R. norvegicus showed co-infection by H. hammondi-like and Besnoitia sp., while one R. norvegicus exhibited co-infection by H. hammondi-like and Sarcocystis gigantea. In the case of Mus musculus, co-infection by T. gondii and H. hammondi-like was detected. The sole analysed S. araneus was found positive for T. gondii (100% sequence similarity with KX007999-KX008033) in heart tissue.
The results of the Sarcocystidae generic PCR, conducted on the 18S rDNA, are presented in Table 2, Table 3, Table 4, along with species confirmation achieved through specific PCR assays (ITS rDNA for T. gondii and H. hammondi and B. besnoiti and 18S rDNA for S. gigantea).
Table 2.
List of the 26 R. norvegicus positive at 18S rDNA PCR and sequencing along with species confirmation achieved through specific PCR assays.
| ID number | Sex | Weight | Brain | Tongue | Heart |
|---|---|---|---|---|---|
| 20 | F | 390 | – | – | Besnoitia sp. |
| 82 | M | 40 | H. hammondi-like | – | – |
| 83 | F | 320 | – | H. hammondi-like | – |
| 84 | M | 155 | – | – | Besnoitia sp. |
| 87 | F | 320 | T. gondiia | – | – |
| 135 | M | 74,5 | – | Besnoitia sp. | na |
| 136 | F | 240 | H. hammondia | – | na |
| 137 | F | 370 | – | Besnoitia sp. | – |
| 140 | M | 265 | H. hammondia | – | – |
| 144 | M | 470 | – | Besnoitia besnoitib | – |
| 216 | M | 191 | H. hammondia | H. hammondi-like | H. hammondia |
| 217 | F | 156 | – | – | S. giganteac |
| 219 | F | 185,7 | H. hammondia | – | – |
| 220 | F | 86,19 | H. hammondia | – | H. hammondi-like |
| 221 | M | 36,6 | – | H. hammondi-like | H. hammondi-like |
| 3 | F | 180 | – | H. hammondi-like | H. hammondia |
| 4 | F | 314 | H. hammondia | H. hammondi-like | H. hammondia |
| 19 | M | 96 | T. gondiia | – | – |
| 22 | F | 45 | H. hammondia | – | T. gondiia |
| 25 | F | 80 | H. hammondia | – | Besnoitia sp. |
| 26 | M | 294,5 | H. hammondia | – | S. giganteac |
| 69 | M | 48 | H. hammondia | – | – |
| 71 | F | 220 | Besnoitia sp. | – | H. hammondia |
| 78 | F | 94,7 | H. hammondia | – | H. hammondia |
| 79 | M | 82 | – | – | H. hammondia |
| 81 | M | 132 | – | – | T. gondiia |
species confirmed by ITS specific PCR.
species confirmed by ITS specific PCR.
species confirmed by 18S specific PCR.
Table 3.
List of the 13 R. rattus positive at 18S rDNA PCR and sequencing along with species confirmation achieved through specific PCR assays.
| ID number | Sex | weight | Brain | Tongue | Heart |
|---|---|---|---|---|---|
| 76 | F | 5,2 | – | T. gondiia | – |
| 77 | F | 6 | – | – | H. hammondia |
| 148 | M | 91 | – | T. gondiia | – |
| 215 | F | 82 | H. hammondia | T. gondiia | H. hammondia |
| 218 | F | 97 | H. hammondia | – | – |
| 1 | M | 104 | H. hammondia | H. hammondia | H. hammondia |
| 2 | M | 90 | H. hammondi-like | H. hammondi-like | H. hammondi-like |
| 21 | F | 139,22 | T. gondiia | – | – |
| 30 | M | 162,95 | – | T. gondiia | – |
| 31 | M | 141,3 | H. hammondia | – | H. hammondi-like |
| 38 | M | 59,6 | – | – | H. hammondia |
| 51 | M | 140 | H. hammondia | H. hammondi-like | H. hammondia |
| 75 | M | 45 | – | – | T. gondiia |
species confirmed by ITS specific PCR.
Table 4.
List of the 13 M. musculus positive at 18S rDNA PCR and sequencing along with species confirmation achieved through specific PCR assays.
| ID number | Sex | Weight | Brain | Tongue | Heart |
|---|---|---|---|---|---|
| 101 | F | 17 | – | – | T. gondiia |
| 109 | F | 25 | – | H. hammondi-like | – |
| 111 | M | 17 | – | – | Besnoitia besnoitib |
| 112 | F | 22 | – | – | H. hammondia |
| 113 | F | 15 | H. hammondi-like | – | – |
| 115 | M | 14 | H. hammondi-like | – | H. hammondia |
| 117 | M | 23 | H. hammondi-like | – | – |
| 119 | M | 7,9 | – | H. hammondi-like | H. hammondi-like |
| 120 | M | 7 | H. hammondia | – | H. hammondi-like |
| 122 | M | 14,7 | H. hammondi-like | – | – |
| 7 | M | 21 | – | – | H. hammondi-like |
| 32 | M | 9,7 | – | H. hammondi-like | na |
| 80 | M | 18,5 | T. gondiia | H. hammondi-like | – |
species confirmed by ITS specific PCR.
species confirmed by ITS specific PCR.
The alignment of all T. gondii obtained in the present study (plus the T. gondii type I, RH reference strain) with H. hammondi/H. hammondi-like showed the presence of two transitions C/T and A/G as the only differences between the two species over 297 bp of the 18S rDNA. Moreover, the A/G transition further separate the “true” H. hammondi from the H. hammondi-like group. The p-distance observed between T. gondii and the group of H. hammondi is very low ranging from 0% to 0.1%, due to the low genetic resolution of the 18S rDNA.
The ML tree obtained (tree not reported), despite with a low bootstrap support, showed 3 separated clusters, one composed by our T. gondii together with the reference strain, the second by the “true” H. hammondi (having 100% identity with AH008381) and the latter by H. hammondi-like group.
The sequences obtained in this study have been deposited in GenBank under the accession numbers PP500534-PP500612 (18S rDNA) and PP502867-PP502868 (ITS rDNA).
4. Discussion
Rattus spp. and M. musculus exemplify species capable of coexisting within anthropogenically influenced environments. This coexistence raises concerns for potential human health risks owing to the close proximity between these species and human habitats. Rodents constitute a notably significant group of mammals, particularly in terms of serving as reservoirs for various pathogens, some of which are of zoonotic concern (Han et al., 2015). Their biological attributes, characterized by elevated reproductive rates, opportunistic behaviours, adaptability, and worldwide distribution, position them strategically, thereby enhancing the likelihood of disease transmission among wildlife, domestic animals, and human populations (Han et al., 2015; Luis et al., 2013)
Within the scope of this research, we examined the occurrence of Sarcocystidae infections in synanthropic rodents using a broad-spectrum PCR assay targeting the 18S rRNA of Coccidia, and subsequent specific PCR confirmation. This molecular method, involving a single PCR assay followed by Sanger sequencing, enabled us to reveal the presence of various protozoan species, namely T. gondii, H. hammondi/H. hammondi-like, Besnoitia sp., and S. gigantea, within the studied host species (R. norvegicus, R. rattus, M. musculus, and S. araneus).
In the current study, T. gondii DNA was detected in all the rodent host species, with an overall prevalence rate of 6.1%. Notably, among these species, R. rattus exhibited the highest infection rate, with 9% prevalence. The worldwide seroprevalence is approximately 6%, with the highest rates recorded in Africa (24%) and South America (18%), and the lowest in Europe (1%) (Galeh et al., 2020). It's important to note that serological tests cannot definitively predict the presence/absence of the parasite. This limitation has been previously observed (Dubey, 2022), as viable T. gondii has been isolated from rodents that tested seronegative (AraùJo et al., 2010). Hence, molecular studies appear to offer more epidemiological reliability.
Rodents play a pivotal role in the perpetuation of the T. gondii life cycle and the epidemiology of toxoplasmosis. They are recognized as reservoirs and carriers of the disease, serving as the primary source of infection for cats and their related species (Dabritz et al., 2008). This role becomes particularly significant in species inhabiting close proximity to human habitats due to the profound implications for both the environment and human health. The establishment of the infection transmission cycle through rodents results in the release of oocysts from infected felids, leading their dissemination into environment. Consequently, this amplifies the infection risk for various hosts including humans (Mercier et al., 2013). The importance of rodents in maintaining the lifecycle of T. gondii has been further strengthened and highlighted following studies involving the neuroanatomical interaction of chronical established CNS cysts in the behavioural pattern of these hosts. Numerous studies indicate that T. gondii alters rodent behaviour, making them more susceptible to predation by cats (Webster, 2007). These alterations include increased activity, reduced neophobia (fear of novelty), and decreased predator vigilance (Webster, 1994, 2001, 2007; Webster and Brunton, 1994; Berdoy et al., 1995; Lamberton et al., 2008). These changes likely facilitate parasite transmission from the intermediate host to the feline host. (Berdoy et al., 2000; Vyas et al., 2007; Kaushik et al., 2014).
Hammondia hammondi (including H. hammondi-like), molecularly found in the 17% of the analysed rodents, is a non-zoonotic coccidian parasite that bears a close resemblance to T. gondii (Frenkel and Dubey, 1975). The life cycles of these two parasites also share similarities like in the definitive hosts (Felidae) but differently from T. gondii, H. hammondi follows an obligatory two-host lifecycle, consequently, only sporulated oocysts are infective to rodents, and solely bradyzoite cysts are infective to cats (Frenkel and Dubey, 2000). Intermediate hosts include mice (Mus musculus), rats (Rattus norvegicus), and other rodents but also, rabbits, hamsters, goats, dogs, and pigs (Shimura and Ito, 1987). The prevalence of H. hammondi remains undisclosed in both definitive and intermediate hosts. Only few research reported the sporadically presence of H. hammondi in cat and dog faeces (Schares et al., 2005, 2008; Dubey et al., 2013) and in intermediate host tissues (Cabral et al., 2021).
In our study the consistent presence of H. hammondi/H. hammondi-like DNA in the rodent hosts are not comparable with other data, since no previous report are, to the best of our knowledge, present in available literature.
The distinction between T. gondii and H. hammondi is of paramount importance, particularly because the latter lacks zoonotic relevance, despite its close genetic relationship. In our investigations, the 18S rDNA despite being highly conserved, emerged as a sensitive genetic marker within the Apicomplexa subphylum, able to distinguish between the two parasites. Notably, there are only a few nucleotide variations between the complete 18S rDNA sequences of T. gondii and H. hammondi (Jenkins et al., 1999). Within our sequences, a single nucleotide variation, represented by C/T and A/G transition, effectively separated T. gondii from H. hammondi samples, leading to the formation of two distinct clusters among our positive sample set. Moreover, the latter transition (A/G) further delineated these samples into two sub-clusters, “H. hammondi” and “H. hammondi-like organism”. In light of the limited knowledge regarding the global population structure of H. hammondi, it remains conceivable that yet undiscovered lineages of H. hammondi may exist, displaying potential genetic diversity (Schares et al., 2021).
Within our study, 3.7% of the rodents exhibited DNA sequences associated with Besnoitia sp. However, it's noteworthy that only two sequences matched with 100% identity to B. besnoiti. The other specimens exhibited a sequence similarity of 94–95% with sequences of Besnoitia spp. available in GenBank, and lower similarity with other Sarcocystidae parasites. This outcome renders the accurate assignment of a species identification for these positive samples challenging, thereby impeding our ability to make informed epidemiological inferences.
This parasite is responsible for bovine Besnoitiosis, a chronic and debilitating disease that has been causing significant economic losses in cattle and has been considered endemic in Italy since 2011 (Gentile et al., 2012). In Europe, no definitive hosts for this parasite have been identified (Basso et al., 2011). The presence of parasite cysts in the skin suggests that the primary way of transmission is likely mechanical, with hematophagous flies serving as the carriers of the parasite (Bigalke, 1968). The intriguing aspect is the molecular identification of this parasite in the internal organs of rodent hosts, particularly in the heart, which raises questions about the epidemiology and potential host range of this Sarcocystidae. Notably the two subjects in which B. besnoiti was detected, were collected from two areas where a consistent population of domestic ruminant is present. This discovery hints the possibility that rodent hosts could serve as competent hosts in the life cycle of Besnoitia. It's worth noting that other Besnoitia species, such as B. jellisoni and B. wallacei, have been previously described in rodents but have not been documented in Europe until now (Alvarez-García et al., 2013).
Finally, our research has unveiled the occurrence of S. gigantea in the heart muscles of two R. norvegicus. The genus Sarcocystis comprises apicomplexan protozoa forming cysts, with a life cycle obligatorily entailing two hosts (Dubey et al., 2015). These cysts are typically located in the striated muscles of herbivorous or omnivorous intermediate hosts, while carnivores serve as the definitive hosts. Remarkably, more than 40 Sarcocystis species have been identified to use rodents as their intermediate hosts, including S. microti (Votýpka et al., 1998; Mugridge et al., 1999), S. muris (Mugridge et al., 1999; Gajadhar et al., 1991), S. myodes (Rudaitytė-Lukošienė et al., 2022), and S. ratti (Zeng et al., 2020; Prakas et al., 2019). An apparent gap in research exists concerning the global prevalence of Sarcocystis spp. in small mammals. Researchers have proposed that infection rates of various Sarcocystis species are contingent upon factors such as the specific parasite species, intermediate host species, geographical region, as well as the presence and abundance of definitive hosts within the studied area (Rudaitytė-Lukošienė et al., 2022; Hu et al., 2022). Sarcocystis gigantea infection is considered to be mildly pathogenic yet relatively common in sheep, with the cat as the definitive host (Dubey et al., 2015). While S. gigantea has not been documented in rodent hosts to date, recent findings have indicated its capacity to cause infections in horses, which serve as non-specific intermediate hosts (Veronesi et al., 2020). This observation suggests that in peri-urban settings where definitive hosts, such as cats, are abundant and the parasite is disseminated within the environment, non-specific intermediate hosts, such as rodents, may potentially develop bradyzoite cysts within their muscle tissue. Our findings support the hypothesis that certain Sarcocystis spp. may possess a broader range of intermediate hosts than was previously recognized (Veronesi et al., 2020).
5. Conclusions
In conclusion, the findings of this study highlight that synanthropic rodents sampled in urban and peri-urban environments serve as valuable indicators of environmental contamination by oocysts of apicomplexan parasites with cat as definitive host. This applies not only to parasites like T. gondii and H. hammondi, which are closely related apicomplexans with distinct epidemiological implications but both having cat-rodent cycle as a robust framework. This pattern is also applicable for S. gigantea, recovered in the hearts of two R. norvegicus, and recognizing cats as the definitive host. This species has never been recorded previously in rodents. Lastly, the presence of B. besnoiti in R. norvegicus and M. musculus, parasite with an unidentified definitive host in Europe, sheds light on the potential role of these hosts as infection sentinels.
CRediT authorship contribution statement
Filippo Maria Dini: Writing – original draft, Methodology, Investigation. Monica Caffara: Writing – review & editing, Methodology. Alice Magri: Investigation, Methodology. Alessia Cantori: Methodology, Investigation. Valentina Luci: Methodology, Investigation. Antonio Monno: Investigation. Roberta Galuppi: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors would like to thank social cooperative For.B and dr. Guglielmo Pampiglione for kindly providing rodent carcasses from the provinces of Ferrara, Forlì-Cesena and Ravenna of Emilia-Romagna Region.
References
- Alvarez-García G., Frey C.F., Mora L.M., Schares G. A century of bovine besnoitiosis: an unknown disease re-emerging in Europe. Trends Parasitol. 2013;29:407–415. doi: 10.1016/j.pt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- AraùJo J.B., Da Silva A.V., Rosa R.C., Mattei R.J., Da Silva R.C., Richini-Pereira V.B., Langoni H. Isolation and multilocus genotyping of Toxoplasma gondii in seronegative rodents in Brazil. Vet. Parasitol. 2010;174:328–331. doi: 10.1016/j.vetpar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Basso W., Schares G., Gollnick N.S., Rütten M., Deplazes P. Exploring the life cycle of Besnoitia besnoiti - experimental infection of putative definitive and intermediate host species. Vet. Parasitol. 2011;178:223–234. doi: 10.1016/j.vetpar.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Baumann C. The paleo synanthropic niche: a first attempt to define animal's adaptation to a human made micro environment in the Late Pleistocene. Archaeol. Anthropol. Sci. 2023;15:63. doi: 10.1007/s12520-023-01764-x. [DOI] [Google Scholar]
- Berdoy M., Webster J.P., Macdonald D.W. Fatal attraction in Toxoplasma-infected rats: a case of parasite manipulation of its mammalian host. Proc. R. Soc. A B. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M., Webster J.P., Macdonald D.W. Parasite altered behaviour: is the effect of Toxoplasma gondii on Rattus norvegicus specific? Parasitology. 1995;111:403–409. doi: 10.1017/s0031182000065902. [DOI] [PubMed] [Google Scholar]
- Bigalke R.D. New concepts on the epidemiological features of bovine besnoitiosis as determined by laboratory and feld investigations. Onderstepoort J. Vet. Res. 1968;35:3–137. [PubMed] [Google Scholar]
- Cabral A.D., Su C., Soares R.M., Gennari S.M., Sperança M.A., da Rosa A.R., Pena H.F.J. Occurrence and diversity of Sarcocystidae protozoa in muscle and brain tissues of bats from São Paulo state, Brazil. Int. J. Parasitol. Parasites Wildl. 2021;14:91–96. doi: 10.1016/j.ijppaw.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention and U.S. Department of Housing and Urban Development . US Department of Health and Human Services; Atlanta: 2006. Healthy Housing Reference Manual.https://www.cdc.gov/nceh/publications/books/housing/housing_ref_manual_2012.pdf [Google Scholar]
- Chiesa F., Muratore E., Dalmasso A., Civera T.A. New Molecular approach to assess the occurrence of Sarcocystis spp. in cattle and products thereof: preliminary data. Ital. J. Food Saf. 2013;2:148–151. doi: 10.4081/ijfs.2013.e41. [DOI] [Google Scholar]
- Cortes H.C., Reis Y., Gottstein B., Hemphill A., Leitão A., Müller N. Application of conventional and real-time fluorescent ITS1 rDNA PCR for detection of Besnoitia besnoiti infections in bovine skin biopsies. Vet. Parasitol. 2007;146(3–4):352–356. doi: 10.1016/j.vetpar.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cucchi T., Vigne J.D., Aufray J.C. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: a zooarchaeological revision of subfossil occurrences. Biol. J. Linn. Soc. 2005;84(3):429–445. [Google Scholar]
- Dabritz H.A., Miller M.A., Gardner I.A., Packham A.E., Atwill E.R., Conrad P.A. Risk factors for Toxoplasma gondii infection in wild rodents from central coastal California and a review of T. gondii prevalence in rodents. J. Parasitol. 2008;94:675–684. doi: 10.1645/GE-1342.1. [DOI] [PubMed] [Google Scholar]
- Dini F.M., Morselli S., Marangoni A., Taddei R., Maioli G., Roncarati G., Balboni A., Dondi F., Lunetta F., Galuppi R. vol. 32. 2023. (Spread of Toxoplasma Gondii Among Animals and Humans in Northern Italy: A Retrospective Analysis in a One-Health Framework Food Waterborne Parasitol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini F.M., Graziosi G., Lupini C., Catelli E., Galuppi R. Migratory wild birds as potential long-distance transmitters of Toxoplasma gondii infection. Pathogens. 2023;12(3):478. doi: 10.3390/pathogens12030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini F.M., Musto C., De Nigris V.M., Bellinello E., Sampieri M., Merialdi G., Barca L., Delogu M., Galuppi R. Sero-epidemiological investigation on Toxoplasma gondii infection in Apennine wolf (Canis lupus italicus) and wild boar (Sus scrofa) in Italy. BMC Vet. Res. 2024;20(1):62. doi: 10.1186/s12917-024-03922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini F.M., Stancampiano L., Poglayen G., Galuppi R. Risk factors for Toxoplasma gondii infection in dogs: a serological survey. Acta Vet. Scand. 2024;66(1):14. doi: 10.1186/s13028-024-00734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Calero-Bernal R., Rosenthal B.M., Speer C.A., Fayer R. second ed. CRC Press; 2015. Sarcocystosis of Animals and Humans. [Google Scholar]
- Dubey J.P., Murata F.H.A., Cerqueira-Cézar C.K., Kwok O.C.H., Su C. Epidemiological significance of Toxoplasma gondii infections in wild rodents: 2009-2020. J. Parasitol. 2021;107(2):182–204. doi: 10.1645/20-121. https://doi-org.ezproxy.unibo.it/10.1645/20-121 [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Tilahun G., Boyle J.P., Schares G., Verma S.K., Ferreira L.R., Oliveira S., Tiao N., Darrington C., Gebreyes W.A. Molecular and biological characterization of first isolates of Hammondia hammondi from cats from Ethiopia. J. Parasitol. 2013;99(4):614–618. doi: 10.1645/12-51.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. third ed. CRC Press; Boca Raton, FL, USA: 2022. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Frenkel J.K., Dubey J.P. Hammondia hammondi gen. nov., sp.nov., from domestic cats, a new coccidian related to Toxoplasma and Sarcocystis. Z. Parasitenk. 1975;46(1):3–12. doi: 10.1007/BF00383662. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K., Dubey J.P. The taxonomic importance of obligate heteroxeny: distinction of Hammondia hammondi from Toxoplasma gondii another opinion. Parasitol. Res. 2000;86(10):783–786. doi: 10.1007/s004360000261. [DOI] [PubMed] [Google Scholar]
- Frynta D., Slabova M., Vachova H., Volfova R., Munclinger P. Aggression and commensalism in house mouse: a comparative study across Europe and the Near East. Behav. Aggress. 2005;31(3):283–293. [Google Scholar]
- Gajadhar A.A., Marquardt W.C., Hall R., Gunderson J., Ariztia-Carmona E.V., Sogin M.L. Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationships among apicomplexans, dinoflagellates, and ciliates. Mol. Biochem. Parasitol. 1991;45:147–154. doi: 10.1016/0166-6851(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Galeh T.M., Sarvi S., Montazeri M., Moosazadeh M., Nakhaei M., Shariatzadeh S.A., Daryani A. Global status of Toxoplasma gondii seroprevalence in rodents: a systematic review and meta-analysis. Front. Vet. Sci. 2020;7:461. doi: 10.3389/fvets.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrt S.D., Brown J.L., Anchor C. Is the urban coyote a misanthropic synanthrope? The case from Chicago. Cities and the Environment (CATE) 2011;4(1) article 3. [Google Scholar]
- Gentile A., Militerno G., Schares G., Nanni A., Testoni S., Bassi P., Gollnick N.S. Evidence for bovine besnoitiosis being endemic in Italy--first in vitro isolation of Besnoitia besnoiti from cattle born in Italy. Vet. Parasitol. 2012;184(2–4):108–115. doi: 10.1016/j.vetpar.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Han B.A., Schmidt J.P., Bowden S.E., Drake J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA. 2015;112(22):7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M.S., Barr B.C., Marsh A.E., Anderson M.L., Rowe J.D., Tarantal A.F., Hendrickx A.G., Sverlow K., Dubey J.P., Conrad P.A. Identification of bovine Neospora parasites by PCR amplification and specific small-subunit rRNA sequence probe hybridization. J. Clin. Microbiol. 1996;34(5):1203–1208. doi: 10.1128/jcm.34.5.1203-1208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok S., Mester A., Takács N., Baska F., Majoros G., Fok É., Biksi I., Német Z., Hornyák Á., Jánosi S., Farkas R. Sarcocystis-infection of cattle in Hungary. Parasites Vectors. 2015;4:69. doi: 10.1186/s13071-015-0685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Sun J., Guo Y., Zeng H., Zhang Y., Tao J. Infection of the asian gray shrew Crocidura attenuata (insectivora: soricidae) with Sarcocystis attenuati n. sp. (Apicomplexa: Sarcocystidae) in China. Parasite. Vector. 2022;15:13. doi: 10.1186/s13071-021-05136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme-Beaman A., Dobney K., Cucchi T., Searle J.B. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol. Evol. 2016;31(8):633–645. doi: 10.1016/j.tree.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Ellis J.T., Liddell S., Ryce C., Munday B.L., Morrison D.A., Dubey J.P. The relationship of Hammondia hammondi and Sarcocystis mucosa to other heteroxenous cyst-forming coccidia as inferred by phylogenetic analysis of the 18S SSU ribosomal DNA sequence. Parasitology. 1999;119:135–142. doi: 10.1017/s0031182099004618. [DOI] [PubMed] [Google Scholar]
- Johnson P.T., Dobson A., Lafferty K.D., Marcogliese D.J., Memmott J., Orlofske S.A., Poulin R., Thieltges D.W. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 2010;25(6):362–371. doi: 10.1016/j.tree.2010.01.005. https://doi-org.ezproxy.unibo.it/10.1016/j.tree.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Kaushik M., Knowles S.C., Webster J.P. What makes a feline fatal in Toxoplasma gondii's fatal feline attraction? Infected rats choose wild cats. Integr. Comp. Biol. 2014;54(2):118–128. doi: 10.1093/icb/icu060. [DOI] [PubMed] [Google Scholar]
- Klegarth A.R. vols. 1–5. 2016. (Synanthropy. The International Encyclopedia of Primatology). [DOI] [Google Scholar]
- Krajcarz M., Krajcarz M.T., Baca M., Golubiński M., Bielichová Z., Bulatović J., Csippán P., Dimitrijević V., Kyselý R., Makowiecki D. The history of the domestic cat in Central Europe. Antiqua. 2022;1–6 doi: 10.15184/aqy.2022.128. [DOI] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberton P.H.L., Donnelly C.A., Webster J.P. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008;135:1143–1150. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- Luis A.D., Hayman D.T.S., O'Shea T.J., Cryan P.M., Gilbert A.T., Mills J.N., Timonin M.E., Willis C.K.R., Cunningham A.A., Rupprecht C.E., Wood J.L.N., Webb C.T. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proceed. Royal Soc. Biol. Sci. 2013;280:1–9. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Médoc V., Beisel J.N. When trophically-transmitted parasites combine predation enhancement with predation suppression to optimize their transmission. Oikos. 2011;120:1452–1458. [Google Scholar]
- Mendoza Roldan J.A., Otranto D. Zoonotic parasites associated with predation by dogs and cats. Parasites Vectors. 2023;16(1):55. doi: 10.1186/s13071-023-05670-y. https://doi-org.ezproxy.unibo.it/10.1186/s13071-023-05670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A., Garba M., Bonnabau H., Kane M., Rossi J.P., Dardé M.L., Dobigny G. Toxoplasmosis seroprevalence in urban rodents: a survey in Niamey, Niger. Mem. Inst. Oswaldo Cruz. 2013;108(4):399–407. doi: 10.1590/S0074-0276108042013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugridge N.B., Morrison D.A., Johnson A.M., Luton K., Dubey J.P., Votýpka J., Tenter A.M. Phylogenetic relationships of the genus Frenkelia: a review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999;29:957–972. doi: 10.1016/s0020-7519(99)00062-4. [DOI] [PubMed] [Google Scholar]
- Prakas P., Kirillova V., Gavarāne I., Grāvele E., Butkauskas D., Rudaitytė-Lukošienė E., Kirjušina M. Morphological and molecular description of Sarcocystis ratti n. sp. from the black rat (Rattus rattus) in Latvia. Parasitol. Res. 2019;118:2689–2694. doi: 10.1007/s00436-019-06393-9. [DOI] [PubMed] [Google Scholar]
- Rubiola S., Civera T., Ferroglio E., Zanet S., Zaccaria T., Brossa S., Cipriani R., Chiesa F. Molecular differentiation of cattle Sarcocystis spp. by Multiplex PCR targeting 18S and COI genes following identification of Sarcocystis hominis in human stool samples. Food Waterborne Parasitol. 2020;18 doi: 10.1016/j.fawpar.2020.e00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaitytė-Lukošienė E., Jasiulionis M., Balčiauskas L., Prakas P., Stirkė V., Butkauskas D. Morphological and molecular description of Sarcocystis myodes n. sp. from the bank vole (Clethrionomys glareolus) in Lithuania. Biology. 2022;1:512. doi: 10.3390/biology11040512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Globokar Vrhovec M., Tuschy M., Joeres M., Bärwald A., Koudela B., Dubey J.P., Maksimov P., Conraths F.J. A real-time quantitative polymerase chain reaction for the specific detection of Hammondia hammondi and its differentiation from Toxoplasma gondii. Parasit vectors. 2021;14:78. doi: 10.1186/s13071-020-04571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Pantchev N., Barutzki D., Heydorn A.O., Bauer C., Conraths F.J. Oocysts of Neospora caninum, Hammondia heydorni, Toxoplasma gondii and Hammondia hammondi in faeces collected from dogs in Germany. Int. J. Parasitol. 2005;35(14):1525–1537. doi: 10.1016/j.ijpara.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Schares G., Vrhovec M.G., Pantchev N., Herrmann D.C., Conraths F.J. Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet. Parasitol. 2008;152(1–2):34–45. doi: 10.1016/j.vetpar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Seppälä O., Valtonen E.T., Benesh D.P. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc Royal Soc B. 2008;275:1611–1615. doi: 10.1098/rspb.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura K., Ito S. Goats as natural intermediate hosts of Hammondia hammondi. Zbl. Bakt. Hyg. A. 1987;264(3–4):348–352. doi: 10.1016/s0176-6724(87)80055-x. [DOI] [PubMed] [Google Scholar]
- Sreekumar C., Vianna M.C., Hill D.E., Miska K.B., Lindquist A., Dubey J.P. Differential detection of Hammondia hammondi from Toxoplasma gondii using polymerase chain reaction. Parasitol. Int. 2005;54(4):267–269. doi: 10.1016/j.parint.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Vangeel L., Houf K., Chiers K., Vercruysse J., D'Herde K., Ducatelle R. Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J. Food Protect. 2007;70:1523–1526. doi: 10.4315/0362-028x-70.6.1523. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Di Palma S., Gabrielli S., Morganti G., Milardi G.L., Middleton B., Lepri E. Sarcocystis gigantea infection associated with granulomatous eosinophilic myositis in a horse. J. Vet.: Diagn. 2020;32(4):611–615. doi: 10.1177/1040638720935847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votýpka J., Hypsa V., Jirků M., Flegr J., Vávra J., Lukes J. Molecular phylogenetic relatedness of frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula stiles 1893: is the genus Sarcocystis paraphyletic? J. Eukaryot. Microbiol. 1998;45:137–141. doi: 10.1111/j.1550-7408.1998.tb05081.x. [DOI] [PubMed] [Google Scholar]
- Vyas A., Kim S.K., Giacomini N., Boothroyd J.C., Sapolsky R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J.P. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr. Bull. 2007;33(3):752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J.P. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology. 1994;109:583–589. doi: 10.1017/s0031182000076460. [DOI] [PubMed] [Google Scholar]
- Webster J.P. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microb. Infect. 2001;3:1–9. doi: 10.1016/s1286-4579(01)01459-9. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Brunton C.F.A. MacDonald DW. Effect of Toxoplasma gondii on neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994;109:37–43. doi: 10.1017/s003118200007774x. [DOI] [PubMed] [Google Scholar]
- Zeng H., Guo Y., Ma C., Deng S., Hu J., Zhang Y. Redescription and molecular characterizationof Sarcocystis cymruensis from Norway rats (Rattus norvegicus) and Sarcocystis ratti from black rats (R. rattus) in China. Parasitol. Res. 2020;119:3785–3791. doi: 10.1007/s00436-020-06883-1. [DOI] [PubMed] [Google Scholar]